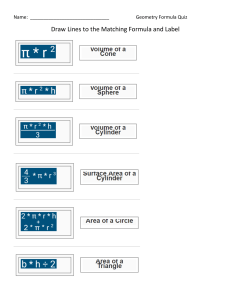
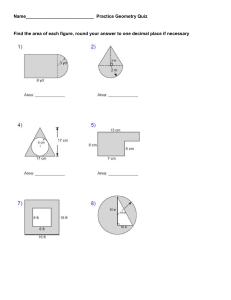
Molecular Geometry 1-Predict the geometry of the following molecules or ions, using the VSEPR model: BeCl2, NO2-, SiCl4 2-Draw the structures of the following molecules state the hybridization of the central atom and predict the molecular geometry: SiF4, AsF5, BH3, TeF6 3-Indicate the type of hybrid orbital’s of the underlined atom, and the molecular geometry: BeCl2 , CCl4 , C2F4 ,SF6,HCN 4-What values of l , ml and ms are permitted for an electron with a principal quantum number equal to 3. 5-Draw electron dot diagrams showing the bonding in the following compounds: CO2, HCN, SiH4, BaF2 6-What is the electron configuration of: Na+, S2- and Fe3+ 7-What positive ion with a double charge in represented by each of the following configurations: 1s22s22p6 1s2 1s22s22p63s23p6 8- a) Write electron configuration using the noble gas that precedes for: Cr3+,Br b) How many p electrons are there in antimony atom Sb? c) Which orbital has the highest energy in 19K atom? d) Which element has the highest first ionization energy C, N, O? 9- what do you expect for the molecular geometry of tellurium tetrachloride, TeCl4? 10- a) How many valence electrons does the halogen have? b) What is the number of 5f orbitals?