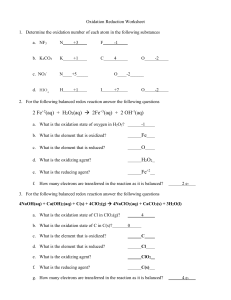
FSC 112-Introductory Chemistry Dr K.O. Abdulwahab 1 Solutions • A solution is a mixture solute (s) and a solvent. • The amount of solute present in a solution is usually expressed as concentration. • The different ways of expressing concentration include:- Mass percent, molality, molarity, mole fraction etc 2 Solution Concentration • Molarity(M): Moles solute/1L solution • Molality (m): Moles solute/1kg solvent • Mole fraction (XA): Moles A* total moles solution • Mass percent: Mass solute total mass of solution x 100 *In some applications, one needs the mole fraction of solvent, not solute. Make sure you find the quantity you need! Concentration Units Continued Molarity (M) M = moles of solute liters of solution Molality (m) m = moles of solute mass of solvent (kg) 4 Molarity • The most commonly used expression of concentration is the molarity (M) • This is defined as :- • The unit is moldm-3 5 Example on Molarity Calculate the molarity of a solution that contains 11.5 g of NaOH dissolved in 1.50 L of solution. [Na = 23, O = 16, H = 1] M= no of moles/volume of solution No of moles (n) = mass/Mm Mm of NaOH= 23+16+1= 40 n= 11.5/40 = 0.2875 M= 0.2875/1.50 Ans = 0.192 moldm-3 6 Questions 1. Calculate the molarity of a solution prepared by dissolving 1.56 g of gaseous HCl in enough water to make 26.8 ml of solution. Ans = 1.59 M 2. How many moles of Ag+ ions are present in 25 ml of 0.2 M Ag2O solution? Hint: First find the number of moles of Ag2O, Then find the no of moles of Ag+ Ans = 0.01 moles 7 Molality (molal) • Molality is a concentration unit based on the number of moles of solute per kilogram of solvent. moles of solute m= kg of solvent in dilute aqueous solutions molarity and molality are nearly equal Molality Calculate the molality of a sulfuric acid solution containing 24.4 g of sulfuric acid in 198 g of water. The molar mass of sulfuric acid is 98.09 g. First, we find the number of moles of sulfuric acid in 24.4 g of the acid, using its molar mass as the conversion factor. The mass of water is 198 g, or 0.198 kg. Therefore, Molality The density of a 2.45 M aqueous solution of methanol (CH3OH) is 0.976 g/mL. What is the molality of the solution? The molar mass of methanol is 32.04 g. M= n/v, n= M x v, = 2.45 M x 1 L = 2.45 moles Molality Next step is to calculate the mass of water in 1 L of the solution, using density as a conversion factor. The total mass of 1 L of a 2.45 M solution of methanol is Because this solution contains 2.45 moles of methanol, the amount of water (solvent) in the solution is Molality The molality of the solution can be calculated by converting 898 g to 0.898 kg: Percent by Mass mass of solute x 100% % by mass = mass of solute + mass of solvent mass of solute x 100% = mass of solution Mole Fraction (X) moles of A XA = sum of moles of all components 13 Percent by mass A sample of 0.892 g of potassium chloride (KCl) is dissolved in 54.6 g of water. What is the percent by mass of KCl in the solution? Mole Fraction B A A A B A B A A B A B A A Mole Fraction () moles of A A A = sum of moles of all components moles of B B B = sum of moles of all components Since A + B make up the entire mixture, their mole fractions will add up to one. A A + + B B A + B = 1.00 Class Work In our glass of iced tea, we have added 3 tbsp of sugar (C12H22O11). The volume of the tea (water) is 325 mL. What is the mole fraction of the sugar in the tea solution? (1 tbsp sugar ≈ 25 g) • Solution First, we find the moles of both the solute and the solvent. • 1 mol C12 H 22 O11 75.g C12 H 22 O11 = 0.219 mol 342 g C12 H 22 O11 1 mol H 2 O 325mL H 2 O = 18.1 mol 18.0 g H 2 O Next, we substitute the moles of both into the mole fraction equation. 0.219 mol sugar moles solute = = 0.012 sugar = (0.219 mol + 18.1 mol) total moles solution χ Types of Chemical Reactions ❖Precipitation reactions ❖Acid-base reactions ❖Oxidation-reduction reactions ❖Decomposition reaction ❖Displacement reaction 18 Precipitation Reactions • Sometimes when two solutions are mixed, an insoluble substance forms i.e. a solid forms and separates from the solution. AgNO3 + HCl → HNO3 +AgCl • Such a reaction is called a precipitation reaction. 19 Reaction of potassium Iodide with lead nitrate led to the formation of a yellow precipitate of PbI2 (lead iodide). 20 Precipitation Reactions In a mixture of K2CrO4(aq) + Ba(NO3)2(aq) to predict the product, we must remember that • When ions form a solid compound, the compound formed must have a zero net charge. Therefore, K+ and Ba2+ cannot combine to form the solid, nor could CrO42- and NO3• The possible combinations of a given cations and anions from the above are: K2CrO4, KNO3, BaCrO4 , Ba(NO3)2. • Note that it cannot be K2CrO4 or Ba(NO3)2. The product will therefore be KNO3 and BaCrO4 21 Precipitation Reactions • Question: which one of these is likely to be the precipitate? Rules for the solubility of salts in water 1. All nitrate (NO3-)salts are soluble. 2. Most salts containing the alkali metal ions (Li+, Na+, K+, Cs+, Rb+) and the ammonium ion (NH4+) are soluble. 3. Most chloride, bromide and iodide salts are soluble except salts containing Ag+ Pb2+ and Hg22+. 22 Precipitation Reactions 4. Most sulphate salts are soluble except BaSO4, PbSO4, HgSO4 and CaSO4 . 5. Most hydroxide salts are only slightly soluble except NaOH and KOH. 6. Most sulphides (S2-), carbonates (CO32-), chromate (CrO42-) and phosphate (PO43-) salts are only slightly soluble. 23 Stoichiometry of Precipitation Reactions Calculate the mass of lead(II) sulphate formed in the reaction of 145 mL of 0.123 M lead(II) nitrate and excess sodium sulphate. • Steps to follow: 1. Write the balanced equation for the reaction 2. Calculate the number of moles of reactant 3. Determine which reactant is limiting 4. Calculate the moles of products as required 5. Convert to grams/kg/mg or other units as required. 24 Example • Calculate the mass of lead(II) sulfate formed in the reaction of 145 mL of 0.123 M lead(II) nitrate and excess sodium sulfate. PbNO3 (aq) + Na2SO4 (aq) → PbSO4 (s) + 2NaNO3 (aq) 1 mole of Pb(NO3)2 will produce 1 mole of PbSO4 From the question no of mole of Pb(NO3)2 = 0.123 x 0.145 (molarity x volume)= = 0.0178 mole 25 Solution (Ctd) Since the ratio is 1:1, the no of moles of PbSO4 is 0.0178 moles. To convert to mass, • 1 mole of PbSO4 = 303.26 g • 0.0178 mole = 303.26 * 0.0178 = 5.40 g 26 Acid-Base Reactions According to Bronsted and Lowry: ⮚An acid is a proton donor ⮚A base is a proton acceptor Prediction of products is just like the precipitation reaction. 27 Acid-Base Titrations • A technique for determining the amount of a certain substance by carrying out a titration experiment is called volumetric analysis. 28 Acid-Base Titrations • A titration experiment involves the titrant (solution in the burette) and the titrand. • The point in the titration where enough titrant has been added to completely react with the titrand is called the equivalence or stoichiometric point. 29 Acid-Base Titrations • This point is often marked by a colour change in the indicator. The point where the indicator actually changes is the end point. • The goal is to choose an indicator such that the end point(where the indicator changes colour) occurs at the equivalence point. 30 Titration a) b) c) Titration of an acid with a base. a) the flask contains acid and a few drops of phenolphthalein, which is colorless in acidic conditions, b) the endpoint has been reached (notice the faint pink color of the solution), finally in c), the solution is well beyond the end point since more base was added. 31 Example (a) 0.5 M hydrochloric acid was titrated against 25cm3 of an unknown concentration of sodium hydroxide. Use the data below to calculate the mean titre. ANS: • Find the mean of the two concordant results • 27.6 + 27.6 = 27.6 cm3 2 (b) Calculate the concentration of the sodium hydroxide. NaOH + HCl → NaCl +H2O • • • • • Using the mean titre from previous question 27.6 cm3 = 0.0276 dm3 Calculate the moles of HCl used = volume x Molarity. 0.0276 x 0.5 = 0.0138 moles This is equal to the moles of NaOH (mole ratio = 1:1) = 0.0138 Calculate the concentration of NaOH = moles ÷ volume = 0.0138 ÷ 0.025 dm3 = 0.55M or 0.6M Practice Questions 1. What volume of a 0.1000 molar HCl solution is needed to neutralise 25.0 ml of 0.35 molar NaOH (Ans = 87.5 mL) 1. A 10.0 ml sample of sulphuric acid from an automobile battery requires 35.08 ml of 2.12 molar sodium hydroxide solution for complete neutralization. What is the molarity of the sulphuric acid? 34 Oxidation and Reduction Reactions ⮚Oxidation - Addition of oxygen Increase in oxidation number Removal of hydrogen Removal of electron 35 Reduction Reactions ⮚Reduction - Removal of oxygen Decrease in oxidation number Addition of hydrogen Addition of electrons 36 Oxidation and Reduction Reactions • Reducing agent causes reduction to take place and itself is oxidised. • Oxidizing agent causes oxidation to take place and itself is reduced. • The oxidation state / number: - is the charge the atom would have if the shared electrons were divided equally between identical atoms bonded to each other. 37 Oxidation and Reduction Reactions Rules for Assigning Oxidation States 1. The oxidation number of a neutral atom or element is 0 For example Na(s) , O2(g) P4 are all 0 2. (a)The sum of the oxidation numbers of all the atoms in a neutral compound is zero. E.g NaCl = 0. (b) For an ion, the sum of the oxidation number is equal to the charge on the ion e.g The oxidation number of PO33- is -3 38 Oxidation and Reduction Reactions Rules for Assigning Oxidation States 3. The oxidation state of a monoatomic ion is the same as its charge e.g Na+ = +1, Cl - = -1, Ca 2+ = +2. 4. Oxygen in a compound usually has an oxidation number of -2, except in peroxides where oxygen has an oxidation number of -1. 39 Oxidation and Reduction Reactions Rules for Assigning Oxidation States 5. Hydrogen usually has a oxidation number of +1, but when it combines with a metal, it has an oxidation number of -1. 5. All group 1 metals have oxidation number of +1. 7. All group 2 metals have oxidation number of +2. 40 Classwork • Determine the oxidation number of (a) Al in Al2O3 (b) P in H3PO4 (c) S in SO32(d) N in NO3• Determine the reducing and oxidising agents in the reaction below: CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (g) 41 Balancing of Redox Equation • Redox reaction occurring in acidic solutions MnO4- + Fe2+ → Mn2+ + Fe3+ Step 1. Identify oxidising and reducing agents and write half equations • MnO4- gains electrons, it acts as the oxidizing agent as it is reduced MnO4- → Mn2+ (reduction) 42 Balancing of Redox Equation Fe2+ loses electron, it acts as the reducing agent as it is oxidised Fe2+ → Fe3+ (oxidation) • Step 2: For each half-reaction (a)Balance all the elements except hydrogen and oxygen (b)Balance all oxygen using water MnO4- → Mn2+ + 4H2O (c) Balance hydrogen using H+ 8H+ + MnO4- → Mn2+ + 4H2O 43 Balancing of Redox Equation (d) Balance the charge using electrons 5e- + 8H+ + MnO4- → Mn2+ + 4H2O Fe2+ → Fe3+ + e- Step 3: If necessary, multiply one or both balanced half reactions by an integer to equalize the number of electrons transferred in the two half reactions. Fe2+ → Fe3+ + e- (*5) 44 Balancing of Redox Equation Step 4: Add the half reactions and cancel identical species. 5e- + 8H+ + MnO4- + 5Fe2+ → Mn2+ + 4H2O +5Fe3+ +5e5Fe2+ + 8H+ + MnO4- → Mn2+ + 4H2O + 5Fe3+ 45 Example Balancing redox equations Balance the equation showing the oxidation of Fe2+ ions to Fe3+ ions by dichromate ions (Cr2O72-) in an acidic medium Fe2+ + Cr2O72- → Fe3+ + Cr3+ Step 1: Identify oxidising and reducing agents and write half reactions Fe2+ : +2 Fe3+ : +3 Cr2O72- : 2Cr + 7(-2) = -2 2Cr = 12 Cr = +6 Cr3+ : +3 Fe2+ → Fe3+ Iron loses one electron, i.e. it is oxidised. It donates one electron to dichromate, i.e. it is the reducing agent. Half equation: Oxidation reaction: Fe2+ → Fe3+ + eCr26+ → Cr3+ Chromium gains three electrons, i.e. it is reduced. It gains its electrons from iron, i.e. it is the oxidising agent. Half equation: Reduction reaction: Cr26+ + 3e- → Cr3+ Fe2+ + Cr2O72- → Fe3+ + Cr3+ Step 2: Balance each kind of atom other that H and O Fe2+ → Fe3+ + e- Cr2O72- + 3e- → 2Cr3+ Step 3: Balance oxygen atoms by using H2O Fe2+ → Fe3+ + e- Cr2O72- + 3e- → 2Cr3+ + 7H2O Step 4: Balance H atoms by using H+ ions 14H+ + Cr2O72- + 3e- → 2Cr3+ + 7H2O Fe2+ → Fe3+ + e- Step 5: Use electrons as needed to obtain a charge that is balanced Fe2+ → Fe3+ + e- +2 +2 +3 +2 14H+ + Cr2O72- +3e- → 2Cr3+ + 7H2O -1 +14 -2 -3 +9 Add three electrons to the reactant side to balance the charges Fe2+ → Fe3+ + e- 14H+ + Cr2O72- + 6e- → 2Cr3+ + 7H2O +6 0 +6 Step 6: Ensure the number of electrons gained equals the number of electrons lost and add the 2 half reactions together Fe2+ → Fe3+ + e14H+ + Cr2O72- + 6e- → 2Cr3+ + 7H2O ×1 6Fe2+ → 6Fe3+ + 6e14H+ + Cr2O72- + 6e- → 2Cr3+ + 7H2O 14H+ + Cr2O72- + 6Fe2+ + 6e- → 2Cr3+ + 7H2O + 6Fe3+ + 6e- 14H+ + Cr2O72- + 6Fe2+ → 2Cr3+ +7H2O + 6Fe3+ Balanced Check to ensure that all atoms and charges are balanced. ×6 Balancing Redox Equation in acidic medium Questions Cr2O72- (aq) + HSO3- (aq) → Cr3+ (aq) + HSO4- (aq) CuS (s) + NO3 - (aq) Cu2+(aq) + SO42- (aq) + NO (g) Fe2+ + Cr2O72- → Fe3+ + Cr3+ MnO4- + SO2 → Mn2+ + SO42- Balancing of Redox Equation • Redox reaction occurring in basic medium 1. Use the half-reaction method as specified for acidic solutions to obtain the final balanced equation as if H+ were present. 2. To both sides of the equation obtained, add a number of OH- ions that is equal to H + ions. (You want to eliminate H+ by turning is into H2O) 50 Balancing of Redox Equation 3. Eliminate the number of H2O molecules that appear in both sides of the equation. 4. Check that the elements and charges are balanced. 51 Balancing of Redox Equation in Basic solution Write a balanced equation to represent the oxidation of iodide ion (I-) by permanganate ion (MnO4-) in basic solution to yield molecular iodine (I2) and manganese (IV) oxide (MnO2) Skeletal equation: I- + MnO4- I2 + MnO2 Step 1: Identify oxidising and reducing agents and write half equations I- loses an electron: it acts as the reducing agent as it is oxidised I- → I2 + e- Oxidation reaction MnO4- gains 3 electrons: it acts as the oxidising agent as it is reduced MnO4- + 3e- → MnO2 Reduction reaction Step 2: Balance each kind of atom other than H and O 2I- → I2 + eMnO4- + 3e- → MnO2 Step 3: Balance the O atoms by using H2O 2I- → I2 + e- MnO4- + 3e- → MnO2 + 2H2O Step 4: Balance H atoms by using H+ ions 2I- → I2 + e- 4H+ + MnO4- + 3e- → MnO2 + 2H2O Step 5: Since the reaction occurs in a basic medium, for each H+ ion we add an equal number of OH- ions to both sides of the equation. Where H+ and OHions appear on the same side of the equation, they may be combined to form H2O. 4H+ + 4OH- + MnO4- + 3e- → MnO2 + 2H2O + 4OH4H2O + MnO4- + 3e- → MnO2 + 2H2O + 4OH2H2O + MnO4- + 3e- → MnO2 + 4OHStep 6: Use electrons as needed to obtain a charge that is balanced 2I- → I2 + e- -2 0 -2 -1 -1 Add one electron to the product side to balance charges 2I- → I2 + 2e- 2H2O + MnO4- + 3e- → MnO2 + 4OH- 0 Already balanced! -1 -4 -3 0 -4 -4 Step 7: Ensure the number of electrons gained equals the number of electrons lost and add the two half reactions together 2I- → I2 + 2e- ×3 2H2O + MnO4- + 3e- → MnO2 + 4OH- ×2 6I- → 3I2 + 6e4H2O + 2MnO4- + 6e- → 2MnO2 + 8OH2MnO4- + 6I- + 4H2O → 2MnO2 + 3I2 + 8OHBalanced Check to ensure that all atoms and charges are balanced. Question Balance the following redox equation which occurs in basic solution Mn2+ + H2O2 → MnO2 + H2O Step 1: Identify the oxidising and reducing agents and write half reactions Mn2+ → MnO2 Mn2+ : +2 MnO2: Mn +2(-2) = 0 Mn = +4 Mn2+ loses two electrons: it acts as the reducing agent as it is oxidised Mn2+ → MnO2 + 2e- H2O2 → H2O Oxidation reaction H2O2: 2(+1) + 2O = 0 O = -1 H2O: 2(+1) + O = 0 O = -2 H2O2 gains one electron: it acts as the oxidising agent as it is reduced H2O2 + e- → H2O Reduction reaction Step 2: Balance each kind of atom other than H and O. Mn2+ → MnO2 + 2e- Already balanced in this case H2O2 + e- → H2O Step 3: Balance the O atoms by using H2O 2H2O + Mn2+ → MnO2 + 2eH2O2 + e- → 2H2O Step 4: Balance the H atoms by using H+ 2H2O + Mn2+ → MnO2 + 2e- + 4H+ 2H+ + H2O2 + e- → 2H2O Step 5: For each H+ ion, add equal no. of OH- to both sides of equation 2H2O + Mn2+ + 4OH- → MnO2 + 2e- + 4H+ + 4OH2H2O + Mn2+ + 4OH- → MnO2 + 2e- + 4H2O Mn2+ + 4OH- → MnO2 + 2e- + 2H2O 2H+ + 2OH- + H2O2 + e- → 2H2O + 2OH2H2O + H2O2 + e- → 2H2O + 2OHH2O2 + e- → 2OHStep 6: Use electrons as needed to obtain a charge that is balanced Mn2+ + 4OH- → MnO2 + 2e- + 2H2O +2 -4 0 -2 0 -2 -2 H2O2 + e- → 2OH- -1 0 -2 -1 -2 Add one electron to the reactant side to balance charges H2O2 + 2e- → 2OHStep 7: Ensure no. of electrons gained equals no. of electrons lost and add the half reactions together Mn2+ + 4OH- → MnO2 + 2e- + 2H2O H2O2 + 2e- → 2OHMn2+ + H2O2 + 2OH- → MnO2 + 2H2O Questions Write balanced equations to represent the following reactions in a basic solution (a) Fe(OH)2 + MnO4- → MnO2 + Fe(OH)3 (b) Bi(OH)3 + SnO22- → SnO32- + Bi Stoichiometry of Redox Reactions Example 1. A piece of iron wire weighing 0.1568 g is converted into Fe2+ (aq) and requires 26.24 ml of a KMnO4 (aq) solution for its titration. What is the molarity of the KMnO4? • The balanced equation is: 5Fe2+ + MnO4- + 8H+ 5Fe3+ + Mn2+ + 4H2O 5 moles of Fe2+ = 1 mole of MnO460 Solution contd No of moles of Fe = mass/ Mm = 0.1568/ 56 = 0.002808 moles 0.002808 Fe = 1/5* 0.002808 MnO4- = 0.0005616 moles Molarity = mole/volume = 0.0005616/0.02624 = 0.02140 M 61 Practice Questions 2). 24.5 ml of KMnO4 (aq) solution is required to titrate 0.350 sodium oxalate in the redox reaction. What is the molarity of the KMnO4? [Hint balance the equation first] C2O42- + MnO4- + H+ Mn2+ + H2O + CO2 3. A 25.00 ml sample of Fe2+ requires 34.77 ml of 0.05876 molar K2Cr2O7 for its titration. What is the molarity of the Fe in the sample? Fe2+ + Cr2O72Fe3+ + Cr3+ 62 2. Decomposition Example: NaCl Cl Na → General: Cl + Na AB → A + B Compound = Element + Element Ex. Decomposition Reaction Other examples of Decomposition Reactions • • • 2H₂O(I ) → 2H₂(g) + O₂(g) CaCO₃(s) → CaO(s) + CO₂(g) 2KClO₃(s) → 2KCl(s) + 3O₂(g) Single Replacement Reaction Single Replacement Reactions • • Write and balance the following single replacement reaction equations: Zn(s) +2 HCl(aq) → ZnCl2 + H2(g) • 2 NaCl(s) + F2(g) → 2 NaF(s) + Cl2(g) • 2 Al(s)+ 3 Cu(NO3)2(aq)→ 3 Cu(s)+ 2 Al(NO3)3(aq) Double Displacement Example: MgO + CaS Mg + Ca O General: S → Mg S + Ca O AB + CD → AD + CB Double Replacement Reactions • • Think about it like “foil”ing in algebra, first and outer ions go together + inside ions go together Example: AgNO3(aq) + NaCl(s) → AgCl(s) + NaNO3(aq) • Another example: K2SO4(aq) + Ba(NO3)2(aq) → KNO3(aq) + BaSO4(s) 2 Practice • 1. 2. 3. 4. 5. 6. Predict the products: HCl(aq) + AgNO3(aq) → CaCl2(aq) + Na3PO4(aq) → Pb(NO3)2(aq) + BaCl2(aq) → FeCl3(aq) + NaOH(aq) → H2SO4(aq) + NaOH(aq) → KOH(aq) + CuSO4(aq) →