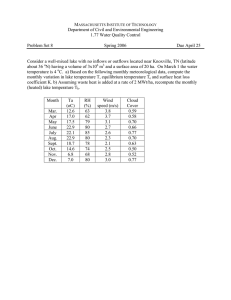
Hydroclimatic variations of the early-to-mid holocene in southwest Iran
advertisement

ABSTRACT HYDROCLIMATIC VARIATIONS OF THE EARLY-TO-MID HOLOCENE IN SOUTHWEST IRAN By Rosemarie H. Wrigley August 2015 A ~2500 year record of hydrologic change from southern Iran is inferred from the mineralogy and stable isotopic composition of bulk and biogenic carbonates archived in Lake Hirom (27º 57’N, 53º52’E). This change is related to regional variations in moisture and to the larger Indian Summer Monsoon circulation (ISM). During the early Holocene, increased summer insolation from ~10,000 to 8,000 yr BP contributed to the intensification of the ISM. This intensification may have increased summer precipitation north of the modern ISM limit. Evidence of wetter lake conditions in Lake Hirom occur from 8,800 to 7,800 yr BP. Drier conditions occur and persist from 7,800 to 6,300 yr BP, until lacustrine marl changes to peat, indicating maximum aridity. The timing of the drying trend in the mid-Holocene of Lake Hirom correlates with cave and lake records in the Arabian Peninsula, and lake records in northern Iran, indicating a regional drying event. HYDROCLIMATIC VARIATIONS OF THE EARLY-TO-MID HOLOCENE IN SOUTHWEST IRAN A THESIS Presented to the Department of Geological Sciences California State University, Long Beach In Partial Fulfillment of the Requirements for the Degree Master of Science in Geology Committee Members: Lora R. Stevens, Ph.D. (Chair) Gregory J. Holk, Ph.D. Matt Becker, Ph.D. College Designee: Robert D. Francis, Ph.D. By Rosemarie H. Wrigley B.S., 2012, California State University, Northridge August 2015 ProQuest Number: 1599197 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. ProQuest 1599197 Published by ProQuest LLC (2015). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, MI 48106 - 1346 ACKNOWLEDGEMENTS I would like to acknowledge a number of people who have helped me with my writing, research, and who have also given me encouragement throughout my time as a graduate student. I would like to express the deepest appreciation to my committee chair, Dr. Lora Stevens who has been an amazing mentor, teacher, and advisor. I would like to thank her for encouraging my research and for allowing me to grow as a research scientist. Her advice on both research as well as on my career have been priceless. I am grateful for her spirit of adventure in regard to research and scholarship, and an excitement in regard to teaching. Without her supervision and constant help this thesis would not have been possible. I would like to thank my committee members, Dr .Gregory Holk and Dr. Matt Becker, for their help. Their feedback and guidance with my research and writing has been very useful to the completion of my thesis. In addition, a thank you to Dr. Gregory Holk and Dr. Benjamin Hagedorn whose help in the laboratory was priceless. A Special thanks to Dr. Christine Whitcraft for allowing me to use her freeze drier at CSU Long Beach, and to Dr. Kathleen Johnson for allowing me to use her laboratory at UC Irvine. I would like to thank everyone in Dr. Lora Steven's laboratory for their help with preparing and analyzing my samples. I also want to thank Gina Oliver, Tracy La Rocco, and Priscilla Miranda for their encouragement and help with my research. iii I am grateful for the financial support of the Carl W. Johnson-Bert Conrey, Graduate Fellowship in Geological Sciences. I would not have been able to complete my degree without the financial support. My research thesis could not have been completed without the research funds provided by NSF-EAR Grant 0903117 awarded to Dr. Lora Stevens. Last but not least; I would like to thank my sister, Veronica Wrigley, my mother, Carmen Wrigley, my father, Christopher Wrigley, and my close friends Luna and Collin Boucher. Their moral support and guidance helped me to complete my classes, research, and thesis. There were countless times when I needed moral support, and they were always there to encourage me and to persevere. iv TABLE OF CONTENTS Page ACKNOWLEDGEMENTS................................................................................................iii LIST OF TABLES.............................................................................................................vii LIST OF FIGURES............................................................................................................ix CHAPTER 1. INTRODUCTION...................................................................................................1 2. BACKGROUND.....................................................................................................5 Site Description............................................................................................5 Regional Geology and Physiography...........................................................5 Regional Climate.........................................................................................7 Holocene Climate in Southwest Asia........................................................12 Hydroclimatic Reconstructions from Geochemical Proxies......................17 Ostracodes as a Geochemical Proxy..............................................19 3. METHODS............................................................................................................21 Methods and Proxies..................................................................................21 Core Retrieval and Sampling.....................................................................21 Chronology................................................................................................22 Ostracodes..................................................................................................22 Ostracode Geochemistry............................................................................23 Bulk Carbonate..........................................................................................25 Mineralogy.................................................................................................26 Carbon Content..........................................................................................26 4. RESULTS..............................................................................................................28 Core Lithology...........................................................................................28 Age Model/Stratigraphy.............................................................................31 Total Organic and Inorganic Carbon.........................................................32 v CHAPTER Page Stable Isotopic Composition of Endogenic Carbonate...............................36 Macrofauna................................................................................................37 Stable Isotopic Composition of Ostracodes...............................................42 Trace Elements in Ostracode Carapaces....................................................44 5. DISCUSSION........................................................................................................48 Brine Evolution and Mineralogy as an Indicator of Effective Moisture....48 Detrital Minerals as Proxies of Climate.....................................................51 Isotope Data of Bulk Carbonates and Ostracodes as Indicators of Effective Moisture.....................................................................................52 Trace Elements of Ostracodes as Indicators of Effective Moisture...........53 Mean Climate Changes..............................................................................54 Earliest Holocene (9,300-8,800 yr BP)..........................................54 Early Holocene (8,800-7,800 yr BP).............................................57 Early-to-Mid Holocene (7,800-6,300 yr BP).................................58 Mean Climate Change-Interval 1-3...............................................60 Regional Climate Change..........................................................................61 6. CONCLUSION......................................................................................................65 APPENDICES...................................................................................................................66 A. OSTRACODE OXYGEN AND CARBON ISOTOPE DATA...............................67 B. OSTRACODE TRACE ELEMENT DATA............................................................69 C. CARBONATE OXYGEN AND CARBON ISOTOPE DATA...............................71 D. TOTAL CARBON AND INORGANIC CARBON DATA....................................75 REFERENCES..................................................................................................................79 vi LIST OF TABLES TABLE Page 1. Radiocarbon Dates for Lake Hirom........................................................................33 vii LIST OF FIGURES FIGURE Page 1. Regional map of Asia................................................................................................4 2. Google Earth® image of Lake Hirom. ....................................................................6 3. Geologic map of southeast Fars, Iran. .....................................................................8 4. Measured precipitation in mm and calculated evapotranspiration in data over a year...........................................................................................................................9 5. Image depicting the climatic regimes influencing southwest Iran's climate. ........10 6. SW Asia with the locations of Lake Hirom (star), lake sites (filled circles), speleothem sites (open circles), and marine site (closed triangle). .......................14 7. Oxygen isotope record from Qunf cave, Hoti cave, and interpreted lacustrine phases of Lake Awafi. ...........................................................................................15 8. Image of the entire 7 meters of core. .....................................................................27 9. Stratigraphic column for the entire core taken from Lake Hirom. ........................29 10. Mineral abundances of unsieved sediment by XRD. ...........................................30 11. Age–depth model of calibrated 14C dates with error bars. ...................................34 12. Total organic (TOC) and inorganic (TIC) carbon found within the marl section of the core, grey bars indicate mean values.............................................35 13. Stable isotopic composition of the lake carbonates.. ...........................................38 14. Covariance of oxygen and carbon isotopes. ........................................................39 15. Covariance of oxygen and carbon isotopes. ........................................................40 16. Macrofuana found within the marl section of the core. .......................................41 viii FIGURE Page 17. The adult ostracode species count. .......................................................................43 18. Stable isotopes of the ostracod carapaces, values are reported in permil. ...........45 19. Trace elements in the ostracod carapaces, values are reported as molar ratios. ..47 20. Comparison of carbonate and ostracod carapace isotopic trends. .......................55 21. Comparison of trace element ratios Mg/Ca and Sr/Ca for C. torosa (female), gypsum, celestine, and intermediate and high-Mg calcite (%). ............................56 22. Comparison of dry and wet climates in Lake Hirom- Iran, Lake Awafi-SE Arabia, Hoti cave (N, north) and Qunf cave (S, south)- Oman, and Lake Mirabad-Iran..........................................................................................................63 ix CHAPTER 1 INTRODUCTION The weakening and strengthening of weather systems is affected by variability in the Earth's climate. One of the causes of the intensification in weather systems is the global increase in temperature (Stephens and Hu, 2010). Climate model projections predict an increase in the intensity of precipitation in wet regions and a decrease in arid regions. Due to anthropogenic forcings we are currently experiencing global warming and in effect we are seeing intensification of precipitation events, with strong spatial variations (Chou and Lan, 2012). The economics of many societies are reliant on the timing and amount of precipitation. Thus, it is important to understand how significant these changes in precipitation are and will become, by placing these changes in the longer-term context of Holocene climate change. Reconstructing past hydroclimatic conditions of a region can provide links between water availability and variations in climatic forcings, such as greenhouse gas levels, insolation, and ocean circulation. The precession of the equinoxes, a 23,000-year cycle, changes the amount of insolation received on Earth. Approximately 11,000 years ago the northern hemisphere received maximum summer insolation, which caused an increase in global summer temperatures (Ruddiman, 2000). Although not a perfect analog for today's temperature increase, the warmer than modern temperatures during the early- to mid-Holocene. Thus, 1 this time period can be used to look at the relationship among increased annual temperatures and effective moisture (precipitation minus evaporation). Monsoon rains are economically vital for many countries because they are a main source of precipitation during the summer months. Because the monsoon system responds to the differential heating of a continent and an ocean, an increase in global temperature and/or an increase in insolation will strengthen and potentially prolong the monsoon. The modern Indian summer monsoon (ISM) penetrates the most southern tip of the Arabian Peninsula near Oman in response to both the Northern Hemisphere heating and the summer shift of the Intertropical Convergence Zone (ITCZ). Today, the ISM does not extend past Oman and into Iran. However, the ISM was stronger in the early Holocene (Burns et al., 2001; Parker et al., 2006; Fleitmann et al., 2007) and may have had a greater influence in Iran. This thesis focuses on the hydroclimatic changes during the early to mid Holocene in SW Iran as inferred from environmental proxies archived in the lake sediment from Lake Hirom, Iran (FIGURE 1). Lake Hirom (27º 57’N, 53º52’E) is the most southern perennial lake site in Iran identified to date, making it potentially sensitive to the influence of the ISM. All paleoclimatic research in Iran (e.g., Stevens et al., 2001, 2012; Griffiths et al., 2001; Djamali et al., 2009) has occurred further north beyond the possible influence of the Indian monsoon system. Changes in moisture balance in Lake Hirom during the early-to mid-Holocene are identified with numerous climatic proxies, including pollen, mineralogy, sediment, and micro- and macro fauna. Geochemical proxies are particularly useful in reconstructing 2 changes in moisture balance. The oxygen isotopic composition of endogenic carbonates and ostracod carapaces, and the trace elements in the ostracod carapace record reflect changes in lake water residence time and therefore variations in effective moisture. Thus, drier times, when effective moisture is low (i.e., excessive evaporation), result in an increase of the δ18O value of the lake water, and an increase in the relative concentration of trace elements, both of which are ultimately recorded in the endogenic carbonate and ostracod carapaces. These inferred changes in effective moisture can be linked to larger changes in synoptic climate, principally the strength of the ISM and changes in the strength of the westerly winds. This thesis will use the proxy evidence from Lake Hirom to indicate the changes in strength of the ISM and related climatic regimes in the early-tomid Holocene. 3 FIGURE 1. Regional map of Asia. Star denotes location of Lake Hirom in SW Iran. 4 CHAPTER 2 BACKGROUND Site Description Lake Hirom (27º 57’N, 53º52’E), Iran is located in the southwestern region of the Asian continent. This region is part of the Near East, which also includes countries along the eastern shores of the Mediterranean and Northeastern Africa (FIGURE 1). Lake Hirom lies within an intermontane basin in the southwestern portion of the Zagros Mountains (FIGURE 2). The lake is ephemeral with large sections drying out each year. The area with the most persistent water body is located on the northern edge of the basin. The intermontane basin is spring fed from the base of the mountains to the north and east (Djamali, pers. comm.). The aerial images shows that the lake can extend ~ 2 km south from the highway along the northern edge and can have an E-W width of ~2 km. The overall basin is much larger (~120km2 ) and thus during wetter intervals in the past, the lake may have been substantially larger in area and volume. An alluvial fan has built across the lake basin significantly limiting the size of the basin. Its role in the water balance of the lake is unknown. Regional Geology and Physiography The collision of the Arabian and the Eurasian plates formed the Zagros fold and thrust belt that trends NW- SE along the western border of Iran in the late Cretaceous. 5 FIGURE 2. Google Earth® Image of Lake Hirom. The basin is filled in the center by an alluvial fan. The eastern edge of the basin has several small springs. The main spring is along the northern edge of the basin (arrow indicates main spring). Black dot represents coring site. The topographic lines were created using ASTER GDEM, which is a product of METI and NASA. 6 The Zagros Mountains consist mainly of sedimentary rocks, with marine carbonates as the dominant lithology (Talebian and Jackson, 2002) (FIGURE 3). Salt diapirs have been found throughout the Zagros Mountains, with several occurring near Lake Hirom (Talbot and Jarvis l984; Motamedi et al., 2010). The mountains form an orographic barrier between the Mesopotamian lowlands to the west and the Iranian plateau to the east. As a result, the Zagros Mountains have higher precipitation than the surrounding areas, although precipitation decreases to the south (van Zeist and Bottema, 1977). Regional Climate Iran has a continental climate and is mainly arid or semi-arid, with an annual temperature range of -3 to 35 degrees Celsius, with a large daily range. The Lars weather station approximately 55 km southeast of Lake Hirom shows the annual potential evapotranspiration and precipitation in mm (FIGURE 4). A positive moisture balance occurs during the months of December through March, while a negative moisture balance occurs from April through November. The wettest and driest months of the year are December, and May/November, respectively. During July there is a slight increase in the precipitation values. Precipitation is controlled by the interplay of three dominant pressure systems: an anticyclonic ridge over Asia, travelling depressions which track through the Mediterranean Sea and the Persian Gulf, and the monsoon Asiatic low (FIGURE 5) (Taha et al., 1981). These pressure systems strengthen and diminish according to the season, with the first two mentioned strengthening during the winter, and the last one strengthening during the summer. 7 FIGURE 3. Geologic map of southeast Fars, Iran. Rectangle around the Lake Hirom Basin, star denotes Lake Hirom. 8 FIGURE 4. Measured precipitation in mm and calculated evapotranspiration in data over a year. Most of the year has moisture deficit. 9 A B FIGURE 5. Image depicting the climatic regimes influencing southwest Iran's climate. A. The winter pressure systems, the depressions (low pressure, LP) tracking with the westerly winds and the anticylonic ridge (AR), are shown in black bold font. B. The monsoon Asiatic low (AL) forms during the summer with the northward shift of the ITCZ. The modern ISM extent and summer ITCZ position are shown in black bold font. 10 The anticylconic ridge is made up by the Siberian anticyclone (northern part of the ridge), and the subtropical high-pressure belt (southern part of the ridge, 30°N). The Siberian anticyclone is an area of high pressure formed by an accumulation of cold air masses (Gong and Ho, 2001). The subtropical high-pressure belt is formed by cool sinking air from the equatorial Hadley cell (Starr, 1948). During the winter, polar maritime air masses follow the passing Atlantic low-pressure systems through Europe, and invade Asia Minor and the Black Sea. This leads to small depressions forming over the Mediterranean Sea which track with the westerly winds (Kendrew, 1961). The average frequency of the travelling depressions in the winter currently does not exceed three a month. The extent to which these storms penetrate into the interior of Iran is limited in part by the winter strengthening of the Siberian high-pressure system (Gong and Ho, 2001). For most of Iran, as with the Near East in general, precipitation falls during winter and spring (Taha et al, 1981). Prior to the monsoon Asiatic low forming in June, the pre-monsoon circulation consists of a split in the jets, with the polar-front jet (northern jet) and the subtropical jet (southern jet) flowing north and south of the Tibetan plateau, respectively (Chang, 1967). In May and June the Tibetan plateau heats up, and the subtropical jet moves south and dissipates, leaving just the polar-front jet (Chang, 1967). The monsoon Asiatic low forms during the summer as the heating of the Tibetan plateau replaces the subtropical high-pressure belt. In June the Intertropical Convergence Zone (ITCZ) shifts northward, and separates the monsoon currents from the easterlies to the north (Chang, 1967). The convergence zone is referred to as the monsoon trough, and 11 persists until August. The western arm of the monsoon trough is the westward extension of the low-pressure belt over India and Pakistan centered at 30°N during the summer (Taha et al., 1981). In the summer the warmer air temperatures allows for an increased amount of water vapor to be stored in the atmosphere, which ultimately produces the classic "monsoon rains"(Chang, 1967). The relative high pressure that forms over the Arabian Sea and low pressure over the Asian continent allows for this moisture to move northward. The modern Indian summer monsoon (ISM) penetrates the most southern tip of the Arabian Peninsula near Oman, and does not extend into Iran. The Shamal winds occur during the winter and summer in eastern Arabian Peninsula and the Arabian Gulf. The winter Shamal winds are northwesterly winds that follow the cold frontal passage over the Persian Gulf (Ali, 1992). The summer Shamal winds are northwesterly winds that are associated with the Indian and Arabian thermal lows, and are more likely to be dry and create dust and sand storms (Ali, 1992). Holocene Climate in Southwest Asia The evolution of the Holocene climate of Southwest Asia is complex and not well understood. The early Holocene climate of the Near East (~6,000 -11,000 yr BP), in particular, has been the focus of many paleoclimatic studies. During this time summer insolation was greater than present with a maximum value at 523.16 W/m2 at 60°N. Concomitantly winter insolation was lower, and the combined effect was enhanced seasonality. The greater summer insolation caused higher summer temperatures in many regions, and greater global climate variability. The greater summer temperatures in the 12 subtropics of the northern Hemisphere resulted in the intensification of monsoons (Sirocko et al., 1996). Several studies indicate that the Southwest Indian summer monsoon (ISM) intensified and may have reached more northerly latitudes in the early Holocene (FIGURE 6) (Fleitmann et al., 2007; Overpeck et al., 1996; Burns et al., 2001; Parker et al., 2006). In northern Iran several lake studies (FIGURE 6) have explored the climate of the Holocene (Stevens et al., 2001, 2006, 2012; Bottema, 1986; van Zeist and Bottema, 1977; Griffiths et al., 2001; Djamaili et al., 2009). However, these sites are believed to be well beyond the reach of an enhanced monsoon and are interpreted in terms of westerly airflow (Stevens et al., 2001, 2006). In southern and northern Oman proxies indicate a rapid increase in precipitation from approximately 10,000 yr BP to 9600 yr BP (FIGURE 7) (Fleitmann et al., 2007). The lag between the peak solar insolation (~ 11,000 yr BP) and the peak precipitation is attributed to glacial boundary forcings. During the deglacial at ~11,000 yr BP, pulses of melt water weakened the North Atlantic thermohaline circulation, resulting in colder North Atlantic air temperatures and increased snow cover in Eurasia. The increased albedo due to Eurasian snow cover suppressed the increased summer insolation effects which in turn delayed monsoonal precipitation until the beginning of the Holocene (Overpeck et al., 1996; Fleitmann et al., 2007). Sediment records from Awafi in Saudi Arabia (FIGURE 7) suggest greater precipitation relative to evaporation beginning at 8,500 yr BP (Parker et al., 2006). Between 12,000 and 9,000 yr BP, proxies in Awafi indicate dry conditions despite the evidence for an intensified ISM in Oman. Parker et al. (2006) argue that the delayed 13 FIGURE 6. SW Asia with the locations of Lake Hirom (star), lake sites (filled circles), speleothem sites (open circles), and marine site (closed triangle). Records of wetter conditions due to the ISM are denoted with squares. The maximum summer extent of the modern ITCZ is shown by the black line, and ISM airflow by the black arrows. 1. Marine core (Sirocko et al., 1996) 2. Southern Oman Qunff cave (Fleitmann et al., 2007) 3. Rub a Khali lake (Parker et al., 2006) 4. Southern Oman Defore cave (Fleitmann et al., 2007) 5. Northern Oman Hoti cave (Fleitmann et al., 2007) 6. Awafi Lake (Parker et al., 2006) 7. Lake Mirabad (Stevens et al., 2006) 8. Lake Zeribar (Stevens et al., 2001) 9. Lake Urmia (Stevens et al., 2012). 14 FIGURE 7. Oxygen isotope record from Qunf cave, Hoti cave, and interpreted lacustrine phases of Lake Awafi (Parker et al., 2006; Fleitmann et al., 2007). 15 wetter conditions are due to the dominance of the Northwesterly Shamal winds, driven by stronger westerlies. These conditions are explained by the termination of high latitude glaciers in the early Holocene, which both weakened global wind systems and allowed them to migrate north (Glennie and Singhvi, 2002), initiating the Shamal winds in Southwest Asia. The increase in precipitation at 8,500 cal yr BP is attributed to the northern penetration of the ISM (Parker et al., 2006). Precipitation remained high in northern and southern Oman with slight decreases at 7,900 and 7,600 yr BP. Similarly, sediment records from Awafi indicates periods of decreased precipitation at 8,200, 7,900, and 7,600 yr BP. The weakened monsoon activity may be due to a larger amount of snow cover in the Tibetan Plateau caused from a melt water pulse in the North Atlantic. The higher albedo suppressed the insolation and may have weakened the monsoon activity (Parker et al., 2006). At 6,300 yr BP proxies in northern and southern Oman indicate a shift from monsoon moisture to a westerly moisture source (Fleitmann et al., 2007). At ~6,000 yr BP Awafi indicates a switch from monsoonal precipitation to cyclonic winter precipitation from systems in the Mediterranean (Parker et al., 2006). Northern Iran is beyond the influence of the modern ISM, however there is a pronounced shift in hydroclimate in all records between 6000-6500 yr BP. In northern Iran the transition to the early Holocene (~10000 yr BP), while slightly wetter than the glacial period, is interpreted as drier or similar to present. The early Holocene evolution is controversial with oxygen isotopes suggesting wet conditions and pollen suggesting dry conditions. A proposed shift in the seasonality of moisture (winter precipitation as 16 opposed to spring precipitation, which would cause lower oxygen isotope values) was used to resolve this controversy (Stevens et al., 2001, Griffiths et al, 2001). However, new records from the Neor peatland (Arashi et al., in press) indicate lower dust concentrations in the early Holocene relative to the late Holocene, arguing for wetter than present conditions. These new records suggest that the oxygen isotope values in previous studies may have indicated wetter conditions rather than a shift in seasonality that was proposed. Hydroclimatic Reconstructions from Geochemical Proxies Geochemical proxies are useful in reconstructing changes in effective moisture (i.e., precipitation minus evaporation). Oxygen isotopes of calcite from either endogenic carbonates or ostracod carapaces can be used to indicate the effective moisture of the region (Stevens et al., 2001; Holmes, 1996). In addition, the elemental ratios of Sr and Mg, which substitute for Ca in the ostracod carapace, can also be used as an indicator of salinity, and thus effective moisture as well (Chivas et al., 1986; Xia et al., 1997; Holmes, 1996; Stevens et al., 2006). These three main proxies, with additional evidence from pollen (Djamali, pers. comm.) and mineralogy, will be used to reconstruct the hydroclimatic evolution at Lake Hirom. The baseline δ18O value (i.e., long-term average, prior to evaporation effects) of the lake water is controlled by precipitation amount and type (Araguás-Araguás et al., 2000), air mass trajectory, and temperature (Dansgaard, 1964). Seasonal timing of precipitation (i.e., winter versus summer precipitation) can also influence the δ18O value of lake water, where the colder temperature winter storms result in lower δ18O values 17 (Stevens et al., 2001). The trajectory of air masses feeding the lake's catchment can alter the δ18O value of precipitation through Rayleigh distillation processes (Araguás-Araguás et al., 2000). The further an air mass travels from the vapor source, the lower the δ18O value of precipitation will be due to preferential rain out of the heavier 18O. Calculations of simple Rayleigh distillation of an air mass, however, are complicated due to entrainment of new water vapor as an air mass passes over a continental water body (ex. Black Sea) and/or soil moisture. The monsoons create an additional complication to the δ18O value of precipitation via a process known as the "amount effect" (Araguás-Araguás et al., 2000). The amount effect is related to sudden and large rain out events, commonly associated with monsoonal downpours (Rozanski et al., 1992). Thus, moisture derived from the ISM that reaches into Iran may be strongly depleted in the δ18O due to the rain out effect (Araguás-Araguás et al., 2000). Once the water reaches the lake's catchment it may be further modified by evaporative enrichment, in which the lighter 16O is preferentially removed. Evaporation is a kinetic (non-equilibrium) process that is very difficult to quantify isotopically. Hydrologic models of this interplay point to average temperature, relative humidity and wind speed as critical controls on amount of evaporation (Benson, 1994). Evaporation flux may be quantified but the isotopic enrichment due to evaporation is much more difficult to estimate. Essentially, a comparative assessment is the only option for past climate studies. Thus drier times, when effective moisture is low (i.e., excessive evaporation), result in an increase of the δ18O value of the lake water, which is ultimately recorded in ostracod carapaces and lake carbonate sediment. 18 Ostracodes as a Geochemical Proxy Ostracodes are bivalved Crustacea that occur in either marine or terrestrial environments. They are either benthic, living in the sediment surface or subsurface, or periphytic, living on the surface of rooted aquatic plants. Their carapaces are constructed with low-Mg calcite and are preserved in the sediment. There are three main lineages of recent non-marine ostracodes belonging to the order Podcopida (Martens et al., 2008). The largest family of this order is Cypridoedea. Two genera, Cyprididae and Candonidae, belong to the family Cypridoedea, and are found in the paleartic zone, which is a biogeographic ecozone that includes the terrestrial region of Europe, northern Africa, Asia north of the Himalaya foothills, and the northern and central parts of the Arabian Peninsula (Martens et al., 2008). Ostracodes can reproduce sexually and asexually, and show sexual dimorphism (Butlin et al., 1998). Ostracodes, like all arthropods, molt up to nine times, and reach lengths of approximately 0.5 to 2.5 mm long (Holmes, 1996). Both strontium and magnesium content of simple closed-basin lakes (those with no outflow save evaporation) can be sensitive indicator of salinity as their concentrations increase with increasing evaporative concentration of water. Thus, the molar ratios of Sr/Ca and Mg/Ca in non-marine ostracodes have been used in a number of studies to reconstruct temperature and salinity changes in lakes (Chivas et al., 1986; Xia et al., 1997; Holmes, 1996; Stevens et al., 2006). Ostracodes precipitate their shells from ions taken directly from the water, which makes the ostracod carapace mineral composition reflect that of the host water chemistry (Caporaletti, 2011). Because the carapaces are secreted over a short amount of time (days), and not incrementally, their shell chemistry 19 represents the chemistry of the host water at a specific time (Holmes, 1996; Chivas et al., 1986). During the formation of the shell, Mg and Sr will substitute for Ca in ratios commensurate with those of the host water. Experiments have shown that the partitioning of Sr is virtually temperature-independent (De Decker and Forester, 1988) and is related to solely to the concentration of Sr in the host water, which typically increases with evaporative concentration. Thus, increases in Sr/Ca ratios in ostracodes are interpreted as increasing salinity due to a decrease of effective moisture (precipitation minus evaporation) (Chivas et al., 1986). In contrast Mg partitioning is dependent on both temperature and Mg/Ca of the host water (De Decker and Forester, 1988; Chivas et al., 1986). So, changes in Mg/Ca ratios in ostracodes are more difficult to interpret. An increase in the Mg/Ca ratio may result from decreasing water temperatures and/or an increase in salinity. Separating the two effects is often not possible. The partitioning coefficient for Sr and Mg is also species-dependent (Chivas et al., 1986). Because of the molting process, juvenile ostracod shells may form during different seasons and thus in geochemically different water than adults. Furthermore, juvenile ostracodes preferably substitute Mg and discriminate against Sr leading to anomalously high Mg levels and low Sr levels (Chivas et al., 1986). Therefore, adult ostracodes are preferred for geochemical analysis. 20 CHAPTER 3 METHODS Methods and Proxies Proxies are used as a substitute for an indirect measurement of our past climatic changes. In order to constrain interpretations, multiple proxies found within the marl were investigated. The primary proxy used to infer changes in hydroclimate was the stable-isotopic compositions of endogenic calcite and/or ostracodes. Other proxies were used to support interpretations based on these data. All analyses, except the stableisotopic composition of ostracodes, were performed at CSULB in either the Institute for Integrated Research in Materials, Environment and Society (IIRMES) or the Department of Geological Sciences. Core Retrieval and Sampling In 2010 Morteza Djamali (IMBE-CNRS, France) collected a 6-m core at the northern edge of Lake Hirom in approximately 15 cm of water. The core was composed of six contiguous 1-m drives (FIGURE 8). Cores from deeper water further into the basin were not possible without a boat, which was unavailable. The core was split in half longitudinally, and stored in PVC pipes. Photographs were taken at 10 cm intervals for the entire core, and a stratigraphic column was made noting the sediment structure and type (FIGURE 9). Samples from the lower three meters of the core were collected at 5 21 cm increments for pollen analysis by M. Djamali. Pollen preservation is poor, but select taxa are presented in this thesis. Additional samples, 5 mm in thickness, were collected at 5 cm increments for the lower 225 cm of marl sediment. Where layering is visible, samples were collected parallel to these layers. Contiguous samples, 5mm in thickness, were collected in the upper 25 cm of this lower sequence to better characterize the change in climate during the transition to the peat interval (150-375 cm). The marl samples collected from the core were cut into two pieces, one for stable-isotope analysis and the other for carbon content. Chronology A total of eight samples were collected for radiocarbon analysis (TABLE 1). The basal peat date of 9300 cal yr BP was measured by M. Djamali at Poznań Radiocarbon Laboratory. Six samples of plant material within the marl from 53 cm, 156 cm, 250 cm, 450 cm, 507 cm, and 600 cm and one sample within the peat from 396 cm were dated at the Keck Radiocarbon Lab at the University of California-Irvine. All radiocarbon dates were calibrated with Calib 7.1 (Stuiver and Reimer, 1993) and reported as cal yr BP. Mean dates within the 2 error are used in the age-depth model. Other samples depths were dated using a linear sedimentation rate (FIGURE 11). Ostracodes Sub-samples were weighed wet and dry to calculate the percent water for each sample. This is necessary to calculate the number of ostracodes per dry g of sediment. The subsamples were then wet sieved at 63 µm to separate ostracodes from the very fine sediment. The ostracodes are picked from the sediment with a fine-tipped brush and 22 separated by species and by instar. The adult carapaces were cleaned with HPLC grade ethanol for isotopic and trace element analysis, following the technique of Anadón et al., (2006). Ostracode Geochemistry Two to three adult valves of a single species were selected for δ18O and δ13C analysis. Measurements were made on a Finnigan Mat 253 IRMS coupled to a Kiel IV autosampler at the University of California-Irvine. Three standards, NBS-18, NBS-19, and Oxcal, were used for calibration. The samples are placed into septa free glass vials and phosphoric acid is reacted with the samples at elevated temperatures under temperature control. The CO2 evolves in the septum-free vials and diffuses under medium vacuum pressure into a crypgenic trapping system. The water and noncondensable gases that evolved during the reaction are removed from the CO2 gas phase under high vacuum in the first trap. If there is too much CO2 gas prior to transfer into the microvolume, the CO2 sample size can be reduced by expansion into a defined volume. In the microvolume, the dry CO2 is prepared for analysis in a dual microvolume inlet system, which is connected to the IRMS. The NBS-18 standard has a δ13C value of -5.0‰ relative to V-PDB (Vienna PeeDee Belemnite) and a δ18O value of 6.9‰ relative to V-SMOW (Vienna Standard Mean Ocean Water). The NBS-19 standard has a δ13C value of 2.0‰ relative to V-PDB (Vienna PeeDee Belemnite) and a δ18O value of 28.6‰ relative to V-SMOW (Vienna Standard Mean Ocean Water). The standard Oxcal, an internal laboratory standard, has a δ13C value of -7.49‰ relative to V-PDB (Vienna PeeDee Belemnite) and a δ18O value of 8.18‰ relative to V-SMOW (Vienna Standard 23 Mean Ocean Water). The three standards were used to normalize the raw δ13C and δ18O values of the samples using a three point calibration method. According to the FinniganTM Keil IV manual, the precision of this technique for δ13C is ±0.03‰ and for δ18O is ±0.07‰. Results are reported as per mil (‰) relative to the PeeDeeBelemnnite (VPDB) standard and are listed in Appendix A. The phosphoric acid residue from the isotope procedure was pipetted into 15 ml vials to which 0.5M Trace Element Grade HCl was added to keep the Sr, Mg, and Ca in solution. This solution was analyzed on an Agilent ICP-MS at IIRMES, CSULB. An internal standard, rhodium, is added to each sample before analysis. The sample solution is then pumped into the inlet system from the 15ml vial. It is nebulized into a fine sample aerosol. The aerosol is then carried into a high temperature argon plasma, which atomizes and then ionizes the sample to produce a cloud of positively charged ions. The sample ions are extracted from the plasma into a vacuum system containing a quadrupole analyzer. The analyzer can scan the mass range, so multi element analysis can be performed on the sample. The ions are focused into the analyzer, where they are separated by their mass-to-charge ratio (m/z). The ion concentration of a specific mass-to charge ratio is measured by an electron multiplier detector. The count rate obtained for a particular ion is compared with a calibration plot to give the concentration for that element in the sample. The precision of the instrument is between ±4.65% for a mass range 7 and ±8.48% for mass range of 89. Concentrations were converted to molar ratios and values are listed in Appendix B. 24 Bulk Carbonate The fine sediment (<63) µm was freeze dried and crushed for bulk isotopic analysis. Approximately 0.3 mg of sample was analyzed with a Finnigan MAT deltaplus IRMS coupled to a GasBench II carbonate device. Two international standards, NBS-18, NBS-19, and one internal standard, STD A, were used for calibration. The standards and samples are placed in glass vials with a rubber septa. The vials are placed in sample trays where they are flushed with helium, which removes trapped air from the glass tube. After the samples are purged anhydrous phosphoric acid is injected into each vial. The liberated CO2 gas and helium enters the Gas Bench II through a diffusion trap system, which removes the water. The CO2 is then transported to a gas chromatograph. The isothermal gas chromatograph concentrates the CO2 gas. A reference gas injection system is used with the sample gas for comparison. The NBS-18 standard has a δ13C value of -5.0‰ relative to V-PDB (Vienna PeeDee Belemnite) and a δ18O value of 6.9‰ relative to V-SMOW (Vienna Standard Mean Ocean Water). The NBS-19 standard has a δ13C value of 2.0‰ relative to V-PDB (Vienna PeeDee Belemnite) and a δ18O value of 28.6‰ relative to V-SMOW (Vienna Standard Mean Ocean Water). The Standard A, the laboratory standard, has a δ13C value of -39.9‰ relative to V-PDB (Vienna PeeDee Belemnite) and a δ18O value of 11.6‰ relative to V-SMOW (Vienna Standard Mean Ocean Water). The three standards were used to normalize the raw δ13C and δ18O values of the samples using a three point calibration method. According to the FinniganTM Gas Bench II manual (2005 edition), the precision of this technique for δ13C is ±0.06‰ and 25 for δ18O is ±0.08‰. The Results are reported as per mil (‰) relative to VPDB and are listed in Appendix C. Mineralogy The mineralogy of both the coarse (>63 µm) and fine (<63 µm) was determined with a Rigaku Miniflex® x-ray diffractomoter (XRD) equipped with a sealed CuKα tube and diffracted beam monochrometer to reduce β-rays. Peak identification was performed with X’Pert HighScore Plus 3.0. The relative percentage of minerals were calculated using Rietveld Refinement, which was performed using the structural models from the PDF-4 minerals database with the X’Pert HighScore Plus 3.0 software. Carbon Content Unsieved, but crushed, sub-samples were measured for total carbon (TC) and total inorganic carbon (TIC) analysis on a UIC™ CM5014 CO2 Coulometer. The UIC coulometer contains a cell setup that undergoes a titration reaction when a CO2 stream is introduced. The CO2 is quantitatively absorbed in the cell solution, monoethanolamine, while a current generated by platinum and silver electrodes in the cell electrochemically generates a base. The samples were weighed in ceramic and plastic boats to approximately 11 mg. Total carbon was measured by combusting the subsample in a furnace heated to 950° C. The total inorganic carbon was measured through acidification of the subsample with 70% perchloric acid. The TOC is calculated by subtracting the total inorganic carbon from the total carbon. The standard used is STD A, an internal laboratory standard, and is ~ 12% TIC and TC, the precision of the technique to measure 26 TC is ±0.86% and TIC is ±0.80%. The TIC and TOC values are reported in percent carbon and are listed in Appendix D. FIGURE 8. Image of the entire 7 meters of core. 27 CHAPTER 4 RESULTS Core Lithology The core consists of alternating layers of peat and marl (FIGURE 8, FIGURE 9). The basal section of the core consists of ~ 30 cm of peat that gradually transitions to marl. The lower marl section is ~ 300 cm thick and abruptly transitions to an upper layer of peat. The upper peat section is ~250 cm thick and abruptly transitions to the uppermost marl section, which is ~150 cm thick. The marl sections have clear banding with some laminated intervals. Marl sediment color ranges from rust to grey. Visual organic matter in the marl was rare, except in the lowermost marl section. X-ray diffractometry of the marl indicated that calcite was the dominant mineral phase (Figure 10). The calcite exhibited varying degrees of Mg substitution. Low-Mg calcite (<3% Mg) occurred from approximately 8,840 to 7,150 cal yr BP (630-460 cm). Abrupt and short-lived periods of high-Mg-calcite occur after 7,150 cal yr BP, with a higher frequency near the marl/peat transition (6,650-6,300 cal yr BP) (410-376.5 cm). Calcite with an intermediate Mg composition occurred infrequently in the lower marl section (8,840-7,700 cal yr BP) (630-515 cm) and increases near the marl peat transition (6,650-6,300 cal yr BP) (410-376.5 cm). Dolomite occurred sporadically throughout the marl section. However, it never exceeded 25% and rarely exceeded 15% of the total 28 FIGURE 9. Stratigraphic column for the entire core taken from Lake Hirom. The locations where dates were taken are denoted with stars, age inversions are included. 29 FIGURE 10 Mineral abundances of unsieved sediment by XRD. Asterisk denotes intermediate-Mg Calcite. Figure 10 30 mineral fraction. Periods with elevated dolomite were from 8,450 to 8,200 cal yr BP (590-565 cm) and 7,850 to 6,300 cal yr BP (530-376.5 cm). Halite was ubiquitous with background levels around 10%. Periods with higher concentrations occurred between 8,750 and 8,350 cal yrs BP (620-580 cm), 7,650 and 7,350 yrs (510-480 cm), and 6,550 and 6,300 years (400-376.5 cm). Gypsum was found in high percentages from 8,8407,850 cal yr BP (630-545 cm), with only very minor amounts in the uppermost marl section. The gypsum occurred as large discoids (>63 µm) and most, if not all, is sieved out with the macrofauna. Celestine was found throughout the core, however only in low percentages. A single notable peak in celestine occurred at approximately 6,300 cal yr BP (376.5cm). Quartz occurred often, with high percentages at 8,150 cal yr BP (560cm), 7,300 cal yr BP (475cm), and near the marl/peat transition at 6,550 cal yr BP and 6,300 cal yr BP (400-376.5). Age Model/Stratigraphy Eight dates were collected for the entire core. The marl section that is the focus of this study was bounded by two dates on the upper and lower peat layers and three dates on organic matter within the marl (TABLE 1). All radiocarbon ages were calibrated with Calib 7.1 (Stuiver and Reimer, 1993). The dates at 507 cm (11,443 cal yr BP) and 600 cm (4,665 cal yr BP) were excluded from the age model as they represent age reversals. These two dates were excluded rather than the other dates for two reasons. 1) The peat above and below the marl is in situ. Thus, the ages retrieved using the peat sections at 699 cm (9,300 cal yr BP) and 396 cm (6,290 cal yr BP) are the most reliable dates. 2) The average sedimentation rate for the lower marl section, when age reversals 31 were excluded, is similar to the upper marl section (0.11 and 0.07 cm/year, respectively) (FIGURE 11). The explanation for the age reversals at 600 cm (4,665 cal yr BP) is that it must have been contaminated with modern carbon, and the sample from 507 cm (11,443 cal yr BP) is that it may have been re-worked. An age/depth plot (FIGURE 11) shows the average sedimentation rates between dates and the position of the reversals. The average sedimentation rate for the entire lower sequence is 1.06 mm/yr. Based on this rate, each sample used in this study integrates ~5 years. The upper contiguous samples span 24.5 cm (~125 years), whereas the rest of the sequence (lower 275 cm) have a sample spacing of every ~50 years. Total Organic and Inorganic Carbon The total organic carbon (TOC) and total inorganic carbon (TIC) values of samples retrieved from the marl are shown in FIGURE 12. TIC ranges from 0.96 to 9.92%, with a mean value of 7.98 % ( FIGURE 12). From 8,840-7,900 cal yr BP, TIC values are generally below the long-term average and have large fluctuations. From 7,900-6,400 cal yr BP, TIC is generally above the long-term average and fluctuations are smaller (between 7.5-10.0 %). Two notable decreases occur at 7,200 cal yr BP (470 cm) and 6,500 cal yr BP (395 cm). In the uppermost part of the marl sequence (6,400-6,330 cal yr BP), TIC values vary significantly (0-10%). In general the TIC values increase from the early to mid-Holocene, then decrease at the marl/peat transition. TOC ranges from 0 to 9.3%, with a mean value of 1 % ( FIGURE 12). From 8,840-6,400 cal yr BP TOC values are low (0-2 %), with frequent variations. Near the top of the sequence in the mid-Holocene (6,400-6,330 cal yr BP), the TOC values show large fluctuations up to 32 TABLE 1. Radiocarbon Dates for Lake Hirom 14 C Years Depth Sample Before Age (cal yr (cm) Lab ID ID present error BP) type 53 UCIAMS 152321 1420 60 1336 Charcoal 156 UCIAMS 103156 2110 104 2090 Charcoal 250 UCIAMS 103157 3305 95 3525 Charcoal 396 UCIAMS 103158 5480 28 6290 Charcoal 450 UCIAMS 152322 5955 20 6767 Charcoal 507 UCIAMS 152323 9985 30 11443 Charcoal 600 UCIAMS 152324 4100 20 4665 Charcoal 699 POZ 31054 8290 50 9300 Charcoal 33 FIGURE 11. Age–depth model of calibrated 14C dates with error bars. The ages at 507 cm (11,443 cal yr BP) and 600 cm (4,100 cal yr BP) were excluded from the model because they were interpreted as age reversals. 34 FIGURE 12. Total organic (TOC) and inorganic (TIC) carbon found within the marl section of the core, grey bars indicate mean values. 35 10%. The TOC variations do not show a significant trend, other than an increase in the mid-Holocene. Stable Isotopic Composition of Endogenic Carbonate The carbon and oxygen isotopic composition of endogenic calcite is shown in FIGURE 13. It should be noted that hi-Mg calcite and low-Mg calcite were not separated, and it is expected that the isotopic fractionation will differ between these two (Tarutani et al., 1969; Land, 1980). The small amounts of dolomite were also not taken into account. No corrections can be made for the dolomite given that it is likely detrital and rock samples were not available. However, there is no coherent offset between the isotopic values of samples with or without dolomite (FIGURE 15). δ18O values range from -4.6‰ to 0.25‰ with a mean of -2.5‰ and a standard deviation of ± 0.9. From 8,840 to 7,800 cal yr BP, the δ18O values become isotopically lighter over time with the lowest values at -4.6‰ at 7,800 cal yr BP. There are distinct positive peaks during the general negative trend that occur at 8,700 and 8,050 cal yr BP. From 7,800-6,300 cal yr BP, δ18O values range from -4‰ to -1.5‰, and vary around the average. δ13C values range from -7.9‰ to -1.5‰ with a mean of -4.22‰ , and a standard deviation of ±1.16. From 8,840 to 7,800 cal yr BP, the δ13C values are generally below the average value, with a distinct and protracted decrease at -7.9‰. There are also positive peaks during the general negative trend that occur at 8,700, 8,200 and 8,050 cal yr BP. The δ13C values increase to -1.5‰ at 7,700 cal yr BP showing greater short term variability, with a range from -2‰ to -7‰. 36 Macrofauna The macrofauna found in the lower marl section consist of gastropods, foraminifera, and ostracodes (FIGURE 16). Gastropods are abundant in the top section of the marl (greater than 20 per interval) (7,800-6,300 cal yr BP) (525-376.5 cm), but less abundant in the lower section of the marl (1-5 per interval) (8,840-8,550 cal yr BP) (630600 cm). They are absent between 8,500 and 7,800 cal yr BP (595-530 cm). A single species of foraminifera is found in abundance in the lower-most, middle, and top most section of the marl (greater than 20 per interval) (8,840-8,800, 7,600-7,550, and 6,6506,300 cal yr BP) (630-625, 510-500, 410-376.5 cm). The species is tentatively identified as Ammonia tepida, a saline, non-marine benthic species found in Egypt and Israel (Hayward et al., 2004). Ostracodes do not occur in the lower-most section of the core, unlike gastropods. They appear around 7,800 cal yr BP and increase in abundance to 6,300 cal yr BP (525376.5 cm) (FIGURE 17). Ostracodes show poor diversity and are represented by two main species, Cyprideis torosa (Jones, 1850) and Candona candida (O.F. Muller, 1776) with rare occurrences of Eucypris inflata (Sars, 1903). Identifications of C. candida and E. inflata are tentative. Cyprideis torosa exhibits clear sexual dimorphism, so the female and male species were grouped separately. Females were more abundant than males in many intervals. The tentatively identified Candona candida was found in several intervals where Cyprideis torosa did not occur. E. Inflata occurs only twice in the marl section in the upper-most section of the early Holocene segment, though they are the dominant species in the youngest marl sections above the peat (Stevens, pers. Comm.). 37 FIGURE 13. Stable isotopic composition of the lake carbonates. The grey bar denotes the average values for the carbon and oxygen isotopes. 38 F FIGURE 14. Covariance of oxygen and carbon isotopes. The lack of a pattern between low- and high-Mg calcite indicates that fractionation has not caused significant changes in isotopic values. 39 FIGURE 15. Covariance of oxygen and carbon isotopes. The lack of pattern for dolomite indicates that fractionation did not cause a significant change in the isotopic values. 40 FIGURE 16. Macrofuana found within the marl section of the core. Circles indicate the presence of the macrofauna within the specific interval. 41 Stable Isotopic Composition of Ostracodes Carbon and oxygen isotopic values of ostracod carapaces are shown in FIGURE 18. The entire ostracode record represents ~160 years, thus changes are considered very abrupt and short-lived. Limited data are due to the lack of ostracodes in the lower marl section, thus the ostracode record is significantly smaller than the sediment record. Analyses were done on individual species and, in the case of Cyprideis torosa, by gender. The data are not continuous, providing only windows of information. Data often do not overlap, but where they do usually do not track well between the different ostracodes. The δ18O values of Candona candida have an average value of 0.47‰. The δ18O values of Cypredies torosa male have an average value of -0.86‰, with much greater variability than C. candida. Peaks occur at 6,510, and 6,450-6,440 cal yr BP (395-393, and 390-387 cm). A pronounced increase occurs from 6,430 to 6,400 cal yr BP (387-384 cm). The δ18O values of C.torosa female have an average value of -1.83‰. From 6,480 to 6,430 cal yr BP (392-387 cm) the δ18O values are lower than average. They return to their average value by 6,430 cal yr BP and are roughly constant with only small variations. The carbon isotope pattern is quite different. As with the 18O values, there is offset of about 3 to 5 ‰ between the C. candida and C. torosa ostracodes. However, unlike the 18O values, the torosa male and female 13C values track very well. The δ13C values of Candona candida vary between about -2 and -6‰, but there are too few data for a profile interpretation. The δ13C values of Cypredies torosa male have an average value of 7.1‰, with a standard deviation of ± 2.0‰. The δ13C values of Cypredies torosa female have an average value of -6.60‰, with a standard deviation of ± 3.20‰. The most 42 FIGURE 17. The adult ostracode species count. 43 marked feature of the record is a decrease in δ13C values for both C. torosa between 6,520 and 6,490 cal yr BP (395 and 393cm). Values are steady around -8 ‰ until 6,410 cal yr BP (385 cm), and then decrease again. At 6,400 cal yr BP (384 cm) the carbon isotopes increase and remain around -8‰. Trace Elements in Ostracode Carapaces The molar ratios for Mg/Ca and Sr/Ca in the ostracodes are shown in FIGURE 19. The molar ratios are multiplied by103 to better depict the changes in the values. It is not expected that the different species or gender will have the same ratios. The Mg/Ca ratios for both ostracode species generally have the same trend. Candona candida has an average Mg/Ca value of 9.7. Cyprideis torosa (male) has an average Mg/Ca value of 9.5. Cyprideis torosa (female) has an average Mg/Ca value of 10.33, with a standard deviation of ±2.47. The Mg/Ca values for both species stay around the average from 7,600 to 6,390 cal yr BP (510-383cm). An increase in the Mg/Ca values occurs from 6,400 to 6,330 cal yr BP (384-379 cm). The Sr/Ca values for both species are variable. Candona candida has an average Sr/Ca value of 16.39 that generally increases over time. Cyprideis torosa (male) has an average Sr/Ca value of 22.32. The Sr/Ca values for Cyprideis torosa male fluctuate around the average value, although at first the values begin slightly lower from 6,500 to 6,490 cal yr BP (394-393cm). Cyprideis torosa (female) has an average Sr/Ca value of 22.90. The Sr/Ca values for Cyprideis torosa female fluctuate around the average value, except at 6,370 to 6,360 cal yr BP (381380cm), where there is a large increase. 44 FIGURE 18. Stable isotopes of the ostracod carapaces, values are reported in permil. 45 Mg/CaWater and Sr/CaWater molar ratios were derived using a partitioning coefficient value for the species of ostracod Cyprideis from De Decker and Forester, 1988. Cyprideis torosa (male) indicates the host water had an Mg/Ca molar ratio of 2.16 with a standard deviation of ±0.82, and Sr/Ca molar ratio of 0.05 with a standard deviation of ±0.008. Cyprideis torosa (female) indicates the host water had an Mg/Ca molar ratio of 2.25 with a standard deviation of ±0.54, and Sr/Ca molar ratio of 0.05 with a standard deviation of ±0.011. 46 FIGURE 19. Trace elements in the ostracod carapaces, values are reported as molar ratios. 47 CHAPTER 5 DISCUSSION Changes in hydroclimate can cause lakes to change geochemically and physically. These changes affect the sediment, macrofuana, and vegetation within the lake. Changes in the lithology and faunal assemblages can be used as paleoclimate proxies themselves, but also archives additional proxies. Both lithology and macrofauna suggest a lake of moderate salinity. Thus, multiple lines of evidence can be used to infer climatic changes. Brine Evolution and Mineralogy as an Indicator of Effective Moisture There are three dominant types of sediment found along the edge of Lake Hirom: peat, marl, and evaporites. Today, vegetation is concentrated at ground water seeps along the edge of the lake, and thus the peat likely indicates periods with lower lake levels and a water table just below ground level (Djamali, Pers comm.). Lake Hirom appears to be a closed basin as seen in FIGURE 2, which suggests that drainage did not control peat development, rather changes in lake level did. Ultimately peat at the coring site, as opposed to marl or evaporites, suggests a lack of open water and thus drier conditions. The first transition of marl to peat is at 6300 cal BP, thus indicating the onset of drier conditions in the mid-Holocene relative to the early Holocene. The sediment of Lake Hirom shows significant mineralogical changes that suggest some type of brine evolution. Much of the sediment record is dominated by marl 48 with evaporite minerals occurring several times mixed in with the marl. The marl is often low-Mg calcite but can include high-Mg calcite and even minor amounts of dolomite. The presence of high-Mg calcite implies an evolution of the lake water to more saline conditions. Evaporation of a lake closed to surface water outflow, like Lake Hirom, will lead to a relative enrichment of solutes. The sequence of minerals that precipitate out of the water depends on the composition of the water entering the lake and saturation of different minerals (Hardie and Eugster, 1970). There are no current data available on the water chemistry of either the lake or the springs feeding the lake. So conclusions regarding brine evolution are only tentative. The dominant carbonate phase in the lake is low-Mg calcite (<3 molar %). Both the calcite and bicarbonate are derived from the surrounding mountains, which have a dominant carbonate lithology (FIGURE 3). The precipitation of intermediate-Mg calcite* (~3molar %) and high-Mg calcite (> 5 molar %) occurs when Ca removal via calcite precipitation is significant (Müller et al., 1972). The removal of Ca causes an increase in the Mg/Ca ratio, driving the increased Mg substitution into the calcite lattice. Increases in Mg/Ca ratio will continue to occur with an increase in the residence time of the lake water and lack of renewed Ca input. Thus, intermediate and high-Mg calcite are interpreted as drier conditions (Müller et al., 1972). As the residence time increases, bicarbonate may also be exhausted causing the precipitation of gypsum (Hardie and Eugster, 1970). Should the Ca used in gypsum precipitation be consumed, it may be possible to precipitate (or co-precipitate) another sulfate mineral or even halite. Celestine, SrSO4, may precipitate once Ca is consumed 49 (McCarten et al., 1988). Halite is able to precipitate once little Ca remains in the brine as well (Müller et al., 1972). The lower-most part of the marl contains low and intermediate-Mg calcite, gypsum, and halite. Though the halite is ubiquitous throughout the core it does generally increase when gypsum increases in the lower-most part of the marl. The upper part of the core has intermediate and high-Mg calcite, celestine, and halite. There are no relationships found between the minerals in the upper part of the core, so no clear explanation of brine evolution can be proposed. The frequent occurrence of halite suggests that there is a constant supply of sodium and chloride to the lake. Likely there is a salt dome nearby—perhaps below ground level. Salt domes are common in the Zagros Mountains (Motamedi et al., 2011). Thus halite is likely not a useful proxy of effective moisture indicator for this region. The presence of peat suggests prolonged dry conditions. As opposed to marl, which indicates wetter conditions. The sporadic occurrence of gypsum in the lower part of the core suggests large and abrupt droughts, which caused the lake to have an excessive brine evolution. The mineralogy of the lower part of the core suggest a transition from prolonged droughts (peat) to a wetter climate that was punctuated by large but short duration droughts (marl and evaporites). The mineralogy in the upper part of the core suggest increased aridity until peat occurs, which indicates prolonged dry conditions. 50 Detrital Minerals as Proxies of Climate In Lake Hirom, quartz and dolomite are considered to be detrital in origin. The quartz, which is more resistant to physical and chemical breakdown may have been transported long distances (Dearing, 1999). Quartz is found frequently throughout the core, but rarely occurs in high percentages. Given that most of the quartz is less than 63 µm, it is entirely possible that it is windblown and related to dust from the Shamal. However, relative increases in percentage of quartz occurs with increases in 18O values, which suggest that the dust is greater, with greater aeolian deposition, during drier conditions, although the source maybe somewhat local. Drier conditions would destabilize vegetation and wind would entrain the quartz. The co-occurrence of quartz with celestine confirms that quartz is likely indicative of drier windier conditions. The occurrence of dolomite is problematic. Dolomite in lake sediment can be a primary precipitate (Warren, 1999), diagenetic (Mees et al., 2011), or detrital (Sabins, 1962). Precipitation of dolomite directly from water requires special conditions and excessively high Mg/Ca ratio (Müller et al., 1972). The fact that the lake precipitates hi-Mg calcite indicates that this could be possible—but the hi-Mg calcite does not correlate with the dolomite. Furthermore if the dolomite were endogenic it would be precipitated in larger quantities during periods interpreted as drier, which it was not. Thus, dolomite is not believed to be a primary precipitate. Diagenetic alteration of calcite to dolomite is common in sabhkas (Kendall, 1979). The alteration of calcite to dolomite would cause an increase in Ca in the pore water, and therefore a possible increase in diagenetic Gypsum precipitation. However, dolomite and gypsum do not increase concurrently at 51 Lake Hirom. Thus, dolomite is considered to be detrital, which is plausible as it is less soluble than low-Mg calcite. Dolomite, derived from the Mesopotamian lowlands, is a common constituent of windblown dust into the Arabian Sea (Cullen et al. 2000). As with quartz, dolomite may be derived from long distances and its source is unknown. The quartz and dolomite mineral percentages do not have a relationship suggesting a pattern with detrital mineral input. The detrital minerals are therefore just a common background pulse of dust and unhelpful in establishing drier conditions Isotope Data of Bulk Carbonates and Ostracodes as Indicators of Effective Moisture The δ18O and δ13C values of certain hydrologically closed basins have been shown to have a positive covariance (Talbot, 1990; Li and Ku, 1997), although the relationship is equivocal. Evaporation in closed basins results in an increase in δ18O values. Simultaneously the uptake of 12C via algae causes an increase in δ13C values of DIC from which the carbonates precipitate. Interestingly the carbonate carbon and oxygen isotopes in Lake Hirom do not show a strong covariance (FIGURE 14). The lack of covariance can be a result of three factors. The first is that the lake is hydrologically open, which is physically not obvious (FIGURE 2). The second is that different 18O values contributed from the fraction of dolomite and high-Mg calcite disrupted the covariance. The third is that covariance is not a robust indicator of closed basins. If for example, carbonate precipitation is triggered by degassing rather than photosynthetic uptake of CO2 by algae (Kelts and Hsu, 1978), then there is no a prior reason, particularly in a low productivity lake, that the two would increase simultaneously. 52 Bulk sediment carbonates are often avoided for stable isotopes analysis in paleoclimate studies because it may be difficult to distinguish between authigenic and allogenic carbonates (Leng and Marshall, 2004). Because the mountains that surround Lake Hirom are limestone dominated mountains (Talebian and Jackson, 2002), there is a possibility that some of the carbonates used for isotope analysis were detrital. Because the ostracodes use the water to construct their carapaces, endogenic carbonate should mirror the isotopic trend of the ostracodes. The given caveat is that there are strong vital effects in the fractionation of isotopes into ostracodes (Xia et al., 1997). In FIGURE 20 we see that the isotopic values of the ostracodes and carbonates follow similar trends, within reason. They will not track each other perfectly as the carbonates may precipitate in different seasons than the ostracodes precipitate their shells. But larger trends should follow and they do. Thus, it is inferred that the isotopic signature from the bulk carbonates are derived predominantly from endogenic carbonates. Trace Elements of Ostracodes as Indicators of Effective Moisture The concentration of Sr and Mg of the lake water can be used to infer the moisture balance, because excess evaporation will decrease lake volume, which causes an increase in the relative concentration of various solutes. While excess precipitation will increase lake volume, which causes a decrease in the relative concentration of various solutes in the water. An increase in Sr and Mg will occur concomitantly with an increase in celestine, and/or intermediate-and high Mg calcite. A comparison of Sr/Ca and celestine shows that the Sr/Ca ratios do increase as the celestine occurrence increases (Figure 21). The Mg/Ca ratios of the lake water extrapolated from the ostracodes were 53 approximately 2.2, and values above 2 should result in high-Mg calcite precipitating (Mueller, 1972). There is no relationship between increased Mg/Ca ratios and high-Mg calcite. However, there is a relationship between increased intermediate-Mg calcite and increased Mg/Ca ratios. The co occurrence of increased Sr/Ca and Mg/Ca ratios and celestine and intermediate -Mg calcite, respectively, suggest that these proxies are a good indicator of effective moisture. Mean Climate Changes The early Holocene lacustrine history is divided into three intervals, which are defined by changes in the sedimentology, mineralogy, and general trend of the isotopes. Names are given to each interval for discussion purposes only. These names are not formal designations. The three intervals are Interval 1: 9300-8800 yr BP (Earliest Holocene), Interval 2: 8800-7800 yr BP (Early Holocene), and Interval 3: 7800-6300 yr BP (Early-to-mid Holocene). Earliest Holocene (9,300-8,800 yr BP) The earliest Holocene (Interval 1) is composed of peat, which is interpreted as drier than present conditions. Today, vegetation is concentrated at ground water seeps along the edge of the lake, and thus the peat indicates either extremely low lake levels or a water table below surface (Djamali, Pers comm.). Both support generally drier conditions. The gradual change from peat to marl indicates that the transition to wetter conditions was not abrupt. 54 FIGURE 20. Comparison of carbonate and ostracod carapace isotopic trends. The oxygen trends for both proxies correlate indicting that carbonate is largely authigenic. 55 Mg/Ca and Sr/Ca for C. torosa (female), gypsum, celestine, and intermediate and high-Mg calcite (%). Grey bars show regions where Mg/Ca and Sr/Ca concentrations have increased. 56 FIGURE 21 Comparison of trace element ratios of Mg/Ca and Sr/Ca for C. torosa (female), gypsum, celestine, and intermediate and high-Mg calcite(%). Grey bars show regions where Mg/Ca and Sr/Ca concentrations have increased. Figure 221 Comparison of trace element ratios Early Holocene (8,800-7,800 yr BP) The sediment transition from peat to marl is interpreted as a transition to a permanent or semi-permanent water body due to wetter conditions. The sediment is dominated by low-Mg calcite with punctuated occurrences of gypsum. There are some foraminifera and gastropods in the lowest part of Interval 2, but there is a distinct lack of macrofossils , for reasons that are unclear. So isotopic values are restricted to those from calcite. The lack of high-Mg calcite and infrequent dolomite suggests that isotopic shifts due to mineralogic changes are minor. A long-term trend to more negative 18O values during this interval suggests that the overall climate was getting wetter. However, six abrupt drought events superimposed on this trend are inferred from increased 18O values (8,700, and 8,050 cal yr BP), decreased TIC% (8,700, 8,500, 8,400, and 8,050 cal yr BP), and peaks in gypsum % (8,700, 8,500, 8,400, 8,200, 8,050, and 7,950 cal yr BP). The latter suggests draw down of the lake for multiple years with the resultant increase in 18O values. The disappearance of gastropods and foraminifera occurring between 8,800 and 7,800 cal yr BP is concurrent with several drought events in Interval 2. Drought events may cause lake desiccation, which is coupled with a decrease in macrofauna diversity and/or removal of less adapted species (Hunt and Jones, 1972). The fact that macrofauna do not occur frequently after the first drought event can be interpreted several ways. 1) The macrofauna were not able to recover after the drought events. However, if the macrofauna were able to recover after a drought, as they did in the first drought event, they were not preserved during the subsequent lake desiccation events. 2) Macrofauna 57 prefer to live in submergent vegetation (Hunt and Jones, 1972), and therefore the overall wetter trend of the lake may have caused the lake to grow in size, and any submergent vegetation had followed the shoreline away from the coring site. Early-to-Mid Holocene (7,800-6,300 yr BP) The transition between Intervals 2 and 3 is not obvious in the stratigraphy but is defined by changes in mineralogy. At the beginning of Interval 2 the disappearance of gypsum suggests an end or decrease in the drought events. However, the occasional occurrence of celestine may signify shorter drought events as water becomes more concentrated with Sr. Celestine forms in evaporate-carbonate environments when reacted with Sr-rich fluids (Hanor, 2000), but it is not clear why Sr would be the dominant cation over Ca, save for the fact that Mg is also increasing in the calcite at this time. In Interval 3 TIC% and TOC% fluctuations around the mean. Like the mineralogy the TIC% and TOC% have large fluctuations near the end of Interval 3, although the rapid timing of these changes is likely due to the contiguous sampling interval. The decrease in TIC% indicates a transition from calcite to a more concentrated brine mineral, celestine in this case. The increase in the TOC% indicates an increase in vegetation, which is corroborated by an increase in the aquatic pollen found in this section of the core (Djamali, Pers comm.). The 13C values in Interval 3 are more positive relative to Interval 2. The increase in the 13C values also indicate an increase in vegetative productivity. The 18O values of the bulk carbonate in this interval are more positive relative to Interval 2. The 18O values fluctuate around the average 18O value of -2.5‰, and this is 58 interpreted as the lake fluctuating between drier and wetter stages. Increased 18O values do not correlate with increased intermediate- and- hi-Mg calcite percentages, which indicate that fractionation between low and high-Mg calcite does not cause a large difference in the 18O values (FIGURE 14). However, it is somewhat perplexing if both are driven by evaporative concentration. The appearance of macrofauna at the beginning of Interval 3 suggests that either a suitable substrate developed at this time, allowing for habitation and preservation, or that climatic/aquatic conditions became more suitable. Macrofauna are primarily affected by the salinity of lakes (Geddes et al., 1981), but their distribution can also be affected by organic matter content and substrate (Rieradevall and Roca, 1995). Rieradevall and Roca (1995) documented that with increasing salinity there is a decrease in the diversity of macrofuana. However the diversity of ostracodes in Lake Hirom is already quite poor. Salinity does not seem to influence the diversity of macrofauna as seen by the general increase in the benthic ostracodes and gastropods occurring concurrently with a change in mineralogy, a decrease in TIC%, and an increase in trace elements. This indicates that submergent vegetation and a stable substrate is the more likely driver in presence/absence of macrofuana in this lake. The dominant ostracod, Cyprideis torosa, is a euryhaline species and does not offer much information concerning salinity (Heip, 1976). However, both the female and male forms are useful for isotopic and trace element evaluations of water quality. From 6,510 to 6,440 cal yr BP the decrease in the 18O value of C. torosa indicates a shift toward wetter conditions. The 18O values increase after 6,440 cal yr BP to 6,330 cal yr 59 BP to 6,330 cal yr BP indicating that the lake is getting more concentrated and the climate drier. The 13C values C. torosa can be used to indicate lake vegetative productivity, and in turn effective moisture. From 6,510 to 6,480 cal yr BP the decreasing 13C values indicates a decrease in lake productivity. After 6,480 cal yr BP the 13C values increase until 6,420 cal yr BP indicating increased lake productivity. The 13C values slightly decrease again from 6,420 cal yr BP to 6,330 cal yr BP, indicating a slight decrease in lake productivity. The changes in productivity indicate a sporadic occurrence of aquatic plants, which suggests a transition to more peat like conditions. High Sr/Ca values for C.torosa occur concomitantly with the increase of celestine around 6,400-6,330 cal yr BP, which is consistent with concentration of Sr in the water relative to Ca. Similarly the increase in high-Mg calcite and moderate-Mg calcite near the end of Interval 3 coincides with an increase in C. torosa Mg/Ca values as well. The presence of increased Mg/Ca and Sr/Ca values, celestine, and intermediate and high-Mg calcite suggest that there is excess evaporation, indicating drier conditions. Mean Climate Change-Interval 1-3 The occurrence of peat in the earliest Holocene (Interval 1) suggest that the lake had a prolonged dry climate. In the early Holocene (Interval 2) the lack of macrofossils and increased evaporites indicate short duration, but large scale drought events. Although, the overall oxygen isotope signature in Interval 2 indicates increasingly wetter conditions. The early-to-mid Holocene (Interval 3) was marked by an increase in lake water concentration as seen by the mineralogy and ostracod Sr/Ca and Mg/Ca ratios. 60 Also, an increase in aquatic plants and TOC% indicates a transition to peat. The proxies in Interval 3 suggest that the Lake Hirom is getting drier as it approaches the midHolocene. Regional Climate Change Southwest Asia (Iran, Turkey, Iraq, Saudia Arabia) is unique because it is on the nexus of several synoptic climate systems. The interplay of these systems determines when and how much precipitation the region will receive. A major question concerns whether SW Iran was dominated by the ISM, or by westerly rainfall during the early Holocene. A comparison of the results of Lake Hirom with other climatic records can help elucidate this question (FIGURE 22). During the early Holocene (8000-10000 yr BP), increased summer insolation and decreased winter insolation caused greater seasonality. Lake and speleothem records in the Arabian Peninsula suggest that the ISM reached more northerly locations as early as 10,000 yr BP (Overpeck et al., 1996; Burns et al., 2001; Fleitmann et al., 2007; Parker et al., 2006). However, it is unlikely that monsoonal rains would have penetrated as far north as Hirom, given the occurrence of peat and overall dry conditions. The strength of the ISM is inferred to have increased gradually from 10500-9500 cal yr BP in Oman (Fleitmann et al., 2007). But the prevailing dry conditions from 9300-8800 yr BP at Lake Hirom and the similarly dry conditions at Awafi suggest that if the ISM did strengthen, its effects were not dramatic at either location. In Oman the monsoon strength remained high until ~6300 yr BP, where northern Oman began to see a decrease in precipitation from ISM. In contrast Awafi had a dry climate at 9000 cal yr BP and a change to a 61 wetter climate at 8500 cal yr BP, suggesting that if monsoonal rains did penetrate this far north it was until well into the Holocene (Parker et al., 2006). Alternatively, Awafi could be fed by westerly precipitation, although Parker et al. do not support this model. At ~6000 yr BP the ISM weakens, but Awafi is still has marl mixed with sand after ~6000 yr BP and only intensifies to present (Parker et al., 2006). Thus, this region of Saudia Arabia became more arid at 6000 yr BP at nearly the same time as Hirom. Lake Hirom has a similar oxygen isotope trend to Lake Mirabad in northern Iran. Given the much more northerly position of Mirabad, the trend was hypothesized to indicate an increase in winter storms (snow) rather than an increase in overall precipitation. This leads to two possibilities to explain the lower 18O values in Interval 2 (the early Holocene).: either the ISM penetrated as far north as Lake Hirom or increased winter storms occurred as far south as Lake Hirom. At 8,500 cal yr BP both Awafi and Lake Hirom developed a permanent water body, which is consistent with either the northern penetration of the ISM or the southward penetration of the westerlies. Parker et al. (2006) favor the former, although no concrete evidence is provided to exclude the latter. Given that these two climatic systems operate during different seasons, it is possible that they are not mutually exclusive. If the ISM penetrated to Hirom, then the descending arm could be over northern Iran (dry and possibly early summers). This would mean that the majority of precipitation in Mirabad occurred as winter rain/snow, as suggested by Stevens et al., (2006) and Griffiths et al., (2001). However, a dust record form Lake Neor (37°57'37" N, 48°33'19" E), northern Iran suggests that during the early Holocene this region was 62 FIGURE 22. Comparison of dry and wet climates in Lake Hirom- Iran, Lake Awafi-SE Arabia, Hoti cave (N, north) and Qunf cave (S, south)- Oman, and Lake Mirabad-Iran. The asterisks indicate drought events, the likely source of precipitation is written above each site. 63 indeed wet, not dry (Al Sharifi et al., in press). This would suggest that the shamal were less active and that the westerlies were stronger. Wet winters do not preclude summer rain at Lake Hirom, but do not support it either. If the model of a northward displaced ISM is valid, then its constancy can be examined by the isotopic record. Drought events, or precipitation minima, are considered to be times when the ISM is weakened. With the tentative chronology, five significant droughts 8,500, 8,400, 8,200, and 7,950 cal yr BP events occurred at Hirom. Two of these 8,200, and 7,950 cal yr BP are in agreement with droughts documented in Awafi. The 8,200 cal yr BP event is also correlative with a precipitation minima at Oman, suggesting a link between the ISM and all three sites. However, it also corresponds to Bond Event 5, which is a cooling event from a melt water pulse in the North Atlantic, which would have impacted all synoptic climate patterns (Bond et al., 1997). A cessation of speleothem formation in Oman at 6.3 ka BP signals the retreat of the ISM to the south. This effectively moved the ITCZ and may have impacted all synoptic systems in the region. Awafi, Hirom, and Mirabad all experience a fundamental climatic shift at this time—to a drier mean climate state. Not all are linked to the ISM, so whether the drying at Hirom is due to loss of summer monsoon rains or a large scale shift in westerlies still cannot be resolved. 64 CHAPTER 6 CONCLUSION This thesis adds to the collection of records that document climatic changes in Southwest Asia. The Lake Hirom proxy record includes carbon content, sediment type, minerology, trace elements of ostracod carapaces, and stable isotopes of ostracod carapaces and carbonates. The earliest Holocene (9,300 cal yr BP) is marked by peat formation and indicative of dry conditions at a time when the ISM was increasing in strength in north and south Oman. The dry conditions in the earliest Holocene coincide with dry conditions in Awafi Lake in south east Arabia. The stable isotope record from 8,800 to 7,800 cal yr BP has decreasing δ18O values, which indicates wetter conditions. The timing of the wetter conditions is in good correlation with a permanent water body forming in Awafi, a site interpreted as being influenced by the strengthened ISM penetrated (Parker et al., 2006). One drought event in Lake Hirom at 8,200 cal yr BP has been recorded in all the lake and speleothem records in the Arabian peninsula and corresponds to Bond Event 5 (Overpeck et al., 1996; Burns et al., 2001; Fleitmann et al., 2007; Parker et al., 2006). The formation of peat in Lake Hirom at 6,300 cal yr BP indicates a transition into prolonged dry conditions. The timing of the drier climate coincides with drier conditions occurring in northern lake sites in Iran, and in Awafi. 65 APPENDICES 66 APPENDIX A OSTRACODE OXYGEN AND CARBON ISOTOPE DATA 67 Depth δ13C cm C.candida 379 379.5 -5.3703 380 380.5 -5.0912 381 -3.1588 383.5 384 384.5 385 386 386.5 387 -4.9284 387.5 -6.2400 388 -6.0956 389 -2.3745 390 392 393 394 395 480 505 δ18O 0.2813 -0.7924 -0.2630 -0.6020 -0.7865 -1.0558 -0.0551 δ13C C.torosa (m) δ18O -7.2715 2.0471 -9.6003 -1.2391 -8.6609 0.8576 -10.2475 -9.2858 -7.3726 -7.4070 -6.4099 3.1917 -0.3577 -1.5980 -1.6748 -2.9420 -8.2073 -8.6408 -7.2007 -7.3474 -4.0937 -0.2680 -0.4570 -2.8941 -9.4257 -2.8941 -6.1169 2.9699 -3.7256 -0.3920 68 δ13C C.torosa (f) δ18O -8.5972 -9.0553 -8.0056 -9.6287 -11.4802 -9.3541 -0.0287 -0.7436 -0.1434 -1.4036 -0.7177 0.2912 -7.5010 -7.8438 -6.6283 -0.0666 -1.4525 -1.5342 -8.1229 -9.1664 -8.9420 -4.4266 -5.5423 -3.9478 -8.5901 -10.2390 -3.0015 -2.9154 0.0129 -2.2724 -0.7679 -1.2467 -5.6854 -2.7392 APPENDIX B OSTRACODE TRACE ELEMENT DATA 69 Depth cm Mg/CaOstracod x103 Sr/CaOstracod x103 C.candida Mg/CaOstracod x103 Sr/CaOstracod x103 C.torosa (m) Mg/CaOstracod x103 Sr/CaOstracod x103 C.torosa (f) 379 19.4903 19.4903 16.8622 21.2503 9.9605 20.9786 379.5 6.7181 19.4903 16.8622 21.2503 8.7815 22.4782 2.8196 22.6033 11.4692 30.6594 7.7577 23.8133 9.7793 32.1006 13.8607 31.3694 6.4466 23.0029 8.9902 19.5922 380 380.5 7.3186 19.2174 381 8.3479 20.2182 383.5 384 6.7768 20.3948 384.5 10.1986 30.3912 385 9.6361 20.8262 7.9315 22.8011 386 13.0643 23.1999 12.9030 17.8580 386.5 12.0738 26.1915 11.8921 24.6785 387 8.8768 13.7874 387.5 9.4574 13.7113 11.3155 26.7013 11.6029 25.3941 388 10.7371 12.6731 8.7461 14.5262 9.4072 26.6901 389 6.5629 12.4958 6.6383 23.5603 7.6459 25.2276 8.9327 21.9978 7.4213 19.5171 390 392 393 7.2709 21.9978 8.7319 13.8956 394 10.3261 16.1100 14.8135 22.1929 14.1709 16.0327 10.1253 17.7908 395 480 505 70 APPENDIX C CARBONATE OXYGEN AND CARBON ISOTOPE DATA 71 depth δ13Ccarbonate δ18Ocarbonate(VPD) cm ‰ ‰ 376.5 -4.51 -2.48 377 -4.67 -2.53 377.5 -4.25 -2.87 378 -4.20 -2.60 378.5 379 -3.87 -2.53 379.5 -3.89 -2.52 380 -3.89 -2.52 380.5 -3.85 -2.23 381 -3.65 -3.08 381.5 382 -4.46 -1.75 382.5 -4.09 -1.11 383 -4.00 -1.54 383.5 -4.06 -2.41 384 -2.89 -2.69 384.5 -2.44 -2.47 385 -2.58 -2.48 385.5 -2.69 -3.02 386 -3.71 -3.40 386.5 -4.26 -3.30 387 -4.02 -3.61 387.5 -3.98 -3.36 388 -4.61 -4.08 388.5 -4.58 -3.65 389 -6.59 -3.92 389.5 -3.91 -3.01 390 -4.16 -2.37 390.5 -3.89 -2.64 391 -4.26 -2.98 391.5 -4.05 -2.51 392 -3.86 -2.58 392.5 -3.09 -1.86 393 -3.26 -2.36 72 depth δ13Ccarbonate δ18Ocarbonate(VPD) cm ‰ ‰ 393.5 -3.34 -1.50 394 -3.64 -1.65 394.5 -3.54 -1.12 395 -3.60 -1.16 395.5 -3.61 -1.44 396 -2.79 -0.49 396.5 -2.95 -1.17 397 -4.38 -1.45 397.5 -3.96 -2.68 398 398.5 -3.38 -2.60 399 -4.06 -2.56 399.5 -3.77 -2.97 400 -4.13 -2.57 400.5 -4.48 -2.13 401 -4.07 -2.27 405 -3.58 -2.61 410 -2.26 -3.58 415 -4.50 -1.57 420 -4.09 -3.55 425 -2.74 -3.00 430 -3.97 -1.14 435 -4.01 -2.90 440 -6.78 -3.26 445 -4.00 -2.34 450 -4.40 -3.04 455 -3.69 -2.23 460 -3.58 -2.39 465 -5.71 -3.38 470 -4.63 -1.30 475 -4.27 -1.43 480 -3.69 -2.81 485 -4.62 -1.81 490 -5.64 -2.27 495 -5.30 -3.32 500 -3.81 -2.99 73 depth δ13Ccarbonate δ18Ocarbonate(VPD) cm ‰ ‰ 505 -3.78 -2.84 510 515 -1.61 -2.50 520 -3.76 -4.64 525 530 -7.10 -3.92 535 -7.04 -4.13 540 -6.89 -3.61 545 550 -4.16 -1.55 555 -6.29 -3.48 560 -7.87 -2.60 565 -2.64 -3.23 570 -4.14 -2.44 575 -4.73 -3.21 580 585 590 595 600 605 610 -6.22 -0.41 615 -5.03 0.22 620 -6.47 -1.05 625 630 -5.60 -2.45 74 APPENDIX D TOTAL CARBON AND INORGANIC CARBON DATA 75 depth cm 376.5 377 377.5 378 378.5 379 379.5 380 380.5 381 381.5 382 382.5 383 383.5 384 384.5 385 385.5 386 386.5 387 387.5 388 388.5 389 389.5 390 390.5 391 391.5 392 392.5 393 TIC % 7.70 7.98 3.79 8.63 2.30 9.35 8.03 8.40 0.01 8.78 8.83 9.21 8.13 8.28 8.61 8.42 7.75 8.45 9.24 9.58 8.27 9.75 9.14 9.59 9.63 9.81 9.52 9.43 9.93 9.44 9.33 8.57 6.51 6.25 TOC % 0.00 0.00 1.70 0.00 6.71 0.00 1.17 0.00 9.31 0.00 1.89 0.96 1.65 2.22 1.28 1.15 2.35 1.33 0.52 0.53 1.96 0.86 1.19 0.00 0.00 0.87 1.32 1.01 0.65 1.33 0.82 1.34 2.00 1.46 76 depth cm 393.5 394 394.5 395 395.5 396 396.5 397 397.5 398 398.5 399 399.5 400 400.5 401 405 410 415 420 425 430 435 440 445 450 455 460 465 470 475 480 485 490 495 500 TIC % 7.19 7.34 7.84 8.08 8.48 7.96 8.56 8.54 9.23 9.39 9.59 9.45 9.24 9.28 9.59 9.10 8.33 9.53 9.16 9.36 8.38 8.67 9.00 8.87 7.84 9.60 9.38 8.79 8.87 5.29 8.36 8.71 8.04 8.92 8.77 7.58 TOC % 0.00 0.93 1.65 0.58 1.28 2.44 0.47 1.86 0.33 0.21 0.10 0.64 0.97 0.00 0.00 0.74 1.53 0.00 0.00 0.89 0.98 0.00 1.93 1.02 0.00 0.29 0.66 0.67 0.40 0.34 1.40 0.39 0.81 0.98 1.11 0.00 77 depth cm 505 510 515 520 525 530 535 540 545 550 555 560 565 570 575 580 585 590 595 600 605 610 615 620 625 630 TIC % 8.56 8.62 7.81 9.58 7.78 9.07 8.05 6.83 9.75 2.84 4.88 6.39 8.10 7.21 9.20 6.83 3.85 8.97 5.41 8.04 8.10 3.21 0.96 3.42 7.22 8.02 TOC % 1.45 0.09 0.50 0.99 0.84 0.00 0.00 1.03 0.56 0.00 0.08 0.72 0.26 0.70 2.08 2.26 0.58 1.03 1.10 0.21 0.68 0.47 0.15 0.93 1.26 1.26 78 REFERENCES 79 REFERENCES Abu-Zeid, M.M., Baghdady, A.R., and El-Etr, H.A., 2001, Textural attributes, mineralogy and provenance of sand dune fields in the greater Al Ain area, United Arab Emirates: Journal of Arid Environments, v.48, p.475-499. Ali, A.H.A., 1992, Wind meteorology of the summer shamal in the Arabian Gulf region, M. A. thesis, Boston University. Anadón P, Moscariello A, Rodrı´guez-La´zaro J, and Filippi M.L, 2006, Holocene environmental changes of Lake Geneva (Lac Le´man) from stable isotopes (δ13C, δ18O) and trace element records of ostracod and gastropod carbonates: Journal of Paleolimnology, v.35, p.593–616. Araguás-Araguás, L., Froehlich, K., and Rozanski, K., 2000, Deuterium and oxygen-18 isotope composition of precipitation and atmospheric moisture: Hydrologic Processes, v.14, p.1341-1355. Benson, L.V., 1994, Stable isotopes of oxygen and hydrogen in the Truckee RiverPyramid Lake surface-water system. 1. Data analysis and extraction of paleoclimatic information. Limnology and Oceanography, v.39.2, p.344-355. Bond, G.C., Showers, W., Cheseby, M., Lotti, R., Almasi, P., deMenocal, P., Priore, P., Cullen, H., Hajdas, I., and Bonani, G., 1997, A pervasive millennial-scale cycle in North Atlantic Holocene and Glacial climates: Science, v.278, p.1257-1266. Bottema, S., 1986, A late Quaternary pollen diagram from Lake Urmia (Northwestern Iran): Review of Palaeobotany and Palynology, v.47, p.241-261. Burns, S.J., Fleitmann, D., Matter, A., Neff, U., and Mangini, A., 2001, Speleothem evidence from Oman for continental pluvial events during interglacial periods: Geology, v.29.7, p.623-626. Butlin, R., Schön, I., and Martens, K., 1998, Asexual reproduction in nonmarine ostracods: Heredity, v.81, p.473-480. 80 Caporaletti, M., 2011, Ostracods and stable isotopes: proxies for palaeoenvironmental reconstructions: Joannea Geologie und Paläontologie, v.11, p.345-359. Chang, J.H., 1967, The Indian summer monsoon: Geographical Review, v. 57.3, p.373396. Chou, C., and Lan, C.W., 2012, Changes in the annual range of precipitation under global warming: Journal of Climate, v.25, p.222-235. Chivas, A.R., De Deckker, P., and Shelley, J.M.G., 1986, Magnesium and strontium in non-marine ostracod shells as indicators of paleosalinity and paleotemperature: Hydrobiologia, v.143, p.135-142. Cullen, H. M., deMenocal. P.B., Hemming, S., Brown, F.H., Guilderson, T., and Sirocko. F., 2000, Climate change and the collapse of the Akkadian Empire; Evidence from the deep sea: Geology, v.28, p.379–382. Dansgaard, W., 1964, Stable isotopes in precipitation: Tellus, v.16, p.436-468. De Decker, P., and Forester, R.M., 1988, The use of ostracods to reconstruct continental paleoenvironmental records in De Deckker, P., Colin, J.P., Peypouquet, J.P., (eds.), Ostracoda in the Earth Sciences, Elsevier, Amsterdam, p.175-199. Dearing, J., 1999, Magnetic susceptibility in Walden, J., Oldfield, F. and Smith, J. P. (eds.), Environmental magnetism: a practical guide. Technical Guide, No. 6, p. 35-62, Quaternary Research Association, London, UK. Djamali M., De Beaulieu, J.L., Miller, N.F., Andrieu-Ponel, V., Ponel, P., Lak, R., Sadeddin, N., Akhani, H., and Fazeli, H., 2009, Vegetation history of the SE section of the Zagros Mountains during the last five millennia; a pollen record from the Maharlou Lakes, Fars Province, Iran: Vegetation History Archaebotany, v.18. p.123-136. Fleitmann, D., et al., 2007, Holocene ITCZ and Indian monsoon dynamics recorded in stalagmites from Oman and Yemen (Socotra): Quaternary Science Review, v.26, p.170–188. Geddes, M. C., De Deckker, P., Williams, W. D., Morton, D. W. and Topping, M., 1981, On the chemistry and biota of some saline lakes in Western Australia: Hydrobiologia, v.82, p.201-222. 81 Glennie, K.W., and Singhvi, A.K., 2002, Event stratigraphy, paleoenvironment and chronology of SE Arabian deserts: Quaternary Science Reviews, v.21, p.853–869. Gong, D.Y., and Ho., C.H., 2001, The Siberian high and climate change over middle to high latitude Asia: Theoretically Applied climatology, v.71, p.1-9. Griffiths, H.I., Stevens, L.R., and Schwalb, A., 2001. Environmental change in the south western Iran: the Holocene ostracod fauna of Lake Mirabad: The Holocene, v.11, p.757-764. Hanor, J.S., 2000, Barite–celestine geochemistry and environments of formation. In Alpers, C.N., Jambor, J.L., Nordstrom, D.K. (Eds.), Reviews in Mineralogy and Geochemistry vol. 40, Sulfate Minerals: Cristallography, Geochemistry and Environmental Significance, p. 193–275. Hardie, L.A., and Eugster, H.P., 1970, The evolution of closed basin brines: Minerological Society of America Special Publication, v.3, p.273-290. Hayward, B.W., Grenfell, H.R., Nicholson, K., Parker, R., Wilmhurst, J., Horrocks, M., Swales, A., Sabaa A.T., 2004, Foraminiferal record of human impact on intertidal estuarine environments in New Zealand's largest city: Marine Micropaleontology, v.53, p.37-66. Heip, C., 1976, The life-cycle of Cyprideis torosa (Curstacea, Ostracoda): Oecologia (Bed), v.24, p.229-245. Holmes, J.A., 1996, Trace-element and stable-isotope geochemistry of non-marine ostracod shells in Quaternary palaeoenviromental reconstruction: Journal of Paleolimnology, v.15, p.223-235. Hunt, P.C., and Jones J.W., 1972, The effect of water level fluctuation on littoral fauna: Journal of Fish Biology, v.4, p.385-394. Kendall, A.C., 1979, Continental and supratidal (sabkha) evaporites in Facies Models: Geosciences Canada, Canada, Reprint Set., v.1, p.145-158. Kendrew, W.G., 1961, The Climate of the Near East in The Climates of the Continents: 5th ed. Oxford University Press, London. p.608. Kelts K. and Hsu K. 1978, Freshwater carbonate sedimentation in Lerman A. (ed.), Lakes: Physics, Chemistry and Geology. Springer, New York, p.295–323. 82 Land, L.S., 1980, The isotopic and trace element geochemistry of dolomite: the state of the art: The Society of Economic Paleontologist and Mineralogist, Special Publication, v. 28, p.87-110. Leng, M.J., and Marshall, J.D., 2004, Palaeoclimate interpretation of stable isotope data from lake sediments: Quaternary Science Reviews, v.23, p.811-831. Li, H.C., and Ku, T.L., 1997, δ13C–δ18O covariance as a paleohydrological indicator for closed-basin lakes: Palaeogeography, Palaeoclimatology, Palaeoecology, v.133, p.69–80. Martens, K., Schön, I., Meisch, C. and Horne, DJ., 2008, Global biodiversity of nonmarine Ostracoda (Crustacea): Hydrobiologia, v.595, p.185-193. McCarten, L., Plummer, L.N., Hosterman, J.W., Busenberg, E., Dwornik, E.J., Duerr, A.D., Miller, R.L., and Kiesler, J.L., 1988, Celestine(SrSO4) in Hardee and De Soto Counties, Florida: U.S. Geological Survey Circular 1059, p.129-137. Mees, F., Castaňeda, C., and Van Ranst, E., 2011, Sedimentary and diagenetic features in saline lake deposits of the Monegros region, northern Spain: Catena, v. 85, p. 245–252. Motamedi H,, Sepehr M,, Sherkati S,, Pourkermani, M., 2011, Multiphase hormuz salt diapirism in the Southern Zagros, SW Iran: Journal of Petroleum Geology, v.34, p.29-44. Müller G., Irion, G., and Főrstner, U., 1972, Formation and digenesis of inorganic Ca-Mg carbonates in the lacustrine environment: Naturwissenschaften, v.59, p. 158-164. Overpeck, J., Anderson D., Trumbore S., and Prell W., 1996, The southwest Indian Monsoon over the last 18,000 years: Climate Dynamics, v.12, p.213-225. Parker A., Goudie, A.S., Stokes, S., White, K., Hodson, M.J., Manning, M., and Kennet, D., 2006, A record of Holocene climate change from lake geochemical analyses in southeastern Arabia: Quaternary Research, v. 66, p. 465-476. Rieradevall, M., and Roca, J.R., 1995, Distribution and population dynamic of ostracodes (Crustacea, Ostracoda) in a karstic lake: Lake Banyoles (Catalonia, Spain): Hydrobiologia, v.310, p. 189-196. 83 Rozanski, K., Araguás- Araguás, L, and Gonfiantini, R., 1992, Relation between longterm trends of oxygen-18 isotope composition of precipitation and climate: Science, v.258, p.981-985. Ruddiman, W., F., 2000, Orbital-Scale Climate Change in Earth's Climate: Past and Future, 2nd edition, W. H. Freeman, New York, p. 388. Sabins, F.F., 1962, Grains of detrital, secondary, and primary dolomite from Cretaceous Srata of the Western Interior: Geological Society of America Bulletin, v.73, p. 1183-1196. Schubert, W.H., and Ferreira, R.N., 1997, Barotropic Aspects of ITCZ breakdown: American Meteorological Society, v.54, p.261-285. Sirocko, F., Garbe-Schonberg, D., McIntyre, A., and Molfino, B., 1996, Teleconnections between the subtropical monsoons and high-latitude climates during the last deglaciation: Science, v. 272, p. 526–529. Starr, V.P., 1948, An essay on the general circulation of the Earth's atmosphere: Journal of Meteorology, v.5, p. 39-44. Stephens, G.L., and Hu., Y., 2010, Are climate-related changes to the character of globalmean precipitation predictable?: Environmental Research Letters. v.5, p.1-7. Stevens L.R., Wright, H.E., and Ito, E., 2001, Proposed changes in seasonality of climate during the late glacial Holocene at Lake Zeribar, Iran: The Holocene, v.11, p.747755. Stevens, L.R., Ito, E., Schwalb, A., and Wright Jr., H.E., 2006, Timing of atmospheric precipitation in Zagros Mountains inferred from a multi-proxy record from Lake Mirabad, Iran: Quaternary Research, v.66, p.494-500. Stevens, L.R., Djamali, M, Beaulieu, J.L, and Andrieu-Ponel, V., 2012, Hydroclimatic variations over the last two glacial/interglacial cycles at Lake Urmia, Iran: Paleolimnology, v.47, p. 645-660. Stuiver, M., and Reimer, P. J., 1993, Extended 14C database and revised CALIB radiocarbon calibration program: Radiocarbon, v.35, p.215-230. Taha, M.F., Harb, S.A., Nagib, M.K. and Tantawy, A.H.,1981., The climate of the Near East in Climates of Southern and Western Asia; World Survey of Climatology, v.9, eds Takahasi, K. & Arakawa, H. Elsevier, Amsterdam, p.183-255. 84 Talbot, M.R., 1990, A review of the palaeohydrological interpretation of carbon and oxygen isotopic ratios in primary lacustrine carbonates: Chemical Geology (Isotope Geoscience Section), v.80, p. 261-279. Talbot, C.J., and Jarvis, R. J., 1984, Age, budget and dynamics of an active salt extrusion in Iran: Journal of Structural Geology, v.6, p.521-533. Talebian, M., Jackson, J., 2002, Offset on the main recent fault of NW Iran and implications for the late Cenozoic tectonics of the Arabia-Eurasia collision zone: Geophysical Journal International, v.150, p.422-439. Tarutani T., Clayton R.N., and Mayeda T.K., 1969, The effect of polymorphism and magnesium substitution on oxygen isotope fractionation between calcium carbonate and water: Geochimica et Cosmochimica Acta, v.33, p.987-996. van Zeist, W., and Bottema, S., 1977, Palynological investigations in western Iran: Palaeohistoria, v.19, p.19-95. Warren, J.K., 1999, Evaporites in Their Evolution and Economics: Blackwell, Oxford, UK, 438 pp. Xia, J., Ito, E., and Engstrom, D.R., 1997, Geochemistry of ostracode calcite: Part 1. An experimental determination of oxygen isotope fractionation: Geochimica et Cosmochimica Acta, v.61, p.377-382. 85