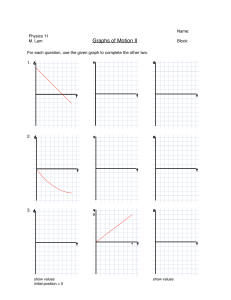
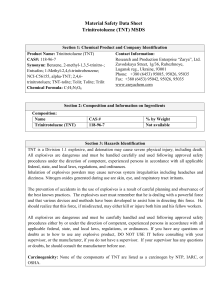
FirePhysChem 3 (2023) 281–291 Contents lists available at ScienceDirect FirePhysChem journal homepage: http://www.keaipublishing.com/cn/journals/firephyschem/ A mathematical model for estimating the Gurney velocity of chemical high explosives Dany Frem FREM Co., Beirut, Lebanon a r t i c l e i n f o Keywords: Gurney velocity High explosives Fragmentation warheads Insensitive explosives Weapons a b s t r a c t The Gurney velocity is an important performance parameter that characterizes the metal pushing capability of conventional chemical explosives. Herein, this study proposes a mathematical model that aims to provide a simple and effective means by which the Gurney velocity of pure and mixed CHNO-based explosives can be accurately determined using as input information the volumetric heat of detonation, the parameter psi (𝛹 ) and an adjustable parameter (𝜆) that accounts for the type of the explosive being studied. The new model proved adequate for evaluating the Gurney velocity of sensitive and insensitive explosives of military interest, including melt-castable and plastic-bonded explosives (PBXs) and showed superior predicting performance compared to benchmark models. It is believed that the Gurney velocity obtained by the new method along with the Gurneytype equations would be very helpful for ordnance engineers for calculating the peak fragment deployment velocity from various warhead geometries, including omnidirectional and directed energy warheads for use in various weapons systems. 1. Introduction Chemical high explosives derive their unique properties from the ultrafast and energetic chemical decomposition reactions induced by external stimuli such as shock, friction, or heat. What makes explosives particularly useful is that these chemical reactions are always accompanied by a sudden volume change. For instance, the detonation of one kg of HMX liberates more than 900 liters of gaseous products within a short time of a few microseconds [1]. The expansion of the detonation products is fast enough to induce shock waves in the surrounding medium, leading to destructive effects on nearby materials and structures. When the explosive charge is in intimate contact with a piece of metal of suitable thickness, like in the case of fragmenting warheads, large numbers of high-velocity fragments are produced, capable of inflicting serious injuries even at very long distances from the epicenter of the detonation. In fact, fragmentation warheads have found an important role in air-, seaand land-based weapon systems whose purpose is to deter and neutralize stationary and moving soft non-hardened targets which include, combat equipment, drones and loitering munitions, radar and communication installations, lightly protected vehicles and aircraft in the open. The effectiveness and lethality of such warheads depend upon many factors, among which are, the type of explosive, fragments material, size, shape, number and mass, fragment spray angle, the initial ejection fragments velocity and the velocity at target impact [2,3]. Wave shaping and the method of initiation have also been cited as significant factors that improve warhead performance [4]. Of particular importance, is the ability to produce optimal fragment size, shape and weight, tailored to the desired terminal effects. This requires means of controlling the breakup of the warhead casing which can be achieved, for example, by introducing grooves on the inner surface of the casing which weakens the structure and constitutes predetermined fracture points. Controlled fragmentation can also be readily obtained by using preformed fragments in the form of cubes or spheres made from steel or alloys of tungsten [5,6] placed in layers around the explosive charge in a polymer matrix. For a warhead designer, it is especially valuable to predict the velocity of the fragments ejected by a given warhead design. It comes as no surprise that the higher initial velocity corresponds to the higher kinetic energies of the fragments for target penetration. Higher kinetic energies also widen the lethal fragment range of the projectile [7]. During the 1940s, physicist Ronald W. Gurney provided simple equations from which the peak fragment velocity (𝑉0 ) of explosively driven cylinders and spheres can be obtained [8]. In deriving these equations, Gurney’s assumed that a fixed amount of chemical energy released during the explosive detonation is converted into the kinetic energy of the fragments and the expanding detonation products. It is also assumed that these products have uniform density and linear velocity profiles from the center of the explosive/metal system to the interface with the metal. The Gurney model predicts the velocity of the fragments √ in terms of the charge-to-metal ratio (𝐶∕𝑀) and the Gurney velocity ( 2𝐸G ). Other investigators have extended and applied the Gurney model to include complex metal/explosive geometries used in practical applications. Some of E-mail address: frem.dany@gmail.com https://doi.org/10.1016/j.fpc.2022.11.002 Received 17 August 2022; Received in revised form 8 November 2022; Accepted 13 November 2022 Available online 14 November 2022 2667-1344/© 2022 Xi’an Modern Chemistry Research Institute. Publishing services by Elsevier B.V. on behalf of KeAi Communications Co. Ltd. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/) D. Frem FirePhysChem 3 (2023) 281–291 using simple correlations and analytical models [9–14] or the output of complex thermochemical codes [15–17]. However, these methods have varying degrees of success and many of these will generally fail to predict the Gurney velocity when applied to certain classes of explosives, especially insensitive ones such as 1,3,5-triamino-2,4,6-trinitrobenzene (TATB) or 3-nitro-1,2,4-triazol-5-one (NTO). List of symbols 𝛽 𝐶 𝐶E 𝐶∕𝑀 𝐷 Δ𝐻f0 𝐸c 𝐸G 𝐸7𝑣0 𝐹 (𝑟) 𝑓 √𝑥 2𝐸G (𝐼) 𝜅𝑖 𝑘T 𝐿 𝜆 𝑀 𝑀arb 𝑀w 𝑁 𝑁arb 𝑁E 𝑛𝑖 Φ𝑖 𝛹 𝛹𝑖 𝛹mix 𝑄 𝑄arb 𝑄𝑖 𝑄mix 𝑅 𝑅0 𝑅e 𝜌 𝜌cu 𝑟𝑖 𝑉0 𝑉0 (𝑟) 𝑣∕𝑣0 𝑉7𝑣0 Model parameter that accounts for gas leakage Mass per unit length of the explosive charge (kg) Effective weight of explosive C (kg) Charge-to-metal ratio Detonation velocity (km/s) Standard heat of formation (kcal/mol) Specific cylinder energy [(mm/μs)2 /2 or MJ/kg] Gurney energy (MJ/kg) Specific cylinder energy at seven volumes expansion [(mm/μs)2 /2 or MJ/kg] Modification factor Velocity reduction coefficient Gurney velocity (km/s) A factor that accounts for gas leakage between preformed fragments Covolume of the ith detonation product Coefficient of tamping material Rod length (cm) Adjustable parameter (𝜆 = 0.25 or 0.5) Mass per unit length of the metal case (kg) Average molecular weight of gaseous detonation products calculated using the [H2 O–CO2 ] arbitrary decomposition assumption (g/mol) Molecular weight of the explosive (g/mol) Weight of tamper N (kg) Number of moles of gaseous detonation products per gram of explosive calculated using the [H2 O–CO2 ] arbitrary decomposition assumption (moles/g) Effective weight of tamper N (kg) Number of moles of the ith detonation product Mass fraction of the ith energetic component in the mixture Parameter psi (mol2 ∙Å3 /g) Parameter psi of the ith energetic component in the mixture (mol2 ∙Å3 /g) Parameter psi of the explosive mixture (mol2 ∙Å3 /g) Heat of detonation (kcal/g or kJ/g) Heat of detonation calculated using the [H2 O–CO2 ] arbitrary decomposition assumption (cal/g) Heat of detonation of the ith energetic component in the mixture (kcal/g or kJ/g) Heat of detonation of the explosive mixture (kcal/g or kJ/g) Radius from the center of the warhead to the outermost ring of rods (cm) Radius of the explosive charge (cm) Radius of the center core explosive (cm) Loading density (g/cm3 or kg/m3 ) Density of the copper cylinder (8.94 g/cm3 ) Radius from the center of the warhead to the ith ring of rods (cm) Peak fragment velocity (km/s) Peak velocity of the preformed fragment at a distance r from the charge centerline (km/s) Volume expansion ratio Cylinder test wall velocity at seven volumes expansion (km/s) 2. A new model for predicting the Gurney velocity In this section, a model is proposed by which the Gurney velocity can be estimated for sensitive and insensitive explosives including nitrogenrich compositions with minimal calculation effort. For a given explosive with the general formula Ca Hb Nc Od , the composition of the formed detonation products can be obtained by applying a set of rules and decomposition pathways (Fig. 1) as dictated by the oxygen balance of the studied explosive [23]. It is then easy to show that the heat of detonation (𝑄) can be computed using the expression [24]: [∑ ( ) ( )] 0 0 − 𝑁 𝑖=1 𝑛𝑖 Δ𝐻f detonation product 𝑖 − Δ𝐻f explosive 𝑄= (1) 𝑀w where 𝑀w is the molecular weight of the explosive, Δ𝐻f0 (detonation product)𝑖 and 𝑛𝑖 are the standard heat of formation and the number of moles of the ith detonation product, respectively. The heat of detonation is not only an important property of explosives but also a major factor used to determine the detonation velocity (𝐷) and pressure (𝑃 ) [25–27]. Furthermore, Akst [28] has shown that the volumetric heat of detonation (𝜌𝑄) directly affects the metal-propelling ability of an explosive as measured in the cylinder expansion test. Xiong [23] introduced the parameter psi (𝛹 ) as a very useful parameter along with the heat of detonation for deriving an empirical equation for estimating the detonation velocity of explosives. Considering the significant impact of the volumetric heat of detonation and the parameter psi on the performance of explosives, the trial-and-error approach was employed to find and optimize a model for predicting the Gurney velocity using (𝜌𝑄) and (𝛹 ) as key parameters: √√ √ ( ) 3 2𝐸G km∕s = 𝛹 𝜌𝑄 − 𝜆 (2) 𝛹= 𝑁 1 ∑ 𝑛𝜅 𝑀w 𝑖=1 𝑖 𝑖 (3) where (𝜌) is the loading density of the explosives in g/cm3 , while (𝑄) is the heat of detonation in kJ/g, 𝑛𝑖 and 𝜅𝑖 are the number of moles and the covolume (Table 2) of the ith detonation product, respectively. In order to reproduce the experimental Gurney velocities, it was necessary to include an additional variable (𝜆) into the model, which can take two values: 0.25 for hydrazinium nitrate-containing compositions and seriously deficient explosives (Type IV and V) and 0.5 for Type I, II and III explosives. For explosive mixtures with or without additional additives like waxes, binders and plasticizers, the heat of detonation (𝑄mix ) and the parameter psi (𝛹mix ) of the mixture are calculated as follows: 𝑄mix = 𝑁 ∑ 𝑖=1 𝛹mix = 𝛷𝑖 𝑄 𝑖 𝑁 ∑ 𝑖=1 𝛷𝑖 𝛹𝑖 (4a) (4b) where (𝛷𝑖 ), (𝑄𝑖 ) and (𝛹𝑖 ) are the mass fraction, the heat of detonation and the parameter psi of the ith energetic component in the mixture. An energetic component is defined as any compound bearing at least one energetic moiety such as nitro, nitramine, furazan, furoxan, tetrazine, etc. Table 3, shows how (𝑄) and (𝛹 ) can be calculated from the heat of formation and the stoichiometry of the explosive. these √ Gurney-type equations are shown in Table 1. The Gurney velocity ( 2𝐸G ) appearing in each of these equations is a characteristic of the explosives and varies with their loading density. There exist several approaches and methods by which the Gurney velocity can be estimated 282 D. Frem FirePhysChem 3 (2023) 281–291 Table 1 Gurney-type equations for peak fragment deployment velocity prediction of various warhead configurations. Warhead type Fragment velocity formula Preformed fragments [18] √ 𝑉0 = 2𝐸G ( 12 + Center core rod [19] 𝑉0 = Jellyroll [20] √ 𝑉0 = 2𝐸G [ Forward-Firing [21] 𝑉0 (𝑟) = √ 2𝐸G √ √ (1+ 𝑅e 𝐿 𝑉0 = 𝛽 = 0.5 (for cylindrical rod fragments) 𝛽 = 0.56 (for rectangular rod fragments) 𝐶 𝑀 𝑅e ∶ radius of the center core explosive (cm) )(1+ 2𝐶𝑀 ) 𝐶 𝑀 𝐶 0.5(1+ 𝑀 ) ] 1 2 𝑟 ( 𝑅𝑖 ) 𝑟𝑖 ∶ is the radius from the center of the warhead to the ith ring of rods (cm). 𝑅: is the radius from the center of the warhead to the outermost ring of rods (cm) √ 𝐶 3 𝐹 (𝑟) 𝑀 2𝐸G [ [(𝐹 (𝑟) LEFPa [22] Remarks 1 𝑀 −2 ) 𝛽𝐶 𝐶 2 𝐶 ) +5𝐹 (𝑟)( 𝑀 𝑀 √ 1+𝐴3 2𝐸G [ 3(1+ + 𝐴) 𝑁E 𝐶E 𝐴2 + )+4] 1 2 1 𝑀 −2 ] 𝐶E ] 𝐹 (𝑟): (1 − 𝐴= 𝑟(𝐼) 2 ) 𝑅0 1+2 𝐶𝑀 1+2 E 𝑁E 𝐶E 𝑁E = 𝑘T 𝑁 a LEFP: Linear Explosively Formed Projectile. Fig. 1. Assumed detonation products based on the type of explosive with the general empirical formula Ca Hb Nc Od . Table 2 The covolumes of the detonation product species. Species 𝜅𝑖 a H2 O 250 CO2 600 CO 390 N2 380 H2 214 O2 350 CH4 528 pansion is observed with a rotating mirror streak camera or its velocity is directly measured using Photonic Doppler Velocimetry (PDV) technique [30]. The wall velocities are reported at a radial displacement of 6 mm, 12.5 mm and 19 mm corresponding to volume expansion ratios (𝑣∕𝑣0 ) of 2, 4, and 7, respectively [31]. the 2 and 7 volume ratios are a figure of merit in explosive applications because it has been shown that for detonations impinging head-on against metals all the energy is transferred to the metal by the time the detonation products reach an expansion ratio of 2 while for grazing detonation the explosion products continue to effectively transfer energy until they reach a volume expansion ratio of 7 [32]. Moreover, the velocity at seven volumes expansion (𝑉7𝑣0 ) is generally assumed to be the terminal wall velocity since at this volume expansion the wall velocity can be reliably obtained before the test breaks up [33,34]. The Gurney velocities collated in Table 4 were primarily obtained from Dobratz & Crawford [35], however, for many other compositions it is the specific cylinder energy (𝐸c ), wall velocity or the Gurney energy (𝐸G ) that are reported in the literature. All of these quantities were evaluated at 𝑣∕𝑣0 = 7 and the Gurney velocity at the cor- C(s)a 46 C(s) stands for solid carbon. 2.1. Source of Gurney velocity data The Gurney velocity of solid and liquid explosives is routinely obtained from the cylinder expansion test. The test is the standard method that provides key hydrodynamic performance information on an explosive’s metal-pushing ability. A typical one-inch cylinder test consists of a fully annealed, oxygen-free high-conductivity (OFHC) copper tube 11.8inch long (300 mm) with a 1 inch inner diameter (25.4 mm) and a wall thickness of 0.1 inch (2.54 mm) [29]. The cylinder is filled with 250– 300 gs of explosive [30] and detonated at one end. The radial wall ex283 D. Frem FirePhysChem 3 (2023) 281–291 Table 3 Equations for the calculation of (𝑄) and (𝛹 ) for different types of explosives [23]. Explosive type Type I (𝑑 − 2𝑏 − 2𝑎 ≥ 0) Type II (𝑑 − 2𝑏 − 2𝑎 < 0) Type III (𝑑 − 2𝑏 − 𝑎 ≤ 0) Type IV (𝑑 − 2𝑏 ≤ 0; 𝑎 ≥ 𝑑 ) Type V (𝑑 − 2𝑏 ≤ 0; 𝑎 < 𝑑) 𝑄 (kcal/g)a 𝛹b 28.9𝑏+94𝑎+Δ𝐻f0 250𝑎+37.5𝑏+190𝑐+175𝑑 𝑀w 𝑀w 2.365𝑏+52𝑑−10𝑎+Δ𝐻f0 𝑀w 1.772𝑏+52𝑑−10𝑎+Δ𝐻f0 𝑀w 54.37𝑑−10𝑎+Δ𝐻f0 𝑀w 57.8𝑑−31.4𝑎+Δ𝐻f0 𝑀w 3. Results and discussion 3.1. Explosive compositions with 𝜆 = 0.5 Table 4 lists Gurney velocity values for more than fifty compositions of pure and mixed explosives many of which are currently used, or have the potential to be used in future ordnance programs. More importantly, the studied compositions have a wide range of loading densities, thermal stabilities and sensitivities toward external insults such as heat, impact, shock, friction, and electrostatic charge. Several interesting models were used to predict the Gurney velocity and their results were compared to the experimental data. The Keshavarz method [9] provides a simple means to obtain the Gurney velocity of an explosive at any loading density with a little computation time while requiring very few or no experimental parameters as input information: √ ( ) 2𝐸G km∕s = 0.404 + 1.020𝜌 − 0.021𝑐 + 0.184(𝑏∕𝑑 ) + 0.303(𝑑∕𝑎) (10) 46𝑎+9.95𝑏+190𝑐+277𝑑 𝑀w 46𝑎+36.75𝑏+190𝑐+277𝑑 𝑀w 46𝑎+107𝑏+190(𝑐+𝑑) 𝑀w 354𝑎+107𝑏+190𝑐+36𝑑 𝑀w a The heat of formation (Δ𝐻f0 ) is in kcal/mol. To apply Eq.(2), the calculated (𝑄) value in kcal/g needs to be. converted to kJ/g by multiplying it by the constant 4.184. b If 𝑎 = 0, 𝛹 ′ = 1.25𝛹 . If 𝑏 = 0, 𝛹 ′ = 1.06𝛹 (except Type I). If 𝑐 = 0, and 𝑑 = 0, 𝛹 ′ = 1.06𝛹 (for Type IV). If 𝑐 = 0, and 𝑑 = 0, 𝛹 ′ = 1.04𝛹 (for Type V). where a, b, c and d are the number of carbon (C), hydrogen (H), nitrogen (N) and oxygen (O) atoms in the explosive composition. The model developed by Locking [12] was chosen for the current analysis simply because the experimental detonation velocities for the majority of the studied explosives are readily available. The Locking model is simple and easy to apply, requiring only two input parameters, the detonation velocity and the loading density of the explosive: responding expansion ratio were calculated using Gurney’s equation for cylindrical geometry: √ √ 2𝐸G = 𝑉7𝑣0 1 + 0.5 𝐶∕𝑀 𝐶∕𝑀 ⎡ ⎤ ⎢ ⎥ 𝜌 1 𝐶∕𝑀 = ⎢ ( ) ⎥ 2 ⎢ OD − 1 ⎥ 𝜌cu ⎣ ID ⎦ √ (5) 𝐸7𝑣0 = 1 𝑉 2 7𝑣0 (6) √ (7) 0.5 2.2. Models performance evaluation The performance and accuracy of the new model as well as other models in estimating the Gurney velocity of explosives were evaluated by mean absolute percent error (MAPE) and root-mean-square error (RMSE) [36]. 𝑁 1 ∑ || 𝑦𝑖 − 𝑦̂𝑖 || × 100% 𝑁 𝑖=1 || 𝑦𝑖 || 0.5 (12) (13) where 𝜑 = 𝑁arb (𝑀arb ) (𝑄arb ) . One interesting point to note is that the majority of the studied compositions belong to Type III explosives whereas, PETN is the sole pure compound that has enough high oxygen balance to be classified as Type II. Moreover, LX-09 and Composition C-3 incorporate small amounts of energetic additives like bis(2-fluoro-2,2-dinitroethyl) formal (FEFO) and mononitrotoluene (MNT) belonging to Type II and Type IV energetic materials, respectively. From the analysis of the data in Table 4, it can be seen that the Gurney velocities calculated using Eq. (2) with 𝜆 = 0.5 are in good agreement with the experimental results in which 83% of the estimated values lie within ±3% of the actual values while the rest (17%) deviate by no more than ±5% (sample calculations can be found in the Appendix section). An important aspect of the new model is its ability to accurately predict the Gurney velocities of √ insensitive explosives. For example, using Eqs. (10)–(13) to estimate ( 2𝐸G ) of known insensitive materials like DNAN, NTO, SK TNBI, TATB and PBX-9502 will result in large deviations which, in some cases, amount to more than 16% (Eq. (10), PBX-9502 and pressed DNAN) whereas the Gurney velocities values obtained from Eq. (2) were within ±3% of the experimental results. Table 4 also shows that compared to the other models, Eq. (2) demonstrates superior prediction performance and better accuracy which is reflected in lower MAPE and RMSE scores of 1.67% and 0.05 km/s, respectively. Gurney energies at 𝑣∕𝑣0 = 7 were determined graphically from the curves of (𝐸G ) as a function (𝑣∕𝑣0 ). MAPE = √ ( ) 2𝐸G km∕s = 0.6 + 0.54 1.44𝜑𝜌 (𝐻 − 𝐾 ) and √ ( ) 2𝐸G km∕s = 0.887𝜑0.5 𝜌0.4 (𝐾 − 𝐹 ) )2 √ √ 𝑁 √1 ∑ ( )2 RMSE = √ 𝑦 − 𝑦̂𝑖 𝑁 𝑖=1 𝑖 (11) where (𝐷) is the detonation velocity (in km/s), (𝜌) is the loading density (in kg/m3 ) and 𝑓𝑥 is the velocity reduction coefficient. Hardesty & Kennedy (H-K) [14] and later Kamlet & Finger (K-F) [13] have related the Gurney velocity to the loading density and to Kamlet’s (𝜑) parameter [64] which in turn depends on the number of moles of gaseous detonation products per gram of explosive (𝑁arb ), the average molecular weight of these gasses (𝑀arb ), and on the heat of detonation (𝑄arb ) (in cal/g), all calculated using the√[H2 O–CO2 ] arbitrary decomposition assumption. The expressions for ( 2𝐸G ) are as follows: where (OD), (ID) and (𝜌cu ) are the outside diameter, internal diameter, and the density of the copper cylinder, respectively, and (𝜌) is the density of the explosive. The relationship between (𝐸7𝑣0 ) and the wall velocity (𝑉7𝑣0 ) at seven volumes expansion is: ( ⎡ 1 + 1.3𝜌 ⎤ ( ) 𝐷 1000 ⎥ 2𝐸G km∕s = and 𝑓x = 18.0467 ⎢ ⎢ 𝜌0 . 4 ⎥ 𝑓x ⎣ ⎦ (8) (9) where 𝑦𝑖 , 𝑦̂𝑖 and N are, respectively, the observed (or experimental) value, the predicted value, and the number of the considered data. The closer RMSD and MAPE are to zero the closer the predicted value of the model is to the experimental value. 284 D. Frem FirePhysChem 3 (2023) 281–291 Table 4 Predicted Gurney velocities for 52 explosive compositions at various loading densities as compared to experimental data. Percent deviations are shown in parentheses. Compositions PETN TNT TNT Tetryl HMX RDX HNS HNS NM DNAN (melt) DNAN (pressed) SK TNBI DAAF NTO NTO TATB FOX-7 Composition A-3 Composition-B Composition-B Composition C-3 Composition C-4 RDX/TNT (50/50) Cyclotol 75/25 Octol 75/25 Cyclotol 77/23 Octol 78/22 RDX/wax (94/6) HMX/wax (96/4) RDX/wax (95/5) PETN/Viton (95/5) LX-09 LX-10 LX-14 PBX-91C PBX-9404 PBX-9501 PBX-9502 QRX080 (FOX-7)vit CL-14/Viton A (97/3) NTO/FOX-7/Kel-F 800 (67/30/3) DNAN/RDX/NTO (40/20/40) TNT/RDX/NTO (40/20/40) KH 30 KH 50 NTO/DAAF/Kel-F 800 (67/30/3) PAX-60-MD PAX-63-MD AFX-521 RX-45-AA AmPicCC RMSE (km/s) MAPE (%) 𝜌 (g/cm3 ) Δ𝐻f0 (kcal/mol)a √ 2𝐸G (km/s) (Exp.)a √ 2𝐸G (km/s) (Eq.(2)) √ √ 𝐷 (km/s)𝜽 2𝐸G (km/s) (Eq.(11)) √ 2𝐸G (km/s) (Eq.(12)) √ 2𝐸G (km/s) (Eq.(13)) 1.76 1.63 1.2 1.62 1.89 1.77 1.6 1.2 1.14 1.45 1.52 1.55 1.691 1.77 1.855 1.854 1.78 1.59 1.71 1.717 1.60 1.60 1.64 1.754 1.821 1.754 1.821 1.65 1.780 1.650 1.710 −128.7 −16.0 −16.0 4.67 17.93 14.71 18.7 18.7 −27.0 −44.61b −44.61b −98.95c 106.12d −24.07b −24.07b −36.85 −32.00b 2.84 1.28 1.28 3.74 3.33 −0.21 3.21 2.78 3.48 3.17 3.10e 4.40f 4.53f −47.58 2.93 2.37 2.16p 2.50 2.97 2.93 2.53q 2.18q 2.41 2.00r 2.02r 2.14c 2.63s 2.37t 2.47s 2.38u 2.66v 2.63 2.70 2.71 2.68 2.66w 2.57t 2.79 2.83 2.79x 2.83x 2.73e 2.93y 2.72y 2.76y 8.26 6.93u 5.470 7.415𝜆 9.11 8.70 6.950 5.740 6.436𝜆 5.690 5.960 6.690 8.020 7.940 8.188 7.675 8.325 7.884𝜆 8.068𝜆 8.094𝜆 7.63 7.917𝜆 7.610 8.340𝜆 8.595𝜆 8.356𝜆 8.622𝜆 8.390 8.730 8.390 8.080 2.93 (0) 2.40 (1.27) 2.12 (−1.85) 2.62 (4.80) 2.95 (−0.67) 2.88 (−1.71) 2.47 (−2.37) 2.20 (0.92) 2.29 (−4.98) 2.04 (2.00) 2.08 (2.97) 2.15 (0.47) 2.51 (−4.56) 2.39 (0.84) 2.44 (−1.21) 2.40 (0.84) 2.64 (−0.75) 2.61 (−0.76) 2.69 (−0.37) 2.70 (−0.37) 2.63 (−1.87) 2.62 (−1.50) 2.61 (1.56) 2.78 (−0.36) 2.82 (−0.35) 2.79 (0) 2.83 (0) 2.70 (−1.10) 2.82 (−3.75) 2.72 (0) 2.81 (1.81) 2.97 (1.37) 2.42 (2.11) 1.98 (−8.33) 2.41 (−3.60) 2.95 (−0.67) 2.87 (−2.05) 2.26 (−10.67) 1.85 (−15.14) 2.43 (0.83) 2.28 (14.00) 2.35 (16.34) 2.29 (7.01) 2.43 (−7.60) 2.70 (13.92) 2.79 (12.96) 2.66 (11.76) 2.93 (10.15) 2.65 (0.76) 2.68 (−0.74) 2.69 (−0.74) 2.60 (−2.99) 2.66 (0) 2.57 (0) 2.78 (−0.36) 2.85 (0.71) 2.79 (0) 2.86 (1.06) 2.73 (0) 2.89 (−1.37) 2.74 (0.74) 2.92 (5.80) 2.77 (−5.46) 2.37 (0) 2.02 (−6.48) 2.54 (1.60) 2.99 (0.67) 2.91 (−0.68) 2.39 (−5.53) 2.12 (−2.75) 2.40 (−0.41) 2.01 (0.50) 2.08 (2.97) 2.32 (8.41) 2.72 (3.42) 2.65 (11.81) 2.70 (9.31) 2.53 (6.30) 2.78 (4.51) 2.72 (3.42) 2.72 (0.74) 2.73 (0.74) 2.63 (−1.87) 2.72 (2.26) 2.60 (1.17) 2.80 (0.36) 2.85 (0.71) 2.80 (0.36) 2.86 (1.06) 2.86 (4.76) 2.91 (−0.68) 2.86 (5.15) 2.73 (−1.09) 2.84 (−3.07) 2.42 (2.11) 2.16 (0) 2.55 (2.00) 2.92 (−1.68) 2.85 (−2.73) 2.41 (−4.74) 2.17 (−0.46) 2.37 (−1.66) 2.21 (10.50) 2.25 (11.39) 2.42 (13.08) 2.59 (−1.52) 2.57 (8.44) 2.62 (6.07) 2.57 (7.98) 2.74 (3.01) 2.63 (0) 2.68 (−0.74) 2.68 (−1.11) 2.63 (−1.87) 2.65 (−0.38) 2.60 (1.17) 2.75 (−1.43) 2.79 (−1.41) 2.76 (−1.08) 2.80 (−1.06) 2.70 (−1.10) 2.80 (−4.44) 2.71 (−0.37) 2.73 (−1.09) 2.90 (−1.02) 2.38 (0.42) 2.10 (−2.78) 2.55 (2.00) 2.98 (0.34) 2.90 (−1.02) 2.36 (−6.72) 2.11 (−3.21) 2.39 (−0.83) 2.12 (6.00) 2.16 (6.93) 2.38 (11.21) 2.59 (−1.52) 2.55 (7.59) 2.60 (5.26) 2.53 (6.30) 2.76 (3.76) 2.65 (0.76) 2.70 (0) 2.70 (−0.37) 2.65 (−1.12) 2.68 (0.75) 2.60 (1.17) 2.78 (−0.36) 2.83 (0) 2.79 (0) 2.84 (0.35) 2.73 (0) 2.85 (−2.73) 2.75 (1.10) 2.77 (0.36) 1.84 1.86 1.835 1.72 1.84 1.841 1.885 1.76 1.790 1.805 1.82 −3.14 1.50 4.07g 0.08 2.28 −20.84 −25.20h −30.99i 2.63j 2.89q 2.92w 2.80q 2.75z 2.90 2.90w 2.377 2.64𝛼 2.65𝛽 2.60s 8.840 8.82a 8.800 8.42 8.80 8.880𝜆 7.691𝜇 8.28 8.320 7.720 2.88 (−0.35) 2.85 (−2.40) 2.84 (1.43) 2.67 (−2.91) 2.86 (−1.38) 2.87 (−1.03) 2.34 (−1.56) 2.55 (−3.41) 2.55 (−3.77) 2.54 (−2.31) 2.99 (3.46) 2.99 (2.40) 2.95 (5.36) 2.76 (0.36) 3.00 (3.45) 2.98 (2.76) 2.76 (16.11) 2.87 (8.71) 2.91 (9.81) 2.62 (0.77) 2.92 (1.04) 2.90 (−0.68) 2.91 (3.93) 2.84 (3.27) 2.91 (0.34) 2.93 (1.03) 2.52 (6.02) 2.77 (4.92) 2.77 (4.53) 2.57 (−1.15) 2.86 (−1.04) 2.82 (−3.42) 2.84 (1.43) 2.70 (−1.82) 2.85 (−1.72) 2.86 (−1.38) 2.51 (5.60) 2.68 (1.52) 2.64 (−0.38) 2.56 (−1.54) 2.92 (1.04) 2.86 (−2.05) 2.89 (3.21) 2.72 (−1.09) 2.90 (0) 2.91 (0.34) 2.46 (3.49) 2.69 (1.89) 2.64 (−0.38) 2.53 (−2.69) 1.854 −23.08i 2.49s 8.141 2.47 (−0.80) 2.86 (14.86) 2.68 (7.63) 2.63 (5.62) 2.61 (4.82) 1.635 −15.09i 2.39r 7.040 2.36 (−1.26) 2.53 (5.86) 2.41 (0.84) 2.48 (3.77) 2.45 (2.51) 1.660 −8.90i 2.45r 7.410 2.47 (0.82) 2.56 (4.49) 2.52 (2.86) 2.54 (3.67) 2.53 (3.27) 1.810 1.825 1.803 i −7.75 −10.83i −1.59k 𝛾 2.76 2.73𝛾 2.47𝛿 8.600 8.480 7.934 2.71 (−1.81) 2.64 (−3.30) 2.42 (−2.02) 2.88 (4.35) 2.86 (4.76) 2.69 (8.91) 2.86 (3.62) 2.81 (2.93) 2.64 (6.88) 2.73 (−1.09) 2.70 (−1.10) 2.57 (4.05) 2.75 (−0.36) 2.71 (−0.73) 2.55 (3.24) 1.780 1.750 1.700 1.752 1.481 10.12l 15.20l −4.05m 3.77n −35.30° 2.85𝜀 2.77𝜀 2.47𝜁 2.31n 2.13𝜂 8.63 8.28 7.202 7.71 6.580 2.84 (−0.35) 2.78 (0.36) 2.45 (−0.81) 2.21 (−4.33) 2.04 (−4.23) 0.05 1.67 2.82 (−1.05) 2.68 (−3.25) 2.46 (−0.40) 2.68 (16.02) 2.40 (12.68) 0.18 5.45 2.88 (1.05) 2.78 (0.36) 2.44 (−1.21) 2.59 (12.12) 2.31 (8.45) 0.11 3.27 2.79 (−2.11) 2.72 (−1.81) 2.43 (−1.62) 2.48 (7.36) 2.26 (6.10) 0.10 3.00 2.83 (−0.70) 2.75 (−0.72) 2.38 (−3.64) 2.43 (5.19) 2.19 (2.82) 0.08 2.31 2𝐸G (km/s) (Eq.(10)) a Heats of formation (HOF) and Gurney velocities, unless otherwise stated, were taken from [35]. b [37]. c [38]. d [39]. e [40]. f HOF of wax was taken from [41]. g HOF of HTPB was taken from [42]. h HOF of FOX-7 and Hytemp were taken from [37] and [43], respectively. i HOF of FOX-7, NTO and DNAN were taken from [37]. j HOF of CL-14 was taken from [44].k HOF of NTO and DAAF were taken from [37] and [39], respectively. l HOF of wax and 3,4-Dinitropyrazole (DNP) were taken from [41] and [45] respectively.m HOF of PYX was taken from [46].n HOF of ANTA was taken from [47]. o HOF of HTPB, BDNPA/F and MDI were taken from [42, 48], and [46], respectively.p Average value from [49]. q [50]. r [51]. s [52]. t [53]. u [28]. v [54]. w [55]. x [14]. y [56]. z [57]. 𝛼 [58]. 𝛽 [59]. 𝛾 [60]. 𝛿 [61]. 𝜀 [45]. 𝜁 [62]. 𝜂 [63]. 𝜃 Unless otherwise stated, the experimental detonation velocity was taken from the same reference as the Gurney velocity. 𝜆 The detonation velocities were calculated using the BKW thermochemical code with the RDX parameter set [41]. 𝜇 The detonation velocity was calculated using the BKW thermochemical code with the TNT parameter set [41]. 285 D. Frem FirePhysChem 3 (2023) 281–291 Fig. 2. Chemical structures of explosives for which the Gurney velocity has been determined experimentally [65]. 3.1.1. Licht’s Gurney velocity data H.H. Licht has compiled an extensive body of data on experimental Gurney velocities performed at the French-German Research Institute of Saint-Louis (ISL) for pure and mixed CHNO-based explosives [65]. Cylindrical charges (16 mm diameter × 145 mm long) with metal confinement were employed to simultaneously obtain the detonation velocity and Gurney velocity at various loading densities. For insensitive explosives like TATB or cast TNT larger charges (25 mm diameter × 250 mm long) were used. Fig. 2 depicts the chemical structures for some of the investigated pure explosives including nitroaromatics, cyclic and acyclic nitramines, nitrate esters as well as hydrogen- and nitrogen-rich compounds like ANQ and TAGN. Equations used previously were once again used to evaluate the Gurney velocity of the compositions shown in Table 5 with one exception, Eq. (10) was excluded because it was found to be the least accurate among all the tested models. It was found from the results in Table 5 that 90% of the estimated Gurney velocities using Eq. (2) lie within ±4% of the experimental values compared to 60%, 67% and 63% for Eqs. (11)–(13), respectively, proving once more the superior performance of the new model in pre286 D. Frem FirePhysChem 3 (2023) 281–291 Table 5 Comparison of experimental and predicted Gurney velocities using Eq.(2) and Eqs.(11)-(13). Percent deviations are shown in parentheses. Compositions HMX PETN RDX TeNHHPm NMP TNAD Tetryl Bis-MNDPy TMNTz 2-MNDPy TNT PETN TATB NTO DADPyOx ADPyOx AMP DINA NITRA HMX/TNT (70/30) RDX/TNT (60/40) HMX/ETN (65/35) ETN/HMX (80/20) RDX/HTPB (85/15) PETN/rubber (89/11) TATB/TNT (60/40) DINA-Dynamite (90/10) NC/DINA (60/40) NTO/HNE/wax (76/19/5) √ 2𝐸G (km/s) (Eq.(2)) √ √ 2𝐸G (km/s) (Eq.(12)) √ 2𝐸G (km/s) (Eq.(13)) 8.773 8.142 8.489 8.368 8.054 7.775 7.573 7.651q 7.228 7.372q 6.913 5.516 7.539 7.959 7.328 6.963 7.876 7.713 7.350 8.319 2.90 (−2.03) 2.90 (−0.68) 2.86 (−0.35) 2.92 (3.55) 2.84 (4.80) 2.63 (−1.13) 2.68 (1.52) 2.63 (0.77) 2.45 (−2.00) 2.53 (1.61) 2.39 (0) 2.32 (−1.28) 2.40 (2.56) 2.41 (3.88) 2.36 (2.61) 2.28 (3.64) 2.67 (2.69) 2.74 (−5.19) 2.24 (−1.75) 2.79 (0) 2.91 (−1.69) 2.74 (−6.16) 2.86 (−0.35) 2.80 (−0.71) 2.70 (−0.37) 2.66 (0) 2.56 (−3.03) 2.62 (0.38) 2.52 (0.80) 2.54 (2.01) 2.38 (−0.42) 2.11 (−10.21) 2.48 (5.98) 2.64 (13.79) 2.44 (6.09) 2.36 (7.27) 2.68 (3.08) 2.65 (−8.30) 2.55 (11.84) 2.76 (−1.08) 2.87 (−3.04) 2.81 (−3.77) 2.82 (−1.74) 2.84 (0.71) 2.79 (2.95) 2.66 (0) 2.61 (−1.14) 2.60 (−0.38) 2.53 (1.20) 2.52 (1.20) 2.41 (0.84) 2.27 (−3.40) 2.57 (9.83) 2.60 (12.07) 2.54 (10.43) 2.44 (10.91) 2.68 (3.08) 2.71 (−6.23) 2.47 (8.33) 2.77 (−0.72) 2.93 (−1.01) 2.87 (−1.71) 2.88 (0.35) 2.90 (2.84) 2.83 (4.43) 2.68 (0.75) 2.61 (−1.14) 2.60 (−0.38) 2.53 (1.20) 2.50 (0.40) 2.36 (−1.26) 2.29 (−2.55) 2.54 (8.55) 2.57 (10.78) 2.50 (8.70) 2.39 (8.64) 2.71 (4.23) 2.76 (−4.50) 2.45 (7.46) 2.80 (0.36) 2.75 7.965 2.71 (−1.45) 2.68 (−2.55) 2.69 (−2.18) 2.71 (−1.45) −8.89l 2.89 8.645q 2.90 (0.35) 2.88 (−0.35) 2.88 (−0.35) 2.95 (2.08) 1.75 −28.11l 2.93 8.160 2.89 (−1.37) 2.74 (−6.48) 2.88 (−1.71) 2.96 (1.02) 1.57 3.55m 2.56 7.897 2.50 (−2.34) 2.73 (6.64) 2.55 (−0.39) 2.56 (0) 1.2 −37.77m 2.38 6.431 2.35 (−1.26) 2.37 (−0.42) 2.35 (−1.26) 2.35 (−1.26) 1.79 −11.38 2.42 7.303 2.42 (0) 2.43 (0.41) 2.53 (4.55) 2.49 (2.89) 1.61 −34.09n 2.85 7.665 2.71 (−4.91) 2.63 (−7.72) 2.69 (−5.61) 2.73 (−4.21) 1.55 −51.76 n 2.49 7.227 2.54 (2.01) 2.51 (0.80) 2.56 (2.81) 2.57 (3.21) 1.64 −14.63° 2.27 7.523 2.20 (−3.08) 2.57 (13.22) 2.57 (13.22) 2.56 (12.78) 1.63 −11.23p 2.32 6.986 2.25 (−3.02) 2.39 (3.02) 2.65 (14.22) 2.68 (15.52) 0.07 2.06 0.14 4.17 0.14 4.28 0.13 3.85 𝜌 (g/cm3 ) Δ𝐻f0 (kcal/mol)a 1.81 1.72 1.73 1.76 1.75 1.64 1.71 1.63 1.53 1.60 1.60 0.98 1.86 1.81 1.80 1.69 1.67 1.62 1.56 1.81 √ 2𝐸G (km/s) (Exp.)b 𝐷 (km/s) 17.93 −128.7 14.71 2.00c −31.55d 17.5e 4.67 36.45f 65.75f 22.44f −16.0 −128.7 −36.85 −24.07 g −31.60h −45.50i 65.73d −73.33j 22.44k 2.13 2.96 2.92 2.87 2.82 2.71 2.66 2.64 2.61 2.50 2.49 2.39 2.35 2.34 2.32 2.30 2.20 2.60 2.89 2.28 2.79 1.74 1.16 1.79 b DINGU/HNE/wax (63/32/5) RMSE (km/s) MAPE (%) 2𝐸G (km/s) (Eq.(11)) a Heats of formation (HOF), unless otherwise stated, were taken from [35]. b Experimental Gurney and detonation velocities were taken from [65] unless otherwise stated. c [66]. d [67]. e [68]. f [69]. g [37]. h [70]. i [71]. j [46]. k [72]. l HOF of ETN was taken from [73]. m HOF of HTPB (or rubber) was taken from [42]. n HOF of DINA was taken from [46] while HOF of NC and dynamite (both considered as Nitrocellulose with 12% nitrogen content) was taken from [35]. o HOF of NTO and HNE were taken from [37] while HOF of wax was taken from [41]. p HOF of DINGU, HNE and wax were taken from [74, 37] and [41], respectively.q The detonation velocities were calculated using the BKW thermochemical code with the RDX parameter set [41]. dicting the Gurney velocities of different classes of explosives at any loading density. Slightly larger deviations (around 5%) were observed for NMP, DINA and DINA-Dynamite. Also, mention should be made that NMP and DINA are the only studied compounds bearing a nitramine and a nitrate ester functionality. On the other hand, however, Eq. (2) correctly reproduces (deviations < 4%) the experimentally observed √ ( 2𝐸G ) for insensitive nitrobenzene and nitroheterocyclic derivatives like TATB, NTO and DADPyOx, which was not the case for the other equations. Recall that all equations, except Eq. (2), had a trend to overestimate the Gurney velocity of most insensitive compositions compared with the measured value. Moreover, Eqs. (11)–(13) have almost double the MAPE and RMSE values, indicating a lower predictive power and accuracy level than the best performing model Eq. (2). observed Gurney velocities, and therefore the value of the adjustable parameter (λ) had to be changed from 0.5 to 0.25 which results in model estimates that are in close agreement with the empirical data. Fig. 3 shows the chemical structure of compounds used in the formulation of the explosives listed in Table 6, which, except for hydrazinium nitrate (HN), fall into Type IV or V explosives. Further, it is noticed in Fig. 3 that these compounds are particularly hydrogen- and nitrogenrich energetic materials and that many of these feature the guanidine moiety substituted with nitro- or amino functionalities like NQ, ANQ and TAGN. One interesting observation that emerges from the analysis of the results provided in Table 6 is that Eq. (11) fails to correctly estimate the Gurney velocity of explosives compositions listed in the first five entries of the table which was not surprising since, as mentioned earlier, these compositions are distinguished by high contents of hydrogen, a fact that contributes to their high detonation velocity despite the low to moderate loading densities as seen, for example, in the case of TAGN and ANQ (1.47 g/cm3 and 1.66 g/cm3 , respectively). The observed high detona- 3.2. Explosive compositions with 𝜆 = 0.25 For a small group of pure and mixtures of explosives, it was found that the use of Eq. (2) with 𝜆 = 0.5 consistently underestimate the 287 D. Frem FirePhysChem 3 (2023) 281–291 Fig. 3. Chemical structures for hydrogen- and nitrogen-rich neutral and ionic high explosives. Table 6 Comparison of experimental and predicted Gurney velocities for explosives compositions with λ = 0.25. Percent deviations are shown in parentheses. Compositions TAGN ANQ X-0535 TKX-50/paraffin (97/3) AFX-902 NQ/TNT (50/50) NQ/TNT (35/65) NQ/HMX/Estane (45.3/50.2/4.5) HMX/HN/TAGN (45/40/15) HMX/HN (70/30) HN/HMX (65/35) RDX/HN (55/45) PETN/HN (45/55) RMSE (km/s) MAPE (%) √ 2𝐸G (km/s) (Eq.(2)) √ √ 2𝐸G (km/s) (Eq.(12)) √ 2𝐸G (km/s) (Eq.(13)) 8.048 8.522 8.453l 8.810h 2.44 (−1.21) 2.51 (−1.57) 2.60 (4.00) 2.69 (1.51) 2.83 (14.57) 2.90 (13.73) 2.82 (12.80) 2.96 (11.70) 2.58 (4.45) 2.66 (4.31) 2.59 (3.60) 2.74 (3.40) 2.61 (5.67) 2.68 (5.10) 2.57 (2.80) 2.76 (4.15) 2.36i 2.45j 8.344i 7.300j 2.26 (−4.24) 2.51 (2.45) 2.80 (18.64) 2.48 (1.22) 2.52 (6.78) 2.52 (2.86) 2.49 (5.51) 2.50 (2.04) −12.02 2.45j 7.050j 2.56 (4.49) 2.40 (−2.04) 2.50 (2.04) 2.47 (0.82) 1.757 −10.86 2.67k 8.561l 2.74 (2.62) 2.87 (7.49) 2.71 (1.50) 2.73 (2.25) 1.73 −23.16f 2.88 9.008 2.91 (1.04) 3.03 (5.21) 2.85 (−1.04) 2.92 (1.39) 1.78 −14.40f 2.95 9.000 3.04 (3.05) 3.00 (1.69) 2.88 (−2.37) 2.95 (0) 1.71 −38.27f 2.88 9.023 2.87 (−0.35) 3.05 (5.90) 2.87 (−0.35) 2.94 (2.08) 1.68 −24.32 f 2.85 8.675 2.93 (2.81) 2.94 (3.16) 2.83 (−0.70) 2.90 (1.75) 1.65 −52.51f 2.71 8.277 2.91 (7.38) 2.82 (4.06) 2.82 (4.06) 2.90 (7.01) 0.09 2.82 0.24 7.86 0.08 2.88 0.10 3.12 𝜌 (g/cm3 ) Δ𝐻f0 (kcal/mol)a 1.47 1.66 1.787 1.750 √ 2𝐸G (km/s) (Exp.)b 𝐷 (km/s) −11.50c 5.27c 21.34d 19.90e 2.47 2.55 2.50 g 2.65h 1.742 1.665 −29.09 −14.15 1.658 b 2𝐸G (km/s) (Eq.(11)) a Heats of formation (HOF), unless otherwise stated, were taken from [35]. b Experimental Gurney and detonation velocities were taken from [65] unless otherwise stated. c [37]. d HOF of LAX-112 was taken from [75]. e HOF of TKX-50 and paraffin (wax) were taken from [76] and [41], respectively. f HOF of TAGN and hydrazinium nitrate (HN) were taken from [37]. g [77]. h [76]. i [28]. j [50]. k [78]. l The detonation velocities were calculated using the BKW thermochemical code with the RDX parameter set [41]. tion velocity for the hydrogen-rich systems may be attributed to their high Chapman-Jouguet (CJ) particle density or the number of moles of gas per gram of explosive [41], however, such an increase in detonation velocity does not necessarily translate into high Gurney velocity which is most likely due to their low heats of detonation. In fact, the calculated heat of detonation for TAGN and ANQ is around 25% lower than that of the conventional explosive TNT (𝑄TNT = 4.329 kJ/g). On the other hand, predictions made by Eq. (2) and Eqs. (12) and (13) that take into account the heat of detonation are in close agreement with experimen- tal data, displaying comparable accuracy in terms of MAPE and RMSE values. 4. Conclusions The new model Eq. (2) has demonstrated a decent prediction accuracy in Gurney velocity determination, compared with the results obtained by Eqs. (10)–(13). With the adjustable parameter (𝜆) set to 0.5, Eq. (2) successfully predicted the Gurney velocity of most of the in288 D. Frem FirePhysChem 3 (2023) 281–291 vestigated explosives including sensitive and insensitive explosives. For explosives belonging to Type IV and V and for hydrazinium nitratecontaining compositions a value of 0.25 for (𝜆) was necessary for Eq. (2) to achieve satisfactory performance compared to the other methods considered. An important implication that emerges from this study is that models based on detonation velocity like Eq. (11) display poor prediction ability when applied to insensitive and hydrogen-rich explosives, in that they tend to overestimate their Gurney velocities which indicate that chemical explosives designed to have high detonation velocity may not necessarily exhibit high Gurney velocity. 𝛹mix = Substituting the value of (𝑄mix ), (𝛹mix ), (𝜌) and (𝜆) into Eq. (2) gives: √√ √ 3 2𝐸G = 14.74 (1.742)(2.373) − 0.25 √ 2𝐸G = 2.26 km∕s Example 2: Cyclotol 77/23 is made of 77wt% RDX and 23wt% TNT. Both RDX and TNT are Type III explosives. The values of (𝑄) and (𝛹 ) for TNT and RDX are calculated as follow: ( ) 1.772(5) + 52(6) − 10(7) − 16 𝑄TNT = = 1.035 kcal/g 4.330 kJ∕g 227 The author declares that there is no conflict of interest concerning this paper. Appendix 𝛹TNT = I. Pure compounds 46(7) + 36.75(5) + 190(3) + 277(6) = 12.06 227 𝑄RDX = Example 1: For pentaerythritol tetranitrate (PETN, C5 H8 N4 O12 , Type II explosive, λ = 0.5) the heat of detonation and the parameter psi are calculated from the equations in Table 3: 𝛹= (0.95 × 15.52) = 14.74 𝑖=1 Declaration of Competing Interest 𝑄 = 𝑁 ∑ 𝛹RDX = ( ) 1.772(6) + 52(6) − 10(3) + 14.71 = 1.384 kcal∕g 5.791 kJ∕g 222 46(3) + 36.75(6) + 190(6) + 277(6) = 14.24 222 (𝑄mix ) and (𝛹mix ) for cyclotol 77/23 are calculated using Eqs. (4a) and (4b): ( ) 2.365(8) + 52(12) − 10(5) − 128.7 = 1.469 kcal∕g 6.147 kJ∕g 316 46(5) + 9.95(8) + 190(4) + 277(12) = 13.90 316 𝑄mix = 𝑁 ∑ (0.23 × 4.330) + (0.77 × 5.791) = 5.455 kJ∕g 𝑖=1 Substituting the value of (𝑄), (𝛹 ), (𝜌) and (𝜆) into Eq. (2) gives: √√ √ 3 2𝐸G = 13.90 (1.76)(6.147) − 0.5 𝛹mix = √ Substituting the value of (Qmix ), (𝛹mix ), (𝜌) and (𝜆) into Eq. (2) gives: √√ √ 3 2𝐸G = 13.74 (1.754)(5.455) − 0.5 Example 2: Pressed 2,4-dinitroanisole (DNAN, C7 H6 N2 O5 , Type III explosive, λ = 0.5) ( ) 1.772(6) + 52(5) − 10(7) − 44.61 𝑄 = = 0.788 kcal∕g 3.297 kJ∕g 198 √ 46(7) + 36.75(6) + 190(2) + 277(5) = 11.65 198 2𝐸G = 2.08 km∕s II. Mixture of explosives Example 1 For AFX-902 (95/5 NQ/Viton A) the heat of detonation and the parameter psi are calculated for NQ (CH4 N4 O2 , Type V explosive, λ = 0.25) only since Viton A is an inert binder: 𝑄 = ( ) 57.8(2) − 31.4(1) − 22.11 = 0.597 kcal∕g 2.498 kJ∕g 104 𝛹 = 354(1) + 107(4) + 190(4) + 36(2) = 15.52 104 (𝑄mix ) and (𝛹mix ) are calculated for the energetic component (NQ) using Eqs. (4a) and (4b): 𝑄mix = 𝑁 ∑ 2𝐸G = 2.79 km∕s List of chemical and atomic compositions of explosive compounds/formulations ADPyOx: 4-Amino-3,5-dinitropyridine-N-oxide (C5 H4 N4 O5 ) AFX-521: 95/5 PYX/Kel-F 800 (C2.697 H1.095 N1.683 O2.448 F0.133 Cl0.036 ) AFX-902: 95/5 NQ/Viton A (C1.047 H3.747 N3.654 O1.827 F0.174 ) AMP: Azidomethyl-trinitrohexahydropyrimidine (C5 H8 N8 O6 ) AmPicCC: 80/12/6.5/1.5 Explosive D/HTPB/BDNPA-F/MDI (C3.043 H3.554 N1.402 O2.519 ) ANQ: 1-Amino-3-nitroguanidine (CH5 N5 O2 ) CL-14/Viton A (97/3): C2.354 H1.572 N2.273 O2.273 F0.104 Composition A-3: 91/9 RDX/wax (C1.87 H3.74 N2.46 O2.46 ) Composition-B: 63/36/1 RDX/TNT/wax (C2.03 H2.64 N2.18 O2.67 ) Composition C-3: 77/4/10/5/1/3 RDX/TNT/Dinitrotoluene/ Mononitrotoluene/ Nitrocellulose/Tetryl (C1.90 H2.83 N2.34 O2.60 ) Composition C-4: 91/5.3/2.1/1.6 RDX/Di(2-ethylhexyl) sebacate/Polyisobutylene/Motor oil (C1.82 H3.54 N2.46 O2.51 ) Cyclotol 75/25: RDX/TNT (C1.78 H2.58 N2.36 O2.69 ) Cyclotol 77/23: RDX/TNT (C1.750 H2.588 N2.385 O2.689 ) DAAF: 3,3′-Diamino-4,4′-Azoxyfurazan (C4 H4 N8 O3 ) DADPyOx: 2,6-Diamino-3,5-dinitropyridine-N-oxide (C5 H5 N5 O5 ) DINA: Dioxyethylnitramine dinitrate (C4 H8 N4 O8 ) DINA-Dynamite (90/10): C1.728 H3.294 N1.586 O3.361 DINGU/HNE/wax (63/32/5): C1.657 H1.800 N2.269 O2.909 DNAN: 2,4-Dinitroanisole (C7 H6 N2 O5 ) DNAN/RDX/NTO (40/20/40): C2.300 H2.368 N2.175 O2.474 ETN/HMX (80/20): C1.330 H2.130 N1.600 O3.719 FOX-7: 1,1-Diamino-2,2-dinitroethylene (C2 H4 N4 O4 ) Substituting the value of (𝑄), (𝛹 ), (𝜌) and (𝜆) into Eq. (2) gives: √√ √ 3 2𝐸G = 11.65 (1.52)(3.297) − 0.5 √ (0.23 × 12.06) + (0.77 × 14.24) = 13.74 𝑖=1 2𝐸G = 2.93 km∕s 𝛹= 𝑁 ∑ (0.95 × 2.498) = 2.373 kJ∕g 𝑖=1 289 D. Frem FirePhysChem 3 (2023) 281–291 (FOX-7)vit: 94/6 FOX-7/Viton A (C1.431 H2.653 N2.541 O2.541 F0.208 ) HMX: 1,3,5,7-Tetranitro-1,3,5,7-tetraazacyclooctane (C4 H8 N8 O8 ) HMX/ETN (65/35): C1.342 H2.452 N2.220 O3.147 HMX/HN (70/30): C0.946 H3.471 N2.839 O2.839 HMX/HN/TAGN (45/40/15): C0.698 H4.130 N3.108 O2.749 HMX/TNT (70/30): C1.871 H2.553 N2.288 O2.685 HMX/wax (96/4): C1.583 H3.166 N2.595 O2.595 HN: Hydrazinium nitrate (H5 N3 O3 ) HN/HMX (65/35): C0.473 H4.367 N2.999 O2.999 HNS: 2,2′,4,4′,6,6′-Hexanitrostilbene (C14 H6 N6 O12 ) KH 30: 66.5/30/3.5 HMX/NTO/Viton A (C1.454 H2.324 N2.720 O2.490 F0.122 ) KH 50: 47.5/50/2.5 HMX/NTO/Viton A (C1.478 H2.100 N2.822 O2.438 F0.087 ) LAX-112: 3,6-Diamino-s-tetrazine-1,4-dioxide (C2 H4 N6 O2 ) LX-09: 93/4.6/2.4 HMX/DNPA/FEFO (C1.43 H2.74 N2.59 O2.72 F0.02 ) LX-10: 95/5 HMX/Viton A (C1.41 H2.66 N2.57 O2.57 F0.16 ) LX-14: 95.5/4.5 HMX/Estane 5702-F1 (C1.52 H2.92 N2.59 O2.66 ) 2-MNDPy: 2-Methylnitramino-3,5-dinitropyridine (C6 H5 N5 O6 ) Bis-MNDPy: 2,6-Bis(methylnitramino)−3,5-dinitropyridine (C7 H7 N7 O8 ) NC/DINA (60/40): C2.032 H3.094 N1.181 O3.499 NITRA: 3-Nitramino-1,2,4-triazole (C2 H3 N5 O2 ) NM: Nitromethane (CH3 NO2 ) NMP: 5-Nitroxymethyl-1,3,5-trinitro-hexahydropyrimidine (C5 H8 N6 O9 ) NQ: Nitroguanidine (CH4 N4 O2 ) NQ/HMX/Estane (45.3/50.2/4.5): C1.345 H3.437 N3.108 O2.307 NQ/TNT (35/65): C2.341 H2.778 N2.205 O2.391 NQ/TNT (50/50): C2.023 H3.024 N2.584 O2.283 NTO: 3-Nitro-1,2,4-triazol-5-one (C2 H2 N4 O3 ) NTO/DAAF/Kel-F 800 (67/30/3): (C1.654 H1.611 N3.193 O1.970 F0.080 Cl0.022 ) NTO/FOX-7/Kel-F 800 (67/30/3): C1.494 H1.856 N2.872 O2.357 F0.08 Cl0.022 NTO/HNE/wax (76/19/5): C1.653 H1.884 N2.718 O2.514 Octol 75/25: HMX/TNT (C1.78 H2.58 N2.36 O2.69 ) Octol 78/22: HMX/TNT (C1.732 H2.593 N2.399 O2.690 ) PAX-60-MD: 62/37/1 HMX/DNP/wax (C1.612 H2.287 N2.612 O2.612 ) PAX-63-MD: 20/79/1 HMX/DNP/wax (C1.842 H1.683 N2.541 O2.541 ) PBX-91C: 90/10 HMX/HTPB (C1.924 H3.497 N2.439 O2.455 ) PBX-9501: 95/2.5/2.5 HMX/Estane/BDNPA-F (C1.47 H2.86 N2.60 O2.69 ) PBX-9502: 95/5 TATB/Kel-F 800 (C2.304 H2.232 N2.208 O2.208 F0.133 Cl0.036 ) PBX-9404: 94/3/3 HMX/NC/CEF (C1.40 H2.75 N2.57 O2.69 Cl0.03 P0.01 ) PETN: Pentaerythritol tetranitrate (C5 H8 N4 O12 ) PETN/HN (45/55): C0.712 H4.034 N2.306 O3.446 PETN/rubber (89/11): C2.186 H3.425 N1.134 O3.404 PETN/Viton (95/5): C1.637 H2.499 N1.203 O3.608 F0.174 QRX080: 95/5 FOX-7/Hytemp C1.557 H3.036 N2.568 O2.646 RDX: 1,3,5-Trinitro-1,3,5-triazacyclohexane (C3 H6 N6 O6 ) RDX/HN (55/45): C0.743 H3.855 N2.908 O2.908 RDX/HTPB (85/15): C2.210 H3.895 N2.307 O2.331 RDX/TNT (50/50): C2.218 H2.453 N2.012 O2.673 RDX/TNT (60/40): C2.044 H2.503 N2.150 O2.679 RDX/wax (94/6): C1.70 H3.39 N2.54 O2.54 RDX/wax (95/5): C1.641 H3.282 N2.568 O2.568 RX-45-AA: 95/5 ANTA/Kel-F 800 (C1.570 H2.233 N3.682 O1.473 F0.133 Cl0.036 ) SK TNBI: Bis(semicarbazidium) 4,4′,5,5′-tetranitro-2,2′-biimidazol1-ide (C8 H12 N14 O10 ) TAGN: Triaminoguanidine nitrate (CH9 N7 O3 ) TATB: 1,3,5-Triamino-2,4,6-trinitrobenzene (C6 H6 N6 O6 ) TATB/TNT (60/40): C2.628 H2.275 N1.923 O2.452 TeNHHPm: 1,1,3,5-Tetranitrohexahydropyrimidine (C4 H6 N6 O8 ) Tetryl: 2,4,6-Trinitrophenyl-N-methylnitramine (C7 H5 N5 O8 ) TKX-50: Dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate (C2 H8 N10 O4 ) TKX-50/paraffin (97/3): C1.036 H3.717 N4.110 O1.644 TMNTz: 2,4,6-Tris(methylnitramino)−1,3,5-triazine (C6 H9 N9 O6 ) TNAD: 1,4,5,8-Tetranitro-1,4,5,8-tetraazadecalin (C6 H10 N8 O8 ) TNT: 2,4,6-Trinitrotoluene (C7 H5 N3 O6 ) TNT/RDX/NTO (40/20/40): C2.119 H2.037 N2.300 O2.521 X-0535: 95/5 LAX-112/Oxy-461 (C1.438 H2.746 N3.958 O1.319 F0.071 Cl0.06 ) References [1] T.M. Klapötke, Chemistry of High-Energy Materials, 4th ed., Walter de Gruyter GmbH, Berlin/Boston, 2017. [2] R.E. Ball, The Fundamentals of Aircraft Combat Survivability Analysis and Design, 2nd ed., AIAA, Reston, 2003. [3] T.C. Ponder, F.A. Roescher, Cylindrical warhead design optimization, Technical Report AFATL-TR-72-42, Air Force Armament Laboratory, Eglin Air Force Base, Florida, 1972. [4] B. Janzon, J. Backofen Jr, R.E. Brown, R. Cayzac, A. Diederen, M. Giraud, M. Held, A.W. Horst, K. Thoma, The future of warheads, armour and ballistics, in: Proceedings of the 23rd International Symposium on Ballistics, Tarragona, Spain, 2007, pp. 3–27. 16-20 April. [5] M.T. Shahraini, Casing toughness effect on anti-air fragmentation warhead performance, in: Proceedings of the 23rd International Symposium on Ballistics, Tarragona, Spain, 2007, pp. 209–213. 16-20 April. [6] C. Bai, H. Wang, C. Feng, Analysis of criteria for assessing safety distance for focused warhead fragments based on CDEM, Math. Probl. Eng. 2019 (2019) 12 Article ID 8735481pages. [7] A. Catovic, An overview of Gurney method for estimating the initial velocities of fragments for high explosive munition, Def. Secur. Stud. 1 (2020) 16–25. [8] R.W. Gurney, The initial velocities of fragments from bombs, shell, grenades, Report No. BRL-405, Ballistic Research Laboratories, Aberdeen Proving Ground, MD, USA, 1943. [9] M.H. Keshavarz, New method for prediction of the Gurney energy of high explosives, Propellants Explos. Pyrotech. 33 (2008) 316–320. [10] M.H. Keshavarz, A. Semnani, The simplest method for calculating energy output and Gurney velocity of explosives, J. Hazard. Mater. 131 (2006) 1–5. [11] D. Frem, Estimating the metal acceleration ability of high explosives, Def. Technol. 16 (2020) 225–231. [12] P.M. Locking, Gurney velocity relationships, in: Proceedings of the 29th International Symposium on Ballistics, Edinburgh, Scotland, UK, 2016, pp. 1291–1300. May 9-13. [13] M.J. Kamlet, M. Finger, An alternative method for calculating gurney velocities, Combust. Flame 34 (1979) 213–214. [14] D.R. Hardesty, J.E. Kennedy, Thermochemical estimation of explosive energy output, Combust. Flame 28 (1977) 45–59. [15] D. Frem, Predicting the Gurney velocity of chemical high explosives: thermochemical code calculations and studies on the detonation products isentrope, J. Energ. Mater. 40 (2022) 99–118. [16] M. Suceska, M. Dobrilovic, V. Bohanek, B. Stimac, Estimation of explosive energy output by EXPLO5 thermochemical code, Z. Anorg. Allg. Chem. 647 (2021) 231–238. [17] D. Frem, A simple relationship for the calculation of the Gurney velocity of high explosives using the BKW thermochemical code, J. Energ. Mater. 33 (2015) 140–144. [18] H.S. Kim, E. Rottenkolber, T. Hartmann, W. Arnold, Modifications of the Gurney equation to account for gas leakage, in: Proceedings of the 28th International Symposium on Ballistics, Atlanta, GA, 2014, pp. 281–288. September 22-26. [19] C. Carrasco, R. Osegueda, O. Melchor-Lucero, L.A. Espino, Modeling hypervelocity impact for kill enhancement of ballistic missile warheads, FAST center for structural integrity of aerospace systems, Report No. AFRL-SR-AR-TR-04-0595, The University of Texas at El Paso, El Paso, Texas, 2004. [20] R.M. Lloyd, Conventional Warhead Systems Physics and Engineering Design, Progress in Astronautics and Aeronautics, 179, AIAA, Reston, 1998 Vol.. [21] R.M. Lloyd, Physics of Direct Hit and Near Miss Warhead Technology, Progress in Astronautics and Aeronautics, 194, AIAA, 2001 Vol.Reston. [22] Z. Akstein, L. Riha, S. Rolc, Correction of Gurney equation for asymmetric sandwich in relation to linear EFP, in: Proceedings of the 26th International Symposium on Ballistics, Miami, FL, 2011, pp. 180–186. September 12-16. [23] W. Xiong, A simple method for calculating detonation parameters of explosives, J. Energ. Mater. 3 (1985) 263–277. [24] M.H. Keshavarz, T.M. Klapötke, Energetic Compounds: Methods for Prediction of Their Performance, 2nd ed., Walter de Gruyter GmbH, Berlin/Boston, 2020. [25] M.H. Keshavarz, Estimating heats of detonation and detonation velocities of aromatic energetic compounds, Propellants Explos. Pyrotech. 33 (2008) 448–453. [26] M.H. Keshavarz, H.R. Pouretedal, An empirical method for predicting detonation pressure of CHNOFCl explosives, Thermochim. Acta 414 (2004) 203–208. [27] M.H. Keshavarz, H.R. Pouretedal, Predicting the detonation velocity of CHNO explosives by a simple method, Propellants Explos. Pyrotech. 30 (2005) 105–108. [28] I.B. Akst, Heat of detonation, the cylinder test, and performance munitions, in: Proceedings of the 9th International Symposium on Detonation, Portland, OR, USA, 1989, pp. 478–488. Vol. 1August 28-September 1. 290 D. Frem FirePhysChem 3 (2023) 281–291 [29] R. Catanach, L. Hill, H. Harry, E. Aragon, D. Murk, Cylinder Test Specification, LA-13643-MS, Los Alamos National Lab, Los Alamos, NM, USA, 1999. [30] C.G. Rumchik, R. Nep, G.C. Butler, B. Breaux, C. Lindsay, The miniaturization and reproducibility of the cylinder expansion test, AIP Conf. Proc. 1426 (2012) 450–453. [31] G.I. Kerley, T.L. Christian-Frear, Prediction of Explosive Cylinder Tests Using Equations of State from the PANDA Code, SAND93-2131, Sandia National Laboratories, Albuquerque, NM, USA, 1993. [32] B.E. Drimmer, Navy Bank of Explosives Data (NAVBED), NSWC MP 83-230, Vol. 1, Naval Surface Weapons Center, White Oak, MD, 1983. [33] B.E. Fuchs, Picatinny arsenal cylinder expansion test and a mathematical examination of the expanding cylinder, Technical Report ARAED-TR-95014, U.S. Army Armament Research, Development and Engineering Center, Picatinny Arsenal, New Jersey, 1995. [34] M. Künzel, O. Němec, J. Pachman, Optimization of wall velocity measurements using photonic doppler velocimetry (PDV), Cent. Eur. J. Energ. Mater. 12 (2015) 89–97. [35] B.M. Dobratz, P.C. Crawford, LLNL Explosives Handbook: Properties of Chemical Explosives and Explosive Simulants, UCRL-52997, Chg.2, University of California, Lawrence Livermore National Laboratory, Livermore, CA, USA, 1985. [36] C. Darab, T. Antoniu, H.G. Beleiu, S. Pavel, I. Birou, D.D. Micu, S. Ungureanu, S.D. Cirstea, Hybrid load forecasting using gaussian process regression and novel residual prediction, Appl. Sci. 10 (2020) 4588. [37] R. Meyer, J. Köhler, A. Homburg, Explosives, 7th ed., Wiley-VCH, Weinheim, 2015. [38] R. Lewczuk, J. Rećko, B. Florczak, The investigation on the properties of TNBI and its salts, in: Proceedings of the 21st Seminar on New Trends in Research of Energetic Materials, Pardubice, Czech Republic, 2018, p. 847. 18-20 April. [39] E.C. Koch, Insensitive high explosives II: 3,3′-Diamino-4,4′-azoxyfurazan (DAAF), Propellants Explos. Pyrotech. 41 (2016) 526–538. [40] D. Mathieu, Prediction of Gurney parameters based on an analytic description of the expanding products, J. Energ. Mater. 33 (2015) 102–115. [41] C.L. Mader, Numerical Modeling of Explosives and Propellants, 3rd ed., CRC Press, Boca Raton, 2008. [42] D. Trache, F. Maggi, I. Palmucci, L.T. DeLuca, K. Khimeche, M. Fassina, S. Dossi, G. Colombo, Effect of amide-based compounds on the combustion characteristics of composite solid rocket propellants, Arabian J. Chem. 12 (2019) 3639–3651. [43] E.C. Koch, High Explosives, Propellants, Pyrotechnics, Walter de Gruyter GmbH, Berlin/Boston, 2021. [44] R.W. Millar, J. Hamid, R. Endsor, P.F. Swinton, J. Cooper, Selection and synthesis of energetic heterocyclic compounds suitable for use in insensitive explosive and propellant compositions, Propellants Explos. Pyrotech. 33 (2008) 66–72. [45] P.E. Anderson, D. Rydzewski, L. Stiel, C. Owens, Next generation high power melt pour explosives, JDR&E 4 (2022) 123–129. [46] , in: P.J. Linstrom, W.G. Mallard (Eds.), NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg MD, 2022, p. 20899, doi:10.18434/T4D303. (retrieved July 26. [47] R.L. Simpson, P.F. Pagoria, A.R. Mitchell, C.L. Coon, Synthesis, properties and performance of the high explosive ANTA, Propellants Explos. Pyrotech. 19 (1994) 174–179. [48] M. Finger, Properties of Bis(2,2-Dinitropropyl)Acetal and bis(2,2-dinitropropyl)formal, Eutectic mixture, UCID -16088, Lawrence Livermore Laboratory, University of California, Livermore, California, 1972. [49] R. Matyáš, S. Zeman, W. Trzciński, S. Cudziło, Detonation performance of TATP/AN-based explosives, Propellants Explos. Pyrotech. 33 (2008) 296–300. [50] H. Hornberg, F. Volk, The cylinder test in the context of physical detonation measurement methods, Propellants Explos. Pyrotech. 14 (1989) 199–211. [51] W. Trzciński, S. Cudziło, S. Dyjak, M. Nita, A comparison of the sensitivity and performance characteristics of melt-pour explosives with TNT and DNAN binder, Cent. Eur. J. Energ. Mater. 11 (2014) 443–455. [52] P.R. Bowden, B.C. Tappan, M.M. Schmitt, R.W. Lebrun, M. Shorty, P.W. Leonard, J.P. Lichthardt, E.G. Francois, L.G. Hill, Synthesis, formulation and performance evaluation of reduced sensitivity explosives, AIP Conf. Proc. 1979 (2018) 100005-1–100005-6. [53] S. Cudzilo, W.A. Trzcinski, A study on detonation characteristics of pressed NTO, J. Energ. Mater. 19 (2001) 1–21. [54] W.A. Trzciński, S. Cudziło, Z. Chyłek, L. Szymańczyk, Detonation properties of 1,1-diamino-2,2-dinitroethene (DADNE), J. Hazard. Mater. 157 (2008) 605–612. [55] T.A.E. Elshenawy, Criteria of design improvement of shaped charges used as oil well perforators, PhD dissertation, University of Manchester, 2012. [56] W.A. Trzciński, S. Cudziło, Characteristics of high explosives obtained from cylinder test data, Hanneng Cailiao 14 (2006) 1–7. [57] K. Tan, Y. Han, G. Luo, M. Yin, S. Wen, F. Huang, Study on the detonation reaction-zone and energy release characteristics of a cast HMX-based PBX, Cent. Eur. J. Energ. Mater. 16 (2019) 380–398. [58] I.G. Cullis, R. Townsley, The potential of FOX-7 explosive in insensitive munition design, J. Appl. Mech. 78 (2011) 051012-1 –051012-8. [59] W.A. Trzciński, S. Cudziło, Z. Chyłek, L. Szymańczyk, Detonation properties and thermal behavior of FOX-7-based explosives, J. Energ. Mater. 31 (2013) 72–85. [60] W.A. Trzciński, L. Szymańczyk, Detonation performances of low-sensitivity NTO/HMX explosives, in: Proceedings of the 7th Seminar - New Trends in Research of Energetic Materials, Pardubice, Czech Republic, 2004, pp. 667–675. April 20-22. [61] B.C. Tappan, P.R. Bowden, J.P. Lichthardt, M.M. Schmitt, L.G. Hill, Evaluation of the detonation performance of insensitive explosive formulations based on 3,3ʹ-Diamino-4,4ʹ-Azoxyfurazan (DAAF) and 3-Nitro-1,2,4-Triazol-5-One (NTO), J. Energ. Mater. 36 (2018) 169–178. [62] M.D. Coburn, D.J. Hufnagle, D.L. Loverro, Development of AFX-511 and AFX-521, two new thermally stable explosives, Report LA-8115-MS, Los Alamos Scientific Laboratory, Los Alamos, NM, USA, 1980. [63] B.C. Tappan, J.M. Budzinski, E.M. Mas, L.M. Hull, L.G. Hill, P.R. Bowden, J.P. Lichthardt, A.M. Schmalzer, M. Shorty, P.I. Miller, D.L. McDonald, M.W. Burkett, Development of low-density explosive formulations based on ammonium picrate with slow detonation velocities, AIP Conf. Proc. 2272 (2020) 050030–050031 –050030-4. [64] M.J. Kamlet, S.J. Jacobs, Chemistry of detonations. I. A simple method for calculating detonation properties of C–H–N–O explosives, J. Chem. Phys. 48 (1968) 23–35. [65] H.H. Licht, Performance and sensitivity of explosives, Propellants Explos. Pyrotech. 25 (2000) 126–132. [66] S. Bourasseau, A systematic procedure for estimating the standard heats of formation in the condensed state of non aromatic polynitro-compounds, J. Energ. Mater. 8 (1990) 416–441. [67] H.S. Jadhav, M.B. Talawar, R. Sivabalan, D.D. Dhavale, S.N. Asthana, V.N. Krishnamurthy, Synthesis, characterization and thermolysis of polynitrohexahydropyrimidines: potential high energy materials, Indian J. Eng. Mater. Sci. 13 (2006) 80–86. [68] R.L. Willer, Synthesis and characterization of high energy compounds. I. trans-1,4,5,8-tetranitro-1,4,5,8-tetraazadecalin (TNAD), Propellants Explos. Pyrotech. 8 (1983) 65–69. [69] H. Ritter, H.H. Licht, Synthesis and characterization of methylnitramino-substituted pyridines and triazines, Propellants Explos. Pyrotech. 18 (1993) 81–88. [70] R.A. Hollins, R.A. Nissan, W.S. Wilson, R.D. Gilardi, 2,6-Diamino-3,5-dinitropyridine-1-oxide - A New Insensitive Explosive, NAWCWPNS TP 8228, Naval Air Warfare Center Weapons Division, China Lake, CA, 1995. [71] Z. Jalový, P. Vávra, New energetic materials of N-heterocyclic group, Sci. Pap. Univ. Pardubice Ser. A 9 (2003) 187–196. [72] H.H. Licht, H. Ritter, New energetic materials from triazoles and tetrazines, J. Energ. Mater. 12 (1994) 223–235. [73] M.H. Keshavarz, Predicting heats of detonation of explosives via specified detonation products and elemental composition, Indian J. Eng. Mater. Sci. 14 (2007) 324–330. [74] M.M. Stinecipher, L.A. Stretz, Sensitivity and performance characterization of DINGU, in: Proceedings of the 8th International Symposium on Detonation, Albuquerque, NM, USA, 1985, pp. 351–356. July 15–19. [75] M.A. Hiskey, D.E. Chavez, D.L. Naud, Insensitive high-nitrogen compounds, Report LA-UR-01-1493, Los Alamos National Laboratory, Los Alamos, NM, USA, 2001. [76] T.M. Klapötke, S. Cudziło, W.A. Trzciński, An answer to the question about the energetic performance of TKX-50, Propellants Explos. Pyrotech 47 (2022) e202100358. [77] J.P. Ritchie, Computational analysis of azine-N-oxides as energetic materials, in: C.H. Reynolds, M.K. Holloway, H.K. Cox (Eds.), Computer-Aided Molecular Design: Applications in Agrochemicals, Materials, and Pharmaceuticals, Eds, American Chemical Society, Washington, DC, 1995, pp. 378–395. [78] R.M. Doherty, J.M. Short, M.J. Kamlet, Improved prediction of cylinder test energies, Combust. Flame 76 (1989) 297–306. 291