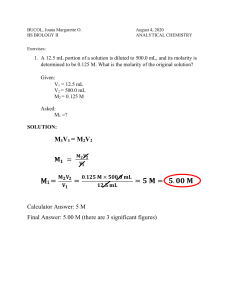
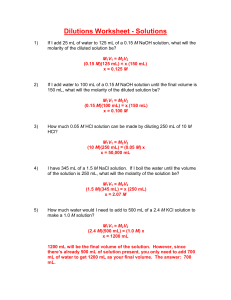
Name: ______________________________________________________ Date: ______________________ Section: _______ Dilutions Dilution is the addition of solvent, which decreases the concentration (molarity) of the solute in the solution. The initial concentration of a solution is always greater than the final concentration. A common example of a dilution is adding more water to a beverage that is too sweet. Formula: M1V1 = M2V2 M= molarity (concentration) 1= initial (start) M1 is always greater than M2 V= volume 2= final (end) V1 is always less than V2 Example: What is the final concentration of a NaOH solution when 100 mL of a 0.15 M solution is diluted to 150 mL? (Unknown = M2) M1= 0.15 M M2= unknown (?) V1= 100 mL V2= 150 mL (V2 > V1) M1V1 = M2V2 0.15 M × 100 mL = M2 × 150 mL Divide both sides by 150 mL to isolate unknown (0.15 M × 100 mL)/150 mL= M2 M2= 0.1 M The final concentration must always be less than the initial concentration. Since the initial was 0.15 M and the final was 0.1 M we can conclude that the answer is accurate. Notice that the final volume was greater than the initial volume. Practice: How much water would need to be added to 600 mL of a 0.63 M KBr solution to give a final concentration of 0.25 M? (Unknown = _____) Name: ______________________________________________________ Date: ______________________ Section: _______ Assignment: Solve the following dilutions word problems. For each problem label the variables, identify unknown, show all work, and include units. 1.) 50.0 mL of a HCl solution was diluted to 350 mL to give a final concentration of 0.80 mL, what was the initial concentration? (Unknown = _____) 2.) 1400 mL of water was added to 100 mL of a KCl solution to give a final concentration of 0.0150 M, what was the initial concentration? (Unknown = _____) 3.) What is the final concentration of a KOH solution when 45.0 mL of a 4.2 M solution is diluted to 250 mL? (Unknown= _____) 4.) 200 mL of water was added to a 1.0 M solution of NaCl to give a final concentration of 0.20 M, what was the initial volume of the NaCl solution? (Unknown= _____)