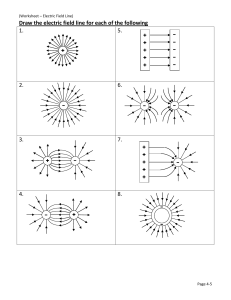
Form 3 worksheet 2018 Module: Nature of matter Topic: Atoms and Molecules and elements 1. (a) Complete Fig. 2.1 to show the electronic structure of 31P. 15 P [2] Fig. 2.1 (a) Name the element shown above? ……………………………………………………………………….. [1] (b) State the atomic number for the above element ……………………………………………………………………………… [1] (c) State the atomic mass for the above element ………………………………………………………………………………. Compiled by Mr Basebi 2018 © [1] Page 1 Form 3 worksheet 2018 2. Fig. 1.1 represents the arrangement of atoms or molecules in four different substances, A, B, C and D. A C (a) Which substance is a diatomic molecule? B D …………………………………………………………………… (i) [1] Explain your answer in (a) ………………………………………………………………………………………………………………………………………… [1] (b) Which substance is an element? ……………………………………………………………………. (i) [1] Explain your answer in (b). ………………………………………………………………………………………………………………………………………… [1] 3. Complete the following by filling in the blanks. (a) An element is a pure substance containing only one kind of ____________. (b) A molecule is a pure substance containing two or more _________________ chemically combined. (c) An element _____________ be separated into simpler materials (except during nuclear reactions). (d) Molecules can only be _____________________ separated, not physically. Compiled by Mr Basebi 2018 © Page 2 Form 3 worksheet 2018 Topic: Periodic table 1. Fig. 1.1 shows the electronic structure of an atom of chlorine. Fig. 1.1 (a) (i) State the Group of the Periodic Table in which chlorine is placed. ................................................................................................................ [1] (ii) Use the information in Fig. 1.1 to give a reason for your answer to (a)(i). ………………………………………………………………………………………………………………………………………. …………………………………………………………………………………………………………………….…………… [1] (b) (i) state the period of the periodic table in which chlorine is placed. ................................................................................................................. (ii) [1] Use the information in Fig 1.1 to give a reason for your answer to (b) (i). ………………………………………………………………………………………………………………………………………… [1] 2. Explain the periodic trend of elements in a periodic table in terms of: (a) Metallic and non metallic properties: ……………………………………………………………………………………………………………………………………….. ………………………………………………………………………………………………………………………………….. [2] (b) Reactivity …………………………………………………………………………………………………………………………………….. Compiled by Mr Basebi 2018 © Page 3 Form 3 worksheet 2018 3. The electronic structures of various atoms are shown below. A B D C E (a) (i) Which one of these structures A to E represents a noble gas? [1] (ii) Which two of these structures represent atoms from the same Group of the Periodic Table? and [1] (iii) Which one of these structures represents an atom with an atomic number of 8? [1] (iv) Which one of these structures is in Period 3 of the Periodic Table? …………………………………………………………………………………………………………………………………. [1] Compiled by Mr Basebi 2018 © Page 4 Form 3 worksheet 2018 4. Table 5.1 shows the elements of the third period of the Periodic Table with their electronic structures. Table 5.1 III Group IV I II Na Mg Al Si 2,8,1 2,8,2 2,8,3 2,8,4 V VI VII 0 P S Cl Ar 2,8,5 2,8,6 2,8,7 2,8,8 (a) Describe how the metallic character of these elements changes across the period. ……………………………………………………………………………………………………… [1] (b) State the relationship between the metallic character of an element and its electronic structure. ………………………………………………………………………………………………………. [1] (c) State and explain which period these elements belong to? ………………………………………………………………………………………………………… ………………………………………………………………………………………………………. [2] (d) Draw the electronic structure of aluminum. Compiled by Mr Basebi 2018 © [1] Page 5 Form 3 worksheet 2018 5. Figure 3 shows the electron arrangement of a different atom from an element in the second row of the periodic table. (a) Give the chemical symbol of this element. ................................................................................................................................................................... [1] (b) Why is this element unreactive? ……………………………………………………………………………………………………………………………………………………………. [1] 6. Figure 2 shows the electron arrangements of three different atoms, X, Y and Z. These atoms are from elements in the second row (lithium to neon) of the periodic table. Figure 2 (a) Which atom is from an element in Group 3 of the periodic table? ………………………………………………………………………………………………………. [1] (b) Explain …………………………………………………………………………………………… ………………………………………………………………………………………………………. [1] Compiled by Mr Basebi 2018 © Page 6 Form 3 worksheet 2018 7. Elements are made of atoms. Table 1 shows the atomic numbers and mass numbers of three atoms. Table 1 Atom Atomic number Mass number 1 12 24 2 12 25 3 12 26 (a) Suggest, in terms of the number of subatomic particles, why the atomic numbers of the three atoms are the same. ………………………………………………………………………………………………………… [1] (b) Explain, in terms of the number of subatomic particles, why the mass numbers of the three atoms are different. ………………………………………………………………………………………………………… [1] (c) What is the common name for elements with the same atomic number? ………………………………………………………………………………………………………… [1] Compiled by Mr Basebi 2018 © Page 7 Form 3 worksheet 2018 Topic: Compounds and Mixtures 1. State which of the following statements best describes a Compound or Mixture. (a) Can be separated into their components by chemical or physical means…………………….... (b) Properties are usually different than the properties of the elements it contains. ……………………………. (c) A pure substance containing two or more kinds of elements. ………………………………………. (d) Properties are similar to the properties of its components. …………………………… [4] 2. which box (A,B,C,D,E) represents an element, compound, molecule and mixture. A B A …………………………………………………. C D E B …………………………………………………. C …………………………………………………. D …………………………………………………. E …………………………………………………... [5] 3. the following are the chemical formulae of some compounds, for each one say what the constituents are? (a) C2H6 …………………………………………………………………………………………………………………………… [1] (b) ZnCl2 …………………………………………………………………………………………………………………………… [1] Compiled by Mr Basebi 2018 © Page 8 Form 3 worksheet 2018 (c) CuCO3 …………………………………………………………………………………………………………………………… [1] Compiled by Mr Basebi 2018 © Page 9 Form 3 worksheet 2018 Topic: Heating and Cooling curve Kagiso was given ice blocks to determine the boiling point of water. He presented his result on a graph below. Study the heating curve below to answer questions that follow. 1. (a) Explain what is happening to the average kinetic energy of the particles in the sample during section 2? ………………………………………………………………………………………………………………………………………………. ……………………………………………………………………………………………………………………………………….. [2] (c) For section 3, explain why the temperature does not remain constant? ………………………………………………………………………………………………………………………………………………. …………………………………………………………………………………………………………………………………………. [2] (d) State the boiling point of this substance? …………………………………………………………………. [1] (e) State the melting point of this substance? ………………………………………………………………… Compiled by Mr Basebi 2018 © [1] Page 10 Form 3 worksheet 2018 Topic: Separation Techniques 1. The diagrams show four methods of purifying substances. ethanol vapour filter paper ethanol heat (c) A B C D Which of these methods, A,B,C or D, is best used for (i) separating the different colours in a sample of ink?......................... [1] (ii) separating two liquids with different boiling points? ………………… [1] (iii) separating mud from water? ………………………………… [1] (iv) making crystals of copper sulphate from copper sulphate solution? …………………….. [1] Compiled by Mr Basebi 2018 © Page 11 Form 3 worksheet 2018 (b) State the name given to the method of separation shown in (i) diagram A, ................................................................................................................. (ii) diagram B. ................................................................................................................. [2] 2. Plants make a variety of coloured pigments. A student extracted red colouring from four different plants, R, S, T and U. The student put a spot of each colouring on a piece of filter paper. The filter paper was dipped into a solvent and left for 30 minutes. The results are shown below. start of experiment result after 30 minutes filter paper R S T U R S T U solvent (i) What is name given to the process shown in the diagram? [1] (ii) Which plant contained the greatest number of different pigments? [1] (iii) Which two plants contained the same pigments? ……………………………………………………………………………………………………………………………… [1] Compiled by Mr Basebi 2018 © Page 12 Form 3 worksheet 2018 The diagram below shows a chromatogram obtained using solutions of three single dyes of colours blue, green and red three other samples H,I, and J. Use it to answer question 9. 3 (i) (a) Which of the samples H, I and/or J contain the following; One dye only? ………………………………………………………………………………………………. (1) (i) A dye other than blue, green or red? ………………………………………………………………………………………………. (1) (b) Which dye is present in all samples? ……………………………………………………………………………………………… (1) (c) Explain why the starting line of the chromatogram should be drawn with a pencil rather than with ink. ………………………………………………………………………………………….……………………………………………………… …………………………………………………………………………………….……………………………………………………… (2) Compiled by Mr Basebi 2018 © Page 13 Form 3 worksheet 2018 Use the information and the diagram below to answer question 4. Traditional alcohol is made by mixing malt powder, sugar and plenty of water. The mixture is allowed to ferment for 2-3 days in a warm room. The mixture is then boiled to separate the traditional alcohol as shown in the set-up below. 4.a) Name the separation technique used above. ………………………………………………………………………………………………………………………………….. (1) b) In this set- up, what is the purpose of the cold water in the trough? ………………………………………………………………………………………………………………….……………... (1) c) Explain why the pipe passing through the trough is at the bottom rather than at the top. …………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………….(3) (g) Drinking water can be obtained by purifying seawater. Explain why distillation rather than filtration is used to purify seawater for drinking. [2] Compiled by Mr Basebi 2018 © Page 14 Form 3 worksheet 2018 4. Petroleum (crude oil) is a fossil fuel consisting of a mixture of different hydrocarbons. Fig. 4.1 shows the industrial apparatus used to separate useful products from petroleum. refinery gases tower naphtha petroleum vapour in bitumen Fig. 4.1 Petroleum is vaporised and passed up a tower. Useful products from petroleum condense at different positions in the tower. (a) State the name of the process shown in Fig. 4.1. ……………………………………………………………………………………………………… [1] Different products from this process have different boiling point ranges. (b) State how the boiling point of a product affects the position in the tower where a product will condense. ………………………………………………………………………………………………………… ………………………………………………………………………………………………………. [1] (c) Three of the useful products obtained from petroleum are shown in Fig. 4.1. State the name of another useful product that is separated from petroleum. ………………………………………………………………………… [1] Compiled by Mr Basebi 2018 © Page 15 Form 3 worksheet 2018 5. The student wanted to separate the solid yeast from the solution. Figure 10 shows the apparatus used. Figure 10 Conical flask Mixture of solution and yeast Funnel Beaker (a) What is missing from the apparatus in Figure 10? ……………………………………………………………………………………………………… [1] (b) Name the solution collected in the beaker. ………………………………………………………………………………………………………. [1] (c) Name the above technique of separation. ………………………………………………………………………………………………………. [1] Compiled by Mr Basebi 2018 © Page 16 Form 3 worksheet 2018 6. Some plants contain oils that can be extracted. Figure 3 shows a process used to separate oils from plants. (a) What is the name of this process? Tick () one box. [1] Displacement Distillation Pressing (b) Describe the change of state at S. …………………………………………………………………………………………………………. [1] (c) Describe the change of state at T. …………………………………………………………………………………………………………. [1] Compiled by Mr Basebi 2018 © Page 17 Form 3 worksheet 2018 7. A student was asked to separate a mixture consisting of copper metal powder and sodium chloride powder. Copper metal powder is insoluble in water. Sodium chloride powder is soluble in water. Using the list of apparatus and materials below, describe a procedure that the student can follow to obtain copper metal from the mixture. You may use a diagram to support your answer. ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… …………………………………………………………………………………………………….. [5] Compiled by Mr Basebi 2018 © Page 18 Form 3 worksheet 2018 8. The set-up below was used to illustrate how salty water can be desalinated. (a) Name the desalination method used. ……………………………………………………………………………………………………… [1] (b) Describe how pure water is obtained in the desalination process illustrated above. …………………………………………………………………………………………………………... …………………………………………………………………………………………………………... …………………………………………………………………………………………………………... ………………………………………………………………………………………………………. [3] (c) Ice- cubes are to be added to water from time to time. Explain what will happen if ice- cubes are not added? ………………………………………………………………………………………………………… ………………………………………………..……………………………………………………. [2] (d) State two physical properties of water. ………………………………………………………………………………………………………….. ……………………………………………………………………………………………………… [2] Compiled by Mr Basebi 2018 © Page 19 Form 3 worksheet 2018 Module: Acids and Bases Topic: Classes of Acids and Bases 1. The diagram shows the changes in pH in a student’s mouth after she has eaten a sweet. 7 6 pH 5 4 3 0 10 20 30 40 50 time / minutes (a) Describe how the acidity in the student’s mouth changes after she has eaten the sweet. [2] (b) (i) Chewing a sweet stimulates the formation of saliva. Saliva is slightly alkaline. Use this information to explain the shape of the graph. [2] (ii) State the name of the type of reaction which occurs when an acid reacts with an alkali. [1] Compiled by Mr Basebi 2018 © Page 20 Form 3 worksheet 2018 2. A student used the apparatus shown below to calculate the concentration of a solution of calcium hydroxide. A hydrochloric acid calcium hydroxide solution (a) State the name of the piece of apparatus labelled A. …………………………………………………………………………………………………………………………………………. [1] (b) Describe how the pH of the solution in the flask changes as the hydrochloric acid is added. ………………………………………………………………………………………………………………………………………………. ……………………………………………………………………………………………………………………………………….. [2] (c) State the colour on the universal indicator for the following solutions: i) Hydrochloric acid ………………………………………………………………………………………….. [1] ii) Calcium hydroxide ………………………………………………………………………………………… [1] (d) Define the term pH ………………………………………………………………………………………………………………………………………………. …………………………………………………………………………………………………………………………………………. [1] Compiled by Mr Basebi 2018 © Page 21 Form 3 worksheet 2018 3. Use the information below to answer questions that follow. 20 % of Sodium hydroxide and 40% of hydrochloric acid will completely ionize in water forming hydroxyl ions and hydrogen ions. 90% of Ammonia and 70% of Ethanoic acid will partially ionize in water to release same ions listed above. (a) What is the concentration and strength of: i. Sodium hydroxide …………………………………………………………………………………… [1] ii. Hydrochloric acid …………………………………………………………………………………… [1] iii. Ammonia ………………………………………………………………..………………….. [1] iv. Ethanoic acid ……………………………………………………………………………………. [1] (b) Complete the word equations below: i. Hydrochloric acid water ___________________ + ____________ [1] ii. Ammonia + water ___________________ + _____________________ [1] 4. State two differences between strong acids and weak acids. Strong acids Weak acids 5. Copper oxide and sodium hydroxide both will turn red litmus paper blue, explain why though having same property the two chemicals are different? ……………………………………………………………………………...……………………………………………………………… …………………………………………………………………………………………………………………………………………….. ………………………………………………………………………..………………………………………………………………. [2] Compiled by Mr Basebi 2018 © Page 22 Form 3 worksheet 2018 Topic: Reaction of Acids with other chemicals 1. Ammonium nitrate can be made by adding nitric acid to a solution of ammonia. (i) What type of reaction is this? [1] (ii) State two application of this type of reaction in everyday life. ……………………………………………………………………………………………...………………………………………... …...……………………………………………………………………………………….…………………………………….. [2] (iii) Complete the word equation for this reaction. Ammonia + nitric acid Ammonium nitrate + ……………………………………………….………….… [1] 2. (i) Complete the following equation for the reaction of calcium carbonate with hydrochloric acid. Calcium carbonate + ………………………… + carbon dioxide + water acid (ii) Describe how you would test for the gas given off in this reaction. Test............................................................................................................................ Result......................................................................................................................... (iii) [3] State any two application of acid-carbonate reaction in everyday life. ………………………………………………………………………………………………………………………………………………. ………………………………………………………………………………………………………………………………………… [2] Compiled by Mr Basebi 2018 © Page 23 Form 3 worksheet 2018 3. The student adds a 2 cm length of magnesium ribbon to a fresh supply of hydrochloric acid. He measures the total volume of gas collected every 30 seconds for 3 minutes. He plots his results as shown in Fig. 6.1. 25 total volume of gas collected 3 / cm 20 15 10 5 0 0 0.5 1.0 1.5 time / min 2.0 2.5 3.0 Fig. 6.1 (a) Describe what happens to the rate of this reaction during the 3 minute period. …………………………………………………………………………………………………………... …………………………………………………………………………………………………….. [2] (b) Suggest a reason for the rate of reaction between 2.5 and 3 minutes. ………………………………………………………………………………………………………… ………………………………………………………………………………………………………. [1] (d) He does a second experiment with a new 2 cm length of magnesium ribbon and the same volume and concentration of acid, but at a higher temperature. Draw on Fig. 6.1 the curve that the student draws. Label your curve T. Compiled by Mr Basebi 2018 © [2] Page 24 Form 3 worksheet 2018 4. (a) Complete the equation for the reaction of hydrochloric acid with zinc. Zinc + hydrochloric acid zinc chloride + ……………….…………… [1] (c) Name the type of reaction above? ……………………………………………………………………………………………………………………………………………….. [1] (d) Describe how you will test for the gas. Test …………………………………………………………………………………………………………………………………… [1] Results ………………………………………………………………………………………………………………………………. [1] (e) State one application of the above type of reaction. ………………………………………………………………………………………………………………………………………………. [1] 4. (a) Fig. 1.1 shows an experiment to compare how three metals react with dilute hydrochloric acid. In two of the test-tubes, bubbles of a gas G are produced. Gas G is an element. (i) State the name of gas G. ……………………………………………………………………………………………...…… [1] Compiled by Mr Basebi 2018 © Page 25 Form 3 worksheet 2018 (ii) Describe a test for gas G. Test ………………………………………………………………………………………………………………………….. [1] Result ………………………………………………………………………………………………………………………. [1] (iii) List the four elements X, copper, magnesium and G in order of reactivity. most reactive least reactive [2] (iv) Suggest the identity of metal X. [1] 5. A sample of soil is mixed with water and filtered. (a) Describe a test that would show that the soil is acidic. .................................................................................................................................. ............................................................................................................................. [2] In order to reduce soil acidity, limestone is sometimes added. Limestone consists mainly of calcium carbonate. (b) Complete the word equation for the reaction occurring between calcium carbonate and dilute hydrochloric acid. Calcium carbonate + hydrochloric carbon acid dioxide + + ................................... (c) The soil treatment described in (b) adds to the amount of carbon dioxide in the atmosphere. (i) Describe how this increase could be affecting the environment. …………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………… [2] Compiled by Mr Basebi 2018 © Page 26 Form 3 worksheet 2018 3. Figure 1 shows apparatus used to investigate the reaction of sulfuric acid with calcium carbonate. (a) Explain any two changes observed in the investigation above. ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… ………………………………………………………………………………………………………… …………………………………………………………………………………………………… [4] Compiled by Mr Basebi 2018 © Page 27 Form 3 worksheet 2018 Topic: Rate of chemical reaction 1. A student investigates the reaction between zinc and hydrochloric acid. The hydrochloric acid is in excess. The student uses the apparatus shown in the diagram. gas syringe gas small test tube held by a piece of cotton zinc powder hydrochloric acid (a) Write a complete word equation for the above reaction. Zinc + Hydrochloric acid …………………………………………….. + ………………………………………. [2] (b) The student reads the volume of gas in the syringe every minute. The results are shown in the table. time in minutes 0 1 2 3 4 5 6 7 volume of gas in cm3 0 23 35 45 50 53 55 55 Compiled by Mr Basebi 2018 © Page 28 Form 3 worksheet 2018 (i) Plot the results on the grid on page 18. 60 50 40 30 20 10 0 (ii) 1 2 3 4 5 6 7 time / min Explain why the volume of gas stays the same after six minutes. ................................................................................................................................................................................................... ............................................................................................................................................................................... [2] (iii) The student does the experiment again. The only difference is that the student uses warm, rather than cold, hydrochloric acid. On the grid, draw the shape of the graph you would expect for the experiment with the warm hydrochloric acid. [2] Compiled by Mr Basebi 2018 © Page 29 Form 3 worksheet 2018 2. A student used the apparatus shown below for investigating the speed of the reaction between iron and sulphuric acid. Sulphuric water Acid iron powder (a) The student repeated the experiment with different concentrations of sulphuric acid. In each experiment the mass of iron powder was the same and the temperature was kept at 30°C. The results are shown in the table. concentration of sulphuric acid / moles per dm3 speed of reaction /cm3 hydrogen per second 0.4 4.2 0.8 8.5 1.6 17.0 (i) Use the information in the table to help you work out how the speed of the reaction is affected by the concentration of sulphuric acid. [2] (d) What will happen to the speed of the reaction if lumps of iron are used instead of iron powder? [1] Compiled by Mr Basebi 2018 © Page 30 Form 3 worksheet 2018 3. Catalyst X was added to 50cm3 of hydrogen peroxide solution at 20oC and the amount of oxygen given off was recorded over a two minute period. The experiment was repeated with the same amounts of catalyst Y and catalyst Z. Apart from the type of catalyst, all conditions were kept the same in the three experiments. A graph of the results is shown below. (i) What is a catalyst? ………………………………………………………………………………………………………………………………… [1] (ii) Which catalyst, X, Y or Z, produced oxygen gas the fastest? Explain your answer. [2] (iii) Why is the final amount of oxygen gas the same in each experiment? [1] Compiled by Mr Basebi 2018 © Page 31 Form 3 worksheet 2018 Use the diagram and the information below to answer question 3 (a) to (d) The diagram shows a set-up used to compare the reaction of calcium with water and that of barium with water. To make the comparison of the reactions fair, the mass of barium and the mass of calcium should be the same. The two metals are found in group 2 in the Periodic Table. 4. (a) Name gas Y. .................................................................................................................................. (1) (b) Suggest two other factors which should be the same to make the comparison fair. ............................................................................................................................................................................................... ...................................................................................................................................................................................... (2) (c) After two minutes from the start of the experiment, the plunger of the syringe in the barium experiment had moved out further than in the calcium experiment. Explain this observation. ............................................................................................................................................................................................... ....................................................................................................................................................................................... (1) (d) Which substance other than water can be used in the experiment? ........................................................................................................................................................................................ (1) Compiled by Mr Basebi 2018 © Page 32 Form 3 worksheet 2018 5. A student investigates the speed of reaction between dilute hydrochloric acid and calcium carbonate. The reaction produces carbon dioxide gas. (d) Fig. 2.1 shows some of the apparatus the student uses. (i) The student wants to measure the volume of gas produced in this reaction every minute for 10 minutes. Complete Fig. 2.1 to show how the student collects and measures the volume of gas. [2] (ii) Describe the test for carbon dioxide gas. test ........................................................................................................................... result .............................................................................................................................. [2] Compiled by Mr Basebi 2018 © Page 33 Form 3 worksheet 2018 (iii) As the reaction proceeds, the acid concentration decreases. Describe what happens to the speed of the reaction. ............................................................................................................................. [1] 6. Fig 4.1 shows apparatus some students use to investigate the effect of temperature on the rate of reaction between calcium carbonate and dilute hydrochloric acid. Table 4.1 shows the results of the investigation. Table 4.1 temperature / °C time taken to fill the gas jar / s 20 156 30 75 40 37 50 20 60 10 Compiled by Mr Basebi 2018 © Page 34 Form 3 worksheet 2018 (a) Use Table 4.1 to state how the rate of reaction changes when the experiment is repeated at higher temperatures. .................................................................................................................................. ............................................................................................................................. [1] (b) Explain your answer to (a) in terms of the collisions between particles. .................................................................................................................................. .................................................................................................................................. .................................................................................................................................. ............................................................................................................................. [2] Compiled by Mr Basebi 2018 © Page 35 Form 3 worksheet 2018 Module: Metals and Non Metals Topic: Properties and uses of Metals and Non Metals 1. State three physical properties which are characteristic of all metals. [3] 2. State three physical properties which are characteristic of all non metals. ………………………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………………… [3] 3. List three most reactive metals. …………………………………………………………… ………………………………………………………….. ………………………………………………………….. [3] 4. List three most unreactive metals. …………………………………………………………… …………………………………………………………… …………………………………………………………… [3] 5. Explain the meaning of the following terms in terms of metals and non metals. (a) Ductile …………………………………………………………………………………………………………………………………… [1] Compiled by Mr Basebi 2018 © Page 36 Form 3 worksheet 2018 (b) Brittle …………………………………………………………………………………………………………………………………… [1] (c) Malleable ………………………………………………………………………………………………………...…………………………. [1] (e) The list below shows some of the substances which are found in the liquid which drains through the waste. Aluminium Oxygen calcium carbonate phosphorus iron lead chlorine magnesium nickel sodium sulphate zinc From this list choose (i) a metal used to galvanise iron. ................................................................................. (ii) a transition metal. ........................................................................................................... (iii) a metal which is in Group IV of the periodic table. .................................................... (iv) a metal which is used to make aircraft bodies. ....................................................... (v) a non-metallic gas that supports burning ……………………………………………… (vi) a non-metallic solid used in matches ……………………………………………………… (vii) a non-metallic gas used to sterilize water ………………………………………………. [7] Compiled by Mr Basebi 2018 © Page 37 Form 3 worksheet 2018 6. A piece of sodium metal attached to a copper wire was left for a few days as shown in the diagram below. Use the diagram to answer question 6. (a) The sodium reacted with one of the gases in the air under the test tube to form an oxide. (i) Name the gas that reacted with sodium? ………………………………………………………………………………………………… [1] (ii) Write the word equation for the reaction ………………………………………………………………………………………………… [2] (iii) What happened to the water level inside the test tube after the reaction? ………………………………………………………………………………………………… [1] (iv) Explain your answer in (iii) ………………………………………………………………………………………………… [1] (b) Give one physical property of water. …………………………………………………………………………………………………….. [1] (c) Name one element found in the same group with sodium in the periodic table. ……………………………………………………………………………………………………... [1] Compiled by Mr Basebi 2018 © Page 38 Form 3 worksheet 2018 7. The diagram below shows a piece of magnesium ribbon burning in air. Use it to answer question 7. (a) Name the substance that reacted with magnesium ribbon to form white powder. ...………………………………………………………………………………………………… [1] (b) What is the chemical name for the white powder formed? ...………………………………………………………………………………………………… [1] (i) Is the white powder an element or compound? …………………………………………………...………………………………………………….. [1] (ii) Explain ………………………………………………………………………………………………………. [1] (c) Magnesium can also react with dilute acids. (i) Name any acid that can react with magnesium. ………………………………………..………………………………………………………… [1] (ii) Write the word equation for the reaction between magnesium and the acid you named above. ………………………………………………………………………………………………….. [2] Compiled by Mr Basebi 2018 © Page 39 Form 3 worksheet 2018 Topic: The chemical process of rusting 1. When Group I elements react with water, hydrogen gas is given off. The diagram shows the reaction of lithium, potassium and sodium with water. A B C (a) Which one of these elements A, B or C is lithium? [1] (b) (i) Write the equation for the reaction of sodium with water by completing the right-hand side. sodium + water ………………………….. + ……………………………. [1] (ii) Apart from fizzing, describe two things that you would see when sodium reacts with water. [2] (iii) After the sodium had reacted with the water, the solution was tested with red litmus paper. What colour did the litmus paper turn? Give a reason for your answer. colour Reason Compiled by Mr Basebi 2018 © [2] Page 40 Form 3 worksheet 2018 2. The diagram shows an experiment to investigate the rusting of some iron nails. A B C Air air Air iron nail iron nail iron nail coated with Zinc Distilled Water drying agent (calcium chloride) Distilled Water (a) For each tube A, B and C predict whether the nails will rust. In each case give a reason. tube A: does the nail rust? reason tube B: does the nail rust? reason tube C: does the nail rust? reason [3] 3. Complete the word equation for rusting. iron + ……………………….. + oxygen ………………………….. [1] 4. State two conditions needed for rusting to occur. ……………………………………………………………………………….. ……………………………………………………………………………….. Compiled by Mr Basebi 2018 © [2] Page 41 Form 3 worksheet 2018 5. Write the chemical name for rust. ………………………………………………………………………………………………………………………………………… [1] 6. State four ways by which rusting can be prevented. ……………………………………………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………………………………………….. ……………………………………………………………………………………………………………………………………………….. ………………………………………………………………………………………………………………………………………… [4] 7. Define the following terms: (a) Oxidation ……………………………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………………………… [1] (b) Corrosion ……………………………………………………………………………………………………………………………………………….. …………………………………………………………………………………………………………………………………………. [1] (c) Rusting ……………………………………………………………………………………………………………………………………………….. ………………………………………………………………………………………………………………………………………… [1] Compiled by Mr Basebi 2018 © Page 42 Form 3 worksheet 2018 Topic: Carbon 1. Define the term allotrope. ……………………………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………………………… [1] 7 Three forms of carbon are diamond, graphite and Buckminsterfullerene. carbon atom diamond graphite (a) State two differences in structure between graphite and diamond. ………………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………………………. ………………………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………..…………………… [2] (b) State the type of bonding between the carbon atoms in diamond. ………………………………………………………………………………………………………………………………………… [1] (c) Suggest why graphite is used as a lubricant. Refer to the layers in your answer. ........................................................................................................................................... ..................................................................................................................................... [1] (d) Which of the two allotropes conduct electricity? Explain your answer. ..................................................................................................................................................................................... ………………………………………………………………………………………………………………………………… [1] Compiled by Mr Basebi 2018 © Page 43 Form 3 worksheet 2018 Topic: Mining Techniques 1. The iron from the blast furnace contains up to 10% by mass of impurities. Use the blast furnace below to answer questions that follow. oxygen and powdered limestone slag forming molten iron from blast furnace A mixture of oxygen and limestone is blown onto the surface of the molten iron. (a) What is the purpose of blowing oxygen onto the molten iron? [1] i) Write the word equation for the above chemical reaction. Oxygen + ………………………………… heat + ………………………………….. [1] The limestone added breaks down to form a basic oxide. The basic oxide reacts with the impurities in the iron and form a slag. (b) Complete the following word equations about the two chemical reactions explained above. Calcium carbonate ………………………………….. + Carbon dioxide calcium oxide + ………………………………. calcium silicate (c) What information in the diagram suggests that the slag is less dense than the molten iron? …………………………………………………………………………………………………………………………………… [1] Compiled by Mr Basebi 2018 © Page 44 Form 3 worksheet 2018 2. Name at list one ore of iron. ………………………………………………………….. [1] 3. Name three raw materials added into the blast furnace. ……………………………………………………………. ……………………………………………………………. ……………………………………………………………. 4. List at list two impurities found in the carbon ore. ………………………………………………………………. ……………………………………………………………… [2] 5. List two uses of iron. ………………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………………… [2] Compiled by Mr Basebi 2018 © Page 45 Form 3 worksheet 2018 Topic: Extraction of Copper 1. The diagram below shows extraction of copper in Bcl mine. Use it to answer questions that follow. (a) State at list one ore of copper. ……………………………………………………………………… [1] (b) State two impurities in copper ore. …………………………………………………………………….. …………………………………………………………………….. [2] (c) Complete the word equation below. Copper sulphide + copper oxide (d) Label stage X on the diagram. Compiled by Mr Basebi 2018 © Sulphur dioxide + ………………………………… [2] [1] Page 46 Form 3 worksheet 2018 Module: Motion 1 Fig. 1.1 shows a car travelling at 30 m/s on a level road. At this speed the car has to overcome a force opposing the car. opposing force driving force Fig. 1.1 (a) The engine of a motor car is able to deliver a force of 80N to the wheels. If this force is able to accelerate the car at 3.8 m/s2, what is the mass of the motor car? …………………………….Kg [2] (b) Calculate the distance travelled by the car at the speed of 30m/s in 10s. ………………………… m [2] (c) Explain why the car slows down when it climbs a hill, even though the driving force is unchanged. .......................................................................................................................................... .......................................................................................................................................... .......................................................................................................................................... [1] Compiled by Mr Basebi 2018 © Page 47 Form 3 worksheet 2018 2. A soccer ball lies on a grass surface and is given a kick so that it starts rolling at 40 m/s. the ball stops rolling after 3 seconds. Calculate: (a) The deceleration of the ball. …………………. m/s2 [2] (b) Which newton law of motion explains why the ball stops? …………………………………………………………………………………………………………………………………. [1] 3. A rocket’s engines need to accelerate a 1000 kg rocket vertically upwards at a constant acceleration of 5 m/s2 . Take acceleration of force of gravity as 9.8 m/s2 . (a) Draw a free body diagram showing the forces acting on the rocket. [1] (b) Calculate the net force that the rocket’s engines need to produce to overcome the force of gravity and accelerate the rocket at 5 m/s2 . ……………….. N [2] Compiled by Mr Basebi 2018 © Page 48 Form 3 worksheet 2018 4. When Jane drives to work, she always places her purse on the passenger’s seat. Along the way she had a sudden stop and her purse has fell on the floor of the car. (a) Explain what causes the purse to fall from the front seat to the floor? …………………………………………………………………………………………………………………………… ……………………………………………………………………………………………………………………….. [2] (b) Which newton law of motion explains this? ………………………………………………………………………………………………………………………... [1] 5. (a) Which newton law of motion is also known as Action- reaction? ……………………………………………………………………..……………………………………………………..… [1] (c) Give an example of a situation that illustrates newton’s law of motion you named above. …………………………………………………………………………………………………………………………… ………………………………...……………………………………………………………………………………… [1] 6. If you double the force of an object what happens to the acceleration? ……………………………………………………………………………………………………………………………… [1] 7. If you double the mass of an object what happens to the acceleration? ……………………………………………………………………………………………………………………………… [1] 8. Explain the term inertia? …………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………….. [1] Compiled by Mr Basebi 2018 © Page 49 Form 3 worksheet 2018 Module: Machines Topic: Simple Machines 1. The diagram below shows Thabo and Neo playing on a see- saw. Use it to answer question 1. (a) On the diagram clearly label the: (i) Pivot (ii) Load when Neo is moving up [2] (b) Neo has a mass of 55kg. calculate her weight (g= 10N/kg) Weight= ……………………………… N [2] (c) State what Thabo should do to keep Neo up yet applying less effort. .……………………………………………………………………………………………………… [1] (d) What is a machine? ………………………………………………………………………………………………………. [1] Compiled by Mr Basebi 2018 © Page 50 Form 3 worksheet 2018 2. Fig. 2.2 shows a support for a leg in plaster and Fig. 2.3 shows a simplified diagram of the forces acting on the leg. centre of gravity hip joint (pivot) of leg in plaster centre of gravity F force F hip joint 34 cm 46 cm 120 N Fig. 2.2 Fig. 2.3 (a) Calculate the force F needed to keep the leg in a horizontal position. (b) State the principle of moments. F = ....................................... [3] .................................................................................................................................. .................................................................................................................................. ................................................................................................................................... [2] Compiled by Mr Basebi 2018 © Page 51 Form 3 worksheet 2018 3. Fig. 6.1 shows a crane lifting some bricks during the building of a house. arm counterweight P 20 m 12000 N Fig. 6.1 The weight of the bricks produces a turning effect, or moment, on the arm of the crane about the point P. The weight of the bricks is 12000 N. (a) Calculate the moment of this force, using the distance marked on Fig. 6.1. Moment …………………………… [2] (b) Suggest one advantage of being able to move the counterweight along the arm. ................................................................................................................................... ................................................................................................................................... [2] (c) Define the term moment. ……………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………… [1] Compiled by Mr Basebi 2018 © Page 52 Form 3 worksheet 2018 4. The diagram below shows a crane used to lift heavy loads. W is the weight of the counter weight. Use the diagram to answer question 3. (a) The crane is in equilibrium. (b) Calculate the moment of the load about the pivot. Moment ………………………………….[2] (c) Calculate force W of the counter weight. W= ……………………………… N [2] Compiled by Mr Basebi 2018 © Page 53 Form 3 worksheet 2018 5. Fig. 16.1 shows a spanner being used to tighten a nut. 0.15 m 40 N force nut spanner Fig. 16.1 (a) Calculate the moment of the 40 N force about the centre of the nut. ………………………….. [2] (b) Explain what needs to be changed is the mechanic using the spanner wanted to gain more mechanical advantage without increasing force applied. ……………………………………………………………………………………………………………………….….. [1] (c) Which class of levers does the spanner fall in? Explain. ………………………………………………………………………………………………………………………………... ………………………………………………………………………………………………………………………….. [2] Compiled by Mr Basebi 2018 © Page 54 Form 3 worksheet 2018 6. Below are two inclined planes. Use the diagrams to answer questions that follow. A B 5m 4m 2m 3m (a) With calculations of mechanical advantage, Show which of the two ( A and B) inclined planes would be suitable to use? ………………………………………………….. [2] (b) At what cost will it be to move the load along A compared to B? ……………………………………………………………………………………………………………………………… [1] 7. If a machine has an effort of 10N and a load of 100N, what is the mechanical advantage of the machine? ……………………………. [2] 1. ......................................... 2. ......................................... Compiled by Mr Basebi 2018 © Page 55 Form 3 worksheet 2018 Module: Body Systems Topic: Skeletal System 1. Fig. 8.1 shows the bones and muscles of a human leg. (a) Muscles in the leg work antagonistically. (i) State which muscle is antagonistic to muscle A. ……………………………………………………………………………………………………………………………………………… [1] (ii) Explain what is meant by antagonistic ………………………………………………………………….…………………………………………………………………………………. ………………………………………………………………….…………………………………………………………………………………. ……………………………………………………………..……………………………………………………………………………….. [2] (b) Label the following: (i) Bone X: …………………………………………………. (ii) joint Y: ………………………………………………… Compiled by Mr Basebi 2018 © [2] Page 56 Form 3 worksheet 2018 2. Fig. 2.1 shows a section through the spinal cord and also some of the muscles and bones of the arm (not drawn to the same scale). E........................................ F........................................ Fig. 2.1 (c) On the diagram, a. label bones E and F; b. draw in the neurone carrying impulses from the spinal cord to the triceps muscle. [5] (d) Describe how the structures shown in Fig. 2.1 bring about movement of the arm in the direction of the arrow. .......................................................................................................................................... .......................................................................................................................................... .......................................................................................................................................... ......................................................................................................................................[3] Compiled by Mr Basebi 2018 © Page 57 Form 3 worksheet 2018 3. Fig. 2.2 shows the legs of an athlete as he starts a race. G J H I position 1 position 2 Fig. 2.2 (c) Identify, by letter, two extensor muscles in Fig. 2.2. 1. ......................................... 2. ......................................... [2] (d) Briefly explain how the muscles (G, H, I and J) and the bones brings about movement for the athlete? …………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………….. [4] (e) List the tree main components of the musculoskeletal system. ……………………………………………………. ……………………………………………………. …………………………………………………….. Compiled by Mr Basebi 2018 © [3] Page 58 Form 3 worksheet 2018 4. Damaged joints may be replaced with metal or plastic. Fig. 1.2 shows a replacement joint in a person’s arm. KK Fig. 1.2 (i) Name the type of joint above. …………………………………………………………………… (v) [1] State the type of movement allowed by the joint that has been replaced. ................................................................................................................................. [1] (vi) There is a structure that attaches a muscle to point K in Fig. 1.2. Name this structure and explain its importance in the movement of the forearm. name of structure ...................................................................................................... importance ................................................................................................................ ................................................................................................................................... [3] Compiled by Mr Basebi 2018 © Page 59 Form 3 worksheet 2018 5. Diagram 1 shows a bent human arm. Diagram 2 is the cross section of the upper arm taken through the line X- Y to show the humerus bone and structure L and M. (a) What type of tissue is L and M. ………………………………………………………………………………………………………. [1] (b) What actions of structure L and M will make the arm to straighten? L ………………………………………………………………………………………………………… M …………………………………………………...……………………………………………….. [2] (c) Name the type of joint at the elbow. ..……………………………………………………………………………………………………... [1] (d) State the two functions of the human skeleton. ………………………………………………………………………………………………………… ………………………………………………………………………………………………………. [2] Compiled by Mr Basebi 2018 © Page 60 Form 3 worksheet 2018 Module: Electrical Energy Topic: Resistance The diagram below shows a circuit with two bulbs connected in parallel. Use it to answer question 8. 1. (a) (i) State the reason why the current passing through the bulbs is not equal. …………………………………………………………………………………………..…………………………………………. (1) (i) Given that V = IR, where R is the resistance, I is the current and V is the voltage, calculate the resistance of bulb F. …………………………………. (2) (b) On the diagram, draw the voltmeter measuring the voltage across bulb F. (2) (C) The two bulbs are then connected in series. (iv) What will happen to the brightness of the bulbs? …………………………………………………………………………………………………………………………………….……….. (1) (ii) Explain your answer in (c) (i). ……………………………………………………………………………………………………………………………… [1] Use the information and the diagram below to answer question 2. Compiled by Mr Basebi 2018 © Page 61 Form 3 worksheet 2018 The circuit diagram below was used to investigate the electrical resistance of copper and aluminum metals. A copper wire and an aluminum wire of the same size were placed between points X and Y, one at a time. 2. a) How many cells are connected in the circuit above? ……………………………………………………………………………………………………………………………. (1) b) What is meant by electrical resistance? ……………………………………………………………………………………..……………………………………. (1) c) The below shows the amount of current recorded when each of the two wires were connected between points X and Y. Wire Amount of current/A Copper 2.58 Aluminum 2.39 i) What conclusion can be drawn from the results? ……………………………………………………………………………………………………………………….. ii) (1) State one feature of the wires that could be changed to make the wires to transmit the same amount of current. ……………………………………………………………………………………………………………………………. (1) d) State one precaution that should be taken when using electricity. ……………………………………………………………………………………………………………………………………… ………………………………………………………………………………………………..……………………………… (1) Compiled by Mr Basebi 2018 © Page 62 Form 3 worksheet 2018 3. Fig. 8.1 shows an electrical circuit using two resistors. 16 V A 6 S 2 Fig. 8.1 (a) The switch S is open and the ammeter reading is zero. State the value of the potential difference across the 6 resistor. potential difference = ........................................ [1] (b) (i) Switch S is now closed. State the value of the total resistance of the circuit. resistance = (ii) .............................................[1] Calculate the current in the ammeter. State clearly the formula that you use. current = .................................................. (b) Calculate the potential difference across the 6 resistor. p.d. = ....................................................... Compiled by Mr Basebi 2018 © [2] [2] Page 63 Form 3 worksheet 2018 Use the information and the diagram below to answer questions 4 (a) to (c). The diagram shows a set-up that was used in an experiment to investigate the relationship between the length of a wire and its resistance. The voltmeter was used to measure the potential difference across different lengths of the wire. The sliding contact was initially placed at the 15 cm mark from the end of the wire. The experiment was repeated by placing the sliding contact at different lengths of the wire. Some of the results of the experiment are recorded in the table below. Length (cm) 0 15.0 30.0 45.0 60.0 90.0 Voltage (V) 0 2.0 4.0 6.0 8.0 12.0 Resistance (Ω) 0 2 4 8 12 4. (a) The current in the circuit was 1.0 A. The resistance of the wire is calculated using the relationship R=V/I. Complete the table by calculating the resistance of the wire when the sliding contact was placed at the 45.0 cm mark. Compiled by Mr Basebi 2018 © Page 64 Form 3 worksheet 2018 (b) (i) on the grid, plot a graph of resistance against length of the wire. (4) (ii) Using your graph, find the resistance when the sliding contact is placed at the 75.0 mark. Show your working. ………………………………………………………………………………………………………………………..…………… (2) (c) (i) What conclusion can be drawn from the investigation? ………………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………………..… (1) (ii) State one factor which affects the resistance of a wire apart from its length. …………………………………………………………………………………….………………………………………………. (1) Compiled by Mr Basebi 2018 © Page 65 Form 3 worksheet 2018 5. Fig. 5.1 shows an electrical circuit. lamp X 200 Ω Fig. 5.1 (a) State the name of component X. ...................................................................................................................................... [1] 6. Fig. 5.2 shows the graph of current against potential difference for the lamp. 0.07 current/A 0.06 0.05 0.04 0.03 0.02 0.01 0.000 1 2 3 4 5 potential difference/V Fig. 5.2 6 The potential difference across the lamp is 2.5 V. Determine (iv) the current in the lamp, current = ...................................... Compiled by Mr Basebi 2018 © Page 66 Form 3 worksheet 2018 (ii) the current in the 200 Ω resistor, current = ...................................... (iii) the current in component X. current = ...................................... 7. Fig. 6.1 shows a circuit in which a voltmeter is placed across a resistor. B 18 Ω power supply 12 Ω V A Fig. 6.1 The potential difference across the 12 Ω resistor is 4.0 V. The voltmeter has three different ranges: 0 to 3.0 V, 0 to 6.0 V and 0 to 30 V. The best range for use in this circuit is 0 to 6.0 V. (a) (i) Explain why using the voltmeter on the range 0 to 3.0 V is unsuitable, ................................................................................................................................................................................ ......................................................................................................................................................................... [1] Compiled by Mr Basebi 2018 © Page 67 Form 3 worksheet 2018 (ii) using the voltmeter on the range 0 to 30 V is unsuitable. ................................................................................................................................................................................ ......................................................................................................................................................................... [1] 9. (i) Calculate the current in the 12 Ω resistor. State the formula that you use. ………………………………………. [2] (ii)Calculate potential between A and B in figure 6.1. …………………………………… [2] Compiled by Mr Basebi 2018 © Page 68 Form 3 worksheet 2018 8. Fig. 7.1 shows an electrical circuit containing a 12 V power supply and a number of resistors. 12 V 6Ω A 3Ω 2Ω 4Ω Fig. 7.1 (a) Calculate the combined resistance of (i) the 2 Ω and 4 Ω resistors in series, (ii) resistance = ................................................ [1] resistance = ................................................ [2] the 3 Ω and 6 Ω resistors in parallel. Compiled by Mr Basebi 2018 © Page 69 Form 3 worksheet 2018 (b) Calculate the reading of the ammeter in Fig. 7.1. ammeter reading = ................................................ (c) [2] Determine the potential difference across the 4 Ω resistor. p.d. = ................................................ Compiled by Mr Basebi 2018 © [2] Page 70 Form 3 worksheet 2018 9. The diagram below shows an electric circuit. (a) What does instrument P measure? ...………………………………………………………………………………………………… [1] (b) How many cells make up the battery in the circuit? ...………………………………………………………………………………………………… [1] (c) The jockey can be placed at either of the points A, B, or C. (i) At which of the points would the resistance of the circuit be highest? ……………...………………………………………………………………………………. [1] (ii) Explain your answer in (c) (i). ………...……………………………………………………………………………………. [1] (d) State the relationship between the resistance of a conductor and its cross- sectional area. ...………………………………………………………………………………………………… [1] Compiled by Mr Basebi 2018 © Page 71