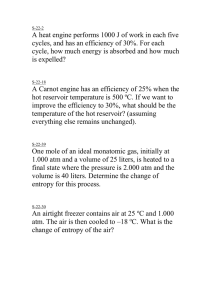
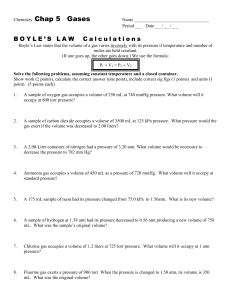
Gas Laws Practice Test 1. Explain how the temperature is related to the kinetic energy and motion of gas particles. 2. If the volume of a gas contained within balloon were to be tripled, what would be the impact upon the pressure if Kelvin temperature is maintained as constant? Directions: Solve the following problems. Show all your work, including units 3. In a mixture of carbon dioxide, oxygen gas, sulfur dioxide and carbon monoxide, the pressure of the carbon dioxide is 0.3 atm, oxygen gas is 0.5 atm, sulfur dioxide is 0.6 atm, and the pressure of the carbon monoxide is 0.1 atm. What is the total pressure in the container? 4. A high-altitude balloon contains 4 Liters of helium gas at 1.35 atm. What is the volume when the balloon rises to an altitude where the pressure is only 1.20 atm? (Assume that the temperature remains constant.) 5. If a sample of gas occupies 27 Liters at 12 Celsius, what will be its volume at 112 Celsius if the pressure does not change? 6. A gas at 10 kPa and 45 Celsius occupies a container with an initial volume of 4 Liters. By changing the volume, the pressure of the gas increases to 25 kPa as the temperature is raised to 190 Celsius. What is the new volume? 7. You fill a rigid steel cylinder that has a volume of 840 milliliters with oxygen gas to a final pressure of 1.1 atmospheres at 145 Celsius. How many moles of oxygen gas does the cylinder contain?(what about mass?) 8. What is the temperature when 4 moles of carbon dioxide occupies a 2 L container and exerts a pressure of torr? 9. What pressure, in atm, will be exerted by 1.25 moles of a gas at 39 Kelvin if it is contained in a 5 Liter vessel? 745 10. What volume will 29 grams of nitrogen gas occupy at 10 Celsius and a pressure of 620 torr? 11. A 35mL sample of hydrogen gas is collected over water at a temperature of 24 oC, the vapor pressure of the water at that temperature is 2.99 kPa, and the atmospheric pressure is 765.5 torr. What is the pressure of the dry hydrogen gas? 12. According to the kinetic molecular theory, molecules increase in kinetic energy when they a. Are mixed with other molecules at lower c. Are condensed into a liquid temperature d. Are heated to a higher temperature b. Are frozen into a solid 13. At STP, 32.0 liters of O2 contain the same number of molecules as a. 22.4 L Ar c. 32. 0 L of H2 b. 28.0 L of N2 d. 44.8 L of He 14. An 8.25 L sample of oxygen is collected at 25°C and 1.022 atm pressure. What volume will the gas occupy 0.940 atm and -15°C? a. 1.78 L c. 10.4 L e. 7.77 L b. 5.00 L d. 8.76 L f. g. The mass of 2.37 liters of a gas is 8.91 grams. What is the density of the gas? 3.76 g/L h. None of these 6.54 g/L i. 0.266 g/L j. 21.1 g/L 15. A 268 cm3 sample of an ideal gas at 18°C and 748 torr pressure is placed in an evacuated container of volume 648cm3. To what Celsius temperature must the assembly be heated so that the gas will fill the whole chamber at 748 torr? a. 431°C c. 704°C e. 324°C b. 120°C d. 597°C 16. If pressure of a gas is increased and its volume remains constant, what will happen to its temperature? a. Increase b. Decrease c. Stay the same 17. One way to increase pressure on a gas is to a. decrease temperature b. increase volume c. d. increase the number of gas particles lower the kinetic energy of the gas molecules 18. If a gases volume is decreased and pressure is constant, its temperature will a. Increase b. Decrease c. Stay the same 19. If the temperature of a gas remains constant but pressure is decreased, the volume will a. Increase b. Decrease c. Stay the same 20. Convert 2.3 atm into mmHg a. 2300 mmHg b. 1750 mmHg c. d. 2.3 mmHg 0.0030 mmHg 21. The pressure of a gas is 750.0 torr when its volume is 400.0 mL. Calculate the pressure (in atm) if the gas is allowed to expand to 600.0 mL at constant temperature. a. 0.660 atm c. 500.0 atm b. 1.48 atm d. 1125 atm 22. The volume of a gas is increased from 150.0 mL to 350.0 mL by heating it. If the original temperature of the gas was 25.0 °C, what will its final temperature be (in °C)? a. - 146°C c. 58.3°C e. 695°C b. 10.7°C d. 422°C 23. Standard temperature and pressure (STP) refers to which conditions? a. 0 oC and 1 kPa c. 0 K and 1 kPa b. 0 oC and 1 mm Hg d. 0 K and 1 atm e. 273 K and 1 atm 24. If 4 moles of a gas are added to a container that already holds 1 mole of gas, how will the pressure change within the container? (Assume volume and temperature are constant.) a. The pressure will be 5 times as great. d. The pressure will not change. b. The pressure will be 2 times as great. e. None of the above are correct. c. The pressure will be 4 times as great. 25. A 4.0 L sample of hydrogen gas at 700 mm Hg would occupy what volume at 250 mm Hg? (Assume temperature and number of particles stays constant.) a. 1.4 x 10 -7 L c. 11.2 L e. 7.0 x 10 5 L b. 1.4 L d. 2.4 L 26. A balloon containing 2.50 L of gas at 1 atm would be what volume at a pressure of 300 kPa? (Assume temperature and number of particles stays constant.) a. 6.33 L c. 0.844 L e. 000833 L b. 8.11 L d. 120. L 27. A syringe containing 75.0 mL of air is at 298 K. What will the volume of the syringe be if it is placed in a boiling water bath (373 K). Assume pressure and the number of particles are held constant. a. 59.9 mL b. 188 mL c. 300. mL d. 8.34 x 106 mL e. None of the above are correct. 28. A gas occupies 40.0 mL at 127 oC. What volume will it occupy at -73 oC? (Assume pressure and number of particles is constant.) a. 182 mL b. 8.80 mL c. 80.0 mL d. 20.0 mL e. None of these is correct 29. If 88.0 grams of solid carbon dioxide evaporates, how many liters of CO 2 gas will be formed at a temperature of 300 K and 2.00 atmospheres of pressure? a. 98.5 liters c. 24.6 liters b. 2170 liters d. 1080 liters 30. A 50.0 mL sample of a gas is at 3.00 atm of pressure and a temperature of 298 K. What volume would the gas occupy at STP? a. 0.00728 mL c. 18.2 mL e. None of these is correct. b. 15.3 mL d. 137 mL 31. A syringe contains 60.0 mL of air at 740 mm Hg pressure and 20 oC. What would be the temperature at which the syringe would contain 30.0 mL at a pressure of 370 mm Hg? (Assume no gas could leak in or out of the syringe.) a. -200 oC c. 5 oC e. None of these is correct o b. 0.0137 C d. 73.3 oC 32. A sample of gas is collected by water displacement. The atmospheric pressure in the room is 757 mm Hg and the vapor pressure of water is 17 mm Hg. What is the partial pressure of hydrogen under these conditions? a. 17 mm Hg c. 757 mm Hg b. 740 mm Hg d. 774 mm Hg e. The question cannot be answered without knowing the temperature of the system. 33. Nitrogen has a molar mass of 28.02 g/mol. What is the density of nitrogen at 1.05 atm and 37°C? a. None of these c. 0.89 g/L e. 4.72 g/cm3 b. 2.82 g/L d. 1.25 g/L 34. How many moles of gas would it take to fill an average man's lungs, total capacity of which is about 4.5 liters? Assume 1.00 atm pressure and 37.0°C. a. 37.0 mol c. 0.75 mol e. 11.2 mol b. 1.24 mol d. 0.18 mole 35. a. b. c. d. Which flask contains the greatest number of molecules? Flask 3 (O2) Flask 1 (NH3) Flask 2 (CH4) Flasks 2 and 3 e. All the same 36. A sealed container contains 1.0 mol of hydrogen and 2.0 moles of nitrogen gas. If the total pressure in the container is 1.5 atm, what is the amount of pressure exerted by each gas? a. H2 = 1.0 atm and N2 = 0.50 atm b. H2 = 0.50 atm and N2 = 1.0 atm c. H2 = 1.0 atm and N2 = 2.0 atm d. H2 = 2.0 atm and N2 = 1.0 atm are e. There is not enough information given to answer the question.