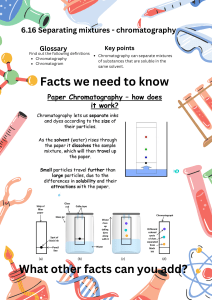
Group: Connor, Arjan, Euan Author: Connor Gunning Date: October 12, 2023 The fractionation of a red, a yellow, a blue, a green, and an unknown dye via Paper Chromatography Objectives: 1. To construct and operate a paper chromatography apparatus proficiently. 2. To gain an understanding of the concept and importance of Rƒ (retention factor) values in chromatographic analysis. 3. To examine and analyze a range of food colorings, determining and recording their respective Rƒ values. 4. To evaluate the obtained Rƒ values in comparison to established standard Rƒ values. 5. To effectively separate complex mixtures of food colorings into their individual constituents using chromatography. 6. To identify the distinct components within these mixtures (green and unknown colors) based on their specific Rƒ values. Materials and Methods: Apparatus: Reagents: 2 scissors set of food colorings (Red, Blue, Yellow, 3 large test tubes (25mm x 200mm) Green, and unknown) 3 Erlenmeyer flasks (250ml) 2 metric rulers 2 pencils 5 chromatography paper strips (2.5cm wide x 66cm long) For methods see Experiment 2D procedure parts I, II, and III. Observations: For quantitative data from the lab see the data tables attached to this document. The qualitative data is as follows, the red food coloring slowly faded in color and spread out as it traveled up the chromatography paper the highest point of the color created an arc rather than a straight line across the paper, the solvent line was difficult to see without proper lighting however it was perfectly straight across the paper. Much like the red coloring the yellow food coloring gradually diminished in intensity and expanded as it ascended the chromatography paper, forming an arc at its Group: Connor, Arjan, Euan Author: Connor Gunning Date: October 12, 2023 peak rather than a straight line across the paper. In contrast, the solvent line, when using proper lighting, appeared as a perfectly straight line across the paper. As with the red and yellow colorings the blue food coloring gradually diminished in intensity and diffused as it ascended the chromatography paper. The apex of the color formed an arc rather than a straight line across the paper. In contrast, the solvent line, while requiring adequate lighting for visibility. The green coloring slowly separated into two distinct fronts, one yellow and one blue the apex of the former was lower than that of the latter and the two fronts created an oval shape on the paper rather than an arc at the apex however they too diminished in intensity as they traveled up the paper. The unknown color similarly separated into three fronts, red, yellow, and blue with the lowest apex being red and then yellow and blue respectively, comparably to the green coloring the constituents of the unknown color also created more of an oval shape on the paper. For visual representation see the attached images of the above-described processes. Questions: 1. a. Which of the colors you tested in Part II of the experiment appeared to contain one or more of the approved dyes listed in table 4? The red coloring, we tested had a retention factor of 0.89 and the average retention factor across all the groups who tested the red coloring was 0.82 which corresponds with the red #2 on table 4 of Laboratory Experiments Experiment 2D which has a retention factor of 0.81. The average retention factor of the yellow coloring tested was 0.81 which seems to correspond with the yellow #6 in table #4 which has a retention factor of 0.77 as it is in the range of +/-0.05. The average retention factor of the blue coloring tested was 0.98 which corresponds to Blue #1 in table 4 of Laboratory Experiments Experiment 2D which has a retention factor of 1.0. b. Which, if any, of the colors you tested did not correspond to any of the approved dyes? All of the primary colors we tested correspond with one of the approved dyes in table 4 of Laboratory Experiments Experiment 2D as they were all within +/- 0.05 of the values on the table. 2. From your results in part III, what are the components of the green food coloring. Support your answer both qualitatively and quantitatively. The green food coloring was a combination of both the yellow and the blue colorings. This could be seen both by its appearance as the green coloring separated into two distinct fronts one yellow and one blue however, this could also be quantitatively verified as the retention factor of the two constituent colors were 0.92 and 1.0 the 0.92 correlates to yellow #5 in table 4 of Laboratory Experiments Experiment 2D which has a retention factor of 0.95 and the 1.0 correlates to blue #1 which also has a retention factor of 1.0. 3. What can you conclude about the identity of the components in the unknown mixture? What qualitative and quantitative evidence supports your answer? Group: Connor, Arjan, Euan Author: Connor Gunning Date: October 12, 2023 The unknown mixture was composed of red, yellow, and blue colorings which is evident both by its appearance and quantitatively. The unknown mixture dispersed into three distinct fronts, one red one yellow and one blue the retention factors of these fronts were 0.77, 0.82, and 1.0 respectively, all of which are within +/-0.05 uncertainty of the values shown in table 4 of Laboratory Experiments Experiment 2D. 4. What might happen if ink, rather than pencil, were used to mark the sample line on the chromatography paper? If ink were to be used for the sample line rather than pencil the ink would most likely travel up the chromatography paper much like the colorings previously discussed. This is due to the fact that the ink is a liquid which means it has a retention factoring meaning it would travel up the chromatography paper most likely screwing up your results. 5. Why should green food coloring be classified as a mixture, whereas yellow, blue, or red should not? The green food coloring we tested should be classified a mixture since we proved that the green coloring was a combination of both yellow and blue coloring, this was proven through observing the two distinct fronts created by the green coloring and by then measuring their retention factors which agreed with the provided retention factors of the yellow and blue colorings. Since no chemical separation method was used and yet the green coloring was observed to be comprised of two separate colors, we can conclude that it must be a mixture. For these same reasons we can conclude that the other two colors are not mixtures as we attempted to separate them physically with paper chromatography and they remained a single substance throughout. Follow-up Questions: 1. Identify the dyes that appear on the chromatogram in Figure 2D-5. (Consult Table 4 of Laboratory Experiments Experiment 2D for Rƒ values.) The original sample was orange food coloring. The dyes that appear on the chromatogram in Figure 2D-5 are red #3 and yellow #6 this can be proven using the values in table 4 of Laboratory Experiments Experiment 2D as the length of the solvent front is 80mm and the first color front is 33mm and the second front is 62mm which correspond to retention factors of 0.41 and 0.78 which correspond to the red #3 and yellow #6 on table 4 of Laboratory Experiments Experiment 2D as there are within +/- 0.05 of the stated values. 2. A pharmaceutical chemist runs a chromatography test on a substance and identifies two of its components by comparing their Rƒ values against certain standards. If the two components have Rƒ values of 1.0 and 0.41, and the solvent front has travelled 12.0cm from the sample’s origin, what is the separation distance on the chromatogram? The equation for the Rƒ value or retention factor of a liquid is the solute front divided by the solvent front. If we know that the Rf value is 1.0 then we know that both the solute front and Group: Connor, Arjan, Euan Author: Connor Gunning Date: October 12, 2023 the solvent front are equal which means that one of the fronts is 12.0cm from the sample’s origin. The next one must have a ratio of 0.41 and we know that denominator is 12.0cm which means the solute front is 0.41 multiplied by 12.0cm which is 4.92cm meaning the separation of these two fronts is 12.0cm-4.92cm or 7.08cm. 3. A chemist performs an Rƒ calculation, obtains a value of 1.2, and decides that the answer is unacceptable. Why? The equation for the Rƒ value or retention factor of a liquid is the solute front divided by the solvent front. The result obtained by the chemist is unacceptable since the solute should not be able to travel farther than the solvent as the solute is meant to travel through the solvent which is impossible if the solute moves farther than the solvent. Discussion: In the experiment conducted with red coloring both the quantitative and qualitative observations agreed with what should have been expected in said experiment. The red coloring did not separate into multiple fronts and traveled with the solvent up the chromatography paper with an average Rƒ value of 0.82 across all groups which is within the +/- 0.05 uncertainty of the value in table 4 of Laboratory Experiments Experiment 2D (0.81 red #2), however the individual experiment done by our group was not within the confines of the accepted uncertainty and this is most likely caused by the lack of accuracy regarding the amount of the solute used which can greatly affect the observed Rƒ value of the solute since the relative concentration of the solute to solvent affects the Rƒ value.² Although both the experiment with the yellow coloring and the experiment with the blue coloring were not performed by our group using the average Rƒ value of the other groups we can see that they too agreed with the expected results provided by table 4 of Laboratory Experiments Experiment 2D. Similarly, to the other experiments, the quantitative and qualitative results from the experiment conducted with the green coloring matched what should have been expected as the two constituent colors separated into distinct fronts whose Rƒ values 0.92 for the yellow and 1.0 for the blue were within the uncertainty of +/- 0.05 of the table 4 values in Laboratory Experiments Experiment 2D (0.95 yellow #5 and 1.0 blue #1). Much like the previously discussed experiments, the unknown mixture behaved as should have been expected both qualitatively and quantitatively. The three colors that comprised the unknown mixture separated into three distinct fronts with Rƒ values of 0.77 for the red coloring, 0.82 for the yellow coloring, and 1.0 for the blue coloring which are all within the range of uncertainty of +/- 0.05 of the values provided in table 4 of Laboratory Experiments Experiment 2D (0.81 Red #2, 0.77 yellow #6 and 1.0 blue #1). As is evident when discussing the Rƒ values calculated by separate groups there is a large margin of error and the results from any individual test may not have been representative of the known Rƒ values for any of the given colors. This may have been caused by many factors one of which has already been discussed which was the difference in the amount of coloring used by each group however another important source of error could have been the testing equipment itself as paper chromatography strips are not all perfect carbon copies of one another and can therefore be a source of error since the interactions of the cellulose within the paper and the polar molecules within the colorings is an important contributor to the observed Rƒ values of the colorings tested.³ If I were to conduct these experiments again I would be sure to have an accurate amount of all of the solutes and solvents used, this could be done by measuring their volumes using a pipette before applying them to the chromatography paper. I would also ensure that all of the experiments had fully completed before taking the Group: Connor, Arjan, Euan Author: Connor Gunning Date: October 12, 2023 chromatography paper out of the solvent as the results may have also been skewed by the fact that the solvents and solutes did not have enough time to travel up the chromatography paper. Conclusion: The components of the two mixtures we tested in this lab were qualitatively and quantitatively identified. We qualitatively identified the components of both mixtures by observing the distinct fronts created as the mixtures traveled up the chromatography paper. The mixtures separated into primarycolored fronts the green separated into a yellow front and a blue front while the unknown mixture separated into a red front a yellow front and a blue front. The individual fronts were then quantitatively proven to match the accepted values of the colorings in table 4 of Laboratory Experiments Experiment 2D by calculating their Rƒ values and observing that they were all within +/- 0.05 of the accepted values. The Rƒ values of the observed fronts for the green coloring and the unknown mixture are as follows, yellow Rƒ 0.92 (corresponds to the 0.95 Rƒ value of yellow #5), and blue Rƒ 1.0 (corresponds to the 1.0 Rƒ value of blue #1) for the green coloring and red Rƒ 0.77 (corresponds to the 0.81 Rƒ value of red #2), yellow Rƒ 0.82 (corresponds to the 0.77 Rƒ value of yellow #2), and blue 1.0 Rƒ (corresponds to the 1.0 Rƒ value of blue #1) for the unknown mixture. As has been made evident throughout this report we successfully used paper chromatography to identify the components of previously unknown mixtures both quantitatively and qualitatively. Group: Connor, Arjan, Euan Author: Connor Gunning Date: October 12, 2023 Works Cited: 1. GeeksforGeeks Title: Retention Factor Formula URL: https://www.geeksforgeeks.org/retention-factor-formula/ Accessed: October 14, 2023 2. LibreTexts Title: Migration Rates of Solutes Source: Analytical Chemistry URL: https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/InstrumentalAnalysis_ (LibreTexts)/26%3A_Introduction_to_Chromatographic_Separations/26.02%3A_Mi gration_Rates_of_Solutes Accessed: October 14, 2023 3. Truman State University Title: Paper Chromatography Source: Truman State University, Chemistry Lab Manual URL: https://chemlab.truman.edu/files/2015/07/Paper-Chromatography-080813.pdf Accessed: October 14, 2023