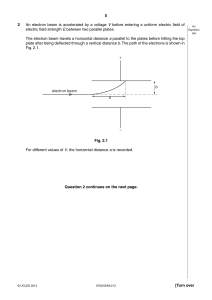
3 2 (a) State the de Broglie relation, explaining any symbols you use. .......................................................................................................................................... .......................................................................................................................................... .................................................................................................................................... [2] (b) An electron of mass m has kinetic energy E. Show that the de Broglie wavelength λ of this electron is given by λ= h . 2mE [2] (c) Calculate the potential difference through which an electron, initially at rest, must be accelerated so that its de Broglie wavelength is equal to 0.40 nm (the diameter of an atom). potential difference = .................................... V [3] © UCLES 2004 9702/04/M/J/04 Page 1 of 11 For Examiner’s Use 10 7 (a) Explain how a line emission spectrum leads to an understanding of the existence of discrete electron energy levels in atoms. .......................................................................................................................................... .......................................................................................................................................... .......................................................................................................................................... .................................................................................................................................... [3] (b) Some of the lines of the emission spectrum of atomic hydrogen are shown in Fig. 7.1. 410 434 486 656 wavelength / nm Fig. 7.1 The photon energies associated with some of these lines are shown in Fig. 7.2. wavelength / nm photon energy / 10–19 J 410 4.85 434 4.58 486 …………… 656 3.03 Fig. 7.2 (i) Complete Fig. 7.2 by calculating the photon energy for a wavelength of 486 nm. [2] © UCLES 2009 9702/42/O/N/09 Page 2 of 11 For Examiner’s Use 11 (ii) Energy levels of a single electron in a hydrogen atom are shown in Fig. 7.3. –0.60 –0.87 –1.36 –2.42 –5.45 energy / 10–19 J –21.80 Fig. 7.3 (not to scale) Use data from (i) to show, on Fig. 7.3, the transitions associated with each of the four spectral lines shown in Fig. 7.1. Show each transition with an arrow. [2] © UCLES 2009 9702/42/O/N/09 Page 3 of 11 For Examiner’s Use 12 7 (a) State an effect, one in each case, that provides evidence for (i) the wave nature of a particle, .............................................................................................................................. [1] (ii) the particulate nature of electromagnetic radiation. .............................................................................................................................. [1] (b) Four electron energy levels in an atom are shown in Fig. 7.1. –0.87 × 10–19 J –1.36 × 10–19 J electron energy –2.42 × 10–19 J –5.44 × 10–19 J Fig. 7.1 (not to scale) An emission spectrum is associated with the electron transitions between these energy levels. For this spectrum, (i) state the number of lines, .............................................................................................................................. [1] (ii) calculate the minimum wavelength. wavelength = ........................................... m [2] © UCLES 2010 9702/41/O/N/10 Page 4 of 11 For Examiner’s Use 24 7 (a) Explain how the line spectrum of hydrogen provides evidence for the existence of discrete electron energy levels in atoms. For Examiner’s Use .......................................................................................................................................... .......................................................................................................................................... .......................................................................................................................................... .......................................................................................................................................... ...................................................................................................................................... [3] (b) Some electron energy levels in atomic hydrogen are illustrated in Fig. 7.1. –0.85 eV –1.50 eV A energy B –3.40 eV Fig. 7.1 Two possible electron transitions A and B giving rise to an emission spectrum are shown. These electron transitions cause light of wavelengths 654 nm and 488 nm to be emitted. (i) On Fig. 7.1, draw an arrow to show a third possible transition. (ii) Calculate the wavelength of the emitted light for the transition in (i). [1] wavelength = ............................................ m [3] © UCLES 2011 9702/41/O/N/11 Page 5 of 11 [Turn over 25 (c) The light in a beam has a continuous spectrum of wavelengths from 400 nm to 700 nm. The light is incident on some cool hydrogen gas, as illustrated in Fig. 7.2. incident light cool hydrogen gas emergent light Fig. 7.2 Using the values of wavelength in (b), state and explain the appearance of the spectrum of the emergent light. .......................................................................................................................................... .......................................................................................................................................... .......................................................................................................................................... .......................................................................................................................................... .......................................................................................................................................... ...................................................................................................................................... [4] © UCLES 2011 9702/41/O/N/11 Page 6 of 11 For Examiner’s Use 30 7 (a) The emission spectrum of atomic hydrogen consists of a number of discrete wavelengths. Explain how this observation leads to an understanding that there are discrete electron energy levels in atoms. .......................................................................................................................................... .......................................................................................................................................... .......................................................................................................................................... ..................................................................................................................................... [2] (b) Some electron energy levels in atomic hydrogen are illustrated in Fig. 7.1. –0.54 eV –0.85 eV –1.5 eV energy –3.4 eV Fig. 7.1 © UCLES 2013 9702/42/M/J/13 Page 7 of 11 For Examiner’s Use 31 The longest wavelength produced as a result of electron transitions between two of the energy levels shown in Fig. 7.1 is 4.0 × 10–6 m. (i) For Examiner’s Use On Fig. 7.1, 1. draw, and mark with the letter L, the transition giving rise to the wavelength of [1] 4.0 × 10–6 m, 2. draw, and mark with the letter S, the transition giving rise to the shortest wavelength. [1] (ii) Calculate the wavelength for the transition you have shown in (i) part 2. wavelength = ............................................. m [3] (c) Photon energies in the visible spectrum vary between approximately 3.66 eV and 1.83 eV. Determine the energies, in eV, of photons in the visible spectrum that are produced by transitions between the energy levels shown in Fig. 7.1. photon energies .................................................................................... eV [2] © UCLES 2013 9702/42/M/J/13 Page 8 of 11 [Turn over 32 7 Some data for the work function energy ĭ and the threshold frequency f0 of some metal surfaces are given in Fig. 7.1. metal sodium zinc platinum ĭ / 10–19 J f0 / 1014 Hz 3.8 5.8 9.0 5.8 8.8 For Examiner’s Use Fig. 7.1 (a) (i) State what is meant by the threshold frequency. .................................................................................................................................. .................................................................................................................................. .............................................................................................................................. [2] (ii) Calculate the threshold frequency for platinum. threshold frequency = ............................................ Hz [2] (b) Electromagnetic radiation having a continuous spectrum of wavelengths between 300 nm and 600 nm is incident, in turn, on each of the metals listed in Fig. 7.1. Determine which metals, if any, will give rise to the emission of electrons. .......................................................................................................................................... .......................................................................................................................................... .......................................................................................................................................... ...................................................................................................................................... [2] (c) When light of a particular intensity and frequency is incident on a metal surface, electrons are emitted. State and explain the effect, if any, on the rate of emission of electrons from this surface for light of the same intensity and higher frequency. .......................................................................................................................................... .......................................................................................................................................... .......................................................................................................................................... ...................................................................................................................................... [3] © UCLES 2013 9702/41/M/J/13 Page 9 of 11 [Turn over 33 7 Electrons, travelling at speed v in a vacuum, are incident on a very thin carbon film, as illustrated in Fig. 7.1. thin carbon film fluorescent screen electron, speed v Fig. 7.1 The emergent electrons are incident on a fluorescent screen. A series of concentric rings is observed on the screen. (a) Suggest why the observed rings provide evidence for the wave nature of particles. .......................................................................................................................................... .......................................................................................................................................... .......................................................................................................................................... ...................................................................................................................................... [2] (b) The initial speed of the electrons is increased. State and explain the effect, if any, on the radii of the rings observed on the screen. .......................................................................................................................................... .......................................................................................................................................... .......................................................................................................................................... ...................................................................................................................................... [3] © UCLES 2013 9702/42/O/N/13 Page 10 of 11 For Examiner’s Use 34 (c) A proton and an electron are each accelerated from rest through the same potential difference. Determine the ratio For Examiner’s Use de Broglie wavelength of the proton . de Broglie wavelength of the electron ratio = .................................................. [4] © UCLES 2013 9702/42/O/N/13 Page 11 of 11 [Turn over