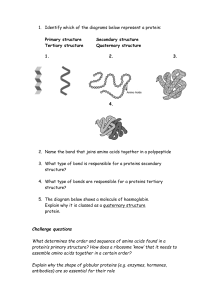
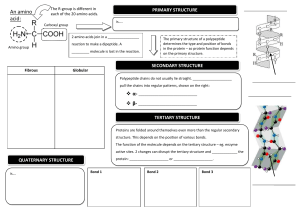
Structure of Amino Acids There are actually thousands of amino acids occurring in nature. But only about 20 amino acids form a part of the proteins in the human body. These twenty acids will be our focus here. Although all these have varied structures, the basic structure of amino acid remains uniform. 1)All amino acids contain a carbon atom in the middle of the molecule, the alphacarbon2)This atom is surrounded by three chemical groups.3)One is an amine group -NH24)The second one is a carboxyl group -OOOH5)The third group is denoted by R. This is the variable radical group and is different for every amino acid. This R group makes the amino acid unique. Classification of amino acids Non-essential amino acid While all amino acids are required for life, not all of them can synthesis in the body. The body can naturally produce 11 out of the 20 amino acids known as non-essential amino acids.For example Alanine, arginine, asparagine, aspartate, cysteine, glutamate, glutamine, glycine, proline, serine and tyrosine are the 11 non-essential amino acids naturally produced by the body. Note A.Non-essential amino acids Non-essential amino acids, except for tyrosine, are derived from products or intermediates of important metabolic pathways. Alanine and aspartate, for example, are formed from compounds produced during cellular respiration. Pyruvate, a byproduct of glycolysis, is used to make alanine. Oxaloacetate, a citric acid cycle intermediate, is used to make aspartate. Six of the non-essential amino acids (arginine, cysteine, glutamine, glycine, proline and tyrosine) are considered conditionally essential, meaning that dietary supplementation may be necessary during illness or in children. Essential amino acid he amino acids that the body cannot produce or synthesise independently, on its own, are known as essential amino acids.For example The nine essential amino acids are: lysine, tryptophan, isoleucine, phenylalanine, leucine, histidine, methionine, threonine and valine. Plants, unlike humans, are able to synthesise all 20 amino acids. Conditional amino acid Conditional amino acids are amino acids that are usually not essential but become so at times of disease or stress. Prematurity in babies, for example, may necessitate the use of these amino acids. Cysteine, arginine, tyrosine, glutamine, ornithine, glycine, serine and proline are among the six conditionally essential amino acids. A peptide linkage, also known as a peptide bond, is a covalent chemical bond that links two consecutive alpha-amino acids from C1 (carbon number one) of one alpha-amino acid and N2 (nitrogen number two) of another1. It is a type of amide bond that is formed between two molecules where an α-amino group of one molecule reacts with the α-carboxyl group of another molecule and a water molecule is being eliminated2. The bond between the two amino acids consists of a nitrogen with one hydrogen bonded to a carbon with a double bonded oxygen: HNC = O Characteristics of Peptide Bonds 1. Peptide bonds are strong with partial double bond character: They are not broken by heating or high salt concentration. They can be broken by exposing them to strong acids or bases for a long time at elevated temperature, and also by some specific enzymes (digestive enzymes). Protein Structure 1. Primary Structure of ProteinThe Primary structure of proteins is the exact ordering of amino acids forming their chains. The exact sequence of the proteins is very important as it determines the final fold and therefore the function of the protein.The number of polypeptide chains together form proteins. These chains have amino acids arranged in a particular sequence which is characteristic of the specific protein. Any change in the sequence changes the entire protein. The following picture represents the primary protein structure (an amino acid chain). As you might expect, the amino acid sequence within the polypeptide chain is crucial for the protein’s proper functioning. secondary structure of protein refers to local folded structures that form within a polypeptide due to interactions between atoms of the backbone.The proteins do not exist in just simple chains of polypeptides. These polypeptide chains usually fold due to the interaction between the amine and carboxyl group of the peptide link.The structure refers to the shape in which a long polypeptide chain can exist.They are found to exist in two different types of structures α – helix and β – pleated sheet structures.This structure arises due to the regular folding of the backbone of the polypeptide chain due to hydrogen bonding between -CO group and -NH groups of the peptide bond. Tertiary Structure of ProteinThis structure arises from further folding of the secondary structure of the protein. H-bonds, electrostatic forces, disulphide linkages, and Vander Waals forces stabilize this structure.The tertiary structure of proteins represents overall folding of the polypeptide chains, further folding of the secondary structure.It gives rise to two major molecular shapes called fibrous and globular.The main forces which stabilize the secondary and tertiary structures of proteins are hydrogen bonds, disulphide linkages, van der Waals and electrostatic forces of attraction. Quateternry The spatial arrangement of various tertiary structures gives rise to the quaternary structure. Some of the proteins are composed of two or more polypeptide chains referred to as sub-units. The spatial arrangement of these subunits with respect to each other is known as quaternary structure. The exact amino acid sequence of each protein drives it to fold into its own unique and biologically active three-dimensional fold also known as the tertiary structure. Proteins consist of different combinations of secondary elements some of which are simple whereas others are more complex. Parts of the protein chain, which have their own three-dimensional fold and can be attributed to some function are called “domains”. These are considered today as the evolutionary and functional building blocks of proteins. .NON-COVALENT INTERACTIONS Hydrophobic Effect-Relese of water molecule from structured solvation layer around the molecule as protein folds increased the net entropy. Hydrogen Bond- Intraction of N-H and C=O of peptide bond leads to local regular structures such as a-helix and B- sheets. London Dispersion- Medium range weak attraction between all atoms contributes significantily to the stablity in the interior protein. Electrostatic Interactions---Long-range strong interactions between permanently charged groups. Protein conformation may be defined as the arrangement in space of its constituent atoms which determine the overall shape of the molecule. The conformation of the protein arises from the bonding arrangements within its structure Peptide Bond Resonance Structure The general structure of the peptide group is rigid and planar. The stability of a peptide bond is because of the resonance of the amide. A resonance structure forms due to the interaction between electrons of the carbonyl group's double bond with those of the C–N bond. This effect is an example of resonance, which can be considered to share electrons between bonds. As a result, the C-N acquires a partial double bond property. The C=O bond acquires a partial single bond property. MOTIF A chain-like biological structure made up of connectivity between secondary structural elements A supersecondary structure of a protein Formed by the connected alpha-helices and betasheets through loops Mainly have a structural function in the protein structure Have similar functions through protein families ot stable independently DOMAIN An independent folding unit of the three-dimensional protein structure. A tertiary structure of the protein Formed by the formation of disulfide bridges, ionic bonds, and hydrogen bonds between amino acid side chains .Mainly have functional importance Have unique functions .Are independently stable The Ramachandran plot provides an easy way to view the distribution of torsion angles of a protein structure. It also provides an overview of allowed and disallowed regions of torsion angle values, serving as an important factor in the assessment of the quality of protein three-dimensional structures. Structure of bases The bases come in two categories: thymine and cytosine are pyrimidines, while adenine and guanine are purines (). Figure %: DNA Bases The pyrimidine structure produced by a six-membered, two-nitrogen molecule; purine refers to a ninemembered, four-nitrogen molecule nucleotideThe chemical composition of nucleotide consists of a phosphate group, a sugar and a nitrogenous base. They are one of the major causes of cancer-causing agents to this very day. Some of the major examples of nucleotides are adenosine, guanosine etc.. nucleoside A nucleoside has a chemical composition that consists of a sugar and a base without the phosphate group. They are used as agents in medicine that are primarily used against viruses and cancer-causing agents. Some of the key examples of nucleosides are the same as nucleotides only with the addition of phosphate groups. The phosphodiester bonds are formed as the result of the condensation reaction between phosphate groups and hydroxyl groups of two sugar groups. For instance, the group that is being formed by the bonding of one oxygen atom and one hydrogen atom is called the hydroxyl group. Such groups are written as -OH or -HO. The “–” represents the carbon to which the hydroxyl group will be attached. Moreover, the molecules containing a single atom of phosphorus covalently bonded to four oxygen atoms are called phosphate groups. The other name for the phosphodiester bond is phosphoester bond. mRNA structure has a linear structure and carries genetic information copied from DNA. tRNA has an L shaped 3D structure. It is specific to each amino acid and carries an amino acid to the growing chain of a polypeptide during the translation process. rRNAs are spherical and provide a structural framework for ribosomes. They associate with tRNA and are essential for protein synthesis. mRNA is formed by transcription process from DNA. They provide a template for protein synthesis. RNA polymerase catalyses the transcription process. In eukaryotic cells, mRNA is formed in the nucleus as hnRNA (heterogenous RNA), which after processing, introns are removed and exons joined together after splicing to form mRNA and move to the cytoplasm to take part in protein synthesis. Trna It has a folded structure, which looks like a cloverleaf. It has three hairpin loops, a small variable loop and the acceptor arm, which carries amino acids. 5’ end has a terminal phosphate group. The acceptor arm – It is present at 3’ end and carries amino acids. It has a terminal sequence of CCA (5’ -> 3’) .The anticodon loop – It has a complementary codon for coding a particular amino acid it carries, during the translation process. And attaches to the codon on mRNA .D loop – has dihydrouridine .TψC loop – has pseudouridine rRNA associates with certain proteins to form ribosomes. They bind to tRNA and accessory proteins and move along mRNA during translation. Large and small ribosomal subunits contain different rRNAs.To conclude, all three rRNAs are essential in protein synthesis mechanism. They differ in their structure and function mono sugar? Simple sugars are called monosaccharides; these are made up of single sugar molecules. The three main monosaccharides that we consume are fructose, galactose and glucose. A disaccharide (also called a double sugar ) is the sugar formed when two monosaccharides (simple sugars) are joined by glycosidic linkage. Like monosaccharides, disaccharides are soluble in water. Three common examples are sucrose, lactose, and maltose. Polysaccharides are major classes of biomolecules. They are long chains of carbohydrate molecules, composed of several smaller monosaccharides. These complex biomacromolecules functions as an important source of energy in animal cell and form a structural component of a plant cell. It can be a homopolysaccharide or a heteropolysaccharide depending upon the type of the monosaccharides.Polysaccharides can be a straight chain of monosaccharides known as linear polysaccharides, or it can be branched known as a branched polysaccharide. An asymmetrical centre in the case of carbon is defined as a carbon atom to which four different groups are attached. In the three-carbon aldose sugar, glyceraldehyde, the asymmetrical centre is located at the central carbon atom.Asymmetrical molecules cannot be divided in half to produce two mirror images. Another name for an asymmetric carbon is chiral carbon. Chiral molecules are asymmetric and typically exist in different stereoisomer forms depending on how the substituent groups are arranged Lipids?These organic compounds are nonpolar molecules, which are soluble only in nonpolar solvents and insoluble in water because water is a polar molecule. In the human body, these molecules can be synthesized in the liver and are found in oil, butter, whole milk, cheese, fried foods and also in some red meats.Lipids are oily or greasy nonpolar molecules, stored in the adipose tissue of the body.Lipids are a heterogeneous group of compounds, mainly composed of hydrocarbon chains.Lipids are energy-rich organic molecules, which provide energy for different life processes.Classification of Lipids :Nonsaponifiable lipids Saponifiable lipid (A nonsaponifiable lipid cannot be disintegrated into smaller molecules through hydrolysis. Nonsaponifiable lipids include cholesterol, prostaglandins, etc Saponifiable Lipids A saponifiable lipid comprises one or more ester groups, enabling it to undergo hydrolysis in the presence of a base, acid, or enzymes, including waxes, triglycerides, sphingolipids and phospholipids. Further, these categories can be divided into non-polar and polar lipids. Nonpolar lipids, namely triglycerides, are utilized as fuel and to store energy. Triglyceride Structure Fatty acids can be metabolised for energy by tissues or stored as energy in the form of triglycerides. The stored triglycerides are digested in response to energy demands, and the unsaturated fatty acids are released into the circulatory system and delivered to the tissues. Developmental biology is the science that investigates how a variety of interacting processes generate an organism’s heterogeneous shapes, size, and structural features that arise on the trajectory from embryo to adult, or more generally throughout a life cycle. It represents an exemplary area of contemporary experimental biology that focuses on phenomena that have puzzled natural philosophers and scientists for more than two millennia. Philosophers of biology have shown interest in developmental biology due to the potential relevance of development for understanding evolution, the theme of reductionism in genetic explanations, and via increased attention to the details of particular research programs, such as stem cell biology Determination, the commitment of a cell to a particular fate, is a process influenced by the action of the extracellular function. This apparent contradiction results from regulation of the expression of various parts of the genome. When the embryo consists of only a few cells, each cell has the potential to develop in many different ways. As development proceeds, however, the possibilities available to individual cells gradually narrow, until each cell's fate is fully determined and the cell has differentiated. Morphogenesis (literally, "creation of form") is the shaping of the multicellular body and its organs. Morphogenesis results from pattern formation, the organization of differentiated tissues into specific structures. In plant development, cells are constrained by cell walls and do not move around the body, so organized division and expansion of cells are the major processes that build the plant body. In animals, cell movements are very important in morphogenesis. And in both plants and animals, programmed cell death is essential to orderly development. Like differentiation, morphogenesis results ultimately from the regulated activities of genes and their products, as well as from the interplay of extracellular signals and their transduction in target cells. During gastrulation, cell movements result in a massive reorganization of the embryo from a simple spherical ball of cells, the blastula, into a multi-layered organism. During gastrulation, many of the cells at or near the surface of the embryo move to a new, more interior location. Sphingolipids are enriched in the Central Nervous System (CNS) and display multiple biological functions. They participate in tissue development, cell recognition and adhesion, and act as receptors for toxins. Terpenes are naturally occurring chemical compounds found in plants and some animals. They're responsible for the aromas, flavors, and even colors associated with various types of vegetation. In terms of cannabis, terpenes are what make certain strains smell or taste different from others. Prostaglandins are a group of lipids with hormone-like actions that your body makes primarily at sites of tissue damage or infection. There are several different types of prostaglandins, and they play several essential roles in regulating bodily processes, including:Blood clot formation at the site of an injury. Blood flow. A wax is a simple lipid which is an ester of a long-chain alcohol and a fatty acid. The alcohol may contain from 12-32 carbon atoms. Waxes are found in nature as coatings on leaves and stems. The wax prevents the plant from losing excessive amounts of water. Steroids reduce redness and swelling (inflammation). This can help with inflammatory conditions such as asthma and eczema. Steroids also reduce the activity of the immune system, which is the body's natural defence against illness and infection. Reducing sugar is one that possesses a free aldehyde or ketonic group.It can reduce Fehling and Tollens reagent.Fehling A is Copper sulfate and Fehling B is an aqueous solution of potassium sodium tartrate Tollens's reagent is the ammoniacal solution of silver nitrate Reducing sugar can act as a reducing agent.It can be further reduced to alcohol.For example maltose and lactose. Non reducing sugar In a basic aqueous solution, a non-reducing sugar is not reduced by a weak oxidising agent (one that oxidises aldehydes but not alcohols, such as Tollen's reagent). There is no free aldehyde or ketonic group. Non-reducing sugars do not have an group attached to the anomeric carbon so they cannot reduce other compounds. E.g. sucrose and Raffinose Sugar alcohols are defined as the sum of saccharide derivatives in which a hydroxyl group replaces a ketone or aldehyde group. Sugar is a crystalline substance generally called sweet. It is a soluble carbohydrate that is used in most foods. It is comprised of oxygen, hydrogen, and carbon. Numerous sugar types are available that are obtained from various sources. The melting temperature (Tm) by definition is the temperature at which one half of the oligo (primer) duplex will dissociate to become single stranded and indicates the duplex stability. Primers with melting temperatures in the range of 52-58 °C generally produce the best results. C0t Curve?While plotting a graph for the renaturation of DNA, the Y-axis is marked as the percentage of DNA that remains single. The percentage is calculated as a ratio of the concentration of ssDNA (C) by the total concentration of DNA (C0). The X-axis is a product of the total or initial concentration of DNA (C0) and the time taken for the reaction in seconds (t). This value comes out as C0t, hence the graph is also known as the C0t curve. While calculating the C0t value, a buffer factor that accounts for the effect of cation is also taken into consideration. What is Haemoglobin? Haemoglobin is a globular protein found in the RBCs, which are responsible for the transport of oxygen from the lungs to the entire body. It is a tetrameric protein made up of two alpha and two beta subunits. Each of the four chains is associated with an iron or heme group which binds with one oxygen molecule each. What is Myoglobin?Myoglobin is a globular protein found in the muscles which are responsible for the storage of oxygen in the muscle cells. This stored oxygen is later used up by us while doing different activities. It is a monomeric protein made of a single type of subunit. It has a higher binding affinity than haemoglobin towards oxygen because myoglobin tends to keep oxygen in an oxidation state of +3, which stabilizes it. Sphingolipids are enriched in the Central Nervous System (CNS) and display multiple biological functions. They participate in tissue development, cell recognition and adhesion, and act as receptors for toxins he endoplasmic reticulum transpires in two forms: a type with a ribosome-studded surface and another with a smooth surface. The latter is called the smooth endoplasmic reticulum, and the former is called the rough endoplasmic reticulum. These membranes form continuous folds, eventually joining the outer layer of the nuclear membrane. Except for sperm cells and red blood cells, the endoplasmic reticulum is observed in every other type of eukaryotic cell. Golgi ApparatusThe Golgi body comprises 5 to 8 cup-shaped, series of compartments known as cisternae. Cisternae is a flattened, disk-shaped, stacked pouches that make up the Golgi apparatus. A Golgi stack mostly contains 4 to 8 cisternae. However, ~60 cisternae are found in some protists. A mammalian cell contains ~40 to 100 stacks of cisternae. Golgi Bodies Functions Its main function is the packaging and secretion of proteins. It receives proteins from Endoplasmic Reticulum. It packages it into membrane-bound vesicles, which are then transported to various destinations, such as lysosomes, plasma membrane or secretion. They also take part in the transport of lipids and the formation of lysosomes. Chloroplasts produce energy through photosynthesis and oxygen-release processes, which sustain plant growth and crop yield. As such, chloroplasts are responsible for the biosynthesis of active compounds such as amino acids, phytohormones, nucleotides, vitamins, lipids, and secondary metabolites