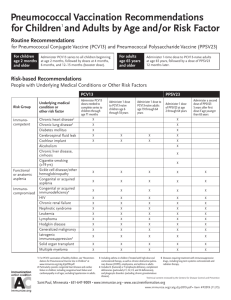
One dose of Prevnar 20 is recommended by the CDC to help protect
ALL eligible adult patients at risk for pneumococcal pneumonial'2
Adults age
55 and older
I
i
J
Aaor portrayal.
Prevnarll0
Pneunrotoccol 20.volenl
(oniugole Vo(ine
Adults age 19-64 with certain underlying
medical conditions,* including:
.
.
Diabetes
COPD
@
Who are pneumococcal vaccine-naiVe
@
/:r/,
Whose previous vaccination history is unknown
.
Chronic heart disease
Who were previously vaccinated with PCV13 onlyl
\l/
Minimum interJal between iaccinations
/?l
\l/
,s at least one year'
to learn
more about eligible adult
patients who could
benefit from one dose
of Prevnar 20.
Scan QR code
Who were previously vaccinated with PPSV23 only
Minimrm interv'al benveen Jaccl.at'o"s
g
s at reast one yea,.
Who were previously vaccinated with PCV13 and PPSV23'
Applies to adulis age >65 (based on shared clinical decision-making) and adults age 19 to 64 with an
immunocompromising condition, cochlear implant, or CSF leak (with incomplete PPSV23 vaccination)
who received their last pneumococcal dose >5 years ago.
Vaccinate with one dose of Prevnar 20 today to help Protect
ALL eligible adult patients against Pneumococcal pneumoniaa
IMFORTANT SAFETY INFORMANON
. Severe allergic reaction (eg, anaphylaxis) to any component of Prevnar 20o or to diPhtheria toxoid is a contraindication
Basei on exoerienc"e with pneumococcal vaccines, individuals with altered immunocompetence may have reduced immune resPonses to Prevnar
Additionally, injection site swelling was also reported (>10%) in adults 18 through 59 years of
age
i
*Alcoholism; chronic hean disease (including CHF and cardiomyopathies); chronic liver disease; chronic lung disease (includrng COPD,
.ochlear rmoldnt: conoenital or acquired as;lenia_; cerebrospinal {luid leaL; drabetes; generalzed maligTancy ; HIV 1{ection ; Hodgk'i
B- (hur"o.ati or T lympioc).te deficiency, complement defic,encies [panicr.Llady C1. C2. C3, and C4 deficienciesl, and phagocytic disor(
,--rno.rppr"rs|on ioiseises req,.:,ring trearmert w th imr-unosuppressive drugs, rncluding long-re/m systemic conrcosteroids and rd
oephrotic iyndrome'; solld organ transPlantr; sickle cel disease or other hemoglobinopathies.l3
Qpf,*
lflmunocorrpro'n srng condil'ons.
References: 1. Centers for Disease Control and Prevention. Advisory Committee on lmmunization Practices (ACIP) rneeting recornmen(
Febr,JarV 6,2023. https://wlvw.coc.gov/vaccines/ac,p/index.ht'nl 2. Kooayasri M, Farrar JL, Grerke R. et al. Use o{ 15_valent onefiocq
vaccineirrong U.5. adults: updateJ recon.nendations ot the Advrsory Committee on lmmunization PIacl ces - United Statet 2022. l'J
ng {or adu rs. PJblisheo April 1, 2022. Accessed December 8. 2022. l'tlps:/
Disease Contr;l and Preve^tion. Pneumococcal vaccine t
4. Prevrar 2@ (Pneurnococcal 20-va.enl Conjugate Vaccr.le) Prescrio 19 Inforrration. Wyeth Pharmaceut cals LLC, 2022.
-
Pl.aaa aoe lndlcadon on rrverr. cde and accomP.nylng Full Pr€r<.iblng lrifornrstion for Prtvnar 20'
I\,4anufactured by Wyeth Pharrraceuticals LLC. Marketed by Pfizer 1n<
PP-PNR-USA-0973-02 @, 2023 Plizer lnc. All riqhts reserved. Pl1ltqd l r the USA/Februarv 2023
20@
Stephanle stone
Vaccine Territory Manager
stephanie.stone@pfi zer.com
Cell 205-9Ai -1647
The CDC recommends one dose of Prevnar 20 to help
protect ALL eligible adult patients, including those
previously vaccinated with PCV1 31'2
Prevna r 2O
Pneumototcol 20-volenl
(oniugote Voccine
On October 2O 2022, the ACIP voted on and provided updated recommendations. These ra<ommendatiors have been adopted by the
CDC Director and arc now official. They will be published in the Morbidity nd Monahy Weeldy Roorlrt (Ml,lfiM) in the coming months.
The CDC recommends Prevnar 20 for ALL of the following eligible adult patients
Eligible adults
PCV13 only
(>l year prior)
>65
'|.9-64
with ccrtain lnd.rlying Dedi(al
.onditionr or othcr ritk {adori
19-64
wtth En itnmunocomp..hiling
.ondft ion, <odtl.ar implant,
or CSF lerk
g @ g g
g @ g
g g g g,
dOne dose of Prevnar 20
can help protect ALL
eligible adults regardlass
of their pneumococcal
vaccination hirtory.
'Based on shared clinlcal decision-making
rWith lncomplete PPSV23 vaccination.
Underlying medica! condifions or other risk factors:
nephrot c ijnorome'; solid organ transplantt; sickle cell disease or other hemoglobinopathies E
tlmmunocompromising conditions.
INDICATION
.
.
Prevnar 204 is indicated for active immunization for the prevention of pneumonia and invasive disease caused by Streptococcus pneumoniae serotypes 1 , 3, 4, 5, 6A, 68,7F, 8,
19F, 22F, 23F, and 33F in adults 1 8 years of age and older
'l
Th is indication for the prevention of pneumonia ca used by S. pneumoniae serotypes 8, 0A, '1 1A, 12F, 158, 22F, and 33F is approved under accelerated approva I based on
9V 10A, 1 1A, 12F, 14, 158, 18C, 194,
benefit in a confirmatory trial
Ploara 3€a lmportant Safaty lniormation on reverlo ridc and accornpanylng Full
Pre*riblng lr orrtlatlon for Prevna.20.