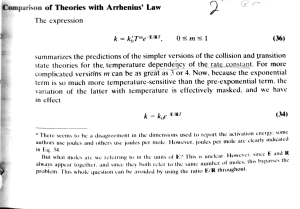
5 2 0 1 4 – S e t D C A T B SECTION 5: SCIENCE PROFICIENCY NAME: ____________________________ Time -– 25 minutes SCHOOL: _________________________ 50 Questions (181 – 230) SIMULATED EXAM – SET B 5 For each item, decide which the best answer choice is, and darken the corresponding circle on the answer sheet. 181. Assume that an Rh- mother has given birth to an 184. In which of the following vessels does most of Rh+ baby and was not given RhoGAM. When the exchange of nutrients and waste products might hemolytic disease of the newborn occur? between the blood and body tissues happens? A. Hemolytic disease of the newborn will never A. arteries B. occur B. At the birth of the first Rh- baby C. veins D. lymphatic vessels C. All pregnancies will show hemolytic disease E. D. During development of the next Rh+ baby E. Hemolytic disease will never occur to an Rh+ binding sites for muscular contraction? A. actin 182. In a certain characteristic of garden peas, tall is B. dominant over short. If two heterozygous plants crossed, what percentage of the D. troponin E. A. 0% 25% of food? D. 75% A. Absorption 100% B. D. Peristalsis blood group? E. A. Type A blood has Type A antigen and Type B antibody. Segmentation 187. Which organelle is found in animal cells but not Type A blood has Type B antigen and Type B in plant cells? antibody. A. ribosomes C. Type A blood has Type A antigen and Type B, B. O antibodies. mitochondria C. nuclei D. Type A blood has Type B, O antigens and D. plastids Type A antibody. E. Digestion C. Mastication 183. Which among the following is TRUE in the ABO B. ATP 186. What process involves the enzymatic breakdown C. 50% E. myosin C. calcium next generation will be homozygous dominant? B. renal tubules 185. Which substance is released in order to expose baby from Rh- mother is normal. are capillaries E. Type A blood has both Type A antigens and centrioles antibody. ACTS ACHI & AHIA, INC. 5-1 744-6250 / 701-7018 5 2 0 1 4 – S e t D C A T 188. After telophase II of meiosis, the chromosomal B 5 192. DNA is to _____ as proteins is to _____ . makeup of each daughter cell is _____. A. nucleus . . . amino acid A. diploid, and the chromosomes are each B. B. nucleotides . . . amino acid composed of a single chromatid. C. nitrogenous bases . . . peptide diploid, and the chromosomes are each D. nucleus . . . peptide composed of two chromatids. E. RNA . . . genotype C. haploid, and the chromosomes are each composed of a single chromatid. 193. In a particular plant, leaf color is controlled by D. haploid, and the chromosomes are each E. gene locus D. Plants with at least one allele D composed of two chromatids. have dark green leaves, and plants with the haploid, and the chromosomes are each homozygous recessive dd genotype have light composed of half of the chromatid green leaves. A true-breeding dark-leaved plant is crossed with a light-leaved one, and the F1 189. What is the important animal hormone offspring is allowed to self-pollinate. The responsible for the control of metabolic functions predicted outcome of F2 is diagrammed in the in the blood particularly for iodine synthesis? Punnett square shown in the figure below where A. thyroxine 1, B. corresponding to each box within the square. cortisol 2, 3, and 4 represent the genotypes C. prostaglandin D d D 1 2 d 3 4 D. insulin E. parathormone 190. What phylum is commonly known as round worms? Which of the boxes marked 1-4 correspond to A. Mollusca B. plants with homozygous genes? Echinodermata A. 1 and 2 C. Platyhelminthes B. D. Nematoda E. C. 3 and 4 Porifera D. 1 and 4 E. 191. A sexually reproducing animal has two unlinked genes, one for head shape (H) and one for tail functioning together make up organs. Which of following parent genotypes is a possible gamete the following is composed of different organs? to form this organism? A. organelles A. Hhtt x hhtt B. HhTT x HHTT D. organisms D. HHTT x HHTt E. hhTt x hhtt ACTS organ membranes C. organ system C. HhTt x HhTt E. 2, 3 and 4 194. Tissues are composed of cells, and tissues length (T). Its genotype is HhTt. Which of the B. 2 and 3 ACHI & AHIA, INC. 5-2 cytoplasm 744-6250 / 701-7018 5 2 0 1 4 – S e t D C A T 195. Which part of the flower will develop into seeds? B 5 200. Two hundred milliliters of water is mixed with A. stigma 720 grams of HCl. What is the molarity of the B. solution if the density of water is 1.00 grams per stamen C. ovary milliliter? (Cl= 35 amu, H=1 amu) D. ovule A. 100 M E. B. anther 0.278 M C. 0.1 M 196. Matter consists of _____. D. 129.6 M A. atom B. E. 20 M molecule C. cell 201. Consider the following reaction: D. organelle E. AlCl3 + Ca(OH)2 Al(OH)3 + CaCl2 tissue How many moles of aluminum hydroxide is produced when 10 moles of aluminum chloride is 197. Matter is defined as anything that has _____. reacted to 12 moles of calcium hydroxide? A. mass and shape A. 10 moles B. B. mass and density 8 moles C. mass only C. 20 moles D. mass and volume D. 12 moles E. E. volume and energy 198. If 25 mL of water at 40C is added to 100 mL of water at 50C, what would be the 4 moles 202. What are the representative elements in the final periodic table? temperature of the mixture? A. Elements that belong to IIIB to IIB. A. 56C B. B. 48C Elements that have last sublevel configuration of s or p. C. 42C C. Elements that have f sublevel configuration. D. 44C D. Elements that belong to IA-VIIIA and IIIB to E. 88C IIB. E. 199. Thirty liters of gas X at 100 K under 2 atm was Elements that have sublevel configuration of s, p, d and f blocks transferred to a certain container where the temperature is doubled and the pressure is 203. Which of the following is a double displacement increased by 4 atm. What is the mass of the gas reaction? if its density at the new container is 0.01 g/L? A. 2HCl + Mg MgCl2 + H2 A. 20 g B. B. C. NaOH + HCl NaCl + H2O 10 g 2AlPO4+ 3Ca Ca3(PO4)2+ 2Al C. 0.2 g D. 2KClO3 2KCl + 3O2 D. 0.05 g E. E. CH4 + 2O2 CO2 + 2H2O 2000 g ACTS ACHI & AHIA, INC. 5-3 744-6250 / 701-7018 5 2 0 1 4 – S e t D C A T 204. How much water must be added to 300 mL of B 208. Given the following: 0.75 M HCl to dilute the solution to 0.25 M HCl I. solution? II. A. 100 mL B. III. 300 mL C. 600 mL IV. D. 900 mL V. E. 1200 mL Which of the following pairs of element is an 205. Copper has two isotopes, 63Cu and 65 Cu. isotope? If the A. I and II atomic mass of copper is 63.5 g/mol, what is the B. % of each isotope? A. 25.0% 63Cu and 75.0% 65Cu B. 33.0% 63Cu and 67.0% 65Cu C. 50.0% 63Cu and 50.0% 65Cu D. 67.0% 63Cu and 33.0% 65Cu E. 63Cu and 25.0% 65Cu 75.0% D. III and IV E. A. 7 protons and 3 electrons B. D. 10 protons and 7 electrons E. A. 2 4 4 protons and 7 electrons 210. Which of the following equations is not balanced C. 6 correctly? D. 7 A. P4 +3O2 -->P4O6 8 B. E. grams of oxygen react to form water? ⃗⃗⃗⃗⃗⃗⃗⃗⃗⃗⃗⃗⃗⃗⃗⃗⃗⃗⃗⃗⃗⃗⃗ 2KCl + 3O2 D. 2KClO3 chemical reaction if 4 grams of hydrogen and 32 2H2 + O2 3CuCl2 + V2O3 3CuO + 2VCl3 C. Cu(NO3)2 -->CuO + 2NO2 +O2 207. What is the limiting reagent for the following 2H2O 2Al2O3 +3C+6Cl2 -->4AlCl3 +3CO2 211. Which of the following is a definition of a base? A. hydrogen A. It is a proton donor. oxygen B. C. water It turns blue litmus paper to red. C. It is usually formed when a metal oxide D. this reaction has no limiting reagent E. 7 protons and 4 electrons C. 7 protons and 10 electrons C4H6 + O2 XCO2 + H2O B. IV and V 209. A nitride ion N3- (Z=7) has _____. equation would be balanced? E. I and III C. II and III 206. What is the value of X in the reaction below if the B. 5 reacts with water. electricity D. Its pOH is above 7. E. ACTS ACHI & AHIA, INC. 5-4 It is an electron pair acceptor. 744-6250 / 701-7018 5 2 0 1 4 – S e t D C A T 212. The periodic trend that refers to the ability of an atom to attract electrons during B 216. A vector quantity has magnitude and direction. chemical Which of the following is a vector quantity? bonding is _____. A. speed A. electronegativity B. B. C. work electron affinity gravitational pull C. atomic radius D. distance D. ionization energy E. E. 5 temperature radioactivity 217. Given 213. A graduated cylinder has a mass of 80.0 g when Vector A = 15 units, west empty. When 20.0 mL of water is added, the Vector B = 8 units, north graduated cylinder has a mass of 100. g. If a stone is added to the graduated cylinder, the Find the magnitude of the resultant vector. water level rises to 45.0 mL and the total mass is A. 7 units now 156 g. What is the density of the stone? B. A. 2.24 g/mL C. 23 units B. D. 24 units 3.47 g/mL C. 1.56 g/mL E. 17 units 25 units D. 2.60 g/mL E. 218. A ball was thrown vertically upward with a speed 7.80 g/mL of 5 m/s. When the ball went back to where it 214. Which of the following is an expected property of was thrown, what was its displacement? sugar (C6H12O6)? A. 10 meters A. It does not turn to black when heated. B. B. C. zero It has melting point greater than 100C. 5 meters, upward C. It is insoluble in water at 35C. D. 5 meters, downward D. Chemical change occurs when it is heated at E. half a meter 80C. E. 219. A projectile is launched at a certain angle with It can be filtered when dissolved in alcohol. the ground at a speed of 20 meters per second. 215. An atom has 7 electrons in its outer shell. How Neglecting air resistance, what is the speed of many unpaired electrons does it have? the projectile when it hits the ground? A. 0 A. 9.8 meters per second B. B. 1 10 meters per second C. 2 C. zero D. 4 D. 20 meters per second E. E. 6 ACTS ACHI & AHIA, INC. 5-5 25 meters per second 744-6250 / 701-7018 5 D C A T 2 0 1 4 – S e t 220. If acceleration is the rate of change in velocity, 224. Which of B the 5 following describes electrical what is the rate of change in position? current? A. Velocity A. the electric potential difference between two B. Distance points in a circuit C. Force B. D. Displacement C. the flow of protons through a material E. D. the movement of voltage Speed E. 221. A golf ball, initially resting on the tee, was hit by the flow of electric charge through a material the movement of electric field through a conductor a club. Where in its path, did the golf ball have maximum kinetic energy? 225. Refer to the circuit below. What would be the A. Just right after it was hit by the club B. reading in the ammeter? A few seconds after it was hit by the club C. On the way up D. On the way down E. 3.0 v At the highest point 1.5 Ω 1.5 Ω A 222. Two objects, X and Y, are being pulled at the same speed. Object X weighs 500 kg and object A. 3.0 amperes Y weighs 2000 kg. What is the ratio of the kinetic B. energy of object Y to the kinetic energy of object C. 4.5 amperes X? D. 4.0 amperes A. 1:1 E. B. 1.5 amperes 1.0 ampere 2:1 C. 4:1 226. What power is supplied to the starter motor of a D. 9:1 car that draws 240 amperes of current from a E. 12-volt battery? 16:1 A. 2.0 101 watts 223. A 50-kilogram girl lifts a 1-kilogram object B. 5.4 103 watts vertically upward to a height of 0.5 meters. How C. 3.5 103 watts much work is done by the girl in lifting the D. 6.0 101 watts object? E. 4.5 103 watts A. 5 joules B. 50 joules 227. A wave that moves from left to right, creating an C. 250 joules up and down displacement of the medium, is a D. 10 joules A. longitudinal wave. E. B. 500 joules transverse wave. C. mechanical wave. D. electromagnetic wave. E. ACTS ACHI & AHIA, INC. 5-6 sound wave. 744-6250 / 701-7018 5 2 0 1 4 – S e t D C A T 228. Refer to the figure below. A ray of light passes 230. In B uniform 5 circular motion, what is the through three different media. Which of the relationship between acceleration and velocity? following is correct about the speed of the light A. Acceleration in these media? is directly proportional to is inversely proportional to velocity. B. Acceleration velocity. medium 1 C. Acceleration is directly proportional to the square of the velocity medium 2 D. Acceleration is inversely proportional to the square of the velocity medium 3 E. Acceleration is directly proportional to the square root of the velocity A. v1 > v2 > v3 B. v2 > v1 > v3 C. v2 > v3 > v1 D. v3 > v2 > v1 E. v3 > v1 > v2 229. A piece of lead tied by string to a spring scale weighs 63 N. When the piece of lead is completely submerged in a certain liquid, the reading on the spring scale is 57 N. What would the reading in the spring scale be if the liquid were replaced by a twice-as-dense liquid? A. 51 N B. 114 N C. 126 N D. 54 N E. 60 N ACTS ACHI & AHIA, INC. 5-7 744-6250 / 701-7018