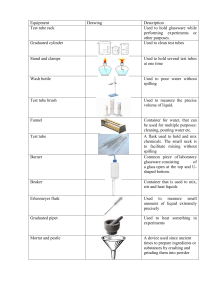
CHM 30: Studio L1 Laboratory Glassware Introduction When you are in a scientific laboratory you need to measure and record the amount of a solid, liquid, or gas that you are using in an experiment. In this class, you will mostly be dealing with liquids and solids. The best option for measuring the amount of a solid is by using a balance to determine its mass. However, when it comes to liquids you have various possibilities, each with varying amounts of uncertainty and ease of use associated with them. Scientists use various terms when describing measurements and equipment. They are often used interchangeably although each is defined in its own way. • Accuracy: How closely a measured value agrees with the true value. • Precision: How closely a set of measured values agree with each other. • Tolerance: The range within which multiple identical measurements will fall when correctly using a piece of equipment. For example, if you were to use a 10 mL volumetric pipet with a stated tolerance of 0.05 mL then you would expect the delivered volume to fall within the range of 9.95 mL to 10.05 mL. This is related to the uncertainty in a measurement: for this class you can treat the two interchangeably. The uncertainty of a measurement is represented by the number of significant figures: the last significant digit represents the uncertain digit. For example, the burets you will use have an uncertainty of ±0.02 mL: therefore, volumes delivered from it are always reported to the hundredth mL (i.e. you would record 21.00 mL instead of 21 mL). There are six types of glassware that you will be using for liquids: beakers, Erlenmeyer flasks, volumetric flasks, graduated cylinders, pipets, and burets. Beakers and Erlenmeyer flasks may not have volume markings on the side. They are typically used as secondary containers and any markings are estimations meaning they have large uncertainty associated with them. Volumetric flasks are used to prepare solutions. For example, if you wanted to make a saltwater solution with a specific concentration you would add the necessary amount of solid salt (or concentrated salt solution), then dilute to the full volume of the flask. Graduated cylinders come in a variety of sizes and the uncertainty in volume increases as the size of the cylinder increases. Therefore, it is advisable to choose a graduated cylinder with a total volume that is comparable to the volumes needed in the experiment. Pipets come in two varieties: a graduated version that can be used to add varying amounts of liquid and a volumetric version that is designed to dispense one volume. You will be using the graduated pipet today. Finally, the buret is used for titrations and adds very accurate volumes. The tolerance of most volumetric glassware is stated on the glass itself (see the end of this section for typical values). You may also see TC for To Contain or TD for To Deliver stamped on the glass. Volumetric flasks are virtually always stamped with TC because they are designed to hold a very specific volume. However, the amount that is poured out will be a little less due to residual drops of liquid left in the flask. Pipets, graduated cylinders, and burets, on the other hand, are usually designed to deliver a set volume. Therefore, they contain slightly more liquid than the stated volume and the amount that is poured out will match the stated volume within the tolerance limit. You will also see the temperature the glassware was calibrated at (usually 20oC). In this class, you will assume that any difference in temperature is irrelevant. 1 For graduated glassware, the markings give you the certain digits. However, you should read between the markings and report one extra digit. This is where the uncertainty lies. For example, if the water level in your graduate cylinder is halfway between the 87mL and 88mL lines then you should record your measurement as 87.5mL. A good rule is to estimate one more place value than what the lines on the glassware indicate. Keep in mind that this does NOT necessarily reflect accuracy. You usually assume that the markings are accurate, but this may not always be the case. Water forms a meniscus, a curve down, when in glass. To correctly read the volume, you need to have your eyes level with the bottom of the meniscus. See diagram for example. In theory you would use the piece of glassware with the smallest amount of uncertainty to give a very high accuracy and precision. However, in some cases it is not necessary and often saves time to use a less accurate/precise piece of equipment (i.e. using a graduated cylinder rather than a pipet). The purpose of this lab is for you to learn the advantages and disadvantages of the types of glassware mentioned above with regards to their accuracy, precision, and uncertainty. You will determine how accurate your volumes are by weighing each volume of liquid then using density to convert your measured mass to a volume. Density is defined as the ratio of the mass to volume. Densities of liquids and gases are commonly expressed as g/mL and g/L, respectively. Densities of solids are normally reported in g/cm3. Note that the units of mL and cm3 are equivalent. Although this experiment focuses upon the density of water at room temperature and one atmosphere of pressure, it is important to recognize that density is a function of both temperature and pressure for all phases of homogeneous substances. The densities of pure liquids and solutions tend to decrease with rising temperatures. For example, mercury thermometers work because heat causes mercury’s atoms to spread out more causing the decrease in density. Densities of gases, at constant pressure, decrease drastically with increasing temperature as is seen with a hot air balloon. Whereas, the densities of inorganic solids, e.g. table salt, at atmospheric pressure do not change greatly with temperature. Liquid water provides a most remarkable example of a change in density with temperature as shown in the figure at the right. The density of water is a maximum of 1.000g/mL at 4oC and decreases at both higher and lower temperatures. For practical purposes, the density of water is estimated as 1.00 g/mL near room temperature. Ice floats because the density of ice at atmospheric pressure is only 90% of that of liquid water. 2 The following are typical tolerances for the glassware in this lab. Use these values only if the glassware does not have its tolerance listed on it. Glassware Tolerance Glassware Tolerance Beakers ± 5% 10 mL Graduated Pipet ± 0.05 mL Erlenmeyer Flasks ± 5% 10 mL Volumetric Pipet ± 0.02 mL 10 mL Graduated Cylinder ± 0.1 mL 50 mL Volumetric Flask ± 0.05 mL 100 mL Graduated Cylinder ± 0.6 mL 50 mL Buret ± 0.02 mL Calculations: You will convert your masses to volumes for each trial (excluding the buret) by using the density you calculate in Part 1. Percent Error: The experimental values are the given class average (in the post lab data) while the theoretical values are the volumes stated in the procedure. For example, for the 100mL beaker you are measuring 50mL but for the 10mL graduated cylinder you are measuring 10mL. A percent error can be a positive or negative value. % 𝑒𝑟𝑟𝑜𝑟 = 𝐸𝑥𝑝𝑒𝑟𝑖𝑚𝑒𝑛𝑡𝑎𝑙 𝐴𝑣𝑒𝑟𝑎𝑔𝑒 − 𝑇ℎ𝑒𝑜𝑟𝑒𝑡𝑖𝑐𝑎𝑙 𝑉𝑎𝑙𝑢𝑒 × 100 𝑇ℎ𝑒𝑜𝑟𝑒𝑡𝑖𝑐𝑎𝑙 𝑉𝑎𝑙𝑢𝑒 Standard Deviation using a TI graphing calculator: 1. Press "STAT." Move the cursor to "EDIT" and press "1." 2. Type your data into one of the columns. It is easier to use column L1 3. Press "STAT" and move the cursor to "CALC." 4. Put the cursor on "1-Var Stats" and press "ENTER" (you may have to press it twice). Your mean and standard deviation (Sx) will appear on the screen. 3 Procedure Material: - 100 mL beaker with volume markings - 125 mL Erlenmeyer flask with volume markings - 10 mL graduated pipet - 10 mL graduated cylinder - 100 mL graduated cylinder - 50 mL buret - 50 mL volumetric flask - A 100 or 150 mL beaker that can be used to hold the liquid that you are dispensing. Note that this lab can be done in any order, but make sure you put the data in the correct spot. Some of the glassware in this experiment will have its uncertainty written on it. Using TD equipment (the pipets in particular) there may be a small amount of residual liquid left in the glassware. This should not be blown out as doing so will deliver an excess volume of solution. Always wipe the outside (and sometimes the inside – see the directions) of any glassware to dry it before weighing. Part I 50 mL buret 1. Weigh a 125 mL Erlenmeyer flask and record the mass under initial trial on page 7. This is what you will be transferring your water into. Does NOT need to be dry. 2. Set up your buret and fill it with water. Make sure there are no air bubbles in the tip. The proper setup and technique will be demonstrated in the prelab briefing. 3. Record the initial volume of water in the buret. • All volume readings must be recorded to ±0.02 mL. 4. Dispense 5-7 mL of water into the flask. • Do NOT try to deliver a specific volume – anyplace in this range is acceptable. 5. Record the final volume of water in the buret. 6. Record the total mass of the flask and water. 7. Do NOT dump the water. Instead, repeat steps 4 through 6 adding an additional aliquot (sample) of 57 mL of water to the flask each time. Do this for a total of 4 trials. Part 1 Calculations: • Volume of water added in each trial • Mass of 5-7mL water aliquot for each trial • Density of water for each trial • Mean (average) density of all the trials • Standard deviation of the four trials. You will use your average density for later calculations so make sure that it is correct. 4 Part II 100 mL beaker 1. Weigh a dry 100 mL beaker using a balance at your lab bench and record the mass in the table provided on page 7. For all subsequent measurements you should use the same piece of glassware and balance. 2. Add 50 mL of water (tap water is fine) to the beaker using the markings on the glassware. Remember to read the water volume with the bottom of the meniscus at eye level. 3. Weigh and record the mass of beaker with water in the table provided. 4. Dump the water and repeat these steps two more times: dry the beaker thoroughly using paper towels between trials. 125mL Erlenmeyer flask 1. Weigh and record the mass of a 125 mL Erlenmeyer flask. Remember to use the same flask and balance for all subsequent measurements. 2. Add 50 mL of tap water to the flask using the markings on the glassware. Remember to read the water meniscus correctly. 3. Weigh and record the mass of flask with water. 4. Dump out the water. Drying the flask is more problematic than drying the beaker due to its shape. Therefore, after you have dumped the water reweigh the flask. Record this weight as the mass of flask for the next trial. 5. Repeat these steps until you have three measurements. 50 mL volumetric flask 1. Weigh and record the mass of a dry 50 mL volumetric flask. 2. Add 50.00 mL of tap water to the flask. • There is a mark on the neck of the flask: the bottom of the meniscus needs to be exactly on this line. • Add tap water until the water level is at the bottom of the neck, then slowly fill it to the mark using the bottle of deionized water at your lab bench. 3. Weigh and record the mass of your flask with water. 4. Dump the water and repeat two more times: you do not have to dry the flask. 10 mL graduated cylinder 1. Weigh and record the mass of a dry 100 or 150 mL beaker. We will be using this as a secondary container to transfer the water into. Use the same beaker as before. 2. Using a 10mL graduated cylinder measure out 10.0 mL of water • Like before, use the deionized water squirt bottles to get the bottom of the meniscus exactly on the 10.0 mL mark. 3. Pour the 10.0mL of water into the pre-weighed beaker and record the mass. 4. Do NOT dump the water. Add an additional 10.0 mL aliquot of water from the graduated cylinder to the beaker and record the mass. Repeat this for a total of three trials. 5 Part II Continued 100 mL graduated cylinder 1. Weigh and record the mass of a dry 100 or 150 mL beaker. 2. Using a 100 mL graduated cylinder measure out 10.0mL of water. 3. Pour the water into your pre-weighed beaker and record the mass. 4. Like in the previous procedure do NOT dump the water: add an additional 10.0 mL sample of water from the graduated cylinder into the beaker and record the mass. Repeat this one more time for a total of 3 trials 10 mL graduated pipet 1. Weigh a dry 100 or 150 mL beaker and record the mass. 2. Pipet 10.00 mL of water into the graduated pipet and dispense it into your pre-weighed beaker and record the mass. This technique will be shown in the prelab briefing. 3. Do NOT dump the water: pipet an additional 10.00 mL aliquot of water into the beaker and record the mass. Repeat this one more time for a total of 3 transfers from your pipet. 1. 2. 3. 4. 5. Clean Up You will lose points if you do not clean up your station. Make sure you recorded all your data. Put your station back the way you found it. (Refer to the picture at your station) Wash your hands. Your TA must sign off on your Post Lab Handout before you can leave the lab. Follow Covid guidelines and the directions at the PPE station when leaving the lab. You must finish and turn in the handout before the end of your lab period. Part II Calculations: These are similar for all glassware in Part II. • Mass of water for each trial or aliquot. • Volume of water for each trial or aliquot o Convert the mass of water to mL by using the calculated average density from part I of this experiment. • Average volume of water • Percent error o Remember to use the given class average as the experimental value and the volume that the procedure told you to measure for each piece of glassware as the theoretical value. 6 CHM 30: Studio L1 Laboratory Glassware NAME:____________________________________________ DATE: ________________________ LAB PARTNER: ____________________________________ TA NAME: ____________________ STUDIO DAY & TIME: _____________________ STATION CLEAN: ___________(TA initials) Post lab You need to complete and turn in pages 7-11 BEFORE the end of your lab session. 50 mL buret ± 0.02 mL Sample No. Buret reading (mL) Volume of water added (mL) Mass of flask + water (g) Mass of water in aliquot (g) initial *No water in this mass* 1 2 3 4 Average value of density = ________________ g/mL *You will use the average density for the remaining volume calculations. * Standard deviation of density = ________________ g/mL 7 Density of water (g/mL) 100 mL beaker ± 5% Trial 1 Trial 2 Trial 3 Mass of dry beaker Mass of beaker + water Mass of water Volume of water *calculated* Average volume of your three trials: _________________ Given class average and standard deviation for volume of water: 53.3 ± 2.9 mL % error: _________________ 125 mL Erlenmeyer flask ± 5% Trial 1 Trial 2 Mass of flask Mass of flask + water Mass of water Volume of water *calculated* Average volume of your three trials: _________________ Given class average and standard deviation for volume of water: 51.2 ± 2.4 mL % error: _________________ 8 Trial 3 50 mL volumetric flask ± 0.05mL Trial 1 Trial 2 Trial 3 Mass of flask Mass of flask + water Mass of water Volume of water *calculated* Average volume of your three trials: _________________ Given class average and standard deviation for volume of water: 49.94 ± 0.11 mL % error: _________________ 10 mL graduated cylinder ± 0.1mL Trial 1 Trial 2 Mass of beaker prior to addition Mass of beaker + water Mass of water Volume of water *calculated* Average volume of your three trials: _________________ Given class average and standard deviation for volume of water: 10.12 ± 0.19 mL % error: _________________ 9 Trial 3 100 mL graduated cylinder ± 0.6mL Trial 1 Trial 2 Trial 3 Mass of beaker prior to addition Mass of beaker + water Mass of water Volume of water *calculated* Average volume of your three trials: _________________ Given class average and standard deviation for volume of water: 9.86 ± 0.34 mL % error: _________________ 10 mL graduated pipet ± 0.05mL Trial 1 Trial 2 Mass of beaker prior to addition Mass of beaker + water Mass of water Volume of water *calculated* Average volume of your three trials: _________________ Given class average and standard deviation for volume of water: 9.89 ± 0.13 mL % error: _________________ 10 Trial 3 Post Lab Questions 1. Percent error and standard deviation were found for each type of glassware in this experiment. How do these calculations relate to accuracy and precision? 2. You are working in a laboratory and need to measure exactly 10.00mL of a solution multiple times for an experiment. Which piece of glassware from Part II would you choose? Explain your choice by discussing the glassware’s accuracy, precision, and uncertainty. 3. When working with liquids in a laboratory, we never pipet directly from a solution bottle because this will cause contamination. We also do not pour directly from a solution bottle into narrow-necked glassware being used for measurements. This can lead to spills and incorrect volumes. Instead, we first pour the solution into a secondary container then into the narrow-necked glassware. Glassware used as secondary containers are typically not very accurate but are easy to pour into and from. Which piece of glassware from Part II would you choose as a secondary container? Explain your reasoning. 11