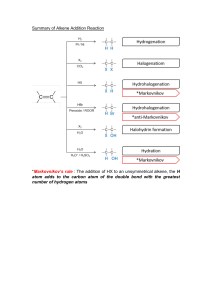
ORGANIC REACTIONS D. F. Palacio, LPT, MA. Learning Outcomes • At the end of the lesson, students are able to: - Identify and discuss the common reactions in organic chemistry - Predict the major and minor product of an organic chemical reaction - Classify organic compounds based on reactivity - Identify the common organic reactions that happen in biological systems Organic Reactions • Reactions are the heart of organic chemistry • It usually begins with electron-rich or deficient sites at functional groups in the reacting molecules. • These are often the location of bonds that might be easily broken. Writing Equations for Organic Reactions • Equations for organic reactions are usually drawn with a single reaction arrow (→) between the starting material and product. • The reagent, the chemical substance with which an organic compound reacts, is sometimes drawn on the left side of the equation with the other reactants. • At other times, the reagent is drawn above the arrow. • Although the solvent is often omitted from the equation, most organic reactions take place in liquid solvent. Ways to Write Organic Reactions • The solvent and temperature of the reaction may be added above or below the arrow. • The symbols “h” and “” are used for reactions that require light or heat, respectively. • When two sequential reactions are carried out, the steps are numbered above or below the reaction arrow. • This convention signifies that the first step occurs before the second step, and the reagents are added in sequence, not at the same time. Substitution Reaction • Substitution is a reaction in which an atom or a group of atoms is replaced by another atom or group of atoms. • In a general substitution, Y replaces Z on a carbon atom • Substitution reactions involve bonds: one bond breaks and another forms at the same carbon atom. • While in some cases Z can be a hydrogen atom, the most common examples of substitution occur when Z is a heteroatom that is more electronegative than carbon. Elimination Reactions • Elimination is a reaction in which elements of the starting material are “lost” and a bond is formed. • In an elimination reaction, two groups X and Y are removed from a starting material. • Two bonds are broken, and a bond is formed between adjacent atoms. • The most common examples of elimination occur when X = H and Y is a heteroatom more electronegative than carbon. Addition Reactions • Addition is a reaction in which elements are added to the starting material. • In an addition reaction, new groups X and Y are added to the starting material. • A bond is broken and two bonds are formed. Alkanes • Combustion: Reaction of alkanes with oxygen to produce carbon dioxide, water, and heat • Halogenation: Reaction of alkane with halogens to produce alkyl halides Alkenes • Reactions of alkenes involves the C=C bond, • Addition of 2 atoms or groups of atoms to a double bond reaction is the key bonding • Hydrogenation – the addition of hydrogen atoms • Halogenation – the addition of halogen atoms • Hydration – the addition of a water molecule • Hydrohalogenation – the addition of a hydrogen halide molecule Addition: General Reaction • A small molecule, AB, reacts with the pi electrons of the double bond • The pi bond breaks and its electrons are used to bond to the A and B pieces • Some additions require a catalyst Types of Addition Reactions 1. Symmetrical: same atom added to each carbon • Hydrogenation - H2 (Pt, Pd, or Ni as catalyst) • Halogenation - Br2, Cl2 2. Unsymmetrical: H and another atom are added to the two carbons • Hydrohalogenation - HCl, HBr • Hydration - H2O (requires strong acid catalyst e.g., H3O+, H2SO4, H3PO4) 3. Self-addition or polymerization Hydrogenation: Addition of H2 Hydrogenation is the addition of a molecule of hydrogen (H2) to a carbon-carbon double bond to produce an alkane • • • • The double bond is broken Two new C-H bonds result Platinum, palladium, or nickel is required as a catalyst Heat and/or pressure may also be required Halogenation: Addition of X2 Halogenation is the addition of a molecule of halogen (X2) to a carbon-carbon double bond to produce an alkane • The double bond is broken • Two new C-X bonds result • Reaction occurs quite readily without a catalyst • Cl and Br are most often the halogen added Hydration A water molecule can be added to an alkene • The addition of a water molecule to an alkene is called hydration Presence of strong acid is required as a catalyst Product resulting is an alcohol Unsymmetrical Addition Two products are possible depending how the 2 groups (as H and OH) add to the ends of the pi bond • The hydrogen will add to one carbon atom • The other carbon atom will attach the other piece of the addition reagent • OH (Hydration) • Halogen (Hydrohalogenation) Markovnikov’s Rule • Dimitri Markovnikov (Russian) observed many acid additions to C=C systems • When an acid adds to a double bond, the H of the acid most often goes to the end of the double bond, which had more hydrogens attached initially • H-OH • H-Cl • H-Br Hydrohalogenation An alkene can be combined with a hydrogen halide such as HBr or HCl The reaction product is an alkyl halide Markovnikov’s Rule is followed in this reaction Polymerization • Reaction of alkene with hydrogen to create polymers • Polymers: A type of long-chain macromolecule linked together by repeated monomers (one unit of organic molecule Some Important Addition Polymers of Alkenes Monomer name Polymer Uses Styrene Polystyrene Styrofoam Acrylonitrile Polyacrylonitrile (Orlon) Clothing Methyl methacrylate Polymethyl methacrylate (Plexiglas, Lucite) Basketball backboards Vinyl chloride Polyvinyl chloride (PVC) Plastic pipe, credit cards Tetrafluoroethene Polytetrafluoroethylene (Teflon) Nonstick surfaces Uses of Polymers Uses of Polymers Aromatic Compounds • The typical reaction is the substitution reaction- hydrogen is replaced by another atom or group of atoms • All benzene reactions we consider require a catalyst • The reactions are: 1. Halogenation 2. Nitration 3. Sulfonation Halogenation A reaction that places a Br or Cl on the ring • The reagent used is typically Br2 or Cl2 • Iron or iron halide are used as catalysts Nitration • A reaction that places the nitro group on the ring • Sulfuric acid is needed as a catalyst Sulfonation Sulfonation places an SO3H group on the ring • Concentrated sulfuric acid is required as a catalyst Alcohols • Acid-catalyzed Dehydration: Elimination of water molecule from alcohols through the aid of concentrated acid and heat as a catalyst to yield alkenes Alcohols • Oxidation: Primary alcohols usually oxidize to carboxylic acids • The symbol [O] represents an oxidizing agent (KMnO4/OH- or H2CrO4) which is used to oxidize a 1 degree alcohol to an aldehyde • Oxidation of primary alcohols • Oxidation of secondary alcohols • Secondary alcohols oxidize to ketones • This reaction is also an elimination of 2H • Tertiary alcohols do not oxidize as there is no H on the carbonyl carbon to remove Oxidation and Reduction in Living Systems • Oxidation is the loss of electrons • Reduction is the gain of electrons • These changes are easily detected in inorganic systems with formation of charged ions • In organic systems it is often difficult to determine whether oxidation or reduction has taken place as there might be no change in charge Organic Oxidation and Reduction In organic systems changes may be tracked: Oxidation: • gain of oxygen • loss of hydrogen Reduction: • loss of oxygen • gain of hydrogen Biological Oxidation-Reduction NAD+ is a coenzyme commonly involved in biological oxidation/reduction reactions: Oxidation and Reduction in Living Systems Thiols • Disulfide Formation • Two cysteine molecules (amino acids) can undergo oxidation to form cystine • Forms a new bond called a disulfide bond • Play a crucial part in proteins Activity Predict the major product in each of the following reactions Name the alkene reactant and the product using IUPAC nomenclature