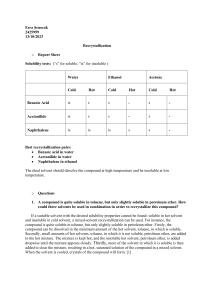
Recrystallization o Report Sheet Solubility tests: (“s” for soluble, “is” for insoluble ) Water Ethanol Acetone Cold Hot Cold Hot Cold Hot Benzoic Acid is s s - s - Acetanilide is s s - s - Naphthalene is is is s s - Best recrystallization pairs: Benzoic acid in water Acetanilide in water Naphthalene in ethanol The ideal solvent should dissolve the compound at high temperature and be insoluble at low temperature. o Questions 1. A compound is quite soluble in toluene, but only slightly soluble in petroleum ether. How could there solvents be used in combination in order to recrystallize this compound? If a suitable solvent with the desired solubility properties cannot be found: soluble in hot solvent and insoluble in cold solvent, a mixed-solvent recrystallization can be used. For instance, the compound is quite soluble in toluene, but only slightly soluble in petroleum ether. Firstly, the compound can be dissolved in the minimum amount of the hot solvent, toluene, in which is soluble. Secondly, small amounts of hot solvents, toluene, in which it is not soluble, petroleum ether, are added to the hot mixture. The mixture is kept hot, and the insoluble hot solvent, petroleum ether, is added dropwise until the mixture appears cloudy. Thirdly, more of the solvent in which it is soluble is then added to clear the mixture, resulting in a hot, saturated solution of the compound in a mixed solvent. When the solvent is cooled, crystals of the compound will form. [1] 2. How can you separate the mixture of meta-nitrobenzoic acid ana para-benzoic acid? To separate a mixture of meta-nitrobenzoic acid and para-benzoic acid, use a technique known as chromatography. By using this technique, two acids are separated according to how polar they are. After being dissolved in a solvent, the mixture is run through a stationary phase-filled column. Typically, silica gel with polar groups on its surface serves as the stationary phase. In comparison to the less polar compound (para-benzoic acid), the more polar compound (meta-nitrobenzoic acid) will interact more strongly with the stationary phase and move through the column more slowly. The two compounds are eventually separated as a result of this. Meta-nitrobenzoic acid and para-benzoic acid can also be separated by converting the mixture into the corresponding methyl ester mixture through the use of diazomethane or other methods. Simple chromatography on silica gel should make it simple to separate the two regioisomeric esters. The separated esters should then hydrolyze with CH3ONa/CH3OH with little difficulty. [2] 3. During recrystallization, a yellow solution of a compound in hot recrystallizayion solvent was treated with acitivated carbon and then filtered through fluted paper. On cooling, the filtrate yielded gray crystals, although the compound is colorless. Explain why the crystals were grey, and how can you prevented this? The crystals' grey colour is most likely caused by the presence of impurities that activated carbon cannot remove. It's possible that impurities existed in the original compound or were added during the recrystallization procedure. During crystallization, impurities may also become trapped in in the crystal's lattice. It is crucial to make sure that the compound is free of all impurities prior to recrystallization in order to prevent this. This can be accomplished by employing an appropriate solvent for recrystallization and making sure it is impurity-free. Additionally, impurities that are still in solution prior to crystallization should be eliminated using activated carbon. Larger, more pure crystals can also be created by slowly cooling the solution. [3] o References http://www.orgchemboulder.com/Labs/Experiments/12%20-%20Recrystallization.pdf [1] http://www.orgsyn.org/demo.aspx?prep=CV1P0391 [2] https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Suppl emental_Modules_%28Physical_and_Theoretical_Chemistry%29/Physical_Properties_of_Matter/Solu tions_and_Mixtures/Case_Studies/RECRYSTALLIZATION [3] Peynircioğlu, N. Bekir (Editor). Organic Chemistry Laboratory Manual. Middle East Technical University Department of Chemistry, 2022.