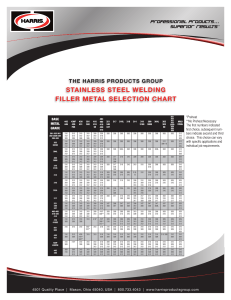
10/3/2020 https://realbeer.com/jjpalmer/Welding.txt Brazing and Welding of 304L Stainless Steel There are many materials and joining processes currently avalailable that can be used in constructing both home and micro breweries. Each material or process has its own limitations and these usually become obvious when the economics of a situation are examined. One of the best beers in the world, Pilsener Urquell, is brewed and lagered in pitch-lined oak barrels. While these materials may be readily available, the care and maintenance of this brewing system is extensive. Because of its relatively low maintenance, stainless steel has become widely used in North America and throughout the world. The stainless steel of choice in the food services industry is the austenitic 300 series. The stainless used for good pots (like Vollrath) is usually 304. Less expensive pots are often made of 303 alloy stainless which is less weldable and is quickly attacked by chlorinated cleaners. Other stainless kitchen equipment, like utensils, are typically Ferritic stainless, which has less Chromium and Nickel and is less acid-neutral. The 300 series of stainless steels was originally developed for use in cryogenics. These steels also perform well at elevated temperatures and are used extensively for steam pipes and exhaust systems. It is the resistance to elevated temperature oxidation and corrosion that makes alloys 304 and 316 the choice for food preparation equipment, including steam-heated boilers and storage tanks. But there is always a cloud for every silver lining and when it comes time to join stainless steel, that cloud is heat. The metallurgy that makes these alloys corrosion resistant and strong also makes welding more difficult than for ordinary steel. What Makes A Steel Stainless? (bold) The alloying addition of Chromium and Nickel to the iron creates a significant percentage of those atoms at the surface, which form tenacious oxides that seal the surface and prevent oxidation of the iron. The process known as "Passivation" for stainless steel, as discussed in the article "Care and Feeding of Stainless Steel" by Micah Millspaw in the July/August issue of Brewing Techniques, is a common means of improving this protective oxide layer through the use of oxidizing acids. Anodizing aluminum alloys is similar in that a solid aluminum oxide barrier is created to prevent further corrosion. Anodized (black) aluminum cookware (Magnalite, Calphalon) is acid neutral / acidic food resistant like stainless steel because of this heavy oxide layer. Plain (bright) aluminum cookware does not have the degree of surface oxides necessary to prevent reaction with corrosive media. Joining of Steel and Brass/Copper (bold) Stainless steel is routinely welded, but it must be done under an inert gas atmosphere. The most reliable method for welding stainless is the Tungsten Inert Gas (TIG) process, aka. GTAW and Helio-Arc. TIG welding has the advantage of a small weld head, lower heat input is required and filler metal is optional. The other common welding methods for stainless steel, Metal Inert Gas (MIG) and Shielded Metal Arc Welding (SMAW), are not as well suited for welding thin sections like beer keg walls. MIG is commonly used for all types of stainless welding but the weld gun must be held close to the work and this decreases its effectiveness in tight areas. MIG equipment will be more available to a do-it-yourselfer and should provide a satisfactory joint. SMAW is commonly used for welding thicker pipe and tanks. It has the disadvantage of obscuring of the weld joint during the pass and the slag must be removed between passes. Equipment and electrode filler rods are readily available, however this welding process is not recommended for this application. The welder does not have the control necessary to insure a good weld. Because 300 series stainless steels are prone to High Temperature Embrittlement and Sensitization, the welder must be careful not to apply too https://realbeer.com/jjpalmer/Welding.txt 1/6 10/3/2020 https://realbeer.com/jjpalmer/Welding.txt much heat for too long during welding. Welding of thin gauge stainless steel requires a definite skill. Producing defect-free welds without overheating the steel takes years of practice, no matter which welding process is used. This is not to say that a serviceable weld cannot be done by a novice. But in my experience, it is better to take stainless steel weld jobs to an experienced welder, rather than attempting it yourself. Bad welds are difficult to correct in stainless steel. It is more economical to get things done right the first time. The scale of welding that a homebrewer would require would most likely not exceed a welder's one hour minimum charge. In fact, to weld 3 pipe nipples into 3 kegs, I was quoted 25 dollars. Not much to pay for a quality job. See Figure 1. In addition an experienced welder will know how to produce a good weld without overheating it. Overheating causes precipitation of the chromium atoms away from the grain boundaries to form chromium carbides, depleting the steel of its corrosion resistance. I should explain some background metallurgy here. All metals are crystalline materials having specific crystal structures which are dependent on temperature. These structures are referred to as "Phases" and are given names such as "Austenite" and "Ferrite". A block of metal is very similar to a block of salt. A block of salt is really a bunch of grains of salt all fused together. These grains are oriented every which-way and the interface to the next grain of salt is called the grain boundary. As you would expect, the grain boundary is weaker than the grain itself. The crystalline structure of metals is exactly the same in this respect. (By the way, if you would like to see a metallic grain structure, go look at an aluminum street-light pole. That mosaic you see is the grain structure.) Because the grain boundaries within a metal are the weakest sites, heat and corrosion usually affect these areas first. The corrosion resistance of stainless steel depends on the chromium. Austenitic stainless is a super-saturated solution of chromium and nickel in iron. It is actually a very high temperature phase that has been quenched to preserve the distribution of elements. Austenitic stainless does not like middling-high heat. It performs well up to 600F (315C), but higher temperatures in the range of 800-1600F (425-870C) cause atom diffusion which causes the properties to change. Temperatures in this range allow the chromium to diffuse away from the grain boundaries to form chromium carbides, the preferred crystalline structure in that temperature range. Exposure to the temperatures that cause diffusion is referred to as being "Sensitized". The diffusion of chromium away from the grain boundaries results in un-stainless grain boundaries surrounded by stainless steel. This situation soon leads to localized corrosion and rapid cracking of the grain boundaries. To correct this, the metal must be heated to at least 1900F (1040C) for a period of time in an inert gas atmosphere and then quenched to retain the austenite crystal structure. Unfortunately, doing this heat treatment to a welded keg would result in a lot of warping and distortion. It is better to get another keg and start over. Welding is a local melting/freezing process that creates high temperature gradients in the metal around the weld. This Heat Affected Zone (HAZ) is the region where unwanted atom diffusion can take place if it is hot enough, long enough. There are time/temperature curves that describe this, and the curve for alloy 304 is shown in Figure 2. This chart shows that for type 304 stainless (nominal carbon content of 0.08%), five minutes at 600C (1110F) or above will cause chromium diffusion that will later cause cracking in service. Type 304L stainless, "L" denoting less carbon (nominal 0.03%), is more weldable and can spend about 6 hours at 600C before becoming Sensitized. Most kegs (in North America) are made from 304L to facilitate the welded construction. Caution must still be taken in service though. I know of one case of a homebrewer that experienced cracking on the bottom of a cut-off keg boiler. The cracks appeared at the flame-line where the flame of the CajunCooker-type propane heater met the keg. This shows that he was running the flame too hot and that, over time, chromium atom diffusion was taking place. Diffusion is cumulative. Once this type of cracking occurs, there is no economical way to correct it. In addition, because modern beer and soda kegs https://realbeer.com/jjpalmer/Welding.txt 2/6 10/3/2020 https://realbeer.com/jjpalmer/Welding.txt are designed thin to save on material, a modified keg should never again be pressurized. Serious injury could result from a rupture at the weld. Welds are always weaker than the base metal. If you wish to do the welding yourself and have access to the necessary equipment, suggested weld schedules for manual TIG and MIG welding of 304L steel are shown in Table 1. The MIG weld setup uses a 97.5%/2.5% mix of Argon and CO2 and 0.030 electrode wire. The TIG welding uses a sharpened (~30¡), Thoriated (2%), 3/32 inch dia. electrode, and 1/16 inch dia. Filler Rod. The shielding gas for TIG welding is 100% Argon. Note that the same Filler metal is used for both processes. As noted in Jeff Donaghue's article, "A Primer on Welding Stainless Steel" in the September issue of BT, vocational welding classes are usually available through Adult Education or Community Colleges. These classes can provide the necessary instruction, equipment and practice material you will want to have before working on your brewing equipment. Table 1 - Manual Welding Parameters for 304L Stainless Steel Welding Type Thickness Current Voltage Filler Rod (inches) (Amps) (Volts) (AWS) MIG 0.063 85 DCEP 21 ER316L TIG 0.045, 0.090 37/70 DCEN 12-14 ER316L Table 1 con't Weld Type Argon Flow (ft^3/hr) MIG 15 TIG 12 Weld Speed in./min. 19 2-4 Wire Feed in/min 184 As Req'd (Shadow Box) A Few Words About Brass (bold) Brass is an alloy of Copper and Zinc with some lead thrown in for machinability. The lead percentage varies, but for the common brass alloys used in plumbing fittings it is 7% or less. Lead is entirely soluble in copper, but the presence of zinc changes this. In Brass, the lead exists as minute globules. These globules act as an intrinsic lubricant during machining. The result is a micro-thin film of lead being smeared over the machined surface. It is this lead (a very small amount) that can be dissolved off by the wort. While this small amount of lead should probably not be a cause of concern, most people would be happier if if wasn't there at all. Well, never let it be said that the Space Program never yields technology applicable to the home. Some chemists working on the International Space Station Alpha program were consulted for an etchant that could safely remove the lead from the surface of brass parts. The chemists determined that a 1-to1 volume ratio of Glacial Acetic Acid (98% by vol.) to Hydrogen Peroxide (30% by vol.) would accomplish this without pitting the brass. This procedure was performed in the lab using the standard laboratory concentrations of these chemicals. The process consisted of a 30 second dunk, swirl and rinse at room temperature, and was successful in removing the lead, as determined by a Lead Home Test Kit (swabs). In addition, the procedure had the added benefit of turning the brass into Pure Gold. (Okay, the color of, anyway.) Because 98% Acetic Acid and 30% Hydrogen Peroxide are not available to the average brewer, the experiment was repeated using the concentrations available in the supermarket. These are 5% Acetic Acid (White Distilled Vinegar) and 3% Hydrogen Peroxide. Due to the difference in concentration, the relative concentration ratio is changed. For the household variety concentrations, a 2to-1 volume ratio of Acetic Acid to H2O2 is needed. The process was expected to take longer with the more dilute solution, so the brass part was immersed for 10 minutes. The results showed the same gold color and the Lead Test swab indicated the lead had been removed. The buttery yellow gold color can be used as an indicator that the process has completed. Home https://realbeer.com/jjpalmer/Welding.txt 3/6 10/3/2020 https://realbeer.com/jjpalmer/Welding.txt Lead Test kits should be available at most hardware stores. This procedure for removing surface lead from brass can easily be conducted at home. A 10-15 minute dunk, swirl, and rinse in a 2/1 volume ratio of 5% Acetic Acid and 3% Hydrogen Peroxide has been shown to be effective. By the way, the solution can be irritating to the skin so either wear gloves or use tongs. (End Shadow Box) Soldering (bold) Soldering and Brazing of stainless steel to itself or to brass or copper works well also. These processes are good alternatives to the welding of stainless steel fittings. They allow the attachment of copper tubing and brass fittings directly onto the stainless steel. There is some potential for galvanic corrosion of the copper or brass in preference to the silver. (In terms of electro-chemical activity: stainless steel is more passive than silver solder, which is more passive than brass/copper.) Available industry service data indicates that the corrosion rate should be quite small. Many people have used silver alloys with these metals and have not experienced any galvanic corrosion problems at all. The difference between soldering and brazing is temperature. The American Welding Society defines Soldering as metal coalescence below 800F. (Brazing being above 800F.) Both processes bond adjoining metal surfaces by completely wetting the surfaces with molten filler metal and maintaining that bond upon solidification. The bond is only as strong as the filler metal, but some braze metals can be very strong indeed. Stainless steel is difficult for solders and braze filler metals to wet. The surface oxides that protect it from corrosion also prevent the filler metals from wetting the surface. Special fluxes are needed to eat through these stainless oxides. The silver solder commonly sold for home plumbing with copper pipe will work on stainless but a different flux is needed. Look for a flux containing hydrochloric acid or one that says it is for fluxing nickel alloys or stainless. The specifics for two common silver solders are listed in Table 2. Table 2 - Common Silver Solders Composition (%) Silver Tin 3 97 2 98 Melting Temperature F C 430 220 450 230 In my experience, getting the steel hot is the big problem. A propane torch can be used, but the flame needs to be slightly reducing in nature to prevent the re-formation of surface oxides. The best method for soldering a copper or brass fitting onto a stainless steel pipe is to "tin" the fitting first with the solder. Flux is then applied to the stainless pipe and the two pieces are fitted together. Then the heat is applied to the joint, and more solder is fed into the joint once its hot. This way the steel surface is protected from the air until it is hot enough to be wetted by the solder. Brazing (bold) Silver-based brazing alloys have lower melting temperatures than Copper/Zinc brazing alloys, so the silver-based alloys are the more practical choice for Do-it-yourselfers. But there are two issues to keep in mind with brazing. The first is that most brazing temperatures are right in the temperature range that causes sensitization of the steel. The braze must be conducted efficiently to ensure that the time limit for the onset of diffusion is not exceeded. Acetylene and propane are two of the most common gases used for Torch Brazing. Use a slightly reducing flame and AWS type 3A flux. This flux has the higher useful temperature range needed for brazing (1050-1600F). Both https://realbeer.com/jjpalmer/Welding.txt 4/6 10/3/2020 https://realbeer.com/jjpalmer/Welding.txt surfaces to be joined must be cleaned and fluxed for best results. As in soldering, it is a good idea to pre-braze the fitting, since it has the higher thermal mass in the localized area. Pre-heating the fitting will help decrease the amount of time that heat is applied to the joint. A friend of mine recently brazed a stainless steel pipe nipple directly onto the side of a stainless steel milk can. See Figure 3. The wall of the can was flattened with a hammer to allow a good flat fit-up with the nipple. The pipe was 1/2 inch NPT with a wall thickness of 1/4 inch. The pipe was heated up first since it had a much higher thermal mass than the milk can wall. It was brazed using flux coated rod and an acetylene torch. The braze was quite strong, allowing him to torque up a connecting threaded fitting such that he later had trouble taking it apart! Silver brazing rod does not contain lead, but some of the alloys contain Cadmium, which is worse. It will cause severe heavy metal poisoning. The American Welding Society alloy designations are listed in Table 3; don't use the alloys containing Cadmium. The best bet is to look for rod that is made for food industry applications. The AWS BAg-5 is recommended for this purpose and is readily available from weld supply shops at about $15.00 an ounce (1/16 inch dia., spooled). Table 3 - Standard AWS Silver-based Brazing Alloys AWS Composition(a), % spec. Ag Cu BAg-l 44.0-46.0 14.0-16.0 BAg-la 49.0-51.0 14.5-16.5 BAg-2 34.0-36.0 25.0-27.0 BAg-2a 29.0-31.0 26.0-28.0 BAg-3 49.0-51.0 14.5-16.5 BAg-4 39.0-41.0 29.0-31.0 BAg-5 44.0-46.0 29.0-31.0 BAg-6 49.0-51.0 33.0-35.0 BAg-7 55.0-57.0 21.0-23.0 BAg-8 71.0-73.0 Rem BAg-8a 71.0-73.0 Rem BAg-13 53.0-55.0 Rem BAg-13a 55.0-57.0 Rem BAg-18 59.0-61.0 Rem BAg-l9 92.0-93.0 Rem BAg-20 29.0-31.0 37.0-39.0 BAg-21 62.0-64.0 27.5-29.5 Zn 14.0-18.0 14.5-18.5 19.0-23.0 21.0-25.0 13.5-17.5 26.0-30.0 23.0-27.0 14.0-18.0 15.0-19.0 ... ... 4.0-6.0 ... ... ... 30.0-34.0 ... Others 23.0-25.0 Cd 17.0-19.0 Cd 17.0-19.0 Cd 19.0-21.0 Cd 16 Cd, 3 Ni 1.5-2.5 Ni ... ... 4.5-5.5 Sn ... 0.25-0.50 Li 0.5-1.5 Ni 1.5-2.5 Ni 10 Sn, .025 max P 0.15-0.30 Li ... 6 Sn, 2.5 Ni Notes: (a) Total maximum allowable impurities in each alloy is 0.15%. Ag is Silver, Cu is Copper, Zn is Zinc, Cd is Cadmium, Ni is Nickel, Sn is Tin, Li is Lithium, and P is Phosphorus. Table 4 - Standard AWS Brazing Alloy Usage Temperatures AWS Brazing spec. temp. F C BAg-l 1145-1400 618-760 BAg-la 1175-1400 635-760 BAg-2 1295-1550 702-843 BAg-2a 1310-1550 710-843 BAg-3 1270-1500 688-816 BAg-4 1435-1650 779-899 BAg-5 1370-1550 743-843 BAg-6 1425-1600 774-871 BAg-7 1205-1400 652-760 BAg-8 1435-1650 779-899 https://realbeer.com/jjpalmer/Welding.txt 5/6 10/3/2020 BAg-8a BAg-13 BAg-13a BAg-18 BAg-l9 BAg-20 BAg-21 https://realbeer.com/jjpalmer/Welding.txt 1410-1600 1575-1775 1600-1800 1325-1550 1610-1800 1410-1600 1475-1650 766-871 857-968 871-982 718-843 877-982 766-871 802-899 Soldering, brazing and welding are all useful methods for joining stainless steel. Consider the joint design when choosing the process. Each process has its own limitations, but the most important is the nature of alloy 304L itself. Soldering is most useful for joining pipes and small fittings. Brazing is useful for making high strength bonds between dissimilar metals. These two methods may be accomplished fairly easily at home. But if the goal is to seal a stainless pipe through the wall of a keg, then welding is the best way to go. If you want to do the welding yourself, ask yourself the following questions: 1. Can I produce a quality weld (no defects or overheating)? 2. What resources do I have available? Weigh the economics of your decision. Time, effort, and equipment investment are some factors to be considered. If you have the interest and knack for learning new skills, then give it a try. Check out vocational welding classes in your area. With some instruction and practice, a serviceable weld is not difficult. If you want to get right to the brewing, then you may want to hit the Yellow Pages. References: M. Jackson, The New World Guide to Beer (Running Press, Philadelphia, Pennsylvania, 1987). ASM Metals Handbook, 9th Ed., Vol. 3, Properties and Selection: Stainless Steels, Tool Materials and Special-Purpose Metals; Fabrication of Wrought Stainless Steels, (American Society for Metals, Metals Park, Ohio, 1980). ASM Metals Handbook, 9th Ed., Vol. 6, Welding, Brazing, and Soldering; Arc Welding of Stainless Steels, (American Society for Metals, Metals Park, Ohio, 1983). ASM Metals Handbook, 9th Ed., Vol. 6, Welding, Brazing, and Soldering; Torch Brazing of Steels, (American Society for Metals, Metals Park, Ohio, 1983). ASM Metals Handbook, 9th Ed., Vol. 6, Welding, Brazing, and Soldering; Brazing of Stainless Steels, (American Society for Metals, Metals Park, Ohio, 1983). ASM Metals Handbook, 9th Ed., Vol. 6, Welding, Brazing, and Soldering; Soldering, (American Society for Metals, Metals Park, Ohio, 1983). ASM Metals Handbook, 9th Ed., Vol. 13, Corrosion; Environmentally Induced Cracking, (American Society for Metals, Metals Park, Ohio, 1987). ASM Metals Handbook, 9th Ed., Vol. 13, Corrosion; Corrosion of Stainless Steel, (American Society for Metals, Metals Park, Ohio, 1987). ASM Metals Handbook, 9th Ed., Vol. 13, Corrosion; Corrosion in the Brewery Industry, (American Society for Metals, Metals Park, Ohio, 1987). John Palmer is a metallurgical and welding engineer for McDonnell Douglas Aerospace in Huntington Beach, California. He is a frequent contributor to the Home Brew Digest on the Internet and the author of "How to Brew Your First Beer", available at several BBSs and FTP computer sites around the world. He is an enthusiastic member of the Crown of the Valley Brewing Club in Pasadena, CA. https://realbeer.com/jjpalmer/Welding.txt 6/6