Biochemistry: Cell Organization & Biomolecules Lecture Notes
advertisement

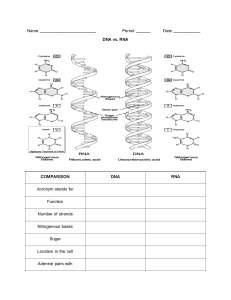
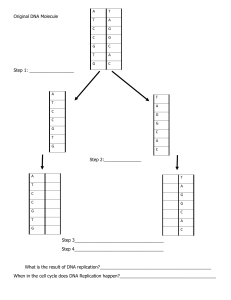
BIOCHEMISTRY Prepared by: Reuelle Kesha Anne A. Margate TOPIC 1: BIOCHEMISTRY AND THE ORGANIZATION OF CELLS 1. Definition of Biochemistry 2. The Cell a. b. Types of Cells: Prokaryotic and Eukaryotic Cells Cell Structures and Functions 3. Overview of the Biomolecules a. b. c. d. Amino acids and Proteins Carbohydrates and Glucose Nucleotides and Nucleic Acids Lipids and Fatty Acids organic chemistry the study of compounds of carbon, especially of carbon and hydrogen and their derivatives Amino Acids The simplest compounds are the amino acids. Under physiological conditions both the carboxyl group and amino group are ionized (COO2 and –NH3+, respectively). Amino acids can be shown in various ways, including a structural formula or a ball and stick formula. Amino acids have a basic structure where a central carbon atom is bonded to a carboxyl group, an amino group, a hydrogen, and a variable group, called Prokaryotes have nuclear region for DNA, ribosomes (for protein synthesis), cell membrane but do not have an internal membrane system. Biochemistry describes the molecular nature of life processes. In living cells, many chemical reactions take place simultaneously. Cells of all types have so many fundamental features in common that it is reasonable to say that they all had a common origin. Life is based on compounds of carbon. This is the subject matter of organic chemistry. The reactions of organic compounds are those of their functional groups, which are specifically linked atoms that react in similar ways under many different conditions. How did living things originate? Both molecules and cells must have arisen ultimately from very simple molecules, such as water, methane, carbon dioxide, ammonia, nitrogen, and hydrogen. Levels of structural organization in the human body Atoms > Molecules or Chemicals > Macromolecules > Organelles > Cell > Tissue > Organ > Organ System Essential Amino acids the body cannot make, and that is needed in the diet. Nucleic Acids: DNA and RNA The strand of DNA is composed of amino acids, it has a purine base backbone and a carbohydrate. DNA is a combination of carbohydrate, proteins, and amino acids. A simple Glucose is C6H12O6, two glucose is called disaccharide, and only glucose can enter the cell, all other carbohydrates cannot enter the cell. All carbohydrate needs to be converted to into glucose (ex. Fructose to Glucose) Only glucose can be converted into energy. (sugar + sugar= polysaccharide, amino acid+ acid= polypeptide) Glucose is converted into a pyruvate, whose byproduct will be NADH (Nicotinamide adenine dinucleotide) plus hydrogen. Glycolysis is the breaking down of glucose in the cell membrane. When NADH is produced in the inner membrane, it will be converted into NAD+ + H+, Hydrogen exits the intermembrane space and accumulates. Hydrogen will create electron charges (electron transport chain process). It will increase electron charges in the intermembrane, thus forcing the H to go back in the inner membrane though ATP synthase. The muscle is shortened through to the act of myosin and tropomyosin by pulling it in both directions. ATP is needed in order for the myosin to pull the tropomyosin going backwards with the muscle fiber and shortening or contracting the muscles. ATP (adenosine triphosphate) will be used as an energy resulting to ADP (adenosine diphosphate) + Pi (inorganic phosphate). ATP can be recycled, as long as there is hydrogen which is abundant in the glucose. High ATP signifies high energy state, and low ATP signifies low energy state. Thus, having a directly proportional relationship. Topic 2: WATER: THE SOLVENT FOR BIOCHEMICAL REACTION The partial negative charge of water is attracted to positively charged ions. Like-wise, the partial positive charge on the other end of the water molecule is attracted to negatively charged ions. PROPERTIES OF WATER Water is composed of two hydrogen (positively charged), and a hydrogen (negatively charged ion) which means water is a dipole. Water is polar, with partial positive charges on the hydrogens, partial negative charge on the oxygen, and a bent overall structure (104.3 degrees). 104.3 is the angle of one hydrogen to another hydrogen in relation to the position of the oxygen. Fats in the body are non-polar, they do not bind easily with water. All the substances in our body are polar, an important characteristic for the body to cleanse the body in the form of urine. Important fat-soluble vitamins: Vitamin A, D, E, K that should not be taken too much as it could overdose that body due to its non-polarity that can cause toxicity. Vitamin C technically has no overdosage as the water could dissolve it, but could be crystalized (kidney stones) if the liver fails to do its part. Thus, water is needed to eliminate substances. electronegativity -measure of the tendency of an atom to attract electrons to it in a chemical bond polar bonds -in which two atoms have an unequal share in the bonding electrons polar bonds- can bind to water nonpolar -refers to a bond in which two atoms share electrons evenly, cannot bind to water dipoles molecules- with positive and negative ends due to an uneven distribution of electrons in bonds salt bridge- an interaction that depends on the attraction of unlike charges Electrostatic attraction between like charges: Hydration shells surrounding ions in solution Unlike charges attract van der Waals radius- the distance between an atom’s nucleus and its effective electronic surface WATER AND OIL- The oil will aggregate itself into a micelle, thus oil with bind together leaving the water around. Sodium palmitate, an amphiphilic molecule: Amphiphilic molecules are frequently symbolized by a ball and zigzag line structure, where the ball represents the hydrophilic polar head and the zigzag line represents the nonpolar hydrophobic hydrocarbon tail. They are said to be immiscible. This is because water is a polar molecule – its structure means that is has a positive charge one end and a negative charge the other end. Water molecules stick together because the positive end of one water molecule is attracted to the negative end of another. (hydro)phobic- loving (hydro)phylic- hating Tail of the fats are non-polar, therefore oil is NON-POLAR molecule. Forces of attraction only exist between the unlike charges. They are said to be immiscible. This is because water is a polar molecule – its structure means that is has a positive charge one end and a negative charge the other end. Water molecules stick together because the positive end of one water molecule is attracted to the negative end of another. Water can be a donor or acceptor, it can influence the structure of a molecule and it can alter the structure of molecule (proton donor or proton acceptor). Carbohydrate- composed of carbon, hydrogen, oxygen (CH2O) Water can alternate the structure of a compound by giving or receiving a H molecule. CONCEPT OF pH Phenolphthalein pH indicator is the widely used indicator in measuring the unknown substance through the addition of base as the known substance. Water is Neutral (it can donate hydrogen or accept hydrogen) Acid is a proton donor Base is a proton acceptor conjugate- means added Acid strength is the amount of hydrogen ion released when dissolved in water the Neutral value of pH (7) as the base cut-off is derived from the Henderson- Hasselbalch equation Rule of thumb- the greater the Ka (dissociation constant), the stronger the acid. +acid= +hydrogen (increase of acid is increase of hydrogen directly proportional) If an acid will dissociate, it will become a conjugate base. If a base will dissociate, it will become a conjugate acid. The greater the dissociation constant, the greater the acid. Hydroxyl is equal to O+H, then Water is hydroxyl + hydrogen when water dissociates, equal number of OH- and H+ will cancel out and free Hydrogen (H+) ions will no longer be present thus the process of hydration of hydrogen occurs. water has a neutral pH because once it dissociates, into a H+O- + H+ , high likely it will for water again leaving no free H+ ions in the solution, thus making it neutral (since higher number of hydrogen implies higher acid strength). Characteristics of acid Buffering- acid is added to the two beakers on the left. The pH of unbuffered H2O drops dramatically while that of the buffer remains stable. Base is added to the two beakers on the right. The pH of the unbuffered water rises drastically while that of the buffer remains stable. The most common buffer is phosphate buffer. No matter how much volume of neutral substances, there will be a resistance of change to pH. Hydrochloric acid is acid, and Sodium hydroxide is a basic. Carbohydrate general molecular formula Cn(H2O)n is metabolized and have a byproduct of ATP’s, water, and carbon dioxide. CHO ATP + H2O + CO2 Bicarbonate also known as HC03 is the byproduct of the body’s metabolism. When humans respire, we produce a byproduct of carbon dioxide, and we do not want CO2 to bind with water in our body. carbon dioxide + water= carbonic acid (pH is below 7, with an acidity of 6 pH, the blood will have acidosis if there is too much carbonic acid in the blood . With homeostasis, carbon dioxide should be eliminated through exhalation. The natural buffer in the blood is bicarbonate buffer system or bicarbonate carbonic acid system. The Carbonic Acid-Bicarbonate buffer system or the Bicarbonate- Carbonic Acid System is the most important buffer for maintaining the pH homeostasis of blood. In this system, gaseous metabolic waste carbon dioxide reacts with water to form carbonic acid, which quickly dissociates into a hydrogen ion and bicarbonate. Blood should be slightly alkaline, otherwise it will be too acidic. Topic 3: THE FLOW OF GENETIC INFORMATION IN THE CELL Prepared by: Reuelle Kesha Anne A. Margate Topic Outline 1. 2. Central Dogma of Life a. Replication b. Transcription c. Translation RNA Synthesis and the Genetic Code a. RNA Synthesis and Regulation b. Basics of gene expression and gene regulation Albumin- most abundant protein in the body that serves as transport protein, and delivers important nutrients needed that is created from the liver. Cells in the liver create these protein replications to produce albumin through protein synthesis. Protein is used for muscle building that is why we should know its synthesis on how it is done within our bodies. Reverse transcription mostly exist in virus cells present in the body. Before cells divide they must synthesize a new copy of DNA though replication. When the DNA is used as a template to synthesize RNA, this process is called transcription. The RNA sequence of messenger RNA (mRNA) is used to direct the synthesis of proteins in a process called translation. It happens in the cytoplasm, specifically in the ribosome. CENTRAL DOGMA OF LIFE When carbonic acid (H2CO3) is dissociated by the buffer system, it will produce a HCO3 + H+, leaving a free hydrogen in the RBC which is a waste product of the body eliminated through urination. If there too much carbonate (CO2), it means there is a high pH then we need the free hydrogen to bind into the carbonate to form bicarbonate. Therefore, in order to achieve normal pH of the body, such system is used for the equilibrium of acid and alkaline. DNA REPLICATION Deoxyribonucleic acid (DNA) helix strands will be separated first through an enzyme called helicase to speed up the process. The DNA will be then transcribed into a mRNA through the help of participating enzymes DNA polymerase I (which binds the nucleotides Adenine, Thymine, Cytosine, and Guanine together). When a molecule of DNA is replicated, each of two strands is used as a template to create a complementary strand. When a cell divides into two, each of the two cells has one of the original template strands and one of the new strands called semiconservative replication. When DNA molecules are replicated, the strands are separated at origins of replications. Synthesis occurs in both directions from the origin along replication forks. origin of replication- the point at which the DNA double helix begins to unwind at the start of replication replication forks in DNA replication- the points at which new DNA strands are formed DNA ligase- the enzyme that links separate stretches of DNA DNA polymerase- the enzyme that forms DNA from deoxyribonucleotides on a DNA template primase- the enzyme that makes a short section Challenges in DNA replication 1. 2. 3. Separating DNA into 2 DNA strands- from a G0,G0 into two pairs: G0,G1 and G1,G0 which are both a semiconservative replication; then into a second replication with G0,G2 & G2, G1 and G1, G2 & G2, G0. Synthesize DNA from 5’ to 3’ end -the polymerase should be able to identify the prime ends to prevent mutation in the gene also known as insults. Guard against errors in replication- to prevent congenital defect caused by mutation in the information of the gene. Replication Proof Reading proofreading- the process of removing incorrect nucleotides when DNA replication is in progress repair- the enzymatic removal of incorrect nucleotides from DNA and their replacement by correct ones mutations- changes in DNA, causing subsequent changes in an organism that can be transmitted genetically DNA polymerase proofreading wherein the 3’ to 5’ (template strand) exonuclease activity of DNA polymerase I removes nucleotides from the 3’ end of the growing DNA chain. 1. 2. 3. Prevents Mutations- error in replication Removal of incorrect nucleotides immediately after they are added to the growing DNA during the replication process DNA polymerase I DNA polymerase removes the mismatch bases of nucleotides through the enzyme exonuclease in the exonuclease site to correct the coding in the DNA. The basics of transcription, RNA polymerase uses the template strand of DNA to make an RNA transcript that has the same sequence as the non-template DNA strand, with the exception that T is replaced by U. If this RNA is mRNA, it can later be translated to protein. Transcription is the process of using a DNA template to produce RNA Many types of RNA are produced such as messenger RNA (mRNA), transfer RNA (mRNA), ribosomal RNA (rRNA), micro-RNA, small inferring RNA, and small nuclear RNA A primer is not needed for RNA synthesis As does all polynucleotide synthesis, the reaction proceeds in the 5’ to 3’ direction. CODING STRAND- 5’ to 3’ TEMPLATE STRAND- 3’ to 5’ mRNA strand- 5’ to 3’ DNA Polymerase I 1. Klenow Fragment- Polymerase and Proof-Reading Activity 2. Repair Activity DNA polymerase I uses 3’ exonuclease activity to remove the incorrect nucleotide. Replication resumes when the correct nucleotide is added, also by DNA polymerase I The 5’ 3’ exonuclease activity of DNA polymerase I can remove up to 10 nucleotides in the 5’ direction downstream from a 3’-OH (3 prime hydroxyl group) single- strand nick. nick translation a type of DNA repair that involves polymerase I using its 5' to 3' exonuclease activity to remove primers or replace damaged nucleotides. DNA polymerase I uses its 3' exonuclease activity to remove the incorrect nucleotide. Replication resumes when the correct nucleotide is added, also by DNA polymerase I. Note that DNA will not undergo Transcription to convert into mRNA if its main goal is to duplicate itself alone. Instead, it will only undergo replication by producing a complementary strand. DNA TRANSCRIPTION If genes do not undergo transcription, it cannot produce proteins. General features of RNA Synthesis 1. 2. This transcription process is the synthesis of RNA through a DNA template, wherein the enzyme to catalyze the process is called DNA- dependent RNA. All four ribonucleic triphosphates: ATP (adenosine triphosphate), GTP (guanosine triphosphate), CTP (cytidine triphosphate), and UTP (uridine triphosphate) are required, as is Mg2+. Note than Magnesium is a cofactor of enzymes. Therefore, if a person has Hypomagnesemia, also known as magnesium deficiency, the enzymes will be inactive and the cells will be unable to have metabolic processes. It happens when you have a lower-than-normal level of magnesium in your blood. It can be mild or severe. Magnesium is an electrolyte that's a key part of many bodily reactions that affect cellular function, nerve conduction and more. 3. 4. 5. 6. A primer is not needed in NA synthesis, but a DNA template is required. Same case with DNA biosynthesis, the RNA chain grows from 5’ 3’ end. The nucleotide at the 5’ end of the chain retains its triphosphate group (abbreviated as PPP). The enzyme uses one strand of the DNA as the template for RNA synthesis. The base sequence of the DNA contains signals for initiation and termination of RNA synthesis. The enzyme binds to the template and moves along it in the 3’ 5’ direction. The template is unchanged. EUKARYOTIC TRANSLATION Steps in Translation 1. Chain Initiation- eIF (eukaryotic initiation factors) 2. Chain Elongation- peptidyl transferase and ribosome translocation 3. Chain Termination- encounter of Stop Codons (by-passed by suppressor RNA) mRNA- AUG (start codon) |UUC|CGA (stop codon: UAA, UGA, UAG) mRNA trinucleotides: AUG, UUC, CGA Methionine (UAC) pairs with AUG, Phenylalanine (AAG) pairs with UUC, Arginine (GCU) pairs with CGA. The decision on what amino acid the tRNA carries will be based on the trinucleotide sequence of the mRNA. Elongation will continue if there is a suppressor RNA. Link: https://www.youtube.com/watch?v=7cn10wayDug STEP 1: Chain Initiation- eIF (eukaryotic initiation factors) Translation occurs after mRNA formed in the nucleus and is transported out of the nucleus and into the cytoplasm where it attaches to the ribosomes. Proteins are assembled on the ribosomes using the mRNA nucleotide sequence as a guide. Thus, mRNA carries a “message” from the nucleus to the cytoplasm. In the Ribosomes in human cells after the mRNA is sent out in the cytoplasm have a ribosomal unit of (S stands for sub unit) 80S 60S 40S. Prokaryotes have 70S and 30 S. 1. Assembly of a 43S preinitiation complex. Methionine- initial amino acid attached to tRNAi The 60S and 40S will be activated by the initiation factors: eIF1A along with IF3 which will activate the 80S into a 43S. IF3 activates 40S that will form the preinitiation complex through the initial Met-tRNA (amino acid Methionine). The ribosome has two structures, overall, it is called 80S, bigger portion is called 60S and smaller portion is 40’. When added together it becomes the 80S complex. The 43S complex therefore contains Met-tRNA, GTP for energy, through eIF2. 2. mRNA is recruited. Scanning Mechanism- 5’ cap orients ribosome to the correct AUG. The presence of the 43S pre-initiation complex triggers the mRNA to do from 48S pre-initiation complex, then proceeds to 60S will be bound and finally turn into a 80S initiation complex. The ribosome will then select the start codon of the trinucleotide sequency which is AUG because of the start codon amino acid Methionine that should start on the 5’ end of the mRNA [at 59:00 on the LECVID]. RNA SYNTHESIS AND GENETIC CODE Summary: 80S is divided into portions 60S and 40S eIF1A and eIF3 initiation factors binds with 40S, forming 43S eIF2 and GTP initiates for the Met-tRNAi (referred as the ternary complex) 43S initiation complexmRNA is initiated with eIF4E,eIF4G,eIF4A,and eIF4B 48S binds with mRNA complex (48S initiation complex) 60S binds with 48S initiation complex with eIFS (eIF3, GDP and eIF2) 80S initiation complex. Enhancer- allows continuous elongation Initiator- used in translation of mRNA to tRNA and synthesis of protein TATA protein- stops the translation, mostly used in mutation of gene otherwise gene will dissolve through a process called sequestration. protein- is a sequence of amino acids peptide-is a short chain of amino acids P (peptidyl) site- the binding site on a ribosome for the tRNA that carries the growing peptide chain What is the function of peptidyl transferase in the ribosome? The ribosomal peptidyl transferase center (PTC) resides in the large ribosomal subunit and catalyzes the two principal chemical reactions of protein synthesis: peptide bond formation and peptide release. Note that you should know the complementary base pairs of the trinucleotide sequence of the mRNA. STEP 2: Chain Elongation peptide bond- an amide bond between amino acids in a protein translocation- in protein synthesis, the motion of the ribosome along the mRNA as the genetic message is being read 2 Eukaryotic Elongation Factors: a. eEF1 b. eEF2 1. Codon Recognition 2. Peptide Bond Formation 3. Translocation Step 3: Chain Termination Encounter of Stop Codons: 1. UAA 2. UAG 3. UGA No tRNA binds to stop codon, Hydrolysis and dissociation of ribosome large and small subunit Suppressor tRNA allows translation to continue resisting the stop codons by inserting selenocysteine residue to the amino acids present in mRNA. Found in mutated genes, cancer cells. chromatin- a complex of DNA and protein found in eukaryotic nuclei histones- basic proteins found complexed to eukaryotic DNA nucleosome- a globular structure in chromatin in which DNA is wrapped around an aggregate of histone molecules Note that: darker color in the nucleus means inactive and unexpressed genes, lighter portion in the nucleus contains active gene. All cells in our body contains similar chromosomes, however the expression of the gene depends because of the selective expression of gene. In order to prevent the gene from being expressed randomly there are 4 ways: 1. Transcription control 2. RNA processing control 3. RNA transport control 4. Translation control Whenever there is methylation or methyl groups attach to the DNA, the DNA will therefore not be expressed. Otherwise, Methyl group will not be found on places that require the gene. Sugar is linked to heterocyclic base by a beta-N-glycosidic bond, almost always to the N-1 of a pyrimidine and to the N-9 of a purine. Video link kay nahurot na akoang ATP: Nucleic acids - DNA and RNA structure https://www.youtube.com/watch?v=0lZRAShqft0 How mRNA Vaccines Work - Simply Explained https://www.youtube.com/watch?v=WOvvyqJ-vwo Cancer Gene Therapy - Aiming Gene Technology at Cancer Specific Molecular Targets https://www.youtube.com/watch?v=DNf2GsjfRQk Topic 4: NUCLEOTIDES AND NUCLEOSIDES Nucleosides Derivatives of purines and pyrimidines that have a sugar lilnked to a ring N Sugar in Ribonucleoside is D-ribose Sugar in Deoxyribonucleoside is 2-deoxy-D-ribose Gout pathophysiology/ Uric Acid Formation https://www.youtube.com/watch?v=2r95ZVqAysQ https://www.youtube.com/watch?v=bznoU5bke4U