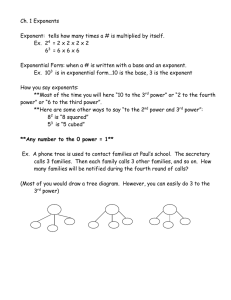
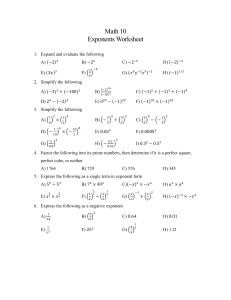
Lesson 12.2 Rate Laws Suggested Reading Zumdahl Chapter 12 Sections 12.2 & 12.3 Essential Question What is a rate law? Learning Objectives: Analyze data from rate experiments. Determine rate laws and rate constants. Review & Preview: Watch the following IsaacsTeach video to review reaction rates and preview rate laws. Watch the following YouTube Video: https://www.youtube.com/watch?v=1jF6yOzZbws The Rate Law By now you know that reaction rate depends on concentration. Consider the reaction of nitrogen dioxide with fluorine to give nitryl fluoride. 2NO2(g) + F2(g) → 2NO2F(g) The rate of reaction is observed to be proportional to the concentration of nitrogen dioxide. Thus, when the concentration of nitrogen dioxide is doubled, the rate doubles. A rate law is an equation that relates the rate of a reaction to the concentration of reactants raised to various powers. The following is the rate law for the previous reaction: Rate = k[NO2][F2] In this rate law, both reactant concentrations have an exponent of 1. Please not the exponents are not related to the coefficients in the balanced chemical equations. Consider the reaction of substances A and B to give D and E, according to the equation aA + bB → cC + dD You could write the rate law in the form Rate = k[A]m[B]n The exponents are often, but not always, integers. They must be determined experimentally and they cannot be obtained from the chemical equation. Once you know the rate law for a reaction and have found the value of the rate constant, you can calculate the rate of a reaction for any values of reactant concentrations. The Rate Constant The rate constant, k, is a proportionality constant in the relationship between rate and concentrations. It has a fixed value at any given temperature. but it varies with temperature. Whereas the units of rate are usually given as M/s, the units of k depend on the form of the rat law. For the previous rate law, Rate = k[NO2][F2], from this you get the following units for k: You are going to need your dimensional analysis skills to study chemical kinetics! Reaction Order In chemical kinetics reactions are classified by their orders. The reaction order with respect to a given reactant equals the exponent from the rate law. For the reaction of nitrogen dioxide with fluorine to give nitryl fluoride, with the rate law, Rate = k[NO2][F2]. The reaction is first order with respect to nitrogen dioxide because the exponent of [NO2] in the rate law is 1. Similarly, the reaction is first order with respect to F2. The overall order of the reaction equals the sum of the orders of the reactants in the rate law. In the example above, the overall order is 2, and we say that the reaction is second order overall. Reactions display a variety of orders. Lets look at some examples. Example 1: When cyclopropane is heated the carbon ring opens up giving propylene, C3H6(g) + Heat → CH2CHCH3(g). This reaction has the following experimentally determined rate law, rate = k[C3H6]. The reaction is first order in cyclopropane and first order overall. Example 2: Nitric oxide, NO, reacts with hydrogen according to the following equation, 2NO(g) + 2H2(g) → N2(g) + 2H2O(g). The rate law is rate = k[NO]2[H2]. Thus, the reaction is second order with respect to NO, first order with respect to H2, and third order overall. Example 3: Acetone, CH3COCH3, reacts with iodine in acidic solution, CH3COCH3(aq)+ I2(aq) + H+ → CH3COCH2I(aq) + HI(aq). The rate law is rate = k[CH3COCH3][H+]. The reaction is first order in acetone, It is zero order in iodine, which means it contains the factor [I2]0 = 1. Therefore, the rate law does not depend on the concentration of iodine, as long as some iodine is present. The reaction is first order in H+, which is a catalyst in this reaction. When catalysts are present, they speed up the rate of reaction and therefore must be included in the rate law. The reaction is second order overall. Determining the Rate Law In order to determine the rate law by experiment, you must determine the order of each reactant and any catalyst. The initial rate method is a simple way to obtain reaction orders. If we are given data from two or more experiments at the same temperature with different concentrations of reactants and different rates we can determine the exponents in the rate law for the reaction as follows: 1. Write the rate law with the concentrations of all species in the equation. Write the coefficients as unknowns: n, m, etc. For example, we might have an equation such as, A + B → C, with the rate law.: 2. Take ratios of the experimental data that give different rates. 3. Cancel common terms and solve for the exponent that does not cancel. An example will help make this clearer. Example: Determining the rate law from initial rates. If we have the following experimental initial rate data for the reaction, A + B → 2C. [A], M [B], M experiment rate = -d[A]/dt 1 0.50 0.50 1.2 2 1.0 0.50 4.8 3 2.0 1.0 38.4 We can write ratios for the data from experiments 1 and 2 Using the data from experiments 1 and 2, we see that the k's cancel as do the concentrations of species B. Solving this equation gives 0.25 = 0.5n , so n = 2 since 0.52 = 0.25 Now we use the known value of n and data from experiments 1 and 3 or from experiments 2 and 3 and solve for m. Here we use experiments 1 and 3: When we substitute the data we get: 0.031 = 0.06 x 0.5m 0.5 = 0.5m , so m = 1 since 0.51 = 0.5 Now that we know the exponents we can write the rate law can be written: HOMEWORK: Finish practice exercises assigned in lesson 12.1 (practice exercises 14.1-14.8)