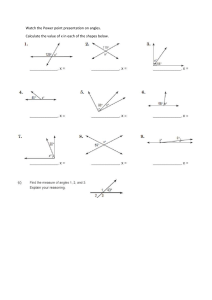
SIT & START Complete exercise 4 and 5 of the book p54-55. 15 minutes Learning Objectives 1. TBAT describe covalent bonding in terms of orbital overlap and the concept of hybridization. [LO 3.2b] 2. TBAT explain the shapes of, and bond angles in, molecules by using the electron-pair repulsion model. [LO 3.2c] Electron pair-repulsion dictates the structure of molecules The shape and bond angles of covalently bonded molecules depend on: 1. The number of pairs of electrons around each atom 2. Whether these pairs are lone pairs or bonding pairs 1. 2. TBAT describe covalent bonding in terms of orbital overlap and the concept of hybridisation. [LO 3.2b] TBAT explain the shapes of, and bond angles in, molecules by using the electronpair repulsion model. [LO 3.2c] Electron pair-repulsion dictates the structure of molecules Order of repulsion: Lone pair-lone pair Lone pair-bond pair Bond pair-bond pair 1. 2. TBAT describe covalent bonding in terms of orbital overlap and the concept of hybridisation. [LO 3.2b] TBAT explain the shapes of, and bond angles in, molecules by using the electronpair repulsion model. [LO 3.2c] Electron pair-repulsion dictates the structure of molecules 1. 2. TBAT describe covalent bonding in terms of orbital overlap and the concept of hybridisation. [LO 3.2b] TBAT explain the shapes of, and bond angles in, molecules by using the electronpair repulsion model. [LO 3.2c] Electron pair-repulsion dictates the structure of molecules Use molecule box! 1. 2. TBAT describe covalent bonding in terms of orbital overlap and the concept of hybridisation. [LO 3.2b] TBAT explain the shapes of, and bond angles in, molecules by using the electron-pair repulsion model. [LO 3.2c] Electron pair-repulsion dictates the structure of molecules 1. 2. TBAT describe covalent bonding in terms of orbital overlap and the concept of hybridisation. [LO 3.2b] TBAT explain the shapes of, and bond angles in, molecules by using the electronpair repulsion model. [LO 3.2c] Electron pair-repulsion dictates the structure of molecules 1. 2. TBAT describe covalent bonding in terms of orbital overlap and the concept of hybridisation. [LO 3.2b] TBAT explain the shapes of, and bond angles in, molecules by using the electronpair repulsion model. [LO 3.2c] Electron pair-repulsion dictates the structure of molecules TBAT describe covalent bonding in terms of orbital overlap and the concept of hybridisation. [LO 3.2b] TBAT explain the shapes of, and bond angles in, molecules by using the electron-pair repulsion model. [LO 3.2c] 1. 2. TBAT describe covalent bonding in terms of orbital overlap and the concept of hybridisation. [LO 3.2b] TBAT explain the shapes of, and bond angles in, molecules by using the electron-pair repulsion model. [LO 3.2c] 1. 2. TBAT describe covalent bonding in terms of orbital overlap and the concept of hybridisation. [LO 3.2b] TBAT explain the shapes of, and bond angles in, molecules by using the electron-pair repulsion model. [LO 3.2c] 1. 2. TBAT describe covalent bonding in terms of orbital overlap and the concept of hybridisation. [LO 3.2b] TBAT explain the shapes of, and bond angles in, molecules by using the electron-pair repulsion model. [LO 3.2c] 1. 2. TBAT describe covalent bonding in terms of orbital overlap and the concept of hybridisation. [LO 3.2b] TBAT explain the shapes of, and bond angles in, molecules by using the electron-pair repulsion model. [LO 3.2c] TASK Complete exercise 4.3 What is left is HW. 1. 2. TBAT describe covalent bonding in terms of orbital overlap and the concept of hybridisation. [LO 3.2b] TBAT explain the shapes of, and bond angles in, molecules by using the electron-pair repulsion model. [LO 3.2c] SIT & START What are the angles of: 1 minute! Instructions Watch the clips. When finished complete the exercises. Update your LO overview. New topic σ-bonds and π-bonds. HW complete 4.5 of hand-out. 1. 2. TBAT describe covalent bonding in terms of orbital overlap and the concept of hybridisation. [LO 3.2b] TBAT explain the shapes of, and bond angles in, molecules by using the electron-pair repulsion model. [LO 3.2c] Mixing of s and p orbitals HYBRIDIZATION https://www.khanacademy.org/science/chemistry /chemical-bonds/hybridization-and-hybridorbitals-chemistry/v/sp3-hybrid-orbital-jay-final https://www.khanacademy.org/science/chemistry /chemical-bonds/hybridization-and-hybridorbitals-chemistry/v/sp2-hybridization-jay-final https://www.khanacademy.org/science/chemistry /chemical-bonds/hybridization-and-hybridorbitals-chemistry/v/sp-hybridization-jay-final 1. 2. TBAT describe covalent bonding in terms of orbital overlap and the concept of hybridisation. [LO 3.2b] TBAT explain the shapes of, and bond angles in, molecules by using the electron-pair repulsion model. [LO 3.2c] Mixing of s and p orbitals HYBRIDIZATION When completed the movies. PRACTICE: https://www.khanacademy.org/science/chemistry /chemical-bonds/hybridization-and-hybridorbitals-chemistry/e/bond-hybridization-quiz 1. 2. TBAT describe covalent bonding in terms of orbital overlap and the concept of hybridisation. [LO 3.2b] TBAT explain the shapes of, and bond angles in, molecules by using the electron-pair repulsion model. [LO 3.2c] Mixing of s and p orbitals SP HYBRIDIZATION 1. 2. TBAT describe covalent bonding in terms of orbital overlap and the concept of hybridisation. [LO 3.2b] TBAT explain the shapes of, and bond angles in, molecules by using the electron-pair repulsion model. [LO 3.2c] Mixing of s and p orbitals sp2 HYBRIDIZATION 1. 2. TBAT describe covalent bonding in terms of orbital overlap and the concept of hybridisation. [LO 3.2b] TBAT explain the shapes of, and bond angles in, molecules by using the electron-pair repulsion model. [LO 3.2c] Mixing of s and p orbitals sp3 HYBRIDIZATION 1. 2. TBAT describe covalent bonding in terms of orbital overlap and the concept of hybridisation. [LO 3.2b] TBAT explain the shapes of, and bond angles in, molecules by using the electron-pair repulsion model. [LO 3.2c] Mixing of s and p orbitals sp3 HYBRIDIZATION 1. 2. TBAT describe covalent bonding in terms of orbital overlap and the concept of hybridisation. [LO 3.2b] TBAT explain the shapes of, and bond angles in, molecules by using the electron-pair repulsion model. [LO 3.2c] Mixing of s and p orbitals sp3 HYBRIDIZATION 1. 2. TBAT describe covalent bonding in terms of orbital overlap and the concept of hybridisation. [LO 3.2b] TBAT explain the shapes of, and bond angles in, molecules by using the electron-pair repulsion model. [LO 3.2c] Other orbital overlaps σ-bonds and π-bonds https://www.khanacademy. org/science/organicchemistry/gen-chemreview/hybrid-orbitalsjay/v/sp3-hybridizedorbitals-and-sigma-bonds https://www.khanacademy. org/science/organicchemistry/gen-chemreview/hybrid-orbitalsjay/v/pi-bonds-and-sp2hybridized-orbitals 1. 2. TBAT describe covalent bonding in terms of orbital overlap and the concept of hybridisation. [LO 3.2b] TBAT explain the shapes of, and bond angles in, molecules by using the electron-pair repulsion model. [LO 3.2c] TASK Exercise of the hand-out 4.5. 1. 2. TBAT describe covalent bonding in terms of orbital overlap and the concept of hybridisation. [LO 3.2b] TBAT explain the shapes of, and bond angles in, molecules by using the electron-pair repulsion model. [LO 3.2c]