Chemistry, Biology, Physics Study Notes: Rate, Membrane, Electromagnetism
advertisement

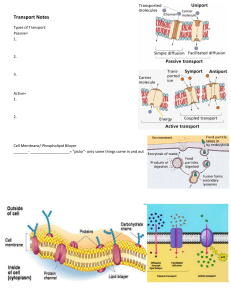
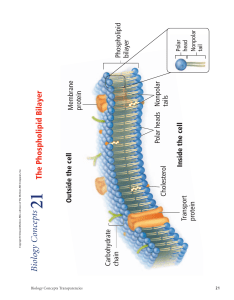
2 DEC 2023 Chemistry Rate of reaction (a must score essay) General definition: - Rate of reaction is the change of quantity of product/reactant per unit time Specific definition: - Question will mention ‘based on the experiment’ - Example of answer: o Rate of reaction is the changes in volume of hydrogen gas produced over time Five factors but usually four tested - Pressure (not been tested yet) - Size: total surface area expose to reaction/(acid used in the experiment) - Temperature: kinetic energy - Catalyst: usually is copper(II)sulphate (remember to write ‘II’) o Lowers activation energy o Particles that collide are easier to achieve activation energy - Concentration: hydrogen ions per unit volume Real life examples that are tested: - French fries and wedges - size - Satay - size - Ice box for storing food – related to kinetic energy Handy tip: - If the question asks to compare between two experiments o Must write ______________ in experiment one is higher than experiment two ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ Biology Chapter 3 Plasma membrane (must know) Phospholipid molecules in phospholipid bilayer: - Polar head (polar like magnet so can attract): attract water (hydrophilic) - Nonpolar tail (no polar so not like magnet): repels water (hydrophobic) In between the phospholipid bilayer, there are cholesterols - Function: make the phospholipid bilayer stronger and more flexible If carbohydrate chain connects with lipid: glycolipid If carbohydrate chain connects with protein: glycoprotein Glycolipid and glycoprotein have same function: - Stabilize the membrane by forming hydrogen bond with water - Act as antigen for cell identification (like passport) Physics chapter 4 electromagnetism F B I Fleming’s Left Hand Rule F B I Fleming’s Right Hang Rule