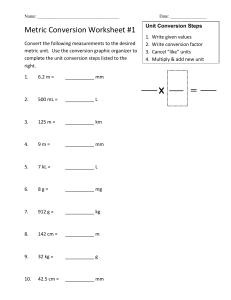
Metric System Length Conversions
advertisement

Lesson 5-4 Length in the Metric System KEY Concept VOCABULARY The metric system is used throughout the world. It is based on powers of ten. The most commonly used units are millimeters, centimeters, meters, and kilometers. The base unit of length in the metric system is the meter . metric system a measurement system based on powers of 10 that includes units such as meter, gram, and liter Unit Abbreviation Number of Meters millimeter mm 0.001 m centimeter cm 0.01 m decimeter dm 0.1 m meter m 1m dekameter dkm 10 m hectometer hm 100 m kilometer km 1,000 m ÷ length a measurement of the distance between two points × To convert between units, multiply or divide by powers of ten. When converting from bigger to smaller units, multiply. When converting from smaller to larger units, divide. A centimeter is one unit smaller than a decimeter. 5.0 dm = __ cm 5 km hm dkm m dm cm 5 0 mm 1 dm = 10 cm Multiplying by 10 moves the decimal point 1 place to the right. 5 · 10 = 50 cm A kilometer is two units larger than a dekameter. 5.0 dkm = __ km 5 km hm dkm 0 0 5 m dm 5 ÷ 102 = 5 ÷ 100 = 0.05 km cm mm meter a base unit in the metric system for measuring length 1 km = 100 dkm Dividing by 100 moves the decimal point 2 places to the left. Example 1 YOUR TURN! Convert. Convert. 8.2 m = ____ mm 1. Are you converting to a smaller unit or a larger unit? 1.3 km = ____ cm 1. Are you converting to a smaller unit or a larger unit? smaller 2. Do you multiply or divide? 2. Do you multiply or divide? multiply 3. How many units are you converting? 3. How many units are you converting? three 4. Use 4. Use 103. 8.2 · 103 = 8.2 · 1,000 = 8,200 8.2 m = 8,200 mm . 1.3 10 1.3 = 1.3 km = Example 2 YOUR TURN! Convert. Convert. 613.5 mm =____ dkm 1. Are you converting to a smaller unit or a larger unit? = 2,234 m = ____ km 1. Are you converting to a smaller unit or a larger unit? larger 2. Do you multiply or divide? 2. Do you multiply or divide? divide 3. How many units are you converting? 3. How many units are you converting? four 4. Use 104. 613.5 ÷ 104 = 613.5 ÷ 10,000 = 0.06135 613.5 mm = 0.06135 dkm 4. Use 2,234 2,234 2,234 m = . 10 = = Guided Practice Convert each measurement. 1 650 mm = ____ dm 2 units A decimeter is than a millimeter. 650 3 10 A dekameter is than a centimeter. 280,900 = 3,467 km = ____ m 4 units A meter is than a kilometer. 3,467 10 280,900 cm = ____ dkm 10 units = 7,431 m = ____ dm A decimeter is than a meter. 7,431 = 10 unit = Step by Step Practice 5 Convert. 80,743 dm = ____ km Step 1 Are you converting to a smaller unit or a larger unit? Step 2 Do you multiply or divide? Step 3 How many units are you converting? Step 4 Use . 10 80,743 = 80,743 = Step 5 80,743 dm = Convert each measurement. 6 10.75 km = ____ hm 7 405 dm = ____ hm by 10 . 10.75 10 = by 10 405 10 = . Convert each measurement. 8 14.4 m = ____ mm 10 14.4 10 9 325 = 6.5 dm = ____ km 6.5 10 325 hm = ____ m 11 10 742 mm = ____ cm 742 = = 10 = Step by Step Problem-Solving Practice Solve. 12 SWIMMING Reece swims 50 meters in a swim meet. How many hectometers did she swim? units Hectometers are by 10 than meters. . place(s) to the The decimal moves . Check off each step. Understand: I underlined key words. Plan: To solve the problem, I will . Solve: The answer is . Check: I checked my answer by . Skills, Concepts, and Problem Solving Convert each measurement. 13 45 dm = mm 14 925 dm = hm Convert each measurement. 15 5,487 m = 17 79 km = 19 631 mm = cm 16 36,725 mm = hm 18 489 dkm = m 20 568,734 dm = dkm dm km Solve. 21 RUNNING Emma ran 11 laps around the track during practice one day. How many kilometers did Emma run during practice? 22 SCHOOL SUPPLIES Ethan has new pencils that are each 140 mm long. How long is each pencil in centimeters? 23 DRIVING Garrett drove 62 kilometers to see his grandmother. How many dekameters did he drive? 1 lap = 400m Vocabulary Check Write the vocabulary word that completes each sentence. 24 The 25 The distance between two points is called the 26 The measuring system based on powers of 10 is the 27 is the base unit for measuring length in the metric system. . Write in your own words how to convert between metric units. Do you think it is easier to convert in the metric system or the customary system? Explain your answer. . Chapter 5 Progress Check 2 (Lessons 5-3 and 5-4) Convert each measurement. 1 80 oz = 3 1.5 lbs = 5 5,000 cm = 7 10 m = 9 200 dkm = lb oz m cm cm 2 2T= lb 4 1T = oz 6 2 km = 8 150 mm = 10 2,500 mm = Solve. 11 ANIMALS In a report on giraffes, Sumintra wrote that one animal weighed 1.5. She didn’t write down the units. What unit would be appropriate for the weight of a giraffe? How many pounds does the giraffe weigh? 12 DISTANCE Aisha lives 2.5 km from her friend Jason. When she walks to his house, how many meters does she have to walk? 13 MODEL CARS Jacob and Carlos had a contest to see how far their model cars would go on one wind up. Jacob’s car went 1.6 m. Carlos’ car went 145 cm. Whose car went farther? By how much? dkm hm m genius PHYSICS by Pradeep Kshetrapal Units, Dimensions and Measurement 1 Notes and exercises by Pradeep Kshetrapal P genius PHYSICS mg by Pradeep Kshetrapal Units, Dimensions and Measurement 7 9Tmt IndirectProported 1.1 Physical Quantity. A quantity which can be measured and by which various physical happenings can be explained and expressed in form of laws is called a physical quantity. For example length, mass, time, force etc. On the other hand various happenings in life e.g., happiness, sorrow etc. are not physical quantities because these can not be measured. Measurement is necessary to determine magnitude of a physical quantity, to compare two similar physical quantities and to prove physical laws or equations. A physical quantity is represented completely by its magnitude and unit. For example, 10 metre means a length which is ten times the unit of length 1 kg. Here 10 represents the numerical value of the given quantity and metre represents the unit of quantity under consideration. Thus in expressing a physical quantity we choose a unit and then find that how many times that unit is contained in the given physical quantity, i.e. anxut Physical quantity (Q) = Magnitude × Unit = n × u NIU Where, n represents the numerical value and u represents the unit. Thus while expressing definite amount of physical quantity, it is clear that as the unit(u) changes, the magnitude(n) will also change but product ‘nu’ will remain same. Notationof Inverse Proportion i.e. n u = constant, or n1u1 n2u 2 constant ; n 1 u i.e. magnitude of a physical quantity and units are inversely proportional to each other .Larger the unit, smaller will be the magnitude. 1.2 Types of Physical Quantity. (1) Ratio (numerical value only) : When a physical quantity is a ratio of two similar quantities, it has no unit. e.g. Relative density = Density of object/Density of water at 4oC Refractive index = Velocity of light in air/Velocity of light in medium Strain = Change in dimension/Original dimension Note : Angle is exceptional physical quantity, which though is a ratio of two similar physical quantities (angle = arc / radius) but still requires a unit (degrees or radians) to specify it along with its numerical value. K (2) Scalar (Magnitude only) : These quantities do not have any direction e.g. Length, time, work, energy etc. Magnitude of a physical quantity can be negative. In that case negative sign indicates that the numerical value of the quantity under consideration is negative. It does not specify the direction. Scalar quantities can be added or subtracted with the help of following ordinary laws of addition or subtraction. Page Vector physical quantities can be added or subtracted according to vector laws of addition. These laws are different from laws of ordinary addition. 7 (3) Vector (magnitude and direction) : e.g. displacement, velocity, acceleration, force etc. Note : 11ft magnitude genius PHYSICS by Pradeep Kshetrapal Units, Dimensions and Measurement 8 vector magnitude with direction There are certain physical quantities which behave neither as scalar nor as vector. For example, moment of inertia is not a vector as by changing the sense of rotation its value is not changed. It is also not a scalar as it has different values in different directions (i.e. about different axes). Such physical quantities are called Tensors. 1.3 Fundamental and Derived Quantities. (1) Fundamental quantities : A few physical quantities which are independent of all other quantities and do not require the help of any other physical quantity for their definition, These quantities are also called fundamental or base quantities. Maes etc Egg censer Time (2) Derived quantities : All other physical quantities can be derived by suitable multiplication or division of different powers of fundamental quantities. These are therefore called derived quantities. If length is defined as a fundamental quantity Area and volume are derived from length and are expressed in term of length with power 2 and 3 over the term of length. 1.4 Fundamental and Derived Units. System of units : A complete set of units, both fundamental and derived for all kinds of physical quantities is called system of units. The common systems are given below – second centimeter gram system (1) CGS system : The system is also called Gaussian system of units. In it length, mass and time have been chosen as the fundamental quantities . corresponding fundamental units are centimetre (cm), gram (g) and second (s) respectively. Jeterkilogram seÉÉ (2) MKS system : The system is also called Giorgi system. In this system also length, mass and time have been taken as fundamental quantities, corresponding fundamental units are metre, kilogram and second. Foot pound system Page 8 (3) FPS system : In this system foot, pound and second are used respectively for measurements of length, mass and time. In this system force is a derived quantity with unit poundal. genius PHYSICS by Pradeep Kshetrapal Units, Dimensions and Measurement 9 (4) S. I. system : It is known as International system of units, and is infact extended system of units applied to whole physics. There are seven fundamental quantities in this system. These quantities and their units are given in the following table Quantity Name of Unit Symbol Length metre m Mass kilogram kg Time second s Electric Current ampere A Temperature Kelvin K Amount of Substance mole mol Luminous Intensity candela cd Besides the above seven fundamental units two supplementary units are also defined – Radian (rad) for plane angle and Steradian (sr) for solid angle. Note : Apart from fundamental and derived units we also use very frequently practical units. These may be fundamental or derived units e.g., light year is a practical unit (fundamental) of distance while horse power is a practical unit (derived) of power. Practical units may or may not belong to a system but can be expressed in any system of units e.g., 1 mile = 1.6 km = 1.6 × 103 m. 1.5 S.I. Prefixes. Prefix Symbol 1018 exa E 1015 peta P 1012 tera T 109 giga G 106 mega M 103 kilo k 102 hecto h 101 deca da 10–1 deci d 10–1 centi c 10–3 milli m 10–6 micro Page Power of 10 9 In physics we have to deal from very small (micro) to very large (macro) magnitudes as one side we talk about the atom while on the other side of universe, e.g., the mass of an electron is 9.1 10–31 kg while that of the sun is 2 1030 kg. To express such large or small magnitudes simultaneously we use the following prefixes : genius PHYSICS by Pradeep Kshetrapal Units, Dimensions and Measurement 10 10–9 nano n 10–12 pico p 10–15 femto f 10–18 atto a / from very small (micro) to very large (macro) magnitudes 1.7 Practical Units. (1) Length : (i) 1 fermi = 1 fm = 10–15 m (ii) 1 X-ray unit = 1XU = 10–13 m (iii) 1 angstrom = 1Å = 10–10 m= Micro 10–8 cm = 10–7 mm = 0.1 mm (iv) 1 micron = m = 10–6 m (v) 1 astronomical unit = 1 A.U. = 1. 49 (vi) 1 Light year = 1 ly = 9.46 1011 m 1.5 1011 m 108 km Macro 1015 m (vii) 1 Parsec = 1pc = 3.26 light year (2) Mass : (i) Chandra Shekhar unit : 1 CSU = 1.4 times the mass of sun = 2.8 1030 kg (ii) Metric tonne : 1 Metric tonne = 1000 kg (iii) Quintal : 1 Quintal = 100 kg (iv) Atomic mass unit (amu) : amu = 1.67 10–27 kg mass of proton or neutron is of the order of 1 amu (3) Time : (i) Year : It is the time taken by earth to complete 1 revolution around the sun in its orbit. (ii) Lunar month : It is the time taken by moon to complete 1 revolution around the earth in its orbit. Page (iii) Solar day : It is the time taken by earth to complete one rotation about its axis with respect to sun. Since this time varies from day to day, average solar day is calculated by taking average of the duration of all the days in a year and this is called Average Solar day. 10 1 L.M. = 27.3 days genius PHYSICS by Pradeep Kshetrapal Units, Dimensions and Measurement 11 1 Solar year = 365.25 average solar day or average solar day 1 the part of solar year 365 .25 (iv) Sedrial day : It is the time taken by earth to complete one rotation about its axis with respect to a distant star. 1 Solar year = 366.25 Sedrial day = 365.25 average solar day Thus 1 Sedrial day is less than 1 solar day. (v) Shake : It is an obsolete and practical unit of time. 1 Shake = 10– 8 sec 1.8 Dimensions of a Physical Quantity. When a derived quantity is expressed in terms of fundamental quantities, it is written as a product of different powers of the fundamental quantities. The powers to which fundamental quantities must be raised in order to express the given physical quantity are called its dimensions. To make it more clear, consider the physical quantity force Force = mass × acceleration mass velocity time mass length/time = mass × length × (time)–2 .... (i) time Thus, the dimensions of force are 1 in mass, 1 in length and – 2 in time. Here the physical quantity that is expressed in terms of the base quantities is enclosed in square brackets to indicate that the equation is among the dimensions and not among the magnitudes. Thus equation (i) can be written as [force] = [MLT–2]. Such an expression for a physical quantity in terms of the fundamental quantities is called the dimensional equation. If we consider only the R.H.S. of the equation, the expression is termed as dimensional formula. Thus, dimensional formula for force is, [MLT – 2]. 1.9 Important Dimensions of Complete Physics. Mechanics (1) Velocity or speed (v) m/s [M0L1T –1] (2) Acceleration (a) m/s2 [M0LT –2] (3) Momentum (P) kg-m/s [M1L1T –1] (4) Impulse (I) Newton-sec or kg-m/s [M1L1T –1] (5) Force (F) Newton [M1L1T –2] (6) Pressure (P) Pascal [M1L–1T –2] (7) Kinetic energy (EK) Joule [M1L2T –2] (8) Power (P) Watt or Joule/s [M1L2T –3] (9) Density (d) kg/m3 [M1L– 3T 0] (10) Angular displacement ( ) Radian (rad.) [M0L0T 0] (11) Angular velocity ( ) Radian/sec [M0L0T – 1] (12) Angular acceleration ( ) Radian/sec2 [M0L0T – 2] (13) Moment of inertia (I) kg-m2 Unit Dimension [M1L2T0] 11 Quantity Page S. N. genius PHYSICS by Pradeep Kshetrapal Units, Dimensions and Measurement 12 S. N. Quantity (14) Torque ( ) Newton-meter [M1L2T –2] (15) Angular momentum (L) Joule-sec [M1L2T –1] (16) Force constant or spring constant (k) Newton/m [M1L0T –2] (17) Gravitational constant (G) N-m2/kg2 [M–1L3T – 2] (18) Intensity of gravitational field (Eg) N/kg [M0L1T – 2] (19) Gravitational potential (Vg) Joule/kg [M0L2T – 2] (20) Surface tension (T) N/m or Joule/m2 [M1L0T – 2] (21) Velocity gradient (Vg) Second–1 [M0L0T – 1] (22) Coefficient of viscosity ( ) kg/m-s [M1L– 1T – 1] (23) Stress N/m2 [M1L– 1T – 2] (24) Strain No unit (25) Modulus of elasticity (E) N/m2 (26) Poisson Ratio ( ) No unit [M0L0T 0] (27) Time period (T) Second [M0L0T1] (28) Frequency (n) Hz Unit Dimension [M0L0T 0] [M1L– 1T – 2] [M0L0T –1] Heat S. N. Quantity Dimension (1) Temperature (T) Kelvin [M0L0T0 (2) Heat (Q) Joule [ML2T– 2] (3) Specific Heat (c) Joule/kg-K [M0L2T– 2 –1] (4) Thermal capacity Joule/K [M1L2T – 2 –1] (5) Latent heat (L) Joule/kg [M0L2T – 2] (6) Gas constant (R) Joule/mol-K [M1L2T– 2 – 1] (7) Boltzmann constant (k) Joule/K [M1L2T– 2 – 1] (8) Coefficient of thermal conductivity (K) Joule/m-s-K [M1L1T– 3 – 1] (9) Stefan's constant ( ) Watt/m2-K4 [M1L0T– 3 – 4] (10) Wien's constant (b) Meter-K [M0L1To 1] (11) Planck's constant (h) Joule-s [M1L2T–1] (12) Coefficient of Linear Expansion ( ) Kelvin–1 [M0L0T0 (13) Mechanical eq. of Heat (J) Joule/Calorie [M0L0T0] (14) Vander wall’s constant (a) Newton-m4 [ML5T– 2] (15) Vander wall’s constant (b) m3 [M0L3T0] Unit 1] –1] Electricity Unit Dimension (1) Electric charge (q) Coulomb [M0L0T1A1] (2) Electric current (I) Ampere [M0L0T0A1] (3) Capacitance (C) Coulomb/volt or Farad [M–1L– 2T4A2] (4) Electric potential (V) Joule/coulomb M1L2T–3A–1 12 Quantity Page S. N. 5-5 Capacity in the Metric System KEY Concept VOCABULARY The base unit of capacity in the metric system is the liter . capacity the amount of dry or liquid material a container can hold Unit Symbol Number of Liters milliliter mL 0.001 L centiliter cL 0.01 L deciliter dL 0.1 L liter L 1L dekaliter dkL 10 L hectoliter hL 100 L kiloliter kL 1,000 L ÷ liter a base metric unit for measuring capacity metric system a measurement system that includes units such as meter, gram, and liter × To convert between units, multiply or divide by powers of ten. When converting from bigger to smaller units, multiply. When converting from smaller to larger units, divide. A milliliter is one unit smaller than a centiliter. 3.0 cL = __ mL 1 cL = 10 cL 3 kL hL dkL L dL cL mL 3 0 Multiplying by 10 moves the decimal point 1 place to the right. 3 · 10 = 30 mL A hectoliter is three units larger than a deciliter. 7.0 dL = __ hL 7 kL hL dkL L dL 0 0 0 7 1 hL = 1,000 dL cL 7 ÷ 103 = 7 ÷ 1,000 = 0.007 hL mL Dividing by 1,000 moves the decimal point 3 places to the left. The most commonly used units are liter and milliliter. The steps for converting between units of capacity are the same as the steps for converting between units of length. GO ON Example 1 YOUR TURN! Convert. 3.62 kL = ____ L Convert. 1. Are you converting to a smaller unit or a larger unit? smaller 1. Are you converting to a smaller unit or 2. Do you multiply or divide? multiply 2. Do you multiply or divide? 3. How many units are you converting? three 3. How many units are you converting? 4. Use 103. 4. Use 61 L = ____ mL a larger unit? 3.62 · 103 = 3.62 · 1,000 61 . 10 = 3,620 = 61 = 61 L = 3.62 kL = 3,620 L Example 2 YOUR TURN! Convert. 5,813 cL = ____ kL Convert. 1. Are you converting to a smaller unit or a larger unit? larger 1. Are you converting to a smaller unit or 2. Do you multiply or divide? divide 2. Do you multiply or divide? 3. How many units are you converting? 3. How many units are you converting? 360 mL = ____ dkL a larger unit? five 4. Use 4. Use 105. 360 5,813 ÷ 105 = 5,813 ÷ 100,000 . 10 = 360 = = 0.05813 360 mL = 5,813 cL = 0.05813 kL Guided Practice Convert each measurement. 1 437 hL = ____ cL 2 unit(s) A centiliter is 175 dkL = ____ L A liter is unit(s) than a hectoliter. 437 10 = than a dekaliter. 175 10 = Step by Step Practice 3 Convert. 7,450 cL = ____ hL Step 1 Are you converting to a smaller unit or a larger unit? Step 2 Do you multiply or divide? Step 3 How many units are you converting? Step 4 Use . 10 = 7,450 = Step 5 7,450 cL = Convert each measurement. 4 384 mL = ____ dL by 10 384 10 5 24,500 dL = ____ kL . by 10 . 24,500 = 10 = Step by Step Problem-Solving Practice Solve. 6 SOCCER Camille’s soccer team drinks 18 liters of a sports drink during a game. How many milliliters of sports drink does the team drink during a game? Milliliters are unit(s) by 10 than liters. . The decimal moves place(s) to the . Check off each step. Understand: I underlined key words. Plan: To solve the problem, I will . Solve: The answer is . Check: I checked my answer by . GO ON Skills, Concepts, and Problem Solving Convert each measurement. 7 175 kL = dkL 8 321 L = mL 9 9.62 cL = hL 10 86 hL = L 11 5,000 kL = L 12 4 mL = cL 13 76,500 dkL = 14 6 hL = mL cL Solve. 15 TRUCKING Brock is a truck driver. He used 2,500 liters of gas on his last trip. How many kiloliters of gas did he use? 16 MEDICINE One dose of a children’s medicine is 5 mL. How many centiliters are in one dose? 17 SWIMMING A school swimming pool holds 375,000 liters of water. How many millimeters of water does it hold? Vocabulary Check Write the vocabulary word that completes each sentence. 18 A(n) 19 is the amount of dry or liquid material that a container can hold. 20 The measuring system that includes milliliters, liters, and kiloliters is the 21 is the base metric unit for measuring capacity. . Compare and contrast the units of liter and meter. Lesson 5-6 Mass in the Metric System KEY Concept VOCABULARY Mass in the metric system is measured using the following units. The base unit of mass in the metric system is the gram . gram a base metric unit for measuring mass Unit Symbol Number of Meters milligram mg 0.001 g centigram cg 0.01 g decigram dg 0.1 g gram g 1g dekagram dkg 10 g hectogram hg 100 g kilogram kg 1,000 g ÷ metric system a measurement system that includes units such as meter, gram, and liter × To convert between units, multiply or divide by powers of ten. When converting from bigger to smaller units, multiply. . . When converting from smaller to larger units, divide. A gram is one unit smaller than a dekagram. 8.0 dkg = __ g 8 kg hg dkg g 8 0 dg cg mg 1 dkg = 10 g Multiplying by 10 moves the decimal point 1 place to the right. 8 · 10 = 80 g A decigram is two units larger than a milligram. 2.0 mg = __ dg 2 kg hg dkg g dg cg mg 0 0 2 mass the amount of matter in an object 1 dg = 100 mg Dividing by 100 moves the decimal point 2 places to the left. 2 ÷ 102 = 2 ÷ 100 = 0.02 dg The most commonly used units for mass are the milligram, gram, and kilogram. Example 1 YOUR TURN! Convert. Convert. 1.7 kg = ____ cg 5,662 g = ____ mg 1. Are you converting to a smaller unit or a larger unit? smaller 1. Are you converting to a smaller unit or 2. Do you multiply or divide? multiply 2. Do you multiply or divide? 3. How many units are you converting? 3. How many units are you converting? a larger unit? five 4. Use 4. Use 105. . 5,662 1.7 · 105 = 10 = 5,662 1.7 · 100,000 = 170,000 = 5,662 g = 1.7 kg = 170,000 cg Example 2 YOUR TURN! Convert. Convert. 483 cg = ____ dkg 3,601.4 mg = ____ g 1. Are you converting to a smaller unit or a larger unit? larger 1. Are you converting to a smaller unit or 2. Do you multiply or divide? divide 2. Do you multiply or divide? 3. How many units are you converting? 3. How many units are you converting? a larger unit? three 4. Use 4. Use 103. . 3,601.4 483 ÷ 103 = 10 483 ÷ 1,000 = 0.483 3,601.4 483 cg = 0.483 dkg 3,6014 mg = = = Guided Practice Convert each measurement. 1 4,324 cg = ____ g A gram is than a centigram. 4,324 10 = 2 units 6.8 dkg = ____ hg A hectogram is than a dekagram. 6.8 10 = unit Step by Step Practice 3 Convert. 345,000 g = ____ kg Step 1 Are you converting to a smaller unit or a larger unit? Step 2 Do you multiply or divide? Step 3 How many units are you converting? Step 4 Use . 10 345,000 Step 5 = 345,000 = 345,000 g = Convert each measurement. 4 640 cg = ____ dkg 5 3.4 dkg = ____ dg by 10 . 640 10 by 10 . 3.4 = 10 = Step by Step Problem-Solving Practice Solve. 6 HEALTH A bottle of vitamins has 250 tablets. Each tablet has 200 mg of Vitamin C in it. How many grams of Vitamin C are in one bottle? Find the total number of milligrams in the bottle. 200 mg · = Convert milligrams to grams. ÷ = Check off each step. Understand: I underlined key words. Plan: To solve the problem, I will . Solve: The answer is . Check: I checked my answer by . GO ON Step by Step Practice 3 Find the area of the figure. 3 in. Step 1 Identify the shape. 10 in. Step 2 Write the formula for the area. Step 3 Name the value for each variable. Step 4 Substitute the values into the formula. Step 5 Simplify. A= ( )= Find the area of each figure. 4 ft 4 5 8 mm 5 ft 3 ft 16 mm 8 ft The figure is a 1 h(b + b ) A = __ 1 2 2 . The figure is a . A= A= A= Step by Step Problem-Solving Practice Solve. 6 ART PROJECT Brianne has to make a collage for art class on a poster board that has dimensions 16 inches by 22 inches. Because Brianne has to use the whole board, what is the total area she needs to cover with her collage? The board is a . A= Check off each step. Understand: I underlined key words. Plan: To solve the problem, I will . Solve: The answer is . Check: I checked my answer by . GO ON -To convert between units of area, multiply or divide by squares of powers of ten When converting from bigger to smaller units, multiply. When converting from smaller to larger units, divide. To convert between units of volume, multiply or divide by cube of powers of ten When converting from bigger to smaller units, multiply. . . When converting from smaller to larger units, divide. Relation between Volume and Capacity 1ml equal to ?????Cm3 1000am 1000mL I 1 ml = 1 g Relation between volume and capacity Measuring Density What is density 1 How to measure density 2 Measuring matter in different systems 3 4 mass 9 Chapter Progress Check 3 5 (Lessons 5-5, 5-6, and 5-7) Convert each measurement. 1 300 cL = L 2 6,000 mg = 3 4 kL = liters 4 16 hL = cL 5 12 g = kg 6 11 hg = g 7 150 kg = 8 19 dL = hL dg g Find the perimeter and the area of each figure. 9 10 15 m 16 ft 5 ft 3 ft 8 ft 25 m 11 12 30 in. 5 ft 32 in. 13 yd 85 yd 84 yd 36 in. Solve. 13 GROCERIES Mehlia bought 6,804 g of fruit at the grocery. How many kilograms of fruit did she buy? 14 HEALTH Doctors recommend that adults drink about 1,900 mL of water every day. About how many liters of water should an adult drink per day? 15 SCIENCE In an experiment, Reina measured 145 mL of hydrochloric acid. How many liters did she have? Chapter Chapter Test 5 Convert each measurement. 1 1 km = hm 1 hm = dkm 1 hg = 10 1 hL = 1 dkm = m 1 dkg = 10 1 1 g = 10 1 L = 10 1m= 4 2 dm 1 kg =10 1 kL =10 3 dkL = 10 1 dm = cm 1 dg = 10 1 dL = 1 cm = mm 1 cg = 10 1 15 dkL = kL hL mL dkL L dL cL 5 mL = 10 mL 4,120 g = kg hg cL kg dkg g dg cg 6 192 in. = ft 7 1.7 km = cm 8 1,200 c = gal 9 4T= 10 16 c = fl oz 11 5 mi = ft 12 72 oz = lb 13 6.7 g = mg 14 800 L = cL 15 328 mm = oz m mg Which are the basic units in the metric system? MAGNITUDE BASIC UNIT SYMBOL LENGTH metre m MASS gram g CAPACITY litre L VOLUME cube metre m3 SURFACE square metre m2 Note: you can write meter or metre, liter or litre; it depends if it is American or British English. Work in groups to find objects you can measure with these units: a) metre: a table, _________________________________________ b) gram: sugar pot, ____________________________________________ c) litre: oil, _______________________________________________ d) cube metre: a swimming pool, __________________________________ e) square metre: a room, ___________________________________________ For multiples and submultiples we use prefixes that multiply or divide the basic unit by powers of 10 Meanings of metric prefixes: 1000 = 103 KiloHecto- 100 = 102 Deca- or (deka-) 10 = 101 Basic Multiples unit Deci- 0,1 = 10–1 Centi- 0,01 = 10–2 Milli- 0,001 = 10–3 Submultiples 1. LENGTH Measurements of 1 dimension How long is your pencil? Measure it using a ruler from one end to another. It is about 12 cm long. Length is a measure of how long or wide something is. For example: the bed is 1,90 m long and 80 cm wide. What’s the perimeter of the bed? We have to add twice the length plus twice the width: Length 1,90 x 2 = 3,80 m Width 80 x 2 = 160 cm To add length plus width we must change the amounts to the same units: m or cm 3,80 m = 380 cm + 160 cm = 540 cm or 160 cm = 1,60 m + 3,80 = 5,40 m The basic unit of length is the metre. Multiples and submultiples are: Kilometre Hectometre Decametre metre decimetre 1km= 1000m 1 hm = 100m 1dm=0,1m 1cm= 0,01m 1 dam= 10m MultIples centimetre millimetre 1mm= 0,001m Submultiples How many dm, cm and mm are in one metre? 1 m = 10 dm, 100 dm and 1000 mm Exercise: 1.Choose the most appropriate unit – km, m, cm, mm – to measure: a) a pen b) a stamp c) a building d) the distance from London to Oxford e) an eraser 2. Choose the most reasonable measurement: 1. Width of your hand a) 95 mm 2. Height of an adult a) 163 mm 3. Length of a pair of scissors b) 9,5 dm c) 95 cm b) 1.63 m a) 20 cm c) 1630 cm b) 2000 mm 1.2 CONVERTION OF METRIC UNITS Metric system is based on multiples of ten. To change from one unit to another, we must multiply or divide by ten. c) 2 m 1.2 CONVERTION OF METRIC UNITS Metric system is based on multiples of ten. To change from one unit to another, we must multiply or divide by ten To change LARGER UNITS TO SMALLER UNITS, multiply by 10 for every place moved to the right. To change SMALLER UNITS TO LARGER UNITS, divide by 10 for every place moved to the left. 1. LENGTH Measurements of 1 dimension To change LARGER UNITS TO SMALLER UNITS, multiply by 10 for every place moved to the right. To change SMALLER UNITS TO LARGER UNITS, divide by 10 for every place moved to the left. For example change 50 cm to mm. 50 x 10 (1 place to the right) = 500 mm. cm (larger) mm (smaller) 700dm to m 70 : 100 (2 places to the left) = 0,7 m dm (smaller) m (larger) Exercise: Complete: a) 1,2 km =_______m d) 0,5 dm= _______mm b) 4 m = _____ cm e) 2 dam = _____km c) 25 cm = ______m f) 1550 mm = _____________hm 1.3 PRACTICES of Length EXERCISE 1 Drawing dictation (materials needed: a ruler) To the whole class: ask them to draw lines or figures using rulers. Give them five minutes Example: A)draw a line of 4,5 cm D) Draw a tree of 40 mm tall B) Draw a line of 30mm C) draw a line of 1,5dm E) Draw an envelope of 5cm of length and 35mm width Correct in class checking in groups or at the blackboard. GAME: Students propose similar dictations in groups or to the whole class as a BINGO. EXERCISES 2 How tall are you? (materials needed: ruler and tape measure) Divide the class in groups of 4 or 5 students. Who is the tallest of the group? Who is the smallest of the group? Use a tape measure and show your answers with a drawing. In my group the tallest is David. He is 1,56 m tall and the shortest is Lucas. He is 1,35 m tall. Lucas David 1,56 Express m the tall measurements in dm, cm, mm. 1,35 m tall 2. MASS Investigate how heavy an elephant, an ant, a cow…are., What units of mass do we use to express their weight? Tons, kilograms, grams Mass is a measurement of how heavy something is. The basic unit of mass in the metric system is gram (g). Units of mass. Multiples and submultiples are: Kilogram Hectogram Decagram 1kg= 1000g 1 hg = 100g gram 1 dag = 10g decigram centigram milligram 1dg = 0,1g 1cg = 0,01g 1mg= 0,001g Multiples Submultiples To measure mass we can use scales (metric balance). Examples: - A dictionary has a mass of about 1 kg - A paper clip has a mass of about 1 g - A grain of salt has a mass of about 1 mg - The mass of heavy things is expressed in tons (t) 1 ton = 1000 kg We use also “quintal” (q) for 100 kg. Search on Internet other units of mass in Spain: like arroba, fanega… Exercise: Choose one of these units (g, mg, kg, t) to express the mass of: a) a bag of potatoes b) a box of cereal c) a feather d) a hamster e) a lorry To convert one unit to another proceed as in length: count the number of places and multiply or divide by powers of 10. Example: 150g to kg there are 3 places to the left, so divide by 1000 150:1000= 0,150kg 150g to mg there are 3 places to the right, so multiply by 1000 150 x 1000 = 150 000 mg Exercise: compare, write <, =, or > a) 754 kg _____754 000g c) 280 000 mg____ 28 g d) 1845hg ____18,45 kg b)876 hg____8.96 kg e) 0,0001 g ____10 mg Propose similar exercises to your teams and correct them together. Complete this converting unit table: 0,854 kg kg hg dag g dg cg mg 0,854 8,54 85,4 854 8540 85400 854000 324,54 g 910 dag 2t 2.1 PRACTICES of Mass EXERCISE 1 Body mass index (materials needed: bathroom scales and tape measure) To calculate the body mass index we use this formula: weight height 2 Use a bathroom scale to measure your weight and a tape measure to measure your height, then divide the weight by the square of your height. 47 47 = = 20,346 1,52 2 2,31 Paula weight 47 kg Height 1,52 m 20,35 to know more visit: http://www.nhlbisupport.com/bmi/bmi-m.htm EXERCISE 2 How heavy is…? (materials needed: scales) Divide the class in groups and ask students to measure the weight of school materials and their lunch. They have to choose 5 items and then change them to different mass units. Item Math book School bag Sandwich Biscuits g kg mg hg 2. MASS Investigate how heavy an elephant, an ant, a cow…are. What units of mass do we use to express their weight? Tons, kilograms, grams Mass is a measurement of how heavy something is. The basic unit of mass in the metric system is gram (g). Metric units of mass are related to each other in the same way that place-value positions within the decimal system of numeration are related. The mass of heavy things is expressed in tons (t) 1 ton = 1000 kg. We use also “quintal” (q) for 100 kg. 3. CAPACITY It’s the amount of liquid a container can hold. (Also volume) We should drink 2 litres of water per day. If we have glasses of ml. How many glasses will we fill? 2 litres = 2000 ml 2000 : 200 = 10 glasses of water. To measure how much a container can hold we use the units of capacity Example: The water in a swimming pool is measured in kilolitres. A tall thermos holds about 1 L. 20 drops of water equals 1 mL To measure capacity we can use a measuring cylinder or a beaker. Practise to measure little things as jewellery or very light things like a feather, medicines, spices… with a special scale: precision balance or scale. 3. CAPACITY It’s the amount of liquid a container can hold. (Also volume) We should drink 2 litres of water per day. If we have glasses of ml. How many glasses will we fill? 2 litres = 2000 ml 200 2000 : 200 = 10 glasses of water. To measure how much a container can hold we use the units of capacity: Example: a carton holds 1 litre of milk: it has the capacity of 1 litre. The litre is the basic unit of capacity in the metric system. It represents what a cube of 1 dm of side can hold. So 1 dm3= 1 L Units of capacity. Multiples and submultiples are: Kilolitre Hectolitre Decalitre 1kL= 1000L 1 hL = 100L 1 daL= 10L Litre L Multiples decilitre 1dL 0,1L centilitre = 1cL = 0,01L millilitre 1mL= 0,001L Submultiples Example: The water in a swimming pool is measured in kilolitres. A tall thermos holds about 1 L. 20 drops of water equals 1 mL To measure capacity we can use a measuring cylinder or a beaker. Give more examples of measurements of capacity. To convert one unit to another: count the number of places and multiply or divide by powers of 10. Covert 2 L to KL = 3 places to the left Divide by 10 raised to 3 2 : 103 = 0,002 kL Example 5L = 0,005kL = 0,05 hL = 0,5 daL = 50 dL = 500cL = 5000 mL Exercise: 1. Convert these units: a) 8,2 kL to L b) 65,4 mL to L c) 45 daL to mL 2. Express in litres the following measurements: a) 3 daL, 6 L and 2 dL b) 7hL, 2 L and 8 mL c) 3 kL, 20cL and 300 mL 2.1 PRACTICES of Capacity EXERCISE 1 Finding the volume of irregularly shaped object. Displacement method. Investigate how Archimedes discovered this method. 1. Pour water into a beaker. Read the water level. 50 mL 2. Drop a stone into the water. Read the new water level. 65mL 3. Subtract 65 – 50 = 15 mL is the volume of the stone. EXERCISE 2 Liquid products Bring to the class labels of different liquid products, for example: empty bottles of medicines, cleaning products, cans of drinks, cartons of milk or juice, yogurt… How much liquid can they hold? Express it in different units and show on a table. Item L kL mL cL A bottle of bleach A bottle of shampoo A can of coke A brick of juice EXERCISE 3 Problems in daily life. 1. I have 36 containers of 15 L of olive oil to sell to the market. rest in bottles of 1 L to s. How many bottles of 1 L will I have? 2 are full and I pour the 3 2. How many glasses of 330 mL do we need to fill a bottle of 2 L? 3. From one orange I get 5 cL of juice. How many oranges will I need for one litre of juice? 4. For a party I bought 5 cans of 33 cL of coke, 2 bottles of 2 litres of lemonade and 10 cartons of 125 mL of juice. How much liquid did I buy? 1 1 2 of orange juice, of lemon juice, of pineapple 8 10 3 juice and the rest of water. If I want to prepare 1 litre, how much of each juice will I use? 5. The recipe for a cocktail says: Solve them in groups and invent similar ones to your class group. -To convert between units of area, multiply or divide by squares of powers of ten When converting from bigger to smaller units, multiply. When converting from smaller to larger units, divide. 5. AREA Measurements of 2 dimensions 10 X 10 = 100 In a flat shape is the space inside the lines. What’s the measure of your blackboard? To find it out we measure the area (green part) using surface units. We have to measure 2 dimensions: Length and width and multiply them: 2m long x 1,20 m wide = 2,40 m2 The basic unit of surface in the metric system is the squared metre, and it is the area of a square of 1 m of side: 1 m2 Units of surface: Multiples and submultiples are: Square Square Square Kilometre Hectometre Decametre 1km2= 1 hm2 = 1000 000 10 000 m2 Square metre 1 dam2= 100 m2 Square Square Square decimetre centimetre millimetre 1dm2 = 1cm2 = 0,001m2 0,000 01 m2 m2 1mm2= 0,0000 001m2 m2 Multiples Submultiples To convert units of surface we multiply or divide by 100 for each place it depends on if we move to the right or to the left. For example to change 2 hm2 to m2, as we change from a greater to a lowest we multiply by 100 as many times as places. Since we move 2 places to the right from hm2 to m2 we should multiply by 10 000 = 2 x 10 000 = 20 000 m2. Example: A table is 120 cm long and 70 cm wide. Its area is 120 x 70 = 8400 cm2 = 8400 : 100 00= 0,84 m2 Área and hectárea en español. ¿y fanega? Other units for large surfaces: area 1(a) = 100 m2 And hectare 1(ha) = 1hm2 =100 areas = 10 000m2 Exercises: 1. What unit of surface will you use to measure: cm2, mm2, km2, m2, dm2? a) Your room floor 2. Complete the table: b) a paper area Km2 hm2 c) The surface of a country dam2 m2 7 5 0,0025 dm2 cm2 70000 mm2 To convert between units of volume, multiply or divide by cube of powers of ten When converting from bigger to smaller units, multiply. . . When converting from smaller to larger units, divide. 2.1 PRACTICES of surface EXERCISE 1 Area of objects (materials needed: ruler and tape measure) Complete this table. In groups they have to measure length and width of these objects to find the area. Remember to use the same units to operate. Compare the results with other groups (give them 10 minutes maximum). AREA in m2 Length Width AREA 2m 70cm 200 x 70 = 14000:100 00= 14000 cm2 1,4 m2 OBJECT Door Window Ruler Math book Classroom Paper sheet EXERCISE 2 Space at home. Measure at home the area of your bedroom and other rooms from your house. Then prepare problems to show to your group or class. For example: My bedroom is 230 cm wide and 4,5 m long. If I want to covert the floor with a blue carpet, how many m2 will I need? EXERCISE 3 Surfaces of places Find out the surface of different countries or towns; express it in m2 and compare. Example: Spain has a surface of 506,990 km2, is it bigger than France? If we have 30% of forest surface, How many hectares are there? 30% of 506,990 = 152,097 km2 = 152,097 x 100 = 15209,7 ha 4. VOLUME Measurements of 3 dimensions 10 X 10 X 10 = 1000 Generally,volume is measurement of solids.In a 3 D shape or solid shape, the volume is how much space something occupies. We use a cube as a measurement unit. The basic unit of volume in the metric system is the cube metre, and it is the space that takes a cube of 1 m of side: 1 m3 Units of volume. Multiples and submultiples are: Cubic Cubic Kilometre Hectometre 1km3= 1 hm3 = 1000 000 1000 000 000 m3 m3 Cubic Decametre 3 1 dam = 1000 m3 Cubic Cubic Cubic metre decimetre centimetre m3 3 3 1dm = 1cm = 0,000 3 0,001m 001 m3 Multiples Cubic millimetre 1mm3= 0,0000 000 001m3 Submultiples To convert units of volume we multiply or divide by 1000 for each place it depends on if we move to the right or to the left. For example to change 2 hm3 to m3, as we change from a greater to a lowest we multiply by 1000 as many times as places. Since we move 2 places to the right from hm3 to m3 we should multiply by 1000 000 = 2 x 1000 000 = 2000 000 m3. Example: An object has a volume of 245 cm3, how many dm3 ? and mm3? 245 : 1000 = 0,245 dm3 245 x 1000 = 245 000 mm3 Exercise: Express in m3 the following volume: a) 0,4 hm3 b) 0,0032 dm3 c) 24 dm3 4.1 RELATING METRIC UNITS Metric units of volume, capacity and mass are related to one another in this way: A cube of 1 dm of side holds 1 litre of water and has a mass of 1 kg (Only for water o similar liquids density, for example not for honey) 1 dm3 = 1L of water = 1 kg Exercise: How many litres are in 3 m3? 1st we convert m3 to dm3 3 x 1000 = 3000 dm3 = 3000 L Solved problem: A child wants to fill a 500 cm3 bucket. How many litres of water does he need to fill it? 500cm3 dm3 litre or 500 mL 1 place to the left, so divide by 1000 = 0,500 dm3 = 0,5 L what is half a 2. On this signpost, the distance to Madrid is given as 9 miles. Show the distance in km. Madrid 9 Airport Invent similar problems to your class and check the results. http://www.math-drills.com/measurement.shtml Metric- imperial conversion exercises. III. MEASURING TEMPERATURE We have three scales to measure temperature: CELSIUS, FAHRENHEIT AND KELVIN. Celsius is used In Spain and many countries in Europe and Fahrenheit is used in U.S.A. http://www.bbc.co.uk/skillswise They settle in different degrees the freezing point of water and the boiling point of water. CELSIUS O C FAHRENHEIT Freezing point of water 0 oC 32 o F Boiling point of water 100 0 C 212 o F Normal Body temperature 37 o C 98,6 o F O F 5 5 = Example 78oF (78 – 32) = 25,5 o C 9 9 Twenty-five point five degrees Celsius or Centigrade To convert o F to o C (oF – 32) 9 + 32 = Example 32 o C 5 Eighty-nine point 6 degrees Fahrenheit To convert o C to o F o C 32 x 9 +32 = 89,6 o F 5 Why the fraction 5/9 and 9/5? The difference between the freezing point and the boiling point in Fahrenheit is 212 – 32 = 180, in Celsius is 100 – 0 = 100. The proportion is o C to o F = 180 9 = + 32 if we reduce it. 100 5 And o F to o C = 100 5 = – 32 180 9 Converting practices and reading temperatures: http://www.mathdrills.com/measurement.shtml Solve these problems and convert the degrees to Celsius. Invent similar ones for the class. Example: At 11 a.m. the temperature outside is 35 o F, and at 11 p.m. is – 13 o F. By how many degrees has the temperature fallen? 35 – (–13) = 35 + 13 = 48 o F (48 –32) 5/9 = 8,8 o C 1. The pool water temperature at 9 a.m. was 62 oF, but by 6 p.m. the temperature was 70 o F, How much has the temperature risen? 2. When Susan was sick, the temperature was 38,8 o C. After she recovered, her temperature was 36,5 o C. How much has her temperature dropped? Exercise 2: Write “R” if the statement is reasonable and “U” if it is unreasonable: a) Your body temperature when you are well is about 37 oC ______ b) Inside a freezer it is 10 oC _______ c) The skating lake is frozen when the temperature is – 5 o C _____ d) You need a coat in 25 o C ______ e) When you boil potatoes the water is about 70o C ______ Exercise 3: Choose a reasonable temperature for: 1) The temperature of your classroom in a warm day: a) 68 o F 2) The temperature of a dish of ice cream: a) 31 o F b) 80 o F b) 0 o F c) 45 o F c) –10 o F PRACTICE 1 Weather report Bring newspapers or documents where the students can read the weather report and temperatures. In groups they choose a country or area and they should convert the temperature in degrees Fahrenheit. Then they will write and read aloud a report about the temperatures from yesterday to tomorrow in Celsius degrees and Yesterday the temperature in In degrees Fahrenheit: Fahrenheit Oxford was 7oC, Today the Yesterday was 44,6oF, degree: o o temperature will be 10 C and Tomorrow it will be 8 oC today 50 F and tomorrow it will be 46,4 oF To convert units of surface we multiply or divide by square of power of ten . For example to change 2 hm 2 to m2 , as we change from a greater to a lowest we multiply by 100 as many times as places. Since we move 2 places to the right from hm 2 to m 2 we should multiply by 10 000 = 2 x 10 000 = 20 000 m2 . Example: A table is 120 cm long and 70 cm wide.