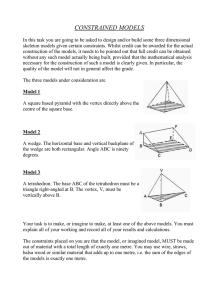
Science Notes (Tuesday 14 March 2023) Physics - measurements 物理学 SI Units: Length = metre 长度 Mass = kilogram 质量 Time = second 时间 1. Other units of length. a. Tera metre = 100000000000 metre (10 *12) b. Giga metre = 100000000 metre (10 *9) c. Mega metre = 100000 metre (10 *6) d. e. f. g. h. i. Kilometre (km) = 100 metre (10 *3) Metre (m) = 1 metre Centimetre (cm) = 0.01 metre (10 *-2) Millimetre (mm) = 0.001 metre (10 *-3) Micrometre (um) = 0.0000001 metre (10 *-6) Nanometre (nm) = 0.0000000001 metre (10 *-9) ***** 如果 10 的隔壁没有 “减”的符号, 就是向后移。如果 “减”的符号,就要向 前移。***** ***** big -> small = multiply***** ***** small ->big = divide***** --------------------------------------------------------------------2. (a) Other units of mass. a. Megatonne (mt) = 1000000000 kg (10 *9) b. c. d. e. Tonne (t) = 100 kg (10 *3) Kilogram (kg) = 1 kg Gram (g) = 0.001 kg (10 *-3) Milligram (mg) = 0.000001 kg (10 *-6) -----------------------------------------------------------------------------------------------------3. Other units of times. a. Day (d) = 86400 seconds / 1440 minutes / 24 hours b. Hour (h) = 3600 seconds / 60 minutes c. Minute (min) = 60 seconds d. Seconds (s) e. Millisecond (ms) = 0.001 seconds Science Notes (Monday 20 March 2023) Biology – Characteristic living things and cells 1. Unique of living things All living things have to perform 7 life characteristics. 2. MRS GREN M = Movement R = Respiration S = Sensitivity G = Growth R = Reproduction E = Excretion N = Nutrition MRS GREN: A. M=Movement An action by an organism causing a change in position or place. Example: A bird flapping its wings. B. R = Respiration The chemical reactions (化学反应) in cells that break down nutrient molecules (oxygen) and release energy. Example: A person breathing. C. S = Sensitivity The ability to detect and respond to change in the environment. Example: 5 senses (五官) Eyes – sight Mouth – tasting Skin – touch Ear – hearing Nose – smell D. G = Growth A permanent increase in size. Example: A bean ---------> a sprout *** after it grows, it won’t shrink or become smaller. *** E. R = Reproduction The processes that make more of the same kind of organism. Example: Dog + dog = puppy Man + woman = baby F. E = Excretion Removal from organisms of toxic materials and substances in excess of requirements. Example: Excreta, urine and sweat. G. N = Nutrition Taking in of materials for energy, growth and development. Example: Salmon fish: omega 3 Vegetables: fibres Fruits: vitamins Grains, breads: carbohydrate Fish, egg, beans: protein Science Notes (Tuesday 21 March 2023) Chemistry 1. Summary of Solid, Liquid and Gas. (1.0) A. Solid: a. Fixed volume b. Has definite shape c. High density d. Arrangement: Particles closely and completely arrange. e. Force of attraction: Very strong (restrict movement, particles held in fixed positions.) B. Liquid: a. Fixed volume b. No definite shape (takes shape of containers.) c. Moderate to high density. d. Arrangement: Less closely and compactly arranged than solid. e. Force of attraction: Weaker than solid. (Particles slide across each other’s.) C. Gas: a. b. c. d. No fixed volume (*expand to fill the container*) No definite shape (*takes shape of container*) Low density Arrangement: Particles not arranged. (Compactly and widely spaced) e. Force of attraction: Weakest (Particles move randomly in all direction at great speed.) ------------------------------------------------------------------------------------------------------ Summary of Solid, Liquid and Gas. (2.0) Main Characters Type of movement Solid Vibrate and rotate at fixed position. Vibrate and slide over each other. Liquid Gas Move rapidly and freely in all direction. Kinetic energy (speed of moving of particles) Low High on average Very high, move at high speed Ability to press / compressibility Difficult (*particles closely packed*) Not easy (*particles packed quite close*) Easy (*particles very far apart*) 2. Effects on matter a. Temperature effects on matter: Solid, Liquid and Gas increase in volume (Expansion) when temperature increase. The decrease in volume (contraction) when temperature is lowered. Effect much bigger for gas than solid and liquid. **** First point and second point means 热胀冷缩。 **** Expansion = 增大 (for volume *体积*) Contraction = 缩小 (for volume *体积*) b. Pressure effects on matter: Volume of gas at fixed temperature can be easily reduced by increasing pressure of gas. (*gas is easy to be compress*) Liquid only slightly compressible. Volume of solid unaffected by changing the pressure. Physics 1. Density – 密度 Mass Density is the mass per unit volume. (*density = mass / volume*) Density = mass ÷ volume Density Or Volume Density D = m ÷ v Volume = mass / density Mass = density * volume 2. Units for density Density = mass (kg) / volume (m *3 / cm *3) (Density) Kg ÷ m *3 Kg ÷ cm *3 **体积 = m *3 / cm *3 (metre cube / centimetre cube) **面积 = m *2 / cm *2 (metre square / centimetre square) 3. Densities of solids, liquids and gases. The density of solids and liquids will be similar. Gases always have a much lower density. 4. Weight and mass Weight is a force. It tells us how heavy something is. It is measured in Newton’s (N). It changes depending upon the size of gravity. (trip to the moon.) Mass tells us how much substance there is in an object. It is measured in kilograms (kg). It never changes. On earth, we multiply mass by 10 to get weight. Example: Weight = mass * 10 70 (mass) * 10 = 700 (weight) ***** weight and mass are NOT THE SAME!!!***** Science Notes (Friday 7 April 2023) Biology – Cells 1. Introducing cells Cells 细胞 -> tissues 组织 -> organ 内脏 -> organ system 身体系统 -> organism 成体 -----------------------------------------------------------------------------------------------------Cell organization 1. Cell – Epithelial cell Smallest unit that can carry out life’s activities. 2. Tissues – Epithelial tissue Group of cells working together to perform specific jobs. 3. Organ – Stomach Group of tissue working together to perform specific jobs. 4. Organ System – Digestive System Group of organs working together to perform specific jobs. 5. Organism The body of the living things. -----------------------------------------------------------------------------------------------------Cell, basic structure of all living creatures. 1. Unicellular Organisms that are made of ONLY ONE CELL. Examples: Bacteria 细菌 Yeast 酵母菌 2. Multicellular Organisms that are made of MORE THAN ONE CELL. Example: Plants & Animals 动植物 Humans 人类 Human eye -> magnifying glass -> light microscope -> electronic microscope. 3. Magnification: Magnification = Size in drawing / size of actual size Examples: 40 mm / 8 mm = 5 times or × 5 30 mm / 10 mm = 3 times or × 3 Physics 1. Irregular objects. The volume of a liquid can be determined using a measuring cylinder. The volume of irregular objects has to be found by displacement. 2. (Q & A) Question: You are asked to find the density of the stone. What will you do? Answer: 1. Finding mass Weigh the stone on a weighing scale 2. Finding volume Put a certain volume of water in a measuring cylinder. Add the stone into the water. Calculate the change in volume. 3. Calculating density Calculate the density by dividing the mass of the stone by the volume of water displaced. Science Notes – Physics (Friday 28 April 2023) Today’s Topic – Energy 1. Unit of energy JOULE: small measurement of energy called The amount of energy in food is measured in kilojoules (KJ) 1 KJ = 1000 J / 1 kilojoule = 1000 joules A calorie can also be used to measure energy. (*1 calorie = 4.2 J*) 2. Types of Energy Magnetic energy: energy in magnets and electromagnets Heat energy: also called thermal energy. Example: A cup of hot tea has thermal energy in the form of kinetic energy from its particles. Potential energy (gravitational): energy in an object above ground due to the pull of gravity. Potential energy (stored): stored energy due to the position of the object. Light energy: also called as radiant energy. Electrical energy: energy in moving charges or static electric charges. Chemical energy: stored energy in fuel, foods and batteries. Some chemical reactions can release energy. Sound energy: energy released by vibrating objects. Nuclear energy: stored in the nuclei of atoms. Light energy: also called as radiant energy. Kinetic energy: the energy in moving objects – also called movement energy. ***** If you can’t remember it, just remember “Mr Harry Potter Needs Electricity and Light to Chase Serious Killers” ***** 3. Change energy Different types of energy can be transferred from one type to another. Energy transfer diagrams show each type of energy, whether it is stored or not, and the processes taking place as energy is transferred. Examples for change energy: Light energy from sun converted into chemical energy by photosynthesis. Kinetic energy (winding up the toy) sound energy (dog toy barking) Science and Mathematics Tuition (Friday 12 May 2023) Today: Science – physics Today’s topic: sounds 1. What causes sound? Sounds are caused by vibrations. A vibration is a fast backwards and forwards movement that repeats many times. Loud sound has more sound energy than soft sound. 2. Describing sounds One Direction, how would you describe sound to a deaf person? I would talk about how loud the sound is or what pitch it is. For example, I’m still young so my voice is quite high pitched ;) The pitch of an object is called its frequency (f) and is measured in Hertz (Hz). Sound wave moving to the right: 3. Adele, what is amplitude? Loud sounds are made by the vibrations that are large. We say the vibrations have a large amplitude. The amplitude of a vibration is how far something moves from its rest position when it’s moving backwards and forwards. Drawing below show’s that sound waves showing amplitudes: Watch out!! Remember that the amplitude shows how far the particle has moved from its starting position in either a forwards or a backwards direction. So the amplitude is half the total height of the wave from its trough to its crest. 4. Q & A Session Question: What is amplitude? Draw one sound wave and label the amplitude. Answer: 5. What is pitch? Sounds can be high or low. The pitch depends on how many vibrations the source of the sound produces in one second. Frequency – number of vibrations per second. Measured in hertz (Hz) 1 Hz = one vibration/second 2000 Hz = 2000 vibrations/seconds Figure 2 shows a low frequency sound Figure 3 shows a high frequency sound 6. Q & A Session Question: a. Which frequency is a high frequency? b. Which frequency represents a guitar? c. Which frequency represents a whistle? A. B. Answer: A. Low frequency (waveform of bass guitar) B. High frequency (waveform of a whistle) 7. Wave patterns Connect a loudspeaker and an oscilloscope to a signal generator. Signal generator produces sounds at different frequencies. The oscilloscope displays a picture of the sound as a wave pattern on a screen. Example: 8. Pitch and amplitude The height of the oscilloscope signal shows the amplitude of the sound. This shows how much energy a sound wave has. How often the wave goes up and down gives the frequency of the vibration. As frequency increases, the waves on the screen get closer together and have a shorter wavelength. Figure 4 shows a quiet sound and figure 5 shows a loud sound of the same frequency. Loud sounds have a bigger amplitude than soft sounds. 9. Summary: Loud sounds have a large amplitude. Example: shout. Soft sounds have a small amplitude. Example: whisper. High pitched sounds have a high frequency. Example: whistle. Low pitched sounds have a low frequency. Example: guitar.