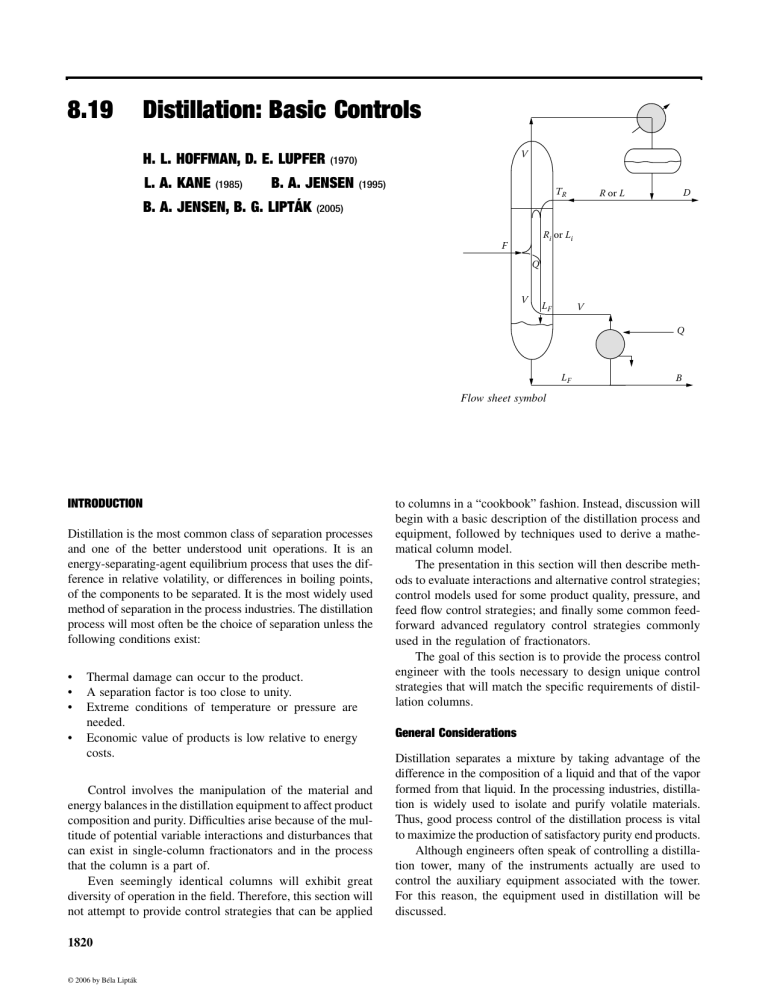
8.19 Distillation: Basic Controls H. L. HOFFMAN, D. E. LUPFER L. A. KANE (1985) B. A. JENSEN B. A. JENSEN, B. G. LIPTÁK V (1970) (1995) TR D R or L (2005) Ri or Li F Q V LF V Q LF B Flow sheet symbol INTRODUCTION Distillation is the most common class of separation processes and one of the better understood unit operations. It is an energy-separating-agent equilibrium process that uses the difference in relative volatility, or differences in boiling points, of the components to be separated. It is the most widely used method of separation in the process industries. The distillation process will most often be the choice of separation unless the following conditions exist: • • • • Thermal damage can occur to the product. A separation factor is too close to unity. Extreme conditions of temperature or pressure are needed. Economic value of products is low relative to energy costs. Control involves the manipulation of the material and energy balances in the distillation equipment to affect product composition and purity. Difficulties arise because of the multitude of potential variable interactions and disturbances that can exist in single-column fractionators and in the process that the column is a part of. Even seemingly identical columns will exhibit great diversity of operation in the field. Therefore, this section will not attempt to provide control strategies that can be applied 1820 © 2006 by Béla Lipták to columns in a “cookbook” fashion. Instead, discussion will begin with a basic description of the distillation process and equipment, followed by techniques used to derive a mathematical column model. The presentation in this section will then describe methods to evaluate interactions and alternative control strategies; control models used for some product quality, pressure, and feed flow control strategies; and finally some common feedforward advanced regulatory control strategies commonly used in the regulation of fractionators. The goal of this section is to provide the process control engineer with the tools necessary to design unique control strategies that will match the specific requirements of distillation columns. General Considerations Distillation separates a mixture by taking advantage of the difference in the composition of a liquid and that of the vapor formed from that liquid. In the processing industries, distillation is widely used to isolate and purify volatile materials. Thus, good process control of the distillation process is vital to maximize the production of satisfactory purity end products. Although engineers often speak of controlling a distillation tower, many of the instruments actually are used to control the auxiliary equipment associated with the tower. For this reason, the equipment used in distillation will be discussed. 8.19 Distillation: Basic Controls DISTILLATION EQUIPMENT Condenser There are some basic variations to the distillation process. One such basic difference is between continuous and batch distillation. The main difference between these processes is that in continuous distillation the feed concentration is relatively constant, while in batch distillation it is rich in light components at the beginning and lean in light components at the end. While batch distillation is also described in this section, the emphasis is on the continuous processes. Another basic difference is in the way the condenser heat is handled. The more common approach is to reject that heat into the cooling water and thereby waste it. This necessitates the use of “pay heat” at the reboiler, which usually is a large part of the total operating cost of the column. An alternate approach, also discussed in this section, is “vapor recompression” (Figure 8.19a), in which the heat taken out by the condenser is reused at the reboiler after a heat pump (compressor) elevates its temperature. While vapor recompression controls are also discussed in this section, the emphasis is on the traditional air- or water-cooled condenser designs. The Column The primary piece of distillation equipment is the main tower. Other terms for this piece of equipment are column and fractionator, and all three terms are used interchangeably. The tower, column, or fractionator has two purposes: First, it separates a feed into a vapor portion that ascends the column and a liquid portion that descends; second, it achieves intimate D Removed heat wasted D M F F B Pay heat added Compr. Work B Recovered heat FIG. 8.19a In contrast with conventional distillation, the vapor recompression system uses recovered heat. © 2006 by Béla Lipták 1821 Column Accumulator Feed pump Reflux pump Preheater Reboiler FIG. 8.19b Distillation equipment. mixing between the two countercurrent flowing phases. The purpose of the mixing is to get an effective transfer of the more volatile components into the ascending vapor and corresponding transfer of the less volatile components into the descending liquid. The other equipment associated with the column is shown schematically in Figure 8.19b. In continuous distillation, the feed is introduced continuously into the side of the distillation column. If the feed is all liquid, the temperature at which it first starts to boil is called the bubble point. If the feed is all vapor, the temperature at which it first starts to condense is called the dew point. The feed entering the column is normally operated in a temperature range that is intermediate to the two extremes of dew point and bubble point. However, some optimization strategies may call for designs where the feed is either superheated or subcooled. For effective separation of the feed, it is important that both vapor and liquid phases exist throughout the column. The separation of phases is accomplished by differences in vapor pressure, with the lighter vapor rising to the top of the column and the heavier liquid flowing to the bottom. The portion of the column above the feed is called the rectifying section and below the feed is called the stripping section. Packing and Trays The intimate mixing is obtained by one or more of several methods. A simple method is to fill the column with lumps of an inert material, or packing, that will provide surface for the contacting of vapor and liquid. Another effective way is to use a number of horizontal plates, or trays, which cause the ascending vapor to be bubbled through the descending liquid (Figure 8.19c). 1 Tray designs are numerous and varied. Tray designs include bubble cap plate unit, valve, sieve plate, tunnel, dual-flow, chimney, disc-and-donut, turbogrid trays, v-grid, Perform-Kontakt, Haselden baffle tray, Kittel trays, and other specialty-type units. Dualflo® trays, Flexitray®, Varioflex®, Bi-Frac®, Max-Frac®, NYE Trays®, Superfrac® trays, Super-Flux® trays, and Ultra-Frac® trays are specialty registered tray designs from different manufacturers that are variations of the aforementioned tray designs. Bubble caps 1822 Control and Optimization of Unit Operations • L • • L • L FIG. 8.19c Intimate contact and therefore equilibrium is obtained as the vapor bubbles ascend through the liquid held up on each tray, as the liquid descends down the column. and sieve trays are the most common designs used in distillation applications. 2 Many different types of packings are available. They are normally classified as random or stacked. Random packings are those that are dumped into the containing shell. Raschig rings, Berl saddles, Intalox saddles, and Pall rings are the most common random packings and come in various sizes from 1/2 to 31/2 in (1.25 to 9 cm). Stacked packings, also known as grid or stacked packing, include large-sized Raschig rings and Lessing rings. Packings generally give lower pressure drops at the cost of higher installation costs. They are made of ceramic, plastic, or metal, depending upon the type of packing and the intended application. Other packings such as Maspac®, HyPak®, Tellerette®, IMTP® FLEXIPAC® KATAMAX®: FLEXIGRID®-2, -3, and -4, and KOCH-GLITSCH GRID® EF-25A are specialty registered packings from different manufacturers that are just variations of the aforementioned packings. When deciding between the use of trays and packing, the 3 following factors should be considered: • • • • • • • Because of liquid dispersion difficulties in packed towers, the design of plate towers is considerably more reliable and requires less safety factor when the ratio of liquid mass velocity to gas mass velocity is low. Towers using trays can be designed to handle wider ranges of liquid rates without flooding. Towers using trays are more accessible for cleaning. Towers using trays are preferred if interstage cooling or heating is needed because of lower installation costs of delivery piping. Towers using trays have a lower total dry weight, though total weight with liquid hold-up is probably equal. Towers using trays are preferred when large temperature changes are expected because of thermal expansion or when contraction may crush packing. Design information for towers using trays is generally more readily available and more reliable. © 2006 by Béla Lipták • Packed towers are cheaper and easier to construct than plate towers if highly corrosive fluid must be handled. Diameters of packed towers are generally designed to be less than 4 ft, while plate tower diameters are designed to be more than 2ft. Packed towers are preferred if the liquids have a large tendency to foam. The amount of liquid hold-up is considerably less in packed towers. The pressure drop through packed towers may be less than for plate towers performing the same service, making packed towers desirable for vacuum distillation. Thus, generally, trays work better in applications requiring high flow, such as those encountered in high-pressure distillation columns, such as depropanizers, debutanizers, xylene purification columns, and the like. Packing works best at lower flow parameters, as the low-pressure drop of structured packing makes it very attractive for use in vacuum columns or ethylbenzene recycle columns of styrene plants. The contacting between the vapor and liquid in a singlestage contacting device will not produce total equilibrium. The relationship between ideal and actual performance is the efficiency that translates the number of ideal separation stages into actual finite stages that must be used to accomplish the desired final separation. Efficiency varies, not only with the type of mixing method used (e.g., packing or trays), but also with fluid rates, fluid properties, column diameter, and operating pressure. The influence of plate efficiency in the operation of the distillation tower becomes important in the control of the overhead composition. Because plate efficiencies increase with increased vapor velocities, the influence of the refluxto-feed ratio on overhead composition becomes a nonlinear relationship. Dynamics Dynamic considerations due to liquid hold-up on the trays comes into play when discussing distillation control. Because the liquid on each tray must overflow its weir and work its way down the column due to tray or packing hydraulics, this change will not be seen at the bottoms of the tower until some time has passed. The exact dynamics depend on column size, type of tray, number of trays, and tray spacing. The hold-up at each tray as shown in Figure 8.19c can be modeled by the LaPlace transform of the form KG ( s ) = where KG(s) K T1 S = = = = K (T1 s + 1) transfer function system gain time constant LaPlace transfer operator 8.19(1) 8.19 Distillation: Basic Controls X(t) Y1 Y 4 Y2 Y 1(t) Y1 Y40 0 X(t) Y 2(t) Y4(t) Y10(t) Y40(t) Y1 Y2 Y4 Y10 Y40 = = = = = 1 Lag 2 Lags 4 Lags 10 Lags 40 Lags Sum of the time constants are equal. Time These lags are cumulative as the liquid passes each tray on its way down the column. Thus, a 30-tray column could be approximated by 30 first-order exponential lags in series of approximately the same time constant. K (T1s + 1)n In that case, the condensers are called partial condensers. In this instance, a vapor product is normally withdrawn as well as a liquid product. A total condenser is usually designed for accumulator pressures up to 215 psia (1.48 MPa) at an operating temper4 ature of 120°F (49°C). A partial condenser is used from 215 psia to 365 psia (1.48 to 2.52 MPa), and a refrigerant coolant is used for the overhead condenser if the pressure is greater than 365 psia (2.52 MPa). Common condensers include fin fans and water coolers. However, in order to improve efficiency of heat recovery, heat exchange with another process stream is often performed. Propane is the most common refrigerant used. A pressure drop of 5 psia (34.4 KPa) across the condenser is often assumed if no measurements are available. The condenser and accumulator are the key pieces of equipment with respect to controlling pressure in the column. Reboilers FIG. 8.19d Response of nth-order lags to unit step change. KG (s) = 8.19(2) The liquid leaving the bottom of the column is reheated in a reboiler. A reboiler is a special heat exchanger that provides the heat necessary for distillation. Part of the column bottoms liquid is vaporized and the vapors are injected back into the column as boil-up. The remaining liquid is withdrawn as a bottom product or as residue. As shown in Figure 8.19e, reboilers come in widely varying designs. They can be internal, but most are external to the column. They can use natural or forced circulation. where n = 30 for a 30-tray column Figure 8.19d shows the response of nth order lags to a unit step change. The effect of increasing the number of lags in series is to increase the apparent dead time and increase the response curve slope. Thus, the liquid traffic within the distillation process is often approximated by using a secondorder lag plus dead time as modeled by the LaPlace transform: KG (s) = Ke − t s (T1s + 1)(T2s + 1) e = e of log to the base e φ = dead time T1, T2 = time constants Condensers The overhead vapor leaving the column is sent to a condenser and is collected as a liquid in a receiver, or accumulator. A part of the accumulated liquid is returned to the column as reflux. The remainder is withdrawn as overhead product or distillate. In many cases, complete condensation is not accomplished. V Q Q L B B Internal 8.19(3) where © 2006 by Béla Lipták 1823 External kettle V V Q Q B Vertical thermosyphon B Horizontal thermosyphon FIG. 8.19e Reboiler design variations. External kettle reboilers often use forced circulation (pump), while the thermosyphon designs depend on natural circulation. The horizontal thermosyphon reboiler takes its liquid from the bottom tray, while the others take it from the column bottoms. 1824 Control and Optimization of Unit Operations The kettle reboiler is the most common external forced circulation design. Vertical and horizontal thermosiphon reboilers operate by natural circulation. In these, flow is induced by the hydrostatic pressure imbalance between the liquid inside the tower and the two-phase mixture in the reboiler tubes. In forced circulation reboilers, a pump is used to ensure circulation of the liquid past the heat transfer surface. Reboilers may be designed so that boiling occurs inside vertical tubes, inside horizontal tubes, or on the shell side. A newer development in reboiler design is the concept self-cleaning shell-and-tube heat exchangers for applications where heat exchange surfaces are prone to fouling by the process fluid. Common heat sources include hot oil, steam, or fuel gas (fired reboilers). Cases where simple heat exchange with another process stream is used for efficiency of heat recovery are common. Thus, the choice of instrumentation to control heat addition to the tower depends upon the type of reboiler used. Interheaters/Intercoolers In some cases, additional vapor or liquid is withdrawn from the column at points above or below the point at which the feed enters. All or a portion of this sidestream can be used as intermediate product. Sometimes, economical column design dictates that the sidestream be cooled and returned to the column to furnish localized reflux. The equipment that does this is called a sidestream cooler, or intercooler. Multiproduct fractionators often have these intercoolers in a pumparound stream. At other times, localized heat is required. Then, some of the liquid in the column is removed and passed through a sidestream reboiler, or interheater, before being returned to the column. Interheaters are usually utilized in cryogenic demethanizers. Often the feed is preheated before entering the column. Common preheat mediums include the bottoms product or low-pressure steam. Preheating is often a convenient method to recover heat that would otherwise be wasted. Column Variables Controlling a fractionator requires the identifying of the controlled, manipulated, and load variables (Figure 8.19f). Controlled variables are those variables that must be maintained at a precise value to satisfy column objectives. These normally include product compositions, column temperatures, column pressure, and tower and accumulator levels. Manipulated variables are those variables that can be changed in order to maintain the controlled variables at their desired values. Common examples include reflux flow, coolant flow, heating medium flow, and product flows. Load variables are those variables that provide disturbances to the column. Common examples include feed flow rate and feed © 2006 by Béla Lipták L Overhead product (D) Feed Steam (V) Bottom product (B) Apparent variables: C1 C2 C3 C4 u1 u2 u3 u4 u5 u6 u7 u8 u9 m = = = = = = = = = = = = = = Independent variables overhead temperature overhead pressure overhead composition overhead flow rate bottom temperature bottom pressure bottom composition bottom flow rate feed temperature feed pressure feed composition feed per cent vapor feed flow rate steam flow rate (heat input) 2 1 2 1 2 1 1 1 11 FIG. 8.19f In a binary distillation process the number of independent variables is eleven (11) and the number of defining equations is two (2). Therefore, the number of degrees of freedom is nine (9), which is the maximum number of automatic controllers that can be used on such a process. composition. Other common disturbances are steam header pressure, feed enthalpy, environmental conditions (e.g., rain, barometric pressure, and ambient temperature), and coolant temperature. To handle these disturbances, column controls can be so designed as to make the column insensitive to these disturbances, or secondary controls can be designed to eliminate the disturbances. It is also important to evaluate the expected magnitude and duration of the likely disturbances, so that proper control system scaling and tuning can be achieved. Feedforward controls are designed to compensate for these disturbance variables and are discussed later in this section. There are other advanced control or optimization methods that can be designed to compensate for these disturbance variables. They are discussed in Section 8.21. Pairing of Variables The variables that should be controlled are usually obvious. They are normally identified when process objectives are defined and understood. Load variables are also easily identified. But identification of the manipulated variables can be more difficult. The general guidelines for identifying which manipulated variables to associate with which controlled variables are 8.19 Distillation: Basic Controls • • • • • Manipulate the stream that has the greatest influence on the associated controlled variable. Manipulate the smaller stream if two streams have the same effect on the controlled variable. Manipulate the stream that has the most nearly linear correlation with the controlled variable. Manipulate the stream that is least sensitive to ambient conditions. Manipulate the stream least likely to cause interaction problems. Unfortunately, the decision on pairing controlled and manipulated variables is complicated by the fact that the above rules may sometimes result in conflicting recommendations. Section 8.20 provides information on relative gain calculations, which can help to optimize the pairing of controlled and manipulated variables. Once the pairings are completed, the equations are then solved for the manipulated variables in terms of the controlled and load variables. In that form, the equations are the mathematical representations of the control systems. MODELING AND CONTROL EQUATIONS The primary application of instruments in distillation is to control the product purity, and secondarily, to minimize upsets to the unit caused by a change in process inputs. The instruments calculate the effects of the input changes and determine the corrective action needed to counteract them. The control actions are implemented by direct manipulation of the final control elements or by alteration of the set points of lower level controllers. A careful analysis of limits and operating constraints is essential to the successful control of distillation columns. If the system is not designed to provide limit checks and overrides to handle operating limits, frequent operator intervention will be required during upsets. This is likely to result in a lack of confidence in the control system and will cause the operators to remove the column from automatic control more often than necessary, thereby not only reducing the effectiveness of the system, but also reducing safety. The first step in the design of a good control system is the derivation of a process model. Knowing the defining equations, the manipulated variables can be selected, and the operating equations for the control system can be developed. The instrumentation is then selected for the correct solution of these equations. The final control system can be relatively simple or can be a complex, interacting, multicomponent, computer-based system. In the discussion that follows, the procedures for designing distillation controls is followed by examples of the more common applications in distillation column control. A more detailed discussion of alternative strategies and advanced distillation column controls will be presented in Section 8.21. © 2006 by Béla Lipták 1825 Steady-State Model The first step in the design of a control system must be the development of a process model. Frequently omitted in simple distillation columns, this step is essential to minimize the need for field reconfiguration of control strategies. Even with easily reconfigurable process automation systems (PASs), the development of the model is essential to fully understanding the process. The model defines the process with equations developed from the material and energy balances of the unit. A common simplifying assumption is that all components of the feed have equal heats of vaporization, which leads to the assumption of equimolal overflow. Most shortcut fractionation calculations are based upon this underlying assumption. The model is kept simple by the use of one basic rule: The degrees of freedom limit the number of controlled variables (product compositions) specified in the equations, as was illustrated in connection with Figure 8.19f. Some of the variables that can be manipulated to control a column are shown in Figure 8.19g. Material Balance For example, for a given feed rate only one degree of freedom is available for material balance control. If overhead product (distillate) is a manipulated variable (controlled directly to maintain composition), then the bottom product cannot be independent but must be manipulated to close the overall material balance according to the following equations: F=D+B Accumulation = Inflow − Outflow Accumulation = F − (D + B) 8.19(4) 8.19(5) 8.19(6) Because accumulation is zero at steady state, B is dependent upon F and D, as expressed by Equation 8.19(4): B=F−D 8.19(7) V Heat removed Pressure Reflux rate (L) Feed temperature, Distillate rate (D) Composition (Y) Composition (Z) and rate (F) Heat added (boilup) Bottom rate (B) Composition (X) FIG. 8.19g Variables that fix the distillation operation. 1826 Control and Optimization of Unit Operations or if the bottoms product is the manipulated variable: D=F−B Di = V − Li 8.19(8) Li = L[1 + (Cp/∆H) × (To − Tr)] If the compositions of the feed, distillate product, and bottoms product are known, then the component material balance can be solved: 8.19(9) 8.19(10) 8.19(11) V = VB + VF × F F (z) where: %LLKF = lighter than light key in the feed (mol%) %LKF = light key in the feed (mol%) %LLKD = lighter than light key in the distillate product VB = QB/∆H (mol%) %LKD = light key in the distillate product (mol%) %HKD = heavy key in the distillate product (mol%) %LKB = light key in the bottoms product (mol%) In the most general case, the feed might have four components, having the concentrations of LLKF , LKF , HKF , and HHKF . Three of these components appear in each of the bottom and overhead products. The separation of the column is fixed by specifying the heavy key component in the overhead product HKD and the concentration of the light key component in the bottom product LKB. Equations 8.19(9) to 8.19(11) assume no heavier than heavy key is found in the distillate and that no lighter than light key is found in the bottoms. Rearranging Equation 8.19(11) gives %LKD = (F • %LKF − B • %LKB)/D 8.19(12) Substituting Equation 8.19(8) into Equations 8.19(10) and 8.19(12) gives %LLKD = (F • %LLKF)/(F − Β) 8.19(13) %LKD = (F • %LKF − B • %LKB)/(F − B) 8.19(14) Substituting Equations 8.19(13) and 8.19(14) into Equation 8.19(9) to eliminate %LLKD and %LKD: B/F = (100 − %HK D − %LLK F − %LK F ) (100 − %HK D − %LK B ) 8.19(15) For a given feed composition and desired product compositions, only one bottoms-to-feed ratio, B/F (product split), will satisfy the overall and component material balances. By fixing the bottoms flow, the distillate flow will be fixed. © 2006 by Béla Lipták D(y) L @ Tr where: F = feed rate (the inflow) D = overhead rate (an outflow) B = bottoms rate (an outflow) 100 = %LLKD + %LKD + %HKD D × %LLKD = F × %LLKF F × %LKF = D × %LKD + B × %LKB QT To B = D = L − Lf − Li − QB − QT − VB − VF − ∆H − ∆HD − ∆HL − ∆HLi − Bi if no accumulation occurs in the column bottoms. Di if no accumulation occurs in the accumulator External reflux Liquid flow below feed tray Internal reflux Heat addition at bottom Heat removal at top Vapor boilup rate Vapor fraction in feed Heat of vaporization in reboiler Heat of condensation of distillate Heat of vaporization of reflux Heat of condensation of internal reflux Lf = Li + (1 − VF) × F QB Bi = Lf − VB B(x) Material balance: F = D + B separation is the energy/feed ratio of a column. For binary y(1 − x) process: S = x(1 − y) Separation should be controlled by the more pure product. FIG. 8.19h Energy balance equations can be used to describe the steady-state heat flow model of a distillation column. However, fixing a value of product split does not fix either the distillate or bottoms composition because many combinations of %LLKF , %LKF , %LKB, and %HKD could yield the same value of B/F. Energy Balance The energy balance and the separation obtained are closely related. Conceptually, product composition control can be thought of as a problem of the rate of heat addition QB at the bottom of the fractionator and the rate of heat removal QT at the top of the column. A series of energy balances produces additional equations. Figure 8.19h shows a steady5 state internal model of these equations. The vapor boil-up rate VB equals the heat QB added by the reboiler divided by the heat of vaporization (∆H) of the bottoms product: VB = QB /∆H 8.19(16) The vapor rate V above the feed tray equals the vapor boil-up rate plus the vapor entering with the feed (feed rate 8.19 Distillation: Basic Controls F times vapor fraction VF , provided the feed is neither subcooled nor superheated): V = VB + F × VF 8.19(17) The internal reflux rate, that is, the liquid at the top tray of the column is derived by a heat balance around the top of the tower. Assuming a steady-state heat balance where the heat into the tower equals the heat out: D × ( ∆ H D + C pD × Tt ) + LI × ( ∆ H LI + C pR × Tt ) being used by the control equation. Also, C pL and ∆HL should be calculated near the existing pressure and temperature of the external reflux. The liquid rate, LF , below the feed tray equals the internal reflux plus the liquid in the feed: LF = LI + (1 − VF) × F D = V − LI 8.19(18) + L × ( ∆ H L + C pL × To ) + LI × (C pL × Tt ) I where Cp = specific heat To = overhead vapor temperature (vapor at its dew point) L = external reflux Tr = external reflux temperature LI = internal reflux Tt = top tray temperature (liquid at its bubble point) Equation 8.19(18) reduces to: D × C pD × (Tt − To ) + LI × ∆H LI − L × ∆H L + L × C pL × (Tr − To ) = 0 The bottoms rate, B, equals the liquid rate, L, minus the boil-up, VB : B = L − VB Because the tower doesn’t always operate at steady state, it is essential to also account for the dynamics of the process. This necessitates extending the steady-state internal flow model and requires additional considerations. Figure 8.19i 6 shows the internal flow model that includes dynamics. Di = V − Li DA= Di − D QT or To or zI = L × [1 + K1 × ∆T] V = VB + VF × F 8.19(22) GT F GT & GB are second order lags GB 8.19(23) VB = QB/∆H 8.19(24) Note: This equation is valid for whatever units are used for C pL or ∆HL. Because specific heat and heat of vaporization are nearly always in mass units, care must be taken to account for density differences whenever volume units are © 2006 by Béla Lipták D Li = L[1 + (Cp/∆H) × (To − TR)] If a total condenser is employed, the composition of the internal reflux and external reflux are the same, i.e., ∆H LI = ∆H L , so the constant K2 = 1.0. Thus, LI [1 + K1 (TO − Tr )] L @ Tr 8.19(21) resulting in the equation L= 8.19(27) The criterion for separation is the ratio of reflux (L) to distillate (D) flows vs. the ratio of boil-up (V) to bottoms (B) flow rates. Manipulating reflux affects separation equally as well as manipulating boil-up, albeit in opposite directions. Consequently, only one degree of freedom exists to control separation. Thus, for a two-product tower, two equations define the process. One is an equation describing separation, and the other is an equation for material balance. LI × ∆H LI = L × ∆H L + L × C pL × (To − Tr ) 8.19(20) LpI /L = K2 × [1 + K1 × (Tpo − Tpr)] 8.19(26) Dynamic Model 8.19(19) Making a simplifying assumption that the tray temperature equals overhead vapor temperature (i.e., the dew point of the vapor equals the bubble point of the liquid; Tt = To) produces: C pL Li ∆H L = ⋅ 1.0 + ⋅ (To − Tr ) L ∆H Li ∆H L 8.19(25) The distillate rate, D, equals the vapor rate, V, above the feed tray minus the internal reflux: I + L × (C pL × Tr ) = D × ( ∆ H D + C pD × To ) 1827 QB FIG. 8.19i Dynamic internal flow model. DA & BA represent accumulations in the accumulator and the column bottoms respectively. Li = GB[GT Li + (1 − VF) × F) Bi = Li − VB BA = Bi − B B 1828 Control and Optimization of Unit Operations Because a change in the reflux rate must work its way down the column due to tray or packing hydraulics, this change will not be seen at the reboiler until some time has passed. The holdup at each tray has previously been modeled by the LaPlace transform of Equation 8.19(1). This Laplace transform can be converted to a simple first-order exponential lag equation of the form, which describes the response to a step change in input: −t Llag = L (1 − e ) 8.19(29) where − t1 −t 2 GB = φ1 (1 − e ) (1 − e ) −t3 −t 4 GT = φ2 (1 − e ) (1 − e ) φ1 and φ2 are the dead times while the orientation of separation for a given degree of separation is defined as Orientation of Separation = %HK D %LK B S= y(1 − x ) x (1 − y) 8.19(33) 8.19(34) where x = mole fraction of the key light component the distillate (%LKD) y = mole fraction of the key light component in the bottoms, (LKB) The relationship between separation (S) and the ratio of boil-up to feed (V/F) over a reasonable operating range is V/F = a + bS 8.19(35) where a and b are functions of the relative volatility, the number of trays, the feed composition, and the minimum V/F. The control system therefore computes V based on the equation: GB and GT are the solution to the LaPlace transform of Equation 8.19(3). Changes in boil-up rates are observed at the condenser in a matter of seconds. Normally, no dynamic terms are necessary for vapor streams, as the value of use of computing resources to that of the benefits by compensating for the dynamics is negligible. The liquid inventory in the condenser or associated accumulator will change during unsteady-state actions. In the unsteady state, the difference DI − D is the rate of accumulation of material in the accumulator. Similarly for the liquid inventory at the bottom of the tower (the kettle), the difference BI − B is the rate of accumulation: 8.19(30) 8.19(31) where DA is the accumulation in the overhead accumulator BA is the accumulation in the tower bottoms Separation Equations The control of product compositions for a fractionator is primarily a matter of control of the internal flows. In considering © 2006 by Béla Lipták (%LK D × %HK B ) 8.19(32) KB ) (%HK D × %LK The relationship between x (the light key component) and 7 the energy balance was developed by Shinskey as a function of separation S: These lags are cumulative as the liquid passes each tray on its way down the column. However, implementation of multiple first-order lags is impractical. Fortunately, it can be shown that multiple lags in series can be approximated by a dead time and a second-order exponential lag as shown by the LaPlace transform of Equation 8.19(3). For this reason, two dynamic terms (GT and GB) are included in Figure 8.19i. Equation 8.19(25) is then rewritten as DA = DII − D BA = BI − B Degree of Separation = ln e 8.19(28) where L is the liquid incoming to the tray Llag is the liquid leaving the tray t is the time constant L = GB[GT LI + (1 − VF) × F] product separation, the degree of separation and the orientation of separation are important. The degree of separation is y(1 − x ) V = F a + b x (1 − y) 8.19(36) Because y is held constant, the bottom composition controller adjusts the value of the parenthetical expression if an error should appear in x. Let V/F = y(1 − x)/(1 − y), and the control equation becomes: V = F(a + b[V/F])/x 8.19(37) where [V/F] = the desired ratio of boil-up to feed. Figure 8.19j illustrates four of the most common basic controls for the flows and levels of a two-product fractionator, where it is assumed that feed flow and tower pressure are held constant. A different set of the above control equations for controlling internal product flow rates will apply, depending upon the configuration of instrumentation used. Scaling The form of the control system equations influences the computing functions required. Boolean operands, such as high and low selectors, and dynamic functions, such as dead times, lead, and lag function, are also used. Most process automation systems have these basic computing function blocks. Implementation in a distributed control system (DCS), programmable 8.19 Distillation: Basic Controls PSP PC PSP PC LC LC D L FC D L FC FC FC DSP F 1829 DSP F LSP LSP QSP FC FC QSP BSP BSP LC LC FC FC Q Q B B Case 1 Case 2 PSP PC LC LC D L FC F D L FC FC FC LSP DSP F LSP DSP QSP QSP FC FC LC BSP LC FC BSP FC Q Q B Case 3 FIG. 8.19j Four cases of conventional distillation control configurations. © 2006 by Béla Lipták PSP PC B Case 4 Control and Optimization of Unit Operations Process values Normalized values Analog signals 1 2 3 4 Volts 50 mA dc 20 mA dc 10 20 30 40 4 8 12 16 1 2 3 4 3 6 9 12 mA dc 15 PSIG 0.2 0.4 0.6 0.8 1.0 bar 0 0.25 0.50 0.75 1.0 0 25% 50% 75% 100% 93 116 138 160 182°C 200 360°F 0 240 280 320 Temperature transmitter 0.85 1.7 2.93 0 225 5 100% L′ = 100% 75 L′ = 75% 50 L′ = 50% 25 L′ = 25% 3.4 m3/h 450 775 Linear flow transmitter 1.7 2.4 2.95 900 GPH 0 450 636 779 Differential pressure flow transmitter 900 GPH 0 2.50 3.75 1.25 Chromatograph output 0 1.143 L′ 5 Multiplier output, normalized manipulated variable (M′) 1830 3/h 3.4 m 0% 50 100% 25 75 Multiplier input, normalized ratio (R′) FIG. 8.19l Multiplier output for the solution of Equation 8.19 (39). % 5.0 FIG. 8.19k Common analog signals and their relationship to process variables. logic controller (PLC), or multivariable digital controllers is vendor-specific. The terms of the equations are sometimes scaled because most analog instruments and some PAS systems act on normalized numbers (0–100%) rather than on actual process values. With digital instrumentation and today’s process automation systems, those occurrences are rare. The calculations become easier for those systems operating in engineering units. Analog, and many digital, transmitters also operate on normalized values of the process variables. That is, the measurement signal will vary from 0 to 100% as the process variable shifts from 0 to its maximum value. Figure 8.19k illustrates the relationship among the various forms of analog signals and some typical process measurements. The actual value of a process measurement is found by multiplying the analog signal by the calibrated full-scale value (meter factor) of the process variable. In the examples of Figure 8.19k, the temperature, represented by a 75% analog signal, is 320°F (160°C), the linear flow is 775 gph 3 (2.93 m /h), the output of the differential pressure transmitter 3 (flow squared) is 779 gph (2.95 m /h), and the composition is 3.75%. Example As an example, let us review a flow ratio system in which the load stream, L, has the range of 0 to 1000 gpm 3 (0 to 3.79 m /h); the manipulated stream, M, has a range of © 2006 by Béla Lipták 3 0 to 700 gpm (0 to 2.65 m /h); and the ratio range, R, is 0 to 0.8 (R = M/L). 700M′ = (1000L′)(0.80R′) 8.19(38) Reducing to the lowest form, M′ = 1.143(L′)(R′) 8.19(39) The number 1.143 is the scaling factor. M′ is plotted as a function of L′ and R′ in Figure 8.19l. In applications such as the constant separation system, exact scaling is not critical. Exact scaling is when scaling constants must be used as calculated from instrument spans. The alternative is flexible scaling, where exact ranges are not needed but some arbitrary range is used to allow internal calculations to remain within range. The flexible scaling cannot be used (1) when compensation for feed composition is part of the model, (2) when narrow spans must be used for reasons of stability, and (3) when transmitter calibrations are inconsistent with material balance ratios. Exact scaling techniques must be used for these cases. MULTIPLE COMPONENT DISTILLATION With binary mixtures, only two products are removed in the distillation column. However, most separations involve multiple components. Even then, most distillations remove only two liquid products. In other applications a vapor product is removed, or multiple liquid products are drawn from the tower. Sometimes only one product is withdrawn at a time. 8.19 Distillation: Basic Controls 1831 Columns with Sidedraw Having a sidestream product in addition to the overhead and bottom products adds a degree of freedom to a control system. The source of this extra degree of freedom can be seen from the overall material balance equation: F=D+C+B 8.19(40) where C is the sidestream flow rate. Two of the product streams can be manipulated for control purposes, and the material balance can still be closed by the third product stream. The presence of this added degree of freedom makes the careful analysis of the process even more essential to avoid mismatching of the manipulated and controlled variables. As in the case of the previously discussed columns, the development of a control system for sidedraw applications also involves developing the process model and determining the relationship among the several controlled and manipulated variables. In this case, for a constant feed rate and column pressure, five degrees of freedom exist: three composition specifications and two levels that can manipulate three product flows, and two heat balances (V and L). Several possible combinations of variables are available and should be explored. The possible combinations of manipulated variables for the column in which the bottom composition and the sidestream composition must be controlled are Distillate and sidestream flows Distillate and bottom flows Distillate flow and heat input Sidestream and bottom flows Sidestream flow and heat input Bottom flow and heat input Similarly, the possible combinations of manipulated variables for the column in which the distillate composition and the sidestream composition must be controlled are: Distillate and sidestream flows Distillate and bottom flows Distillate flow and heat input Sidestream and bottom flows Sidestream flow and reflux Bottom flow and reflux LT X FY FIC V Dynamics FY FT FT FIC L Dynamics FY F, z1, z2 D, y1, y2 FT ARC AT C, c1, c2 Ratio RIC controller FT LT FT FIC LIC FY X ARC AT B, x1, x2 FIG. 8.19m Control of composition in two product streams with a sidedraw. The symbols z1, y1, and c1 refer to the concentrations in the feed, distillate, and sidestream of the component under control in the sidestream. The concentrations of the key component in the bottom are respectively expressed by z2, x2, and c2 for the feed, the bottoms, and the sidestream. The resulting control system is shown in Figure 8.19m. Note that in this configuration the ratio of heat input to feed (and, therefore, boil-up to feed) is held constant. Separate dynamic elements are used for the distillate loop and for the heat input and sidestream loops. Multiproduct Fractionators The equations are © 2006 by Béla Lipták LIC z −c D= F 1 1 y1 − c1 8.19(41) z − x2 C = F 2 c2 − x2 8.19(42) Multiproduct fractionators are most common in the refining industry where multicomponent streams are separated into many fractions. Examples of multiproduct fractionators are crude towers, vacuum towers, and fluidized catalytic cracking unit (FCCU) main fractionators. Product quality controls are used to adjust local column temperatures and sidedraw flow rates to control distillate properties related to the product specifications. An example is true boiling point (TBP) cut points. TBP cut points approximate the composition of a hydrocarbon mixture and are numerically similar to the American Society for Testing and 1832 Control and Optimization of Unit Operations SP FT FRC D PRC PT Accumulator FRC FT D L Q Q FRC FRC FT FT FT Main fractionation Heat balance logic FRC Boiling point calculation C FT FRC C F B FIG. 8.19n Control of product flows and pump-around refluxes. Materials’ (ASTM’s) 95%. The ASTM laboratory distillate evaluation method is the standard used in the petroleum refining industry for determining the value (composition) of the distillation products. A computer is required to calculate the product boiling point specification, such as 95% boiling point or TBP cut point on the basis of local temperature, pressure, steam flow, and reflux data. Local reflux is derived from internal liquid and vapor flows, as discussed previously, and the remaining variables are measured. Boiling point analyzers can be used to provide the measurement signals. If there is no analyzer, the calculated boiling points can be used by themselves, or if there is one, they can be used as a fast inner loop with analyzer trim. Because of the volume of liquid/vapor loads within most multiproduct fractionators, the manipulated variables that provide the greatest sensitivity and the quickest response are generally the product flows. Adjustment of reflux flows, as shown in Figure 8.19n, is an example of a heat balance control. The goal is to 8 maximize heat exchange to feed, subject to certain limits (limits and constraints are discussed as part of the subject of the optimization of distillation towers in Section 8.21). The task of maximizing the heating of the feed often simplifies to recovering heat at the highest possible temperature, which means recovering it as low as possible in the column. © 2006 by Béla Lipták Superfractionators The term superfractionator is applied to towers that are physically large. These distillation units separate streams having their light and heavy key relative volatilities quite close to each other. Included in this classification are deisobutanizers, which separate isobutane from normal butane; propylene splitters, which separate propane from propylene; ethylbenzene towers, which separate ethylbenzene from xylene; and xylene splitters, which separate para- and ortho-xylene from meta-xylene. Sometimes, the number of trays and subsequent height make it necessary to physically divide these towers into two or even three sections. Superfractionators have tremendous internal vapor-liquid rates in order to achieve the separation. Reflux-to-distillate ratios are very high, as are vapor-to-bottoms ratios. A large pressure drop through the tower also exists. Long dead times and lag times are experienced before any response is seen to feed rate or reflux changes. Generally, distillate compositions of superfractionators have to be controlled with material balance equations due to the lack of sensitivity of response. Batch Distillation In batch distillation (see Figure 8.19o), an initial charge of liquid is fed to a vessel, and the distillation process is initiated 8.19 Distillation: Basic Controls TC HIC V + LqL TT FT Yi FY X & Σ mD + Yi SP FC ARC 1833 Y AT D FY Distillate D(y) Reflux, L FRC D2 FT Distillate (D) Receiver Wi - Initial batch quantity yi - Initial product concentration v FC FT Steam, Q Wi( y2) x Figure 8.19p shows the control system that will accomplish this when the vapor rate from the batch column is maintained constant. The equation describing this operation is: L − LqL FIG. 8.19o Batch distillation. Y = mD + yi by turning on the heating and cooling systems. During the distillation process, the initial charge in the vessel continually depletes while building up the overhead product in the distillate receiver. Batch distillations are more common in smaller, multiproduct plants where the various products can only be manufactured at different times, and where a number of different mixtures may be handled in the same equipment. Equation 8.19(43) is the basic equation that describes this operation: W = Wi − Dt 8.19(43) where W = amount remaining in the bottoms Wi = the initial charge D = distillate rate t = time period of operation The basic objective of the control system of this type of separation is to keep the composition of the distillate constant. Other goals include keeping the distillate flow constant or maximizing the total distillate production. The main goal of a batch distillation is to produce a product of specified composition at minimum cost. This often means that operating time must be reduced to some minimum while product purity or recovery is maintained within acceptable limits. If product removal is too fast, separation and the quantity of the product are reduced. Conversely, if the product is withdrawn to maintain separation, its withdrawal rate is reduced and operating time is increased. However, the set point to a composition controller can be programmed so that the average composition of the product will still be within 9 specifications while withdrawal rate is maximized. © 2006 by Béla Lipták FIG. 8.19p Control system for batch distillation. 8.19(44) where y = the fraction of key component in the product m = the rate of change of y with respect to the distillate (D) yi = the initial concentration of the product The only adjustment required is the correct setting of m. The higher its value, the faster y will change and the smaller will be the quantity of material recovered. CONTROL OBJECTIVES AND STRATEGIES Operating objectives include the composition specifications for the top and bottom product streams. Other objectives can include increasing throughput, enhancing column stability, and operating against equipment constraints. Yet other considerations include what product composition is considered most important to maintain during disturbances, what are acceptable variations in product specifications, and what are relative economic values of the product streams and cost of 10 energy used in the separation. The column operating objectives are ultimately governed by economic benefits that are measurable, significant, and 11 achievable. Economics of individual fractionators may continually change throughout the life of the plant. Prices and costs may determine that energy savings are important at one particular time but that recovery is more important at some other time. The economic benefits of fractionator control include shifting of less profitable components into more profitable products, energy conservation, and increased throughput. Other benefits arise, including minimum disturbances propagated to downstream units, minimum rework or recycle of off-spec products, and more consistent product quality. Thus, a given column’s operating economics and, therefore, its objectives may change with time. 1834 Control and Optimization of Unit Operations When minimization of fractionator utilities is an objective, the following guidelines are recommended: • • • • Implement control to achieve composition control on all products of the fractionator Operate the fractionator to produce minimum overseparation Ascertain that the reduction in energy usage is reflected in the energy inflow to the production complex Minimize energy waste from blending of overseparated products Alternative Control Strategies Many choices confront the design engineer when selecting the control variables for a column. The first decision involves configuration of the top or bottom control loops, which directly determines product compositions. Once these strategies are tentatively determined, the control strategies for the remaining variables (e.g., pressure or levels) become easier to select. Pairings of controlled and manipulated variables are normally made according to the single-input single-output (SISO) method. Multivariable control, where multiple-input and multiple-output (MIMO) variables are paired, are discussed in Section 8.21. In these multivariable strategies, although a controlled variable can be affected by several manipulated variables, only one manipulated variable is used to directly affect the controlled variable. The minimum number of controlled variables for a fractionator tower is four. These include: Controlled Variables Manipulated Variables Overhead composition Reflux flow Bottoms composition Reboiler heating media flow Accumulator level Distillate flow Bottoms level Bottoms flow This allows for 24 possible configurations (4 factorial). Of course, most towers include pressure as a controlled variable, with condenser flow or vapor bypass as a manipulated variable. Additional manipulated variables can include feed flow and enthalpy. If a tower includes a sidedraw stream, another control pair is added to the possible combinations. In fact, additional control variables increase the number of possible control configurations factorially (e.g., six variables produce 720 possible configurations). The pairing of controlled and manipulated variables can follow three general control structures: energy balance con12 trol, material balance control, and ratio control. Energy balance control uses reflux and reboiler heating media flow to control compositions, thus fixing the energy inputs. Material balance control uses the distillate and bottoms product flows to control compositions, thus fixing the overall © 2006 by Béla Lipták material balance. Ratio control utilizes a ratio of any two flow rates at each end of the column. The two common examples of ratio control are the control of reflux-to-distillate ratio and the boil-up-to-bottoms ratio. These control configurations perform quite differently depending upon the fractionator characteristics. CONTROL LOOP INTERACTION The selection of which product composition to control (or both, if control of both can be controlled) and the decision on which variables will give better control can be aided by calculation of a relative gain array. The concept of relative 13,14 provides a measure of the interaction that can be gain expected between control loops. This subject is covered in more detail in Chapter 2 in Section 2.12 and in Section 8.20. The concept may be used to find the control configurations that will have the least amount of interaction. Therefore, relative gain analysis should be considered the first step in evaluating alternative composition control strategies. In addition, some pairings can be made heuristically from operating experience and on the basis of a general understanding of column dynamics (Table 8.19q). The following are general rules used to reject some pos15,16 sible control pairings: 1. Overhead composition and bottoms composition should not both be controlled with material balance equations if the objective is to control product specifications at both ends of the fractionator. Because of lack of dynamic response the following loops should not be paired: 1. Accumulator level should not be controlled with reboiler heat if the reboiler is a furnace. 2. Bottoms level should not be controlled with reboiler heat if the reboiler is a furnace. 3. Bottoms level should not be controlled with distillate flow. 4. Accumulator level should not be controlled with bottoms product flow. 5. Overhead composition should not be controlled with bottoms product flow. 6. Bottoms composition should not be controlled with distillate flow. 7. Bottoms level should not be controlled with reflux flow. 8. Bottoms composition should not be controlled with reflux flow if the number of trays is greater than a minimum limit (approximately 20). 9. Bottoms level should not be controlled with reboiler heat if the diameter of the column is greater than a minimum limit (approximately 15–20 ft (4.5–6 m), indicating a high volume of liquid in the bottoms). 8.19 Distillation: Basic Controls 1835 TABLE 8.19q 4 Dynamic Response and Sensitivity Limitations on the Pairing of Distillation Control Variables (Both compositions should not be controlled by material balance (B,D) if both specifications are important) Manipulated Variable Controlled Variable Composition of Overhead Product (ACy) Distillate Flow (D) OK if L/D 6 Note 3 Composition of Bottoms Product (ACx) Accumulator Level (LCa) Note 3 OK if L/D 6 Bottoms Level (LCb) Notes: 1. 2. 3. 4. Bottoms Product Flow (B) OK if V/B 3 Vaporization Rate (V) or Heat Input at Reboiler (O) Reflux Flow Rate (L) Notes 1 and 2 Note 2 Notes 1 and 2 OK if trays 20 Not good with furnace OK if V/B 3 OK if L/D 0.5 Not good if furnace is used OK if diameter at bottom 20 ft Control that concentration (x or y) which has the shorter residence time by throttling vapor flow (v). More pure product should control separation (energy). Less pure product should control material balance. When controlling both x and y, the only choices for possible pairings are: a. Control y by D and x by V. b. Control y by D and x by L. c. Control y by L and x by V. d. Control y by B and x by L. Of these, choice d is not recommended because a y/B combination is not responsive dynamically. 10. Accumulator level should not be controlled with reboiler heat if the control objective is to maintain overhead product specification and the V/B ratio is less than a minimum limit (approximately 3). Because of lack of sensitivity, these loops should not be paired: 1. Overhead composition should not be controlled with reflux flow if the reflux ratio (L/D) is less than a minimum value (approximately 6). 2. Accumulator level should not be controlled with distillate flow if the reflux ratio (L/D) is less than a maximum value (approximately 6). 3. Accumulator level should not be controlled with reflux flow if the reflux ratio (L/D) is less than a maximum value (approximately 0.5). 4. Bottoms composition should not be controlled with sidedraw flow if the sidedraw is a vapor phase. 5. Overhead composition should not be controlled with sidedraw flow if the sidedraw is a liquid phase. 6. Bottoms composition should not be controlled with sidedraw flow if the sidedraw is a liquid phase and the sidedraw tray number is greater than a minimum number (approximately 20). 7. Sidedraw composition should not be controlled with reflux or distillate flow if the difference between the total number of trays and the number of the sidestream tray is greater than a minimum value (approximately 20). 8. Bottoms level should not be controlled with sidedraw flow if the difference between the bottoms and the © 2006 by Béla Lipták number of the sidestream tray is greater than a minimum value (approximately 100). 9. Bottoms level should not be controlled with bottoms flow if the V/B ratio is greater than a minimum limit (approximately 3). Choices for controlling product compositions include (1) controlling top or bottom composition only (generally suitable for constant separation conditions, where specifications for one product are loose or where effective feedforward/ feedback systems can be designed to compensate for load changes) and (2) controlling of both product compositions (minimizes energy use and provides tight specification top and bottom products for columns in which the problems of interaction are small). These choices can be broken down further into considerations such as manipulation of distillate-boil-up, DV configuration (generally suitable for high reflux columns) or manipulation of reflux-boil-up, LV configuration (generally suitable for low reflux columns), and so forth. Further considerations include the use of decoupling control schemes (can present practical problems, such as insensitive control, operating problems, and high sensitivity to errors) and the use of temperature measurements to infer composition or analyzers to measure composition directly (generally an economic decision based on how well a temperature-sensitive control point can be determined and the costs of analyzer hardware and maintenance). These choices are based on operating objectives of the column, expected disturbance variables, and the degree of control loop interaction. 1836 Control and Optimization of Unit Operations PRODUCT QUALITY CONTROL Water Conceptually, product control is a problem of making precise adjustments to the rate of heat addition and the rate of heat removal from the tower. Heat removal determines the internal reflux flow rate, and the internal reflux as measured on the top tray is a direct reflection of the composition of the distillate. Heat added determines the internal vapor rate. These internal vapor and liquid flow rates determine the circulation rate, which in turn determines the degree of separation between two key components. Once interaction of the various variable pairings has been established, and the column’s operating objectives and disturbance variables are considered, the primary composition control loops of the column can be selected. Measurement of these control variables can be either direct or inferred. PRC PT TRC Set TT LT FRC LRC Set FT FRC FT FIG. 8.19s If overhead composition is to be controlled, the reflux flow to the column is throttled by a temperature controller. Inferring Composition from Temperature If the cost of on-line analyzer hardware and maintenance is prohibitive, or if backup is desired in case of analyzer failure or maintenance, and because the results of laboratory analysis take too long to be usable for effective control, temperature measurement often can be used to infer composition. Because distillation separates materials according to their difference in vapor pressures, and because vapor pressure is a temperature-controlled function, temperature measurement has historically been used to indicate composition. This presumes that the column pressure remains constant, or that the temperature measurement is compensated for pressure changes, and that feed composition is constant. Then, any change in composition within a column will be detected as a temperature change. The best point to locate the temperature sensor cannot be established from generalizations. The important consideration is to measure the temperature on a tray that strongly reflects the changes in composition. When composition of the bottom product is important, it is desirable to maintain a constant temperature in the lower section. This can be done by letting the temperature measurement manipulate the reboiler steam supply by resetting the steam flow controller set point (Figure 8.19r). When composition of the distillate is more important, it is desirable to maintain a constant temperature in the upper section, as in Figure 8.19s. In this configuration the sensing point for column pressure control should be located near the temperature control point. Keeping the sensor locations close to each other helps to fix the relation between temperature and composition at this particular point. If column temperature profiles caused by small positive and negative changes in manipulated variables, such as a ±1% change in distillate flow (Figure 8.19t), can be generated, the Stage Sensor location for maximum sensitivity 18 16 14 1% decrease in D 1% increase in D 12 10 TRC TT 8 Set FRC 6 FT 4 Steam LT LIC 2 −0.3 FIG. 8.19r In this configuration the reboiler heat input is throttled by a temperature controller to keep the bottoms product composition constant. © 2006 by Béla Lipták −0.2 −0.1 0 0.1 0.2 0.3 Stage temperature change in °C FIG. 8.19t Example of column temperature profiles resulting from a 1% increase and from a 1% decrease in distillate flow. 8.19 Distillation: Basic Controls 14 TT 6 TT TDRC FRC FT SP 1 1837 FRC FT Steam AT ARC FIG. 8.19u Heat input controlled by temperature difference. FIG. 8.119v Distillate withdrawal controlled by chromatograph. following criteria may be helpful in selecting sensor loca17 tions: (1) The sensitivity of the temperature-manipulated variable pairing should be in the range of 0.1 to 0.5°C/% and (2) equal temperature changes should result when increasing and when decreasing the manipulated variable. For a two-product fractionator, distillation temperature is an indication of composition only when column pressure remains constant or if the temperature measurement is pressurecompensated. When separation by distillation is sought between two compounds having relatively close vapor pressures, temperature measurement, as an indication of composition, is not satisfactory. Fixing two temperatures in a column is equivalent to fixing one temperature and the pressure. Thus, by controlling two temperatures, or a temperature difference, the effect of pressure variations can be eliminated. The assumption used here is that the vapor pressure curves for the two components have constant slopes. Controlling two temperatures is not equivalent to controlling a temperature difference. A plot of temperature difference vs. bottom product composition exhibits a maximum. Thus, for some temperature differences below the maximum it is possible to get two different product compositions. Separation of normal butane and isobutane (in the absence of other components, such as pentanes and heavier substances) can be accomplished very well by using temperature difference control. Figure 8.19u illustrates how the heat input to such a column can be controlled by a temperature difference controller. used. (For details, refer to Chapter 8 of Volume 1 of this handbook.) Once, the time required for a chromatographic analysis (several minutes) was a great barrier to its use for automatic control. Since then, the equipment has been enhanced so that analyses can now be made in less than 5 min, and in many cases for low-volatility hydrocarbons, the analysis can be made continuous. With careful handling, the under 5 min sampling rate will permit closed-loop distillate control. In fact, fractionators are successfully controlled with cycle times as long as 7–10 min by applying dead time compensation algorithms. Light ends fractionators have been satisfactorily controlled by the use of chromatography. Figure 8.19v illustrates the controls of a superfractionator designed to separate isobutane and normal butane. In this case, the chromatograph continuously analyzes a sample from one of the intermediate trays, and this measurement is used by the analyzer controller to modulate the product draw-off valve. Overhead and bottoms analyzers typically measure the loss of a valuable product or the presence of impurities. Impurity components are chosen because small concentration variations can be measured more precisely and with better repeatability, and can provide a more sensitive measure of separation. For example, the change of an impurity from 1.0 to 1.1% can be measured with greater precision than a change of the major component from 99 to 98.9%. When composition analyzers are used in feedback control, several configurations can be considered. These include 1) direct control of a manipulated variable, 2) cascade control adjusting the set point of a slave temperature controller, and 3) analysis control in parallel with temperature control in a selective control configuration. The configuration used depends on the control objective, sensitivity of control, and analysis dead time. Control by Analyzers Analytical or composition control is a way to sidestep the problems of temperature control. Although additional investment is needed for the analytical equipment, savings from improved operation usually results. Several types of instruments are available for composition analysis. Of these, the gas chromatograph is the most versatile and most widely © 2006 by Béla Lipták Direct Control by Analyzers Analyzer controllers in a feedback configuration can be considered when the dead time of each analysis update is less than the response time of the 1838 Control and Optimization of Unit Operations SP FT SP FRC FT Σ ARC ARC PRC AT PRC X TT SP FRC FT Accumulator Fractionation FIG. 8.19w Direct control of overhead product composition by an analyzer controller (ARC) throttling the set point of a reflux flow controller (FRC). process. Because it is the control of the composition of the product, which is often the objective, direct control by an analyzer controller would seem to be better than indirect control by temperature. The composition controller provides feedback correction in response to feed composition changes, pressure variations, and variations in tower efficiencies. Figure 8.19w shows the configuration of a control system, in which a chromatograph analyzes a liquid sample from the condenser rundown line. A sample probe gathers the liquid sample and the sampling system conditions and vaporizes the liquid sample to provide a representative vapor sample to the chromatograph. The analyzer controller (ARC) uses the chromatographic measurement to manipulate the reflux flow by adjusting the set point to the reflux flow controller (FRC). Smith Predictor Often the analyzer is so slow that it introduces a significant delay time that degrades the controllability of the process. In that case, some type of dead time compensation is used (see Section 2.19 in Chapter 2). A Smith predictor compensator can serve to model the process to predict what the analyzer measurement should be between analysis updates. When the actual measurement is completed, the model’s prediction is compared to the actual measurement and the input to the controller is biased by the difference. SP FRC AY FT + AY + − AY AY Lag Dead time AT Accumulator D L F B © 2006 by Béla Lipták TT D L F PT +∆ Fractionation PT AY FRC B FIG. 8.19x Analyzer controller with dead-time compensation cascaded to reflux flow control. Figure 8.19x shows the same configuration as did Figure 8.19w except that the analyzer controller is equipped with a first-order Smith predictor that provides dead time compensation. In Figure 8.19x, the multiplier, lag, and dead time calculations (AY) provide the predicted analysis. (The lag represents the first-order process.) This predicted response is subtracted from the actual measurement to give a differential of the actual process from its own model. This delta is added to the model without dead time to provide a modified pseudomeasurement to the analyzer controller. Thus, the analyzer measurement, which has a significant dead time due to sampling and cycle times, provides a trim to the predicted measurement of the model. Triple Cascade and Selective Control Analyzer control cascaded to temperature control can be used when stable temperature on a particular tray is desired and the tower operates at a constant, maintainable, and controllable pressure. An example is cascading the analyzer controller to the overhead temperature of a tower, which in turn is cascaded to the reflux flow rate. Because temperature is an indicator of composition at this pressure, the analyzer controller only serves as a trim correcting for variations in feed composition. Figure 8.19y shows this triple cascade configuration of an analyzer controller setting the temperature controller setting the reflux flow controller. 8.19 Distillation: Basic Controls 1839 SP FT FRC PRC PT PRC PT TT ARC SP FRC FT AT Accumulator D L FY < SP FRC TRC Fractionation F Absorber stripper SP TRC FT Reboiler ARC Lean oil to absorber B FIG. 8.19y Triple cascade configuration of overhead composition control. Analyzer controls can be used in a high or low select configuration in combination with temperature when a high or low limit based on temperature is important. The temperature controller is a constraint controller (see Section 2.28 in Chapter 2 for details) serving to prevent the temperature from exceeding a limit. An example of using this control configuration is the control of the bottoms of an absorber stripper. Here, the temperature should not exceed a certain value, as no additional stripping of the light component in the bottoms of the column could be accomplished. Even though an analyzer controller may call for more heat, this heat would only increase the bottoms temperature of the recycled oil to the absorber without removing the impurity, thereby reducing the absorption capability at the absorber. Figure 8.19z depicts an analyzer controller in a low select configuration with the temperature constraint controller. Note that both cascade and selective control configurations require external feedback to protect them from reset windup. Figure 8.19aa illustrates how the external feedback (EF) is applied to the master controller in a cascade configuration (TIC) and to both controllers (FIC and PIC) in a selective control configuration. For more details on external feedback, refer to Section 2.28 in Chapter 2. Fractionator Trains A controller may use as its measurement the analysis of a single component, or may use the ratio of two components. A ratio (e.g., ethane-to-propane, C2/C3) © 2006 by Béla Lipták FIG. 8.19z Analyzer control in a low select configuration with a temperature constraint controller. is often used when the fractionator is not the final step in the 18 separation sequence. This often occurs in a natural gas liquids separation train where a de-ethanizer, a depropanizer, a debutanizer, and a deisobutanizer (butane splitter) produce the products ethane, propane, butane, isobutane, and gasoline as shown in Figure 8.19bb. Specifications for the primary overhead products may include limitations on the amount of both light and heavy impurities. For example, the propane product from the overhead of the depropanizer would have limitations on ethane as well as isobutane. The problem is that the light impurity (lighter than light key) cannot be controlled in the tower that produces that product. Rather, it must be controlled in an upstream tower. Set point TIC EF FIC Master Override < FY PIC PT Slave TT FT EF EF A/O FIG. 8.19aa In a cascade configuration the external feedback signal (EF) is the slave measurement, while in selective control configurations, it is the signal that is throttling the control valve. 1840 Control and Optimization of Unit Operations Isobutane product AX C3/IC4 AX C5+ Deisobutanizer Propane product Debutanizer AX C2/C3 AX NC4 AX IC4 Depropanizer NGL Deethanizer AX C3 Ethane product N-butane product AX IC4 Straight run gasoline AX NC4 FIG. 8.19bb Analyzer placement in a fractionator train. The lighter than light key specification in the distillate of the downstream tower can be controlled more easily by controlling the ratio in the bottoms of the upstream tower. That is, the ethane content in the propane product (depropanizer distillate) is maintained by controlling the C2/C3 ratio in the bottoms of the de-ethanizer. Measuring the C2/C3 ratio in the bottoms requires an additional analyzer but eliminates the dead time of obtaining the concentration in the overhead of a downstream tower. A feed analyzer is sometimes included as a part of feedforward control. The feed analysis is used in predicting internal reflux/overhead flow and bottoms/heat input. However, when feed composition changes slowly or when results from the analyzer cannot be obtained faster than the dynamics of the tower, this analyzer is omitted and the burden is placed on feedback control from the product analyzers. In practice, a feed analyzer is the exception rather than the rule. Its use is mainly when the analyzer is already in place, because it is controlling an upstream tower. For example, the NGL separation train in Figure 8.19bb has a deethanizer bottoms analyzer that could also be considered the depropanizer feed analyzer. Analyzer Selection The choice of analyzer control depends upon the analytical equipment available and on the type of separation desired. Each type of separation requires a compromise between the controllability and the delay of the control system. For example, the NGL train (Figure 8.19bb) was studied to determine the best analyzer system. In the © 2006 by Béla Lipták depropanizer (where isobutane was to be measured in the presence of ethane, propane, and normal butane) and in the deisobutanizer (where isobutane was to be measured in the presence of normal butane and isopentane), an infrared analysis was to be preferred. However, in the debutanizer the goal was to measure the combined isopentane plus normal pentane concentrations in the presence of isobutane and normal butane to control the butane-pentane separation. Here, investigation revealed that gas chromatography provides the best solution. Some boiling point analyzers are reliable enough to be used for on-line control (see Section 8.50 in Chapter 8 in Volume 1 of this handbook). Normally, cut points between overhead products and side-cuts are maintained by temperature controllers. These controllers generally influence reflux rate or product draws to achieve the desired results. Laboratory distillation results are used to adjust the set points to the temperature controllers. This method of control, however, is cyclical because of the time lags involved in temperature control. To avoid exceeding the target cut points and to meet required product specifications, the cut point is set below specification. This results in downgrading the more valuable product to the stream of lesser value. This downgrading can be minimized through the use of on-line boiling point analyzers. Justification of a boiling point analyzer depends upon the value of the products, how much downgrading is occurring, and the cost of analyzer maintenance. Figure 8.19cc illustrates an end-point analyzer. Viscosity is another property that can be measured continuously to give faster control corrections. In vacuum distillation, the viscosimeter monitors each of the streams for which viscosity is a specification. Any deviation from the desired viscosity is corrected by a change in the set point of the control loop involved. Deviation from the desired viscosity and the subsequent downgrading of the product can occur because of frequent variations in tower operating conditions and feed composition. In addition to normal operation, the use of a viscosity analyzer minimizes downgrading during major upsets and large feed compositions changes. With such an arrangement, low viscosity vacuum bottoms can be detected quickly and diverted to recoverable feed for profitable reprocessing. Once again, profitability determination requires a thorough analysis of column operation and an assessment of the engineering, operating, and maintenance capabilities at the location where this type of control is to be implemented. Many other analytical instruments are being moved out of the laboratory and into the processing area. Mobile units containing several different kinds of analyzers can be used to learn the best place to locate on-stream analyzers. In cases in which permanent analyzers cannot be justified, the mobile unit is connected to the process long enough to find the best operating conditions. Then, the mobile unit can be moved elsewhere. 8.19 Distillation: Basic Controls Conditioned sample Ok (fast but not repeatable) PSV PC Overhead condenser Percent evaporated temperature controller TT Ok (slow) NG Packed column PI 1841 Distillate product NG (D) TRC Feed (F) To distillation column controls Ok (fast) FO TC Boiler pot Sample outlet valve Float Drain valve Analyzer effluent FIG. 8.19cc The end-point distillation analyzer is a miniature version of the process column. Sampling Proper sampling of material in a column is necessary if analyzers are to control effectively. A poor sampling system often is responsible for the unsatisfactory performance of plant analyzers. For details on sampling system design, refer to Chapter 8, Section 8.2, in Volume 1 of this handbook. The sampling points for composition analysis should be at, or very near, the column terminals for the following reasons: (1) freedom from ambiguity in the correlation of sample composition with terminal composition, and (2) improved control loop behavior as a result of reduction of transport lag (dead time) and of the time constants (lags) describing the sampling point’s compositional behavior. This assumes that the controller applies its manipulation at the same terminal (steam or reflux) where the controlled variable is measured. The factors favoring moving the point of sampling nearer to the feed entry point are (1) improved terminal composition behavior as a result of earlier recognition of composition transients as they proceed from the feed entry toward the column terminals, and (2) less stringent analytical requirements as a result of (a) analyzing the control component at a higher concentration and over a wider range, and (b) simplifying the multicomponent mixture, because nonkey components tend to exhibit constant composition zones in the column. © 2006 by Béla Lipták Bottoms product (B) Heater Ok (slow & low pressure) Ok (slow) FIG. 8.19dd The choices of sampling point locations for analyzers used in distillation column control. Figure 8.19dd shows some of the typical sample locations of a distillation tower. Most analyzers are designed to accept a clean, dry, noncorrosive sample at low temperatures, pressures, and flow rates. Such conditions seldom exist in the process, so the sampling system must be designed and operated to overcome the difference between the conditions in the process and the conditions required by the analyzer. The sample system must provide a current and representative sample of the stream being analyzed. It must transport the sample from the sample point to the analyzer with a minimum of transport lag (preferably less than 30 sec and definitely not greater than 1 min). Transportation times are minimized using high flow rate bypass streams taken from the process sample point and returned to the process at a lower pressure. The sample system must condition the sample to remove traces of foreign materials through filtering, maintain pressure and temperature, and maintain or change phase for introduction into the analyzer. For chromatographs, liquid sample points are generally preferred (Figure 8.19ee). This is because vapor streams have historically not provided representative samples. Vapor samples do not tend to produce repeatable values as consistently 19 and reliably because of condensation at the sample probe and in the sample lines when hydrocarbons of high boiling 1842 Control and Optimization of Unit Operations Analyzer Cooling in Sample Out In Water out Control Self valve cleaning filter FI Sample cooler Shut-off valves Flow FI indicator Check valve To drain Pressure gauge Temperature gauge PI T1 Flow indicator FI with needle valve Flow indicator Pressure regulator Calibration sample Shut-off valves Lab sample take off Coalescer Flow indicator FI with needle Check valve valve Pressure relief valve Pressure gauge PI Shut-off valve FIG. 8.19ee Sampling system for a liquid product in a refinery application. points are present in the sample. When the sample lines are long, some separation between components can also occur. However, vapor samples can be used when warranted and if the proper care can be taken. A satisfactory point for measuring bottoms product composition is at the point of highest pressure. This approach will ensure a representative sample and will provide the pressure drop to return the sample bypass. The point of highest pressure is generally immediately after the product pump. However, if liquid holdup in the reboiler and kettle is large, a long lag is introduced, which slows the transient response of the measurement and control system. Alternative sample points such as a bottoms tray or seal pan may be used, but may require extra expense for the sample system. A satisfactory sample-point location for measuring the distillate is the outlet liquid of the overhead vapor condenser. Sampling the overhead accumulator liquid after the reflux or distillate pump should be avoided because of the tremendous process lag it introduces. Sampling the overhead vapor reduces the process lag of sampling after the condenser if a repeatable, representative sample can be obtained. PRESSURE CONTROL Most distillation columns are operated under constant pressure. However, floating-pressure operation can have advantages in many processes. One reason for the resistance to the use of floating-pressure control is based on the fact that temperature is sensitive to pressure changes, and therefore, © 2006 by Béla Lipták it requires pressure compensation if the pressure varies. As analyzers are increasingly replacing temperature-based controls, the argument favoring constant pressure operation is also lessening. However, even when temperature control is used, the temperature measurements can be compensated for pressure variations. The primary advantage of floating-pressure control is the ability to operate at the minimum column pressure within the constraints of the system. Lower pressure reduces the volatility of distillation components, thereby reducing the heat input required to effect a given separation. Other advantages include increased reboiler capacity and reduced reboiler fouling due to lower tower temperatures. In the following paragraphs, floating-pressure control strategies will be described for the following conditions: (1) liquid distillate withdrawn when noncondensables are present, (2) vapor distillate withdrawn when noncondensables are present, and (3) liquid distillate withdrawn when the amount of noncondensables is negligible. Liquid Distillate and Inerts In some separation processes the problem of pressure control is complicated by the presence of large percentages of inert gases. The noncondensables must be removed, or they will accumulate and blanket off the condensing surface, thereby causing loss of column pressure control. The simplest method of handling this problem is to bleed off a fixed amount of gases and vapors to a lower pressure unit, such as to an absorption tower, if one is present in the system. 8.19 Distillation: Basic Controls Vent used to start opening the purge control valve (VPCV-2), when the opening of PCV-1 reaches some preset limit. This can be done by means of a calibrated valve positioner or by using a valve position controller (VPC-2) in Figure 8.19ff. Partially flooded condenser VPCV 2 VPC 2 PCV 1 Vapor Distillate and Inerts PRC 1 PT 1843 LT LIC FIG. 8.19ff Column pressure control with inerts present. If an absorber is not present, it is possible to install a vent condenser to recover the condensable vapors from this purge stream. Often in refinery applications such noncondensables go to the fuel gas system or to flare. It is recommended that the fixed continuous purge be used whenever economically possible; however, when this is not permitted, it is possible to modulate the purge stream. This might be desirable when the amount of inerts is subject to wide variations over time. As the noncondensables build up in the condenser, the pressure controller will tend to open the control valve (PCV-1 in Figure 8.19ff) to maintain the proper rate of condensation. The controller signal that is throttling PCV-1 could also be In the case where the distillate is in the vapor phase and inerts are present, the overhead product is removed under pressure control as shown in Part A of Figure 8.19gg. In this configuration the system pressure will quickly respond to changes in the distillate vapor flow. In this control system a level controller is installed on the overhead receiver to regulate the cooling water to the condenser, so that it will condense only enough condensate to provide the column with reflux. This control system will operate properly only if the condenser is designed to provide a short residence time for the coolant, which will minimize the level control time lag. If this is not the case, the cooling water flow should be maintained at a constant rate. In this case (Part B in Figure 8.19gg), the level controller can regulate the flow of condensate through a small vaporizer and mix it with the vapor from the pressure control valve. If the cooling water has fouling tendencies, it is preferable to use the control system shown in Figure 8.19hh, where a pressure controller regulates a vapor bypass around the condenser. Liquid Distillate with Negligible Inerts In distillation processes where the distillate is in the liquid phase and the amount of inerts is negligible, the column pressure is usually controlled by modulating the rate of condensation in the condenser. The method of controlling the Dry condenser Dry condenser Water Vaporizer Water PRC PRC PT PT Vapor (D) Vapor (D) LRC LT LT Receiver A. Variable water flow LRC B. Constant water flow FIG. 8.19gg Column pressure control when the distillate is in the vapor phase and contains inerts for variable (A) and constant condenser water flow configurations (B). © 2006 by Béla Lipták 1844 Control and Optimization of Unit Operations Partially flooded condenser ∆P Water PRC ∆P PT PRC Vapor (D) ∆P PT LT ∆P LIC LT LRC Liquid (D) B. Liquid distillate A. Vapor distillate FIG. 8.19hh Column pressure controlled by hot gas bypass throttling in case of vapor (A) and liquid (B) distillate processes. rate of condensation depends upon the mechanical construction of the condensing equipment. Controlling the Cooling Water Flow Figure 8.19ii describes a control configuration where the column pressure is controlled by throttling the cooling water flow from the condenser. This method of control is recommended only when the cooling water is treated with chemicals that prevent the fouling of the tubes in the event of high temperature rise across the condenser tubes. In such configuration, the maintenance costs are low, because the control valve is on the water side and the control performance is acceptable, provided the condenser is properly designed. The best condenser for this service is a bundle-type unit with the cooling water flowing through the tubes. This water Water PT Dry condenser PRC LT LIC FIG. 8.19ii Column pressure control by throttling condenser water. © 2006 by Béla Lipták should be flowing at a rate of more than 4.5 ft/s (1.35 m/s), and the water should have a residence time of less than 45 sec. The shorter the residence time of the water, the better will be the quality of control obtained, owing to the decrease in dead time or lag in the system. With a properly designed condenser, the pressure controller needs only proportional control, because a narrow throttling range is sufficient. However, as the residence time of the water increases, the time lag of the system will increase, and consequently the controller will require a wider throttling range and will need automatic reset to compensate for the load changes. The control obtained by using a wide proportional band is not satisfactory for precision distillation columns because of the length of time required for the system to recover from an upset. Also, the dead time varies with load, and therefore the integral setting of the PRC should be set to match the variation in residence time. Therefore, it is unacceptable to use this control system on a condenser box with submerged tube sections, because there would be a large time lag in the system due to the large volume of water in the box. It would require the passage of a significant amount of time before a change in water flow rate would change the temperature of the water in the box and finally would affect the rate of condensation. Controlling the Condensate Flow To reduce such unfavorable time lags, it becomes necessary to use a different type of control system, one that permits the water flow rate to remain constant and controls the amount of surface exposed to the condensing vapors. This is done by modulating the flow of condensate from the condenser. When the column pressure is dropping, this condensate throttling valve reduces the condensate flow, causing it to 8.19 Distillation: Basic Controls Partially flooded condenser PCV 2 PRC 1 PRC-1>PRC-2 ∆P PRC 2 PCV 1 PRC PT PT 1845 PT ∆P LT PRC Inerts PT LIC LT LRC Liquid (D) Liquid (D) B. With inerts A. No inerts FIG. 8.19jj High-speed column pressure control. build up and flood more tube surface and, consequently, to reduce the condensing surface exposed to the vapors. Thereby, the condensing rate is reduced, and the pressure in the column rises. In such designs, a vent valve should be installed to purge the noncondensables from the top of the condenser if it is expected that noncondensables could build up and blanket the condensing surface. Control of Hot Vapor Bypass A third possible control configuration for applications with liquid distillate containing negligible inerts is used when the condenser is located below the receiver. This is frequently done to make the condenser available for servicing and to save on steel work. It is usual practice to elevate the bottom of the accumulator 10–15 ft (3–4.5 m) above the suction of the pump in order to provide a positive suction head on the pump. In this type of installation the control valve is placed in a bypass of the vapor line to the accumulator (see Figure 8.19hh, Part B). When this valve is open, it equalizes the pressure between the vapor line and the receiver, causing the condensing surface to become flooded with condensate because of the 10–15 ft of head in the condensate line from the condenser back to the receiver. The flooding of the condensing surface causes the pressure to build up because of the decrease in the rate of condensation. Under normal operating conditions, the subcooling that the condensate receives in the condenser is sufficient to reduce the vapor pressure in the receiver. The difference in pressure permits the condensate to flow up the 10–15 ft of pipe between the condenser and the accumulator. A modification of this latter system controls the pressure in the accumulator by throttling the condenser bypass flow © 2006 by Béla Lipták (Figure 8.19jj, Part A). The column pressure is maintained by throttling the flow of vapor through the condenser. Controlling the rate of flow through the condenser provides faster pressure regulation for the column. Part A of Figure 8.19jj shows the operation if there are no inerts, as follows: If column pressure rises, PRC-1 opens PCV-1. This increases the vapor pressure in the condenser, which pushes some of the condensate out of it and increases the condensing surface area exposed to the vapors. Therefore, the rate of condensation is increased, and thereby the column pressure is lowered back to the set point of PRC-1. At this higher rate of condensation, the pressure drop (∆P) across PCV-2 is also reduced (the valve opens). If the column pressure drops, the opposite sequence occurs: PCV-1 closes and the flooding of the condenser increases, reducing the rate of condensation and increasing the pressure drop (∆P) across PCV-2 by slightly closing it. The setting of PRC-1 must always be above that of PRC-2. The most common pressure control configuration is shown in Part B of Figure 8.19jj. Here, the column pressure controller is throttling the hot vapor bypass, as was the case in Part A of Figure 8.19hh, but in addition a second pressure controller is utilized on the accumulator. This PRC is set at about 5 PSIG below the required tower pressure and is used to vent the inert gases that may build up in the system. Vacuum Systems For some liquid mixtures, the temperature required to vaporize the feed would need to be so high that decomposition would result. To avoid this, it is necessary to operate the column at pressures below atmospheric. Steam jet ejectors are often used to create vacuum in distillation systems. These 1846 Control and Optimization of Unit Operations PRC Cold/cold exchanger F Steam Air C3 Propane chiller PIC PT Turboexpander PT PRC Surge bypass SP LIC SC Demethanizer LT FIG. 8.19kk Vacuum column pressure control. can be used singly or in stages, when a wide range of vacuum conditions are required. The acceptance of steam jet ejectors is due to their having no moving parts and requiring very little maintenance. Most ejectors are designed for a fixed capacity and work best at one steam condition. Increasing the steam pressure above the design point will not usually increase the capacity of the ejector; instead, it will sometimes decrease the capacity because of the choking effect of the excess steam in the diffuser throat. Steam pressure below a critical value for a jet will cause the ejector operation to be unstable. Therefore, it is recommended that a pressure controller be installed on the steam to keep it at the optimum pressure required by the ejector. The recommended control system for pressure control in vacuum distillation applications is shown in Figure 8.19kk. Here, a controlled rate of air or gas is bled into the vacuum line just ahead of the ejector. Closing this bleed valve makes the maximum capacity of the ejector available to handle any surges or upsets in the process load. A control valve regulates the amount of bleed air used to maintain the pressure on the reflux accumulator. Using the pressure of the accumulator for control involves less time lag than if the column pressure were used as the control variable. Because ejectors are fixed capacity, the variable load is met by air bleed into the system. At low loads this represents a substantial waste of steam. Therefore, if substantial load variations are expected, operating costs can be lowered by installing a larger and a smaller ejector. This makes it possible to automatically switch to the small unit when the load drops off, thereby reducing the steam demand. Vapor Recompression Vapor recompression is another means of improving energy efficiency of the operation. The overhead vapor from the © 2006 by Béla Lipták Turbine Compressor TT TRC Reboiler Residue gas NGL FIG. 8.19ll Vapor recompression pressure control. distillation column is compressed to a pressure where its condensation temperature is greater than the boiling point is at the pressure of the tower bottoms. The heat of condensation of the overhead can then be used as the source of heat for reboiling the bottoms. This scheme is known as vapor recompression. It is used fairly often when the distillation involves a relatively closeboiling mixture, and the boiling points of the top and bottom products are similar. In cryogenic demethanization processes, illustrated in Figure 8.18ll, the column pressure is controlled by the throttling of the speed of the recompression compressors. The heat of condensation of the overhead is also used as the heat for the reboilers in propylene fractionators. Figure 8.19mm shows the pressure controls needed for the operation of this vapor recompression via the heat pump on this particular tower. FCCU main fractionators and crude towers make use of compressors to “draw” vapors from the tower because operation is essentially at atmospheric pressures. The pressure control system used in this case is shown in Figure 8.19nn. In this configuration, the tower pressure is maintained by controlling the speed of the compressor. This is accomplished by the manipulation of the steam used to drive the compressor turbine. 8.19 Distillation: Basic Controls 1847 Feed Flow Control A flow controller in the feed line can maintain a constant flow rate. In some instances, the feed pump of a distillation unit is a steam-driven pump instead of an electrically driven one. In this case, the controller modulates the steam to the driver. Feed composition has a great influence upon the operation of a distillation unit. Unfortunately, feed composition is seldom subject to adjustment. For this reason, it is necessary to make changes elsewhere in the operation of the column in order to compensate for the variations in feed composition. The corrective steps are discussed later. The discussion below assumes a constant feed composition. PT F Heat pump Propylene tower PRC CW SP TRC FRC Accumulator Variable Column Feed FT Propylene (C3−) Reboiler Propane (C3) FIG. 8.19mm Propylene tower vapor recompression pressure control. FEED CONTROLS One of the best means of stabilizing the operation of almost any continuous-flow-process, including distillation, is to hold the flow rates and operating temperatures constant. Therefore, whenever possible, a flow controller should be used on the feed to maintain a constant rate of flow. SP FT Steam PRC CW PT FRC Accumulator Having constant feed conditions simplifies the amount of control required to achieve stable operation. However, the distillate product is often fed to a second column. Then, any changes that occur in the first column are reflected in the quantity and composition of the feed to the second. If the feed flow to the column is controlled by a liquid level controller of the previous column, that controller can be tuned with a low gain, so that the level can swing over a wide range without drastically upsetting the flow of product. Nevertheless, the second column will receive a varying flow of feed if it is linked to the first column. One way to iron out temporary variations caused by liquid level changes is to cascade the level to a flow controller in the product lines. Flow controllers also serve to smooth out the pressure fluctuations caused by the distillate/reflux pump. With variable feed rates and variable feed compositions, cascade controls are justified. If the feed rate and composition are relatively constant, resetting the major control loop manually is sometimes adequate. In other cases the flow controller is arranged as the cascade slave of the level controller (Figure 8.19oo). The control algorithm for the level controller in Figure 8.19oo is usually selected to be nonlinear to allow the level to float in the surge tank without changing the FRC set point, which would upset the feed to the next column. Therefore, the nonlinear controller is so configured that as long as the level in the surge tank is between 25 and 75%, Main fractionator Turbine Compressor LT Nonlinear LRC Set FRC FT Reboiler FIG. 8.19nn Main fractionator pressure control. © 2006 by Béla Lipták FIG. 8.19oo Feed flow to the next column is kept relatively constant by the use of a nonlinear level controller (LRC) on the surge tank acting as the cascade master of the slave flow controller (FRC). 1848 Control and Optimization of Unit Operations To column TRC Set FRC TT FT Steam Distillate FT Feed FY X Feed FC m FIG. 8.19pp Feedforward control minimizes feed rate disturbances. the set point to the FRC remains constant. This will allow the surge tank to fulfill its purpose and smooth out the load variations between the related processes. If the level drops below 25% or rises above 75%, the FRC set point is reduced or increased respectively to protect it from draining or flooding the tank. If feed rate disturbances must be accepted by the column, a feedforward control system as shown in Figure 8.19pp can 10 be used to minimize the impact of these disturbances. The ratio, m, is selected by performance of a simple material balance around the column. Changing the product flow in proportion to the feed flow minimizes internal column transients and, thus, the quantity of off-spec material during recovery. The value of m, however, is accurate only for one feed composition and will have to be readjusted either manually or automatically for different feed compositions. Dynamic compensation, which will be discussed in more detail in Section 8.21, is also recommended here. Another method of minimizing feed rate disturbances is to use adaptive tuning or other nonlinear level control techniques (see Section 2.36 in Chapter 2) on the level controller. The key is to allow the accumulator, or the tower kettle, to utilize its capacity to accommodate transient material balance accumulations and act as a “surge drum” to minimize feed flow changes to the next unit. Feed Temperature Control The thermal condition of the feed determines how much additional heat must be added to the column by the reboiler. For efficient separation, it usually is desirable to have the feed preheated to its bubble point when it enters the column. Unless the feed comes directly from some preceding distillation step, an outside source of heat is required to achieve that. Steam may be used to heat the feed, and a thermocouple inside a thermowell can detect the temperature inside the feed © 2006 by Béla Lipták FIG. 8.19qq The use of a temperature-flow cascade loop improves the column feed temperature controls provided by a preheater. line. In this configuration, the temperature of the feed leaving this preheater controls the steam flow into the preheater. In such a cascade configuration, the temperature master is usually a three-mode controller. On start-up, the initially large correction provided by rate action of this three-mode controller helps to get the unit lined out faster. A full discussion of the advantages of cascade loops is provided in Section 2.6 in Chapter 2. Figure 8.19qq describes such a control system on a preheater application. An alternate feed preheating configuration is to use an economizer on the feed stream. An economizer is a heat exchanger designed to take advantage of the waste heat to preheat the feed. Often, if the bottoms product is just sent to storage, it must be cooled anyway. Therefore, exchanging its heat content with the feed stream accomplishes both the objective of feed preheating and that of product cooling. Two control configurations are common. If heat from the bottoms product stream is not sufficient, a second exchanger using steam is also installed to augment the heating of the feed, and on this second exchanger, a temperature control system like that previously shown in Figure 8.19qq is used. If the available bottoms product heat is more than sufficient, temperature control is achieved by manipulation of a bypass valve around the economizer, as shown in Figure 8.19rr. Constant temperature feed does not necessarily mean constant feed quality. If feed composition varies, its bubble point also varies. It is common practice to set the temperature control at a point that is equivalent to the bubble point of the heaviest feed. As the feed becomes lighter, some of it will vaporize, but this variation can be handled by subsequent controls. FEEDFORWARD CONTROLS This section discusses the basic single-input–single-output feedback control loops, serving to control the product qualities, feed rate and temperature, and tower pressure. Such feedback systems are capable of compensating for deviations and disturbances only after they have occurred and have been 8.19 Distillation: Basic Controls B Economizer TF F TT TRC Reboiler FIG. 8.19rr The heat content of the bottoms products can be utilized to preheat the feed to the column in an economizer preheater. detected. When using these simple control schemes, the operators are required to manually adjust the set point of these SISO loops in response to changing plant conditions as they occur. This approach is usually sufficient to keep the distillation column in operation, but it is not sufficient to achieve optimal performance. As will be discussed in Section 8.21 in more detail, feedforward strategies attempt to compensate for process disturbances in the shortest time possible by accounting for process dynamics, dead times, time delays, and loop interac1 11 tions. The benefits of better control are: • • • • • • • Increased throughput Increased product recovery Energy conservation Reduced disturbances to other processing units Minimum rework or recycle of off-spec products Reduced operating personnel Increased plant flexibility It has been reported that feedforward-based product composition control of distillation can give energy savings of 5 5–15%. While feedback-based basic distillation controls only aim at running the processing unit at current conditions, the objective of feedforward control is not only to do that, but also to account for conditions that can be anticipated. The challenge is to utilize the technique, the tools, and available resources to design unique feedforward control strategies that will match the specific objectives for the distillation columns. The choice between any of these control techniques depends upon factors such as preference and familiarity, complexity, degree © 2006 by Béla Lipták 1849 of compensation, hardware for application, and number of variables monitored and controlled by a single strategy. Often, additional instrumentation is not necessary when building upon basic feedback control designs to implement feedforward control. However, in many cases, if key measurements are not available and are needed for the feedforward calculation or compensation, the installation of new in-line sensors is also required. Unlike basic feedback control, where much of the control could be implemented by simple analog control devices, feedforward control strategies generally require more sophisticated level computing systems. In this section, the common applications of feedback-based distillation column controls are discussed. Section 8.21 will discuss feedforward, model-based, and other advanced control systems, including optimization. CONCLUSIONS This section deals with some of the more basic control configurations for distillation towers. Section 8.20 describes the calculation of relative gains and Section 8.21 is devoted to the more advanced and optimized control strategies. The separation between these three areas is not very sharp, and some overlap does exist. In this section, the control strategies for some of the more common distillation problems have been described. Although many other system configurations can exist, they usually are combinations of those presented. Control strategies today are no longer hardware dependent. Most modern microprocessorbased systems are designed with control function modules to execute a variety of the basic strategies that were discussed in this section. Multivariable unit operations controllers of both the model-predictive and the model-free variety are also slowly becoming available and will be discussed in Section 8.21. It is important to emphasize that control by feedback methods alone cannot approach the quality of control possible by predictive (feedforward) techniques. This is true even though it is likely that the predictive control equations may need to be updated by feedback. In effect, predictive control tends to substantially reduce the size of the errors that are left to be handled by feedback. Further discussion of feedforward strategies and of other techniques for optimization are provided in more detail in Section 8.21. References 1. 2. 3. Lockett, M. J., Distillation Tray Fundamentals, Cambridge, MA: Cambridge Press, 1986. Strigle, R. J., Jr., Random Packings and Packed Towers, Design, and Applications, Houston, TX: Gulf Publishing Company, 1987. Peters, M. S., and Timmerhaus, K. D., Plant Design and Economics for Chemical Engineers, 3rd ed., New York: McGraw-Hill Book Company, 1980. 1850 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. Control and Optimization of Unit Operations Henley, E. J., and Seader, J. D., Equilibrium-Stage Separation Operations in Chemical Engineering, New York: John Wiley & Sons, Inc., 1981. Smith, D. E., Stewart, W. S., and Griffin, D. E., “Distill with Composition Control,” Hydrocarbon Processing, February 1978. Fenske, M. R., “Fractionation of Straight-Run Pennsylvania Gasoline,” Industrial Engineering Chemistry, May 1932. Shinskey, F. G., Process-Control Systems, Application, Design, Adjustment, 3rd ed. New York: McGraw-Hill Book Company, 1988. Bannon, R., et al., “Heat Recovery in Hydrocarbon Distillation,” Chemical Engineering Progress, July 1978. Converse, A. O. and Gross, G. D., “Optimal Distillate Rate Policy in Batch Distillation,” Industrial Engineering Chemistry, August 1963. Thurston, C. W., “Computer-Aided Design of Distillation Column Controls: Part 1,” Hydrocarbon Processing, July 1981. Jensen, B. A., and Collins, P. L., “Incentives For Tighter Fractionator Control,” Control, November 1990. Yang, D. R., Seborg, D. E., and Mellichamp, D. A., “Combined Balance Control Structure for Distillation Columns,” Industrial & Engineering Chemistry Research, September 1991, pp. 2159–2168. Gordon, L. M., “Practical Evaluation of Relative Gains: The Key to Designing Dual Composition Controls,” Hydrocarbon Processing, December 1982. Shinskey, F. G., “Predicting Distillation Column Response Using Relative Gains,” Hydrocarbon Processing, May 1981. Jensen, B. A., Harper, T., and Likins, M. R., “An Expert System in Fractionator Control System Design,” Proceedings of the AIChE Spring Meeting, Orlando, FL, March 1990. Shinskey, F. G., “An Expert System for the Design of Distillation Controls,” Proceedings of the Third International Conference on Chemical Process Control, Asilomar, CA, January 1986. Thurston, C. W., “Computer-Aided Design of Distillation Column Controls: Part 2,” Hydrocarbon Processing, August 1981. Griffin, D. E., “Fractionator Controls Can Save Energy,” Oil & Gas Journal, March 1978. Oglesby, M. W., and Hobbs, J. W., “Chromatograph Analyzers for Distillation Control,” Oil and Gas Journal, January 10, 1966. Bhullar, R. S., “Advanced Distillation Control,” Control, May 1990. Van Kampen, J. A., “Automatic Control by Chromatograph of a Distillation Column,” Convention on Advances in Automatic Control, Nottingham, England, April 1961. Luyben, W. L., “Distillation Decoupling,” AIChE Journal, Vol. 16, No. 2, pp. 198–203, March 1970. Shinskey, F. G., “The Stability of Interacting Loops with and without Decoupling,” presented at the IFAC Symposium on Multivariable Control, Fredericton, New Brunswick, July 4–8, 1977. Shinskey, F. G., Distillation Control for Productivity and Energy Conservation, 2nd ed., New York: McGraw-Hill Book Company, 1984. Ryskamp, C. J., “New Control Strategy Improves Dual Composition Control,” Hydrocarbon Processing, June 1980. Bibliography 1960 Boyd, D. M., “Fractionator Pressure Control,” Petroleum Refiner., February 1947, 113–15. Boyd, D. M., “Fractionator Instrumentation and Control Part 1,” Petroleum Refiner., October 1948, 115–536. Hollander, L., “Pressure Control of Light-Ends Fractionators,” ISA Journal, May 1957, 185–187. Kirschbaum, E, Distillation and Rectification, Brooklyn, NY: Chemical Publishing Co., 1948. © 2006 by Béla Lipták Whistler, A. M., “Locate Condensers at Ground Level,” Petroleum Refiner., 33(3): 173–174, March 1954. 1960–1969 Denbigh, K., The Principles of Chemical Equilibrium, London: Cambridge University Press, 1961, pp. 63 and 116. Van Kampen, J. A., “Automatic Control by Chromatograph of a Distillation Column,” Convention on Advances in Automatic Control, Nottingham, England, April 1961. Smuck, W. W., “Operating Characteristics of a Propylene Fractionating Unit,” Chemical Engineering Progress, June 1963. Lupfer, D. E. and Johnson, M. L., “Automatic Control of Distillation Columns to Achieve Optimum Operations,” ISA Transactions, April 1964. Rijnsdorp, J. E., “Interaction in Two-Variable Control Systems for Distillation Columns,” in Automatica, Vol. 1, London: Pergamon Press, 1965. Forman, E. R., “Control Systems for Distillation,” Chemical Engineering, November 8, 1965. Perry, E. S., and Weissberger, A., Distillation, 2nd ed., New York: Interscience, 1965. Bristol, E. H., “On a New Measure of Interaction for Multivariable Process Control,” IEEE Transactions on Automatic Control, January 1966. Luyben, W. L., “10 Schemes to Control Distillation Columns with Sidestream Drawoff,” ISA Journal, Vol. 13, No. 7, pp. 37–42, 1966. Van Winkle, M., Distillation, New York: McGraw-Hill Book Company, 1967. Wood, C. E., “Method of Selecting Distillation Column Tray Location for Temperature Control,” presented at the Joint IMIQ-AIChE Meeting, Mexico City, September 24–27, 1967. Owens, W. R., and Maddox, R. N., “Shortcut Absorber Calculations,” Industrial Engineering Chemistry, December 1968. Hengstebeck, R. J., “An Improved Shortcut for Calculating Difficult Multicomponent Distillations,” Chemical Engineering (NY), January 13, 1969. Buckley, P. S., “Controls for Sidestream Drawoff Columns,” Chemical Engineering Progress, Vol. 65, No. 5, 1969, p. 45. Luyben, W. L., “Feedback Control of Distillation Columns by Double Differential Temperature Control,” Industrial and Engineering Fundamentals, Vol. 8, November 1969. McNeill, G. A., and Sacks, J. D., “High-Performance Column Control,” Chemical Engineering Progress, March 1969. 1970 Luyben, W. L., “Distillation Decoupling,” AIChE Journal, Vol. 16, No. 2, pp. 198–203, March 1970. Wahl, E. F., and Harriott, P., “Understanding and Prediction of the Dynamic Behavior of Distillation Columns,” I&EC Process Design and Development, Vol. 9, No. 3, pp. 396–407, 1970. Maarleveld, A., and J. E. Rijnsdrop, “Constraint Control of Distillation Columns,” Automatica, 1970, pp. 51–58. 1971 Doig, I. D., “Variation of the Operating Pressure to Manipulate Distillation Processes,” Australian Chemical Engineering, July 1971. Hall, E. B., “Distillation Control Problems in the Chemical and Petroleum Industries,” Proceedings of the Twelfth Annual ISA Chemical and Petroleum Instrumentation Symposium, Houston, TX, April 1971. Holt, A. B., “Improved Fractionation thru Computer Control,” Instrumentation Technology, January 1971, pp. 43–48. King, C. J., Separation Processes, New York: McGraw-Hill Book Company, 1971. Shinskey, F. G., “Avoiding Reset Windup in Cascade Systems,” Instrument and Control Systems, August 1971. 8.19 Distillation: Basic Controls Shinskey, F. G., “When to Use Valve Positioners,” Instrument and Control Systems, September 1971. Shinskey, F. G., “When You Have the Wrong Valve Characteristic,” Instrument and Control Systems, October 1971. Shunta, J. P., and Luyben, W. L., “Dynamic Effects of Temperature Control Tray Location in Distillation Columns,” AIChE Journal, Vol. 17, No. 1, pp. 92–96, January 1971. 1972 Toijala, K., and Fagervik, K., “A Digital Simulation Study of Two-Point Feedback Control of Distillation Columns,” Kem. Teollisuus, January 1972. Shinskey, F. G., “What to Know about Interaction between Control Loops,” Canadian Controls Instrumentation, March 1972. McFarlane, A. G. J., “A Survey of Some Recent Results in Linear Multivariable Feedback Theory,” Automatica, Vol. 8, No. 4, pp. 455–492, 1972. Shinskey, F. G., “Effective Control for Automatic Start-up and Plant Protection,” Canadian Controls Instrumentation, April 1972. Shinskey, F. G., “Controlling Surge-Tank Level,” Instrument and Control Systems, September 1972. Shinskey, F. G., “Controlling Variable Period Once-Through Processes,” Instrument and Control Systems, November 1972. White, M. H., “Surge Control for Centrifugal Compressors,” Chemical Engineering (NY), December 25, 1972. 1973 Evans, F. L., Equipment Design Handbook for Refineries and Chemical Plants, Vol. I, Houston, TX: Gulf Publishing Co., 1973, p. 89. Perry, J. H., Chemical Engineers’ Handbook, 5th ed., New York: McGrawHill Book Company, 1973. Houghton, J., and McLay, J. D., “Turboexpanders Aid Condensate Recovery,” Oil and Gas Journal, March 5, 1973. Sayles, J. H., “Computer Control Maximizes Hydrocracker Throughput,” Instrumentation Technology, May 1973. Frank, O., and Prickett, R. D., “Designing Vertical Thermosyphon Reboilers,” Chemical Engineering (NY), September 3, 1973. Sisson, B., “How to Determine MEA Circulation Rates,” Chemical Engineering (NY), November 26, 1973. O’Conner, J., and Illing, H., “The Turbine Control Valve. A New Approach to High-Loss Applications,” Instrumentation Technology, December 1973. 1974 Ackley, W. R., “Feedforward Control Strategy for Distillation Towers,” Instrumentation Technology, February 1974. Adiutori, E. F., The New Heat Transfer, Cincinnati, OH: Ventuno Press, 1974, Chapter 7. Kline, P. E., “Technical Task Force Approach to Energy Conservation,” Chemical Engineering Progress, February 1974. Shinskey, F. G., “Values of Process Control,” Oil and Gas Journal, February 18, 1974. Ellerbe, R. W., “Steam Distillation Basics,” Chemical Engineering (NY), March 4, 1974. Lupter, D. E., “Distillation Column Control for Utility Economy,” presented at 53rd Annual GPA Convention, Denver, March 25–27, 1974. Waller, K. V. T., “Decoupling in Distillation,” AIChE Journal, May 1974. Farwell, W., “More Cooling at Less Cost,” Plant Engineering, August 1974. Gallier, P. W., and McCune, L. C., “Simple Internal Reflux Control,” Chemical Engineering Progress, September 1974. Rathore, R. N. S., VanWorme, K. A., and Powers, G. J., “Synthesis of Distillation Systems with Energy Integration,” AIChE Journal, September 1974. © 2006 by Béla Lipták 1851 Buckley, P. S., “Material Balance Control in Distillation Columns,” presented at AIChE Workshop on Industrial Process Control, Tampa, FL, November 11–13, 1974. 1975 Bojnowski, J. H., Crandall, J. W., and Hoffman, R. M., “Modernized Separation Systems Saves More than Energy,” Chemical Engineering Progress, October 1975. Boyd, D. M., “Fractionation Column Control,” Chemical Engineering, June 1975. Buckley, P. S., Cox, R. K., and Rollins, D. L., “Inverse Response in a Distillation Column,” Chemical Engineering Progress, June 1975. Franzke, A., “Save Energy with Hydraulic Power Recovery Turbines,” Hydrocarbon Process, March 1975. Luyben, W. L., “Steady-State Energy Conservation Aspects of Distillation Column Control Design,” I&EC Fundam., Vol. 14, No. 4, pp. 321–325, 1975. Monroe, E. S., “Vacuum Pumps Can Conserve Energy,” Oil and Gas Journal, February 3, 1975. Rademaker, O., Rijnsdorp, J. E., and Maarleveld, A., Dynamics and Controls of Continuous Distillation Units, Amsterdam: Elsevier, 1975. Shinskey, F. G., “Controlling Unstable Processes, Part II: A Heat Exchange,” Instrument and Control Systems, January 1975. Sternlicht, B., “Low-Level Heat Recovery Takes on Added Meaning as Fuel Costs Justify Investment,” Power, April 1975. Tyreus, B., and Luyben, W. L., “Control of a Binary Distillation Column with Sidestream Drawoff,” I&EC Process Design and Development, Vol. 14, No. 4, p. 391, 1975. Tyreus, B., and Luyben, W. L., “Two Towers Cheaper than One?” Hydrocarbon Processing, Jun., 1975. Wolf, C. W., Weiler, D. W., and Ragi, E. G., “Energy Costs Prompt Improved Distillation,” Oil and Gas Journal, September 1, 1975. 1976 ISA Standard S51.1, “Process Instrumentation Terminology,” Research Triangle Park, NC: Instrument Society of America, 1993. Shinskey, F. G., “Energy-Conserving Control for Distillation Units,” Chemical Engineering Progress, May 1976. Wright, R. M., “A Better Approach to Distillation Control,” Instruments and Control Systems, June 1976. Van Horn, L. D., and Latour, P. R., “Computer Control of a Crude Still,” Instrumentation Technology, November 1976, pp. 33–39. VanWinkle, M., Distillation, New York: McGraw-Hill, 1976. 1977 Hadley, K., “Control Objectives Analysis,” National Petroleum Refiners Association Computer Conference, New Orleans, 1977. Hobbs, J. W., “Unifying Concepts for Fractionator Product Composition Control,” Proceedings of the Automatic Control Conference, San Francisco, 1977. Evans, L. B., “Impact of the Electronics Revolution on Industrial Process Control,” Science, Vol. 195, No. 4283, March 18, 1977. Hughart, C. L., “Designing Distillation Units for Controllability,” Instrumentation Technology, May 1977. Lieberman, N., “Instrumenting a Plant to Run Smoothly,” Chemical Engineering, September 12, 1977, pp. 140–154. Shinskey, F. G., “The Stability of Interacting Loops with and without Decoupling,” presented at the IFAC Symposium on Multivariable Control, Fredericton, New Brunswick, July 4–8, 1977. 1852 Control and Optimization of Unit Operations 1978 Buckley, P. S., Cox, R. K., and Luyben, W. L., “How to Use a Small Calculator in Distillation Column Design,” Chemical Engineering Progress, June 1978, pp. 49–55. Buckley, P. S., “Distillation Column Design Using Multivariable Control,” Instrumentation Technology, September/October 1978. Buford, B. N., Bush, B. A., and Staten, H. W., “Computers Conserve Energy in NGL Fractionators,” Oil & Gas Journal, December 1978. Doukas, N., “Control of Sidestream Columns Separating Ternary Mixtures,” Instrumentation Technology, June 1978. Doukas, N. and Luyben, W. L., “Economics of Alternative Distillation Configurations for the Separation of Ternary Mixtures,” I&CE Process Design and Development, Vol. 17, No. 272, 1978. Griffin, D. E., Parsons, J. R., and Smith, D. E., “The Use of Process Analyzers for Composition Control of Fractionators,” Proceedings of the ISA Spring Joint Conference, Houston, TX, May 1978. Latour, P. R., “Composition Control of Distillation Columns,” Instrumentation Technology, July 1978. Lamb, M. Y., “Computer Control of a Propylene Upgrading Unit,” 85th National AIChE Meeting, Philadelphia, PA, 1978. McCoy, R. D., “Adding Capabilities to Process Chromatography with Microprocessor-Based Programmers,” Proceedings of the ISA Joint Spring Conference, Houston, TX, May 1978. Mix, T. J., Dweck, J. S., Weinberg, M., and Armstrong, R. C., “Energy Conservation in Distillation,” Chemical Engineering Progress, April 1978. Painter, J. W., and Gonnella, J. L., “Improved Control of a Distillation Column Using a Minicomputer and an On-Line Gas Chromatograph,” Texas A&M Instrumentation Symposium, January 1978. Tolliver, T. L., and McCune, L. C., “Distillation Column Control Design Based on Steady State Simulation,” ISA Transactions, Vol. 17, No. 3, pp. 3–10, 1978. 1979 Watkins, R. N., Petroleum Refinery Distillation, 2nd ed., Houston, TX: Gulf Publishing Company, 1979. Douglas, J. M., Jafarey, A., and McAvoy, T. J., “Shortcut Techniques for Distillation Column Design and Control, Part 1: Column Design,” I&EC Process Design and Development, Vol. 18, pp. 197–202, April 1979. Van Horn, L. D., “Computer Control. How to Get Started,” Hydrocarbon Processing, September 1979. Chin, T. G., “Guide to Distillation Pressure Control Methods,” Hydrocarbon Processing, October 1979, 145–153. Black, J. W., “Model Estimates Analyzer Payouts,” Hydrocarbon Processing, September 1981. Shinskey, F. G., “Controlling Distillation Processes for Fuel-Grade Alcohol,” Instruments and Control Systems, December 1981. Thurston, C. W., “Computer-Aided Design of Distillation Column Controls,” Hydrocarbon Processing, Part 2, August 1981, p. 5. 1982 Rinne, R., Sunnel, H., Latour, P. R., and Payntex, K. K., “Experience with Distillation Unit Computer Control,” Hydrocarbon Processing, March 1982. Roffel, B., and Rijnsdorp, J. E., Process Dynamics, Control, and Protection, Ann Arbor, MI: Ann Arbor Science, 1982. DiBano, R. J., “Advanced Control: General Purpose Design vs. Working It Out in the Field,” Hydrocarbon Processing, August 1982. Dweck, J. S. and Mix, T. W., Conserving Energy in Distillation, MIT Press, 1982. 1984 Mix, P. E., The Design and Application of Process Analyzer Systems, New York: John Wiley & Sons, Inc., 1984. Stephanopoulos, G., Chemical Process Control, An Introduction to Theory and Practice, Englewood Cliffs, NJ: Prentice Hall, 1984. Barduhn, A. J., “Setting the Pressure at which to Conduct a Distillation,” Chemical Engineering Education, Vol. 18, No. 1, pp. 38–40, 1984. 1985 Deshpande, P. B., Distillation Dynamics and Control, Research Triangle Park, NC: Instrument Society of America, 1985. Buckley, P. S., Luyben, W. L., and Shunta, J. P., Design of Distillation Column Control System, Research Triangle Park, NC: Instrument Society of America, 1985. Gani, R., Romagnoli, J. A., and Stephanopoulos, G., “Control Studies in an Extractive Distillation Process: Simulation and Measurement Structure,” Chem. Engng. Commun., 40, pp. 281–302, 1985. 1986 Tsai, T. H., Lane, J. W., and Lin, C. S., Modern Control Techniques for the Process Industries, Vol. 23, Marcel Dekker, 1986. Hansen, T. T., and Jørgensen, S. B., “Optimal Open Loop Control of Binary Batch Distillation,” Chem. Eng. J., 33 , pp. 151–155, 1986. 1980 1987 Treybal, R. E., Mass-Transfer Operations, 3rd ed., New York: McGraw-Hill Book Company, 1980. Van Horn, L. D., “Crude Unit Computer Control. How Good Is It?” Hydrocarbon Processing, April 1980. Ryskamp, C. J., “New Control Strategy Improves Dual Composition Control,” Hydrocarbon Processing, June 1980. Soderstrom, E. D., ‘‘Computer Control for Energy Savings,” Chemical Engineering Progress, August 1980, pp. 60–62. Black, C., “Distillation Modeling of Ethanol Recovery and Dehydration Process for Ethanol and Gasohol,” Chemical Engineering Progress, September 1980. Andrews, A. J., and Griffin, D. E., “Performance Audits Evaluate Two Distillation Control Projects,” Oil & Gas Journal, December 8, 1980. Christensen, F. M., and Jørgensen, S.B., “Optimal Open Loop Control of Binary Batch Distillation with Recycled Waste Cut,” Chem. Eng. J., 34, pp. 57–64, 1987. 1981 Griffin, D. E., “Tighten Distillation Column Control and Save Energy,” Instruments and Control Systems, March 1981. © 2006 by Béla Lipták 1988 Balchen, J. G., Process Control, Structures, and Applications, New York: Van Nostrand Reinhold, 1988. Birky, G. J., McAvoy, T. J., and Tyreus, B. D., “Expert System for Design of Distillation Controls,” Proceedings of the ISA/88 International Conference and Exhibit, Houston, TX, October 1988. Murrill, P. W., Application Concepts of Process Control Theory, Research Triangle Park, NC: Instrument Society of America, 1988. Nichols, G. D., On-Line Process Analyzers, New York: John Wiley & Sons, Inc., 1988. Roat, S. D., Moore, C. F., and Downs, J. J., “A Steady-State Distillation Column Control System Sensitivity Analysis Technique,” Proceedings IEEE Southeast Con., 1988, pp. 296–300. 8.19 Distillation: Basic Controls 1989 Finco, M. V., Luyben, W. L., and Polleck, R. G., ‘‘Control of Distillation Columns with Low Relative Volatilities,” Ind. Eng. Chem. Res., Vol. 28, January 1989, pp. 75–83. Wassick, J. M. and Tummala, R. L., “Multivariable Internal Model Control for a Full-Scale Industrial Distillation Column,” Control Systems Magazine, January 1989, pp. 91–96. Seborg, D. E., Edgar, T. F., and Mellichamp, D. A., Process Dynamics and Control, New York: John Wiley & Sons, Inc., 1989. Hall, G. F., “Improve Process Performance by Using ChromatographDirected Control,” Proceedings of the ISA/89 International Conference and Exhibit, Part 1, Philadelphia, PA, October 1989. Christie, D. A., “The Top-Down Approach to Successful Process Control Projects,” Control, October 1989. Luyben, W. L., Process Modeling, Simulation, and Control for Chemical Engineers, 2nd ed., New York: McGraw-Hill Book Company, 1989. Kister, H. Z., Distillation Operation, New York: McGraw-Hill Publishing Company, 1989. Riggs, J. B., Sinha, R., “High Purity Distillation Control Using Nonlinear Process Model-Based Control,” ISA89, Philadelphia, PA, October 1989. Riggs, J. B., “Nonlinear Process Model-Based Control of a Distillation Column with a Sidestream Draw-Off,” presented at the annual AIChE meeting, San Francisco, CA, November 1989. Riggs, J. B., Sinha, R., and McDaniel, R., “Comparison of Control Techniques for High Purity Distillation Columns,” AIChE Spring National Meeting, Houston, TX, April 1989. 1853 Riggs, J. B., Watts, J., and Beauford, M., “Advanced Model-Based Control for Distillation,” National Petroleum Refinery Association Meeting, Seattle, WA, October 1990. Riggs, J. B., Watts, J., and Beauford, M., “Industrial Experience with Applying Nonlinear Process Model-Based Control to Distillation Columns,” ISA90, New Orleans, LA, October 1990. Riggs, J. B., “Advanced Model-Based Control of a Sidestream Draw Column,” at ISA 90, New Orleans, LA, October 1990. 1991 Coughanowr, D. R., Process Systems Analysis and Control, 2nd ed., New York: McGraw-Hill Book Company, 1991. Jensen, B. A., “Improve Control of Cryogenic Gas Plants,” Hydrocarbon Processing, May 1991. Papastathopoulou, H. S., and Luyben, W. L., “Control of a Binary Sidestream Distillation Column,” Industrial & Engineering Chemistry Research, April 1991, pp. 705–713. Rijnsdorp, J. E., Integrated Process Control and Automation, Amsterdam: Elsevier, 1991. Sandelin, P. M., Haeggblom, K. E., and Waller, K. V., “Disturbance Rejection Properties of Control Structures at One-Point Control of a Two-Product Distillation Column,” Industrial & Engineering Chemistry Research, June 1991, pp. 1182–1186. Sandelin, P. M., Haeggblom, K. E., and Waller, K. V., “Disturbance Sensitivity Parameter and its Application to Distillation Control,” Industrial & Engineering Chemistry Research, June 1991, pp. 1187–1193. 1990 1992 Papastathopoulou, H. S., and Luyben, W. L., “Turning Controllers on Distillation Columns with the Distillated Bottoms Structure,” Industrial and Engineering Chemistry Research, Vol. 29, September 1990, pp. 1859– 1868. Chien, I.-L., and Fruehauf P. S., “Consider IMC Tuning to Improve Controller Performance,” Chemical Engineering Progress, Vol. 86, No. 10, pp. 33–41, October 1990. Cingara, A., Jovanovic, M., and Mitrovic, M., “Analytical First-Order Dynamic Model of Binary Distillation Column,” Chemical Engineering Science, Vol. 45, No. 12, pp. 3585–3592, 1990. Kister, H. Z., Distillation Operation, New York: McGraw-Hill Book Company, 1990. Li, R., Olson, J. H., and Chester, D. L., “Dynamic Fault Detection and Diagnosis Using Neural Networks,” Proceedings of the Fifth IEEE International Symposium on Intelligent Control, Philadelphia, PA, September 1990, pp. 1169–1174. McGreavy, C., Dynamics and Control of Chemical Reactors, Distillation Columns, and Batch Processes, Pergamon Press, 1990. Papastathopoulou, H. S. and Luyben, W. L., “Potential Pitfalls in Ratio Control Schemes,” Industrial & Engineering Chemistry Research, October 1990, pp. 2044–2053. Pitt, M. J., Instrumentation and Automation in Process Control, New York: Horwood, 1990. Skogestad, S., Lundstrom, P., and Jacobsen, E. W., “Selecting the Best Distillation Control Configuration,” AIChE Journal, Vol. 36, pp. 753–764, 1990. Skogestad, S., Jacobsen, E. W., and Morari, M., “Inadequacy of SteadyState Analysis for Feedback Control. Distillate. Bottom Control of Distillation Columns,” Industrial & Engineering Chemistry Research, December 1990, pp. 2339–2346. Pandit, H. G., and Rhinehart, R. R., “Process Model-Based Control of a Nonideal Binary Distillation Column,” Proceedings of the Annual AIChE Meeting, Chicago, IL, November 1990. Pedersen, N. H., and Jørgensen, S. B., “A GC Subsystem for Fast On-Line Concentration Profile Measurement for Advanced Distillation Control,” Analytica Chemica Acta, 238, pp. 139–148, 1990. Fruehauf, P. S., and Mahoney, D. P. “Distillation Column Control Design using Steady-State Models, Usefulness and Limitations,” in Advances in Instrumentation and Control, Vol. 47, Part 1, Research Triangle Park, NC: Instrument Society of America, 1992, pp. 92–120. Skogestad, S., “Dynamics and Control of Distillation Columns: A Critical Survey,” Preprints IFAC Symposium, DYCORD +92, College Park, MD, pp. 1–25. Luyben, W. L., Practical Distillation Control,” New York: Van Nostrand Reinhold, October 1992. Koggersbøl, A., and Jørgensen, S. B., “Dynamics and Control of a Distillation Column with a Sidestream,” IChemE Symposium Series, 128, pp. A429–A449, 1992. © 2006 by Béla Lipták 1993 Fruehauf, P. S., and Mahoney, D. P., “Distillation Column Control Design Using Steady State Models: Usefulness and Limitations,” ISA Transactions, Research Triangle Park, NC. 1993. Balchen, J. G., Dynamics and Control of Chemical Reactors, Distillation Columns, and Batch Processes, Pergamon Press, April 1993. Ganguly, S., “Model Predictive Control of Distillation,” ISA/93 Technical Conference, Chicago, IL, September 1993. 1994 Gokhale, V., Shukla, N., and Munsif, H., “Analysis of Advanced Distillation Control on a C3 Splitter and a Depropanizer,” 1994 AIChE National Annual Meeting, San Francisco, CA, November 1994. Fruehauf, P. S., and Mahoney, D. P., “Improve Distillation Column Control Design,” Chemical Engineering Progress, March 1994. 1995 Fleming, B., and Sloley, A. W., “Feeding and Drawing Products: The Forgotten Part of Distillation,” Proceedings of the ChemShow and Exposition, New York, December 1995. 1854 Control and Optimization of Unit Operations Musch, H. E., and Steiner, M., “Robust PID Control for an Industrial Distillation Column,” Control System Magazine, Vol. 15, No. 4, 46–55, 1995. Hurowitz, S. E., and Gokhale, V., “A Dynamic Model of a Superfractionator: A Test Case for Comparing Distillation Control Techniques,” DYCORD ‘95, 4th IFAC Symposium, Helsingor, Denmark, June 1995. Lundstrom, P., and Skogestad, S., “Opportunities and Difficulties with 5 × 5 Distillation Control,” J. Process Control, Vol. 5, 249–261, 1995. Rawlings, J. B., “Dynamics and Control of Chemical Reactors, Distillation Columns, and Batch Processes (Dycord+‘95),” a Postprint Volume from the 4th IFAC Symposium on Dynamics and Control of Chemical Reactors, Distillation Columns and Batch Processes (DYCORD ‘95), Helsingor, Denmark, June 1995. Diwekar, U. M., “Batch Distillation: Simulation, Optimal Design, and Control (Series in Chemical and Mechanical Engineering),” Taylor & Francis, September 1995. Banerjee, A., and Arkun, Y., “Control Configuration Design Applied to the Tennessee Eastman Plantwide Control Problem,” Computers & Chem. Eng., 19(4), 453–480, 1995. 1996 Koggersbøl, A., Andersen, R., Nielsen, J. S., Jørgensen, S., “Control Configuration for Energy Integrated Distillation,” Computers & Chem. Eng., 20 (supplement), pp. S853–S858, 1996. 1997 Linsley, J., “New, Simpler Equations Calculate Pressure-Compensated Temperatures,” Oil & Gas Journal, May 24, 1997, 58–64. Ming T. Tham, “Distillation,” Base Document URL: http://lorien.ncl.ac.uk/ ming/distil/distil0.htm, October 1997. Anderson, N. A., Instrumentation for Process Measurement and Control, 3rd edition, Boca Raton, FL: CRC Press, October 1997 Hurowitz, S. E. and Anderson, J. J., “Distillation Configuration Selection for Dual Composition Control,” AIChE Spring National Meeting, Houston, TX, April 1997. Hurowitz, S. E. and Anderson, J. J., “Control of High Purity Distillation Columns,” Control 97 Conference, Sydney, Australia, October 1997. Mahoney, D. P. and Fruehauf, P. S., “An Integrated Approach for Distillation Column Control Design Using Steady-State and Dynamic Simulation,” Aspentech technical articles, March 1997. www.aspeutech.com/ corporate/press/publications. Skogestad, S., “Dynamics and Control of Distillation Columns: A Tutorial Introduction,” Trans. IChemE., Vol. 75, Part A, pp. 539–562, 1997. Riggs, J. R., “Improve Distillation Column Control,” Chemical Engineering Progress, October 1998, 31–47. Stichlmair, J. G. and Fair, J. R., Distillation: Principles and Practices, New York: John Wiley & Sons, 1998. 1999 Eker, I. and Sakthivel, K., “Automation & Lube Oil Additives Blending Plant Using an S88.01 Consistent Batch Software: A Case Study,” Proceedings of the World Batch Forum, San Diego, CA, April 1999. Hurowitz, S., Anderson, J., Duvall, M., and Riggs, J.B., “An Analysis of Controllability Statistics for Distillation Configuration Selection,” presented at the AIChE Annual Meeting, Dallas, TX, November 1999. 2000 Andrew W. Sloley, “Steady Under Pressure: Distillation Pressure Control,” presented at the American Institute of Chemical Engineers Spring Meeting, March 6–9, 2000. Betlem, B.H.L. “Batch Distillation Column Low-Order Models for Quality Control Program,” Chemical Engineering Science, 55, pp. 3187–3194, 2000. Roffel, B., Betlem, B. H. L., and De Ruijter, J. A., “Modeling and Control of a Cryogenic Distillation Column,” Computers and Chemical Engineering, 24, pp. 111–123, 2000. Roffel, B., “Distillation: Instrumentation and Control Systems,” in Encyclopedia of Separation Science, London: Academic Press, 2000. Willis, M. J., “Selecting a Distillation Column Control Strategy (a basic guide),” 2000. http://lorien.ud.cc.uk/ming/control/g 2002 Florez, M., “Batch Distillation: Practical Aspects of Design and Control,” Proceedings of the World Batch Forum, Woodbridge Lake, NJ, April, 2002 Cook, B., Engel, M., Landis, C., Tedeschi, S., and Zehnder, A., “Synthesis of Optimal Batch Distillation Sequences,” Proceedings of the World Batch Forum, Woodbridge Lake, NJ, April 2002. 2003 Kralj, F., “Application of the S88 Model in the Control of Continuous Distillation Facilities,” Proceedings of the World Batch Forum, Woodbridge Lake, NJ, April 2003. Jones, M., and Kilian, A., ‘‘Tricky Pressure Control in Distillation Column,” July, 2003. http://instrumentation.co.za/regulator 1998 2004 Betlem, B. H. L., Krijnsen, H. C., and Huijnen, H., “Optimal Batch Distillation Control Band on Specific Measures,” Chemical Engineering Journal, 71, pp. 111–126, 1998. © 2006 by Béla Lipták Hurowitz, S., Anderson, J., Duvall, M., and Riggs, J. B., “Distillation Control Configuration Selection,” submitted to J. Process Control, March 2004.


