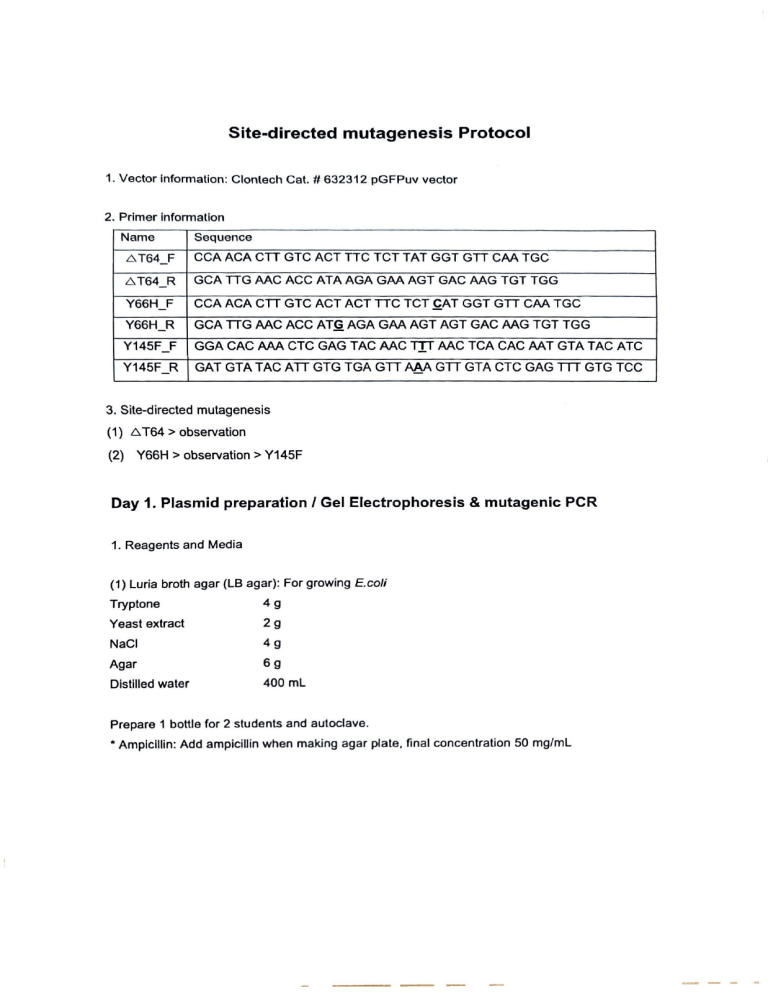
Site-directed mutagenesis Protocol 1. Vector information: Clontech Cat. #632312 pGFPuv vector 2. Primer information Name Sequence AT64_F | CCA ACA CTT GTC ACT TTC TCT TAT GGT GTT CAA TGC AT64_R_ | GCATTG AAC ACC ATA AGA GAA AGT GAC AAG TGT TGG Y66H_F | CCA ACA CTT GTC ACT ACT TTC TCT CAT GGT GTT CAA TGC Y66H_R_ | GCA TTG AAC ACC ATG AGA GAA AGT AGT GAC AAG TGT TGG Y145F_F | GGA CAC AAA CTC GAG TAC AAC TIT AAC TCA CAC AAT GTA TAC ATC Y145F_R | GAT GTA TAC ATT GTG TGA GTT AAA GTT GTA CTC GAG TTT GTG TCC 3. Site-directed mutagenesis (1) AT64 > observation (2) Y66H > observation > Y145F Day 1. Plasmid preparation / Gel Electrophoresis & mutagenic PCR 1. Reagents and Media (1) Luria broth agar (LB agar): For growing E.coli Tryptone 4g Yeast extract 29 NaCl 49 Agar 6g Distilled water 400 mL Prepare 1 bottle for 2 students and autoclave. * Ampicillin: Add ampicillin when making agar plate, final concentration 50 mg/mL 2. Plasmid preparation (Intron DNA-spin plasmid DNA purification kit) (1) Centrifuge bacterial culture at 13,000 rpm for 1 min and discard supernatant. (2) Resuspend cell pellet in 250 uL Resuspension Buffer and vortex. (3) Add_250 uL Lysis buffer to resuspend cells and mix by inverting the tube 10 times and incubate for 3 min at RT. * DO NOT VORTEX. (4) Add 350 wl Neutralization buffer and gently mix by inverting the tube 10 times and incubate the tube in ice for 5 min. (5) Centrifuge at 13,000 rpm for 10 min. While waiting for the centrifugation, insert a column into collection tube. (6) Transfer supernatant into the column carefully. (7) Centrifuge at 13,000 rpm for 1 min. Remove the column from the collection tube, discard filtrate in collection tube. And the place the spin column back in the same collection tube. (8) Add 500 ul Washing buffer A and centrifuge at 13,000 rpm for 1 min. Remove the column from the collection tube, discard filtrate in collection tube. And the place the spin column back in the same collection tube. (9) Add 700 yL Washing buffer B and centrifuge at 13,000 rpm for 1 min. Discard filtrate in the collection tube and place the spin column back in the same collection tube. (10) Centrifuge at 13,000 rpm for 1 min to dry the filter membrane. (11) Place the spin column into a clean, new tube. Add 50 uL Elution buffer to the center of the spin column and wait for 1 min. Centrifuge the tube assembly at 13,000 rpm for 1 min. 3. Mutagenic PCR reaction 10X nPfu enzyme buffer 2 ul nPfu enzyme DNA polymerase(5U) 1 pl dNTP mixture 2 pL Plasmid DNA 1 pl Forward Primer (10 pmole/yL) 1 pl Reverse Primer (10 pmole/uL) 1 pl H20 12 uL Total 20 pL Day 2. Dpn | digestion and transformation 1. Dpn I digestion Prepare the following reaction and incubate the tube at 37°C Dpnl 0.5 pL (10U) 10X Dpn | buffer 2uL PCR product 17.5 uh Total for 1 hr and 30min. 20 uL 2. Transformation (1) Thaw cells on ice and add/5} 10 ul of ligation mix to thawed cells, mix gently, and incubate on ice for minutes. While you're waiting, pre-heat a hot block (with added water) to 42°C. (2) Remove the tubes from the ice and immediately put them in the 42°C hot block for 90 seconds. Return the tubes to ice and incubate for 5 min. (3) Add 0.5 ml of 6 eam to the cells, transfer to a sterile falcon tube, and incubate at 37°C (with shaking) for 1 hour. (4) Plate 100 L of the culture on plates containing the appropriate antibiotic(ampicillin). (5) Incubate overnight at 37°C. The next morning, pick resultant colonies and make a plasmid prep (for PCR and restriction enzyme confirmation that the strain has picked up the correct plasmid). Day 3. Plasmid preparation / Gel Electrophoresis & mutagenic PCR 1. Plasmid preparation (Intron DNA-spin plasmid DNA purification kit) : Same as above 2. Mutagenic PCR reaction 10X nPfu enzyme buffer 2 pL nPfu enzyme DNA polymerase(5U) Tul dNTP mixture 2 pL Plasmid DNA 1 pL Forward Primer (10 pmole/uL) 1 pl Reverse Primer (10 pmole/pL) 1 pL H20 12 pL Total 20 pL Day 4. Dpn I digestion and transformation / Phenotype observation 1. Dpn I digestion Prepare the following reaction and incubate the tube at 37°C for 1 hr and 30min. Dpn I 1 pL (10U) 10X Dpn | buffer 2 yl PCR product 17 uh Total 20 uL 2. Transformation (1) Thaw cells on ice and add 5-10 ul of ligation mix to thawed cells, mix gently, and incubate on ice for 30 minutes. While you're waiting, pre-heat a hot block (with added water) to 42°C. (2) Remove the tubes from the ice and immediately put them in the 42°C hot block for 90 seconds. Return the tubes to ice and incubate for 5 min. (3) Add 0.5 ml of LB medium to the cells, transfer to a sterile falcon tube, and incubate at 37°C (with shaking) for 1 hour. (4) Plate 100 pL of the culture on plates containing the appropriate antibiotics(ampicillin). (5) Incubate overnight at 37°C. The next morning, pick resultant colonies and make a plasmid prep (for PCR and restriction enzyme confirmation that the strain has picked up the correct plasmid). (6) Observe the color change of E. coli on the UV transilluminator.






