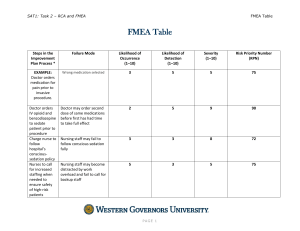
IE 5356 Biomedical Innovation Failure Mode and Effects Analysis (FMEA) Instructor: Dr. George Z. Tan Medical Device Failure – The Bleeding Edge (2018) Failure Mode and Effects Analysis (FMEA): What Begun in the 1940s by the U.S. military, failure modes and effects analysis (FMEA) is a step-by-step approach for identifying all possible failures in a design, a manufacturing or assembly process, or a product or service. •Failure modes - the ways, or modes, in which something might fail. Failures are any errors or defects, especially ones that affect the customer, and can be potential or actual. •Effects analysis - studying the consequences of those failures. FMEA: Why • The purpose of the FMEA is to take actions to eliminate or reduce failures, starting with the highest-priority ones. • Failure modes and effects analysis also documents current knowledge and actions about the risks of failures, for use in continuous improvement. • Ideally, FMEA begins during the earliest conceptual stages of design and continues throughout the life of the product or service. FMEA: When • When a process, product, or service is being designed or redesigned, after quality function deployment (QFD) • When an existing process, product, or service is being applied in a new way • Before developing control plans for a new or modified process • When improvement goals are planned for an existing process, product, or service • When analyzing failures of an existing process, product, or service • Periodically throughout the life of the process, product, or service FMEA: When The Risk categories during the lifecycle of medical device product FMEA: How 1. 2. 3. 4. 5. Break down the system, design, or process into its component elements. Review each element systematically and uncover all possible ways it could fail. Analyze the effects and likely causes of each failure. Rank each potential effect based on user-defined risk criteria. Decide how to detect, eliminate, or minimize the most critical risks. Then, outline a plan of recommended actions to implement to reduce the risks of failure effects. 6. Return to the original risk levels and revise them based on the completed action items in the plan to ensure that the risk goals have been met. The Process of Risk Management FMEA: Key Terms RISK - combination of the probability of occurrence of harm and the severity of that harm HAZARD - potential source of harm HAZARDOUS SITUATION - circumstance in which people, property, or the environment are exposed to one or more hazard(s) HARM - physical injury or damage to the health of people, or damage to property or the environment SEVERITY - measure of the possible consequences of a hazard Risk Factors ◦ Probability of occurrence ◦ Range of possible outcomes (impact or amount at stake ◦ Expected Timing of event ◦ Anticipated frequency of risk events from that source FMEA: Risk Metrics Diagram ● If the potential risk event appears in the critical region or high region, it should be prevented without any care about the cost. ● If the potential risk event appears in the medium region, it should be prevented at a reasonable cost. ● If the potential risk event appears in the low region, it should be prevented following some reasonable steps or some measures should be taken to reduce its negative impacts. Risk Priority Number Three groups of data can be got through FMEA process Severity (S) An assessment of the significance of the failure mode’s effect on item operation Occurrence (O) or Probability (P) The frequency or probability of occurrence of each failure mode Detection (D) The risk that the failure will escape detection. RPN (risk priority number) is a product of these three groups of data RPN=S×O×D Risk Priority Number Score Occurrence Severity Detection 1 Very unlikely to occur Very low—will not affect the process Certain—fault will be caught on test 2/3 Unlikely to occur Low—may affect the process High 4/5 May occur about half of the time Medium—slightly affect the process Moderate 6/7/8 Likely to occur High—mostly affect the process Low 9/10 Very likely to occur Very high—definitely affect the process Fault will be passed to customer undetected The Major Process of FMEA Method Diagramming Techniques Cause and Effect Diagrams ◦ Also known as Ishikawa or fishbone Potential Causes Testing Inadequate Time Effect Project Prioritization Product Delivered Late Personnel Materials Insufficient Resources Bad Specs FMEA Example 15 Risk Register List of ◦ Identified risks ◦ Potential responses ◦ Root causes Updated risk categories (if required) Probability and Impact Plots Rate each risk on scales then plot on matrix Develop mitigation technique for risks above tolerance Add Probability and Impact Matrix results Perform quality check on results Categorize the risks to make them easier to handle Perform urgency assessment to determine which risk need immediate attention Risk Control Overall Strategies Negative Risks (or Threats) ◦ Avoid ◦ Transfer ◦ Mitigate ◦ Acceptance Positive Risks (or Opportunities) ◦ Exploit ◦ Share ◦ Enhance ◦ Acceptance Summary 20