AS & A Level Chemistry Coursebook: Chapter 24 Answers
advertisement

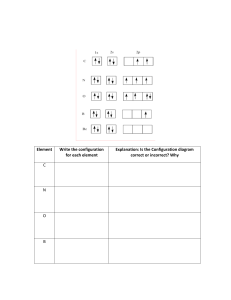
CAMBRIDGE INTERNATIONAL AS & A LEVEL CHEMISTRY: COURSEBOOK Exam-style questions and sample answers have been written by the authors. In examinations, the way marks are awarded may be different. Coursebook answers Chapter 24 Self-assessment questions 1 a i Ti 1s2 2s2 2p6 3s2 3p6 3d2 4s2 ii Cr 1s 2s 2p 3s 3p 3d 4s iii Co 1s2 2s2 2p6 3s2 3p6 3d7 4s2 iv Fe3+ 1s2 2s2 2p6 3s2 3p6 3d5 4s0 v Ni2+ 1s2 2s2 2p6 3s2 3p6 3d8 4s0 vi Cu+ 1s22s2 2p6 3s2 3p6 3d10 4s0 b 2 6 2 6 5 1 For scandium the only observed oxidation state is +3, so the electronic configuration of Sc3+ is 1s2 2s2 2p6 3s2 3p6 4s0. This ion has no d electrons, so does not satisfy the definition of a transition element. The only ion of zinc is Zn2+, with the electronic configuration 1s2 2s2 2p6 3s2 3p6 3d10 4s0. This ion has a completely filled, not a partially filled, d subshell − so zinc is not a transition element. c The + 7 oxidation state involves all of the 3d and 4s electrons in manganese. d Oxidation state of vanadium in a = (VO2+) = +5; b (VO2+) = +4; c (V3+) = +3; d (V2+) = +2. e i ii 2 2 a +4 as this involves all the 4d and 5s electrons, leaving the noble gas electronic configuration of krypton. The positive value indicates that the reaction as written is feasible and its relatively large value suggests that the reaction is likely to occur (although values of E⦵ tell us nothing about the rate of a reaction). d 6 e i 0.0153 × 0.001 = 0.000 015 3 mol ii 0.000 015 3 × 6 = 0.000 091 8 mol iii i +3 ii +2 iii +3 iv +3 v +2 3 4 a [Ni(EDTA)]2− c ethanedioate ion (ox) and ethane-1,2-diamine (en) a i ii H2N Cl NH2 Co Fe2+(aq) → Fe3+(aq) + e− 6Fe2+(aq) → 6Fe3+(aq) + 6e− + H2N − Cr2O72−(aq) + 6Fe2+(aq) + 14H+(aq) → 2Cr3+(aq) + 6Fe3+ + 7H2O(l) E⦵ = +1.33 V + (−0.77 V) = +0.56 V NH2 Cl iii Cr2O7 (aq) + 14H (aq) + 6e → 2Cr3+(aq) + 7H2O(l) c 6 ZrO2 2− = 0.003 67 mol dm−3 b Cr2O72−(aq) + 14H+(aq) + 6e− → 2Cr3+(aq) + 7H2O(l) b 0.000 0918 0.025 b i They are mirror images, which are not superimposable. NC Cl NC Ni Ni Cl cis-isomer 1 2– 2– Cl NC Cl CN trans-isomer Cambridge International AS & A Level Chemistry © Cambridge University Press 2020 CAMBRIDGE INTERNATIONAL AS & A LEVEL CHEMISTRY: COURSEBOOK ii 5 Non-polar, as the charge is distributed perfectly symmetrically around the central nickel (with both cyanide ligands diagonally opposite each other in the square planar structure, and similarly with the two chloride ions). a +2 b [CoCl4]2−(aq) + 6H2O(l) → [Co(H2O)6]2+(aq) + 4Cl−(aq) c A [ PtCl (NH ) (aq)] Cl (aq) [ PtCl ] (aq) [ NH (aq) ] − 6 a i ii iii b i 2 2 4 Ni2+ ...3d8 d Sc3+ ions have electronic configuration [Ar]3d04s0. If d-orbital splitting were to occur in a complex ion containing Sc3+, there would be no electrons in the three 3d orbitals of lower energy, so visible light would not be absorbed in promoting an electron from a lower energy 3d orbital to a higher energy 3d orbital. e Zn2+ ions have electronic configuration [Ar]3d104s0. If d-orbital splitting were to occur in a complex ion containing Zn2+, each of the 3d orbitals would contain two electrons, and would therefore be fully occupied. Visible light could not be absorbed in promoting an electron from a lower energy 3d orbital to a higher energy 3d orbital. 3 Ni ( NH3 )4 ( H2O )2 (aq) Ni ( H2O ) 2+ (aq) NH3 ( aq ) 4 6 2+ [Fe(H2O)6]3+(aq)]: H2O H2O OH2 Fe H2O OH2 H2O 2 3d Cr ( H O ) Cl + (aq) 2 2 4 3+ Cr ( H O ) (aq) Cl – ( aq ) 2 2 6 ii SCN− has a higher value of Kstab than H2O. So the position of equilibrium is shifted to the right. iii [Fe(H2O)5SCN]2+(aq) iv Yes; a colour change is likely / possible. F− has a higher value of Kstab than SCN−. So F substitutes for SCN (and for water) because the position of equilibrium is shifted to the right. 7 c 2 3 2 2− electron from one of the three lower non-degenerate orbitals absorbs that amount of energy (ΔE) and jumps into one of the two higher non-degenerate orbitals. This leaves the transmitted light coloured. a orbitals at the same energy level b The ligands in a complex cause the d orbitals to split, forming two sets of non-degenerate orbitals. The difference in the energy (ΔE) between the non-degenerate d orbitals corresponds to the energy of part of the visible spectrum of light. So when light travels through a solution or a solid containing the complex, an Cambridge International AS & A Level Chemistry © Cambridge University Press 2020