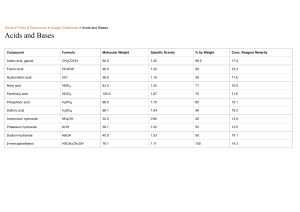
Acid, Bases and Salts CLASS - 10th ONE SHOT 1 What you will get ❖ Premium Lectures ❖ Class notes ❖ PYQs (chapterwise) ❖ Competency based questions Acid bases and salts 3 What is an Acid An acid is a hydrogen containing substance that is capable of donating a proton (hydrogen ion) to another substance. Acidic solution turns blue litmus paper into red. Examples - HCl and HNO3 Apart from the strong acid in our stomach, human bodies are known to produce lactic acid while exercising. What is a Base Bases are the chemical substances which have a bitter taste, are soapy to touch and turn red litmus blue. It dissolves in water to produce hydroxide ions (OH-) in solutions. Basic solution turns red litmus paper into blue. Examples - NaOH → Na(aq) + OH- What are Alkalis Bases that are soluble in water are called Alkalis. Examples - Sodium hydroxide (NaOH), Potassium hydroxide (KOH), Calcium hydroxide [Ca(OH)2]. What are Salts Salts are produced due to the reaction between acids and bases. Chemical properties of acids and bases Acid + Bases → Salt + Water Acid + Metal Acid + Metal Oxide Acid + Metal Carbonate/Metal bicarbonate Chemical properties of acids and bases Acid + Bases → Salt + Water Base + Metal Base + Non - Metal Oxide Reaction of Acid and Base with metals Acid + Metal → Salt + Hydrogen gas EXAMPLES Zn(s) + H2SO4 (aq) → ZnSO4(aq)+ H2 (g) NaOH(aq) + Zn(s) → Na2ZnO2(aq)+ H2(g) Reaction of Metal carbonates and hydrogen carbonates with acids Acid + Metal Carbonate → Salt + Water + Carbon dioxide EXAMPLES Na2CO3(s) + 2HCl(l) → 2NaCl(aq) + H2O + CO2 NaHCO3(s) + HCl(l) → NaCl(aq) + H2O + CO2 Reaction of Metal Oxides with acids The general reaction between a metal oxide and an acid can be written as: Metal Oxide + Acid → Salt + Water EXAMPLES CuO(s) + 2HCl (l) → CuCl2 (aq) + H2O Reaction of Non-Metallic Oxide with bases Non-metal oxides are acidic in nature. When a base reacts with non-metal oxide, both neutralize each other resulting respective salt and water are produced Base + Non-Metal Oxide → Salt + Water EXAMPLES Ca(OH)2(aq) + CO2 (g) → CaCO3 (s) + H2O(l) Do acids and bases have something in common? Both acids and bases are electrolytes which means that they are good conductors of electricity. Acids and bases both produce ions in water solution Acids release hydrogen ions (H+) whereas Bases release hydroxide ions (OH-). The process of mixing acid or base in water is an exothermic one. What happens to an acid or a base in a water solution? Acids in water solution dissociate (H+) ions. Base, when dissolved in water, produce (OH-) ions. Strong and weak Acids/Bases Strong Acid/Base Strong Acid/Base Strong Acid/Base Strong Acid/Base Types of Bases Strong Bases A base which completely ionizes in water thus produce a large amount of hydroxyl ions is called a strong base. Weak Bases A base which is partially ionized in water thus produce a small amount of hydroxyl ions is called a weak base. Strength The solution is considered acidic if the pH of the solution is less than 7 ; the solution is neutral if the pH is around 7 ; if the pH is greater than 7, the solution is called basic. The abundance of hydrogen ions in an acidic solution, then, is greater than that of hydroxide ions. Indicators Those chemical substances which help to detect nature of other chemicals. Example - Litmus solution Examples Indicators Acids Bases Red litmus Remains red Turns blue Blue litmus Turns red Remains blue Turmeric No changes Red Phenolphthalein Colorless Pink Methyl orange Red Yellow Olfactory indicators Those substances whose odour changes in acidic or basic medium are called olfactory indicators. Example - Vanilla, onion clove ( smell of onion and vanilla diminishes in a base and remains as it it in an acid). Universal indicators Those substances which not only detect nature of other chemicals, but also determine their acidic or basic strengths. Example - pH paper Importance of pH in everyday life INTERESTING FACT Rainwater is slightly acidic in nature as in the acid dissolves in the rainwater to form carbonic acid Neutralisation reaction Acid + Bases → Salt + Water Strong Acid + Strong Base → Neutral Salt + Water Strong Acid + Weak Base → Acidic Salt + Water Weak Acid + Strong Base → Basic Salt + Water pH of Salts Salts of a strong acid and a strong base are neutral with pH value of 7. On the other hand, salts of a strong acid and weak base are acidic with pH value less than 7 and those of a strong base and weak acid are basic in nature, with pH value more than 7. Some Important Salts Common salt Bleaching powder Baking soda Washing soda POP (Plaster of Paris) Common salt When electricity is passed through an aqueous solution of sodium chloride ( called brine), it decomposes to form Sodium hydroxide. The process is called the chlor-alkali process. 2NaCl(s) + 2H2O(l) → 2NaOH(aq) + Cl2 + H2 Uses of Sodium hydroxide Sodium hydroxide in its pure form is a white crystalline solid. It is odourless. 2NaCl(s) + 2H2O(l) → 2NaOH(aq) + Cl2 + H2 Uses ● Preparation of soaps and detergents. ● Paper making. ● Used in artificial fibres. Uses of Hydrogen ● Used as fuel. ● Used in margarine. ● Ammonia for fertilisers. Uses of Chlorine ● Used in water treatment. ● Swimming pools. ● PVC, Disinfectants, CFCs, Pesticides Uses of Hydrochloric acid (HCl) ● Cleaning steel ● Ammonium chloride ● Medicines, Cosmetics Uses of Bleach ● Household bleach ● Bleaching clothes Bleaching powder The chemical nature of bleaching powder is calcium oxychloride. Ca(OH)2 + Cl2 → CaOCl2(aq) + H2O Uses ● It is used for bleaching cotton. ● It is used as an oxidizing agent in chemical industries. ● It can be used for disinfection of water. Baking Soda - (NaHCO3) The chemical nature of baking soda is sodium bicarbonate. It is produced on a large scale by treating cold and concentrated solution of sodium chloride with ammonia and carbon dioxide. NaCl + H2O + CO2 + NH3 → NH4Cl + NaHCO3 On heating, it decomposes to give sodium carbonate with the evolution of carbon dioxide. NaHCO3 → Na2CO3 + H2O + CO2 Baking Soda - (NaHCO3) Uses ● Used as an antacid to treat acidity in stomach. ● Used to make baking powder, which is used in preparation of cakes, breads etc. Washing Soda - (Na2CO3.10H2O) The chemical nature of bleaching powder is sodium carbonate. Sodium bicarbonate, on heating decomposes to give sodium carbonate with the release of hydrogen gas. 10H2O Na2CO3 → Na2CO3.10H2O Uses ● Used in glass, soap and paper industries. ● Employed in the manufacture of sodium compounds such as borax. ● Used to remove permanent hardness of water. Plaster of Paris - (CaSO4.1/2H2O) The chemical nature of POP is Calcium sulphate hemihydrate. POP is prepared by heating gypsum at 373 K. On heating, it loses water molecules and becomes calcium sulphate hemihydrate. Uses ● ● ● It is used to fix gaps in the walls/roofs of buildings/houses. Used in making casting for several ornaments as well as decorative material. Used in designing products for fire protection system. Water of Crystallization ● The water of crystallization means having a constant range of molecules existing in one unit of salt. ● Crystal salts with water of crystallization are recognized as hydrates. ● The different names of water crystallization are crystallization water or water of hydration.