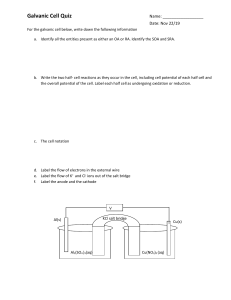
1 The following cell is a chemical cell connected to a light bulb and voltmeter. Salt Bridge V Graphite Electrode A 150cm3 0.6M Fe(NO3)2(aq) + 1M MgSO4(aq) (a) Graphite Electrode B 150cm3 1M K2Cr2O7(aq) + 0.8M AgNO3(aq) Draw the direction of electron flow on the figure. (1 mark) (b) For the above setup, (i) Write the half equations for the reaction occur in the two cells. (1 mark) (ii) Write the overall equation for the cells. (1 mark) (c) Salt bridge in the cell can be prepared by soaking filter paper into NaCl solution. (i) State the function of salt bridge. (2 marks) (ii) Other than NaCl solution, what else can be used. (1 mark) (d) Initial reading of the voltmeter of the experiment is 1.1V and the overall resistance of the circuit is 2Ω. Estimate the initial rate of consumption of the oxidizing agent. (4 marks)