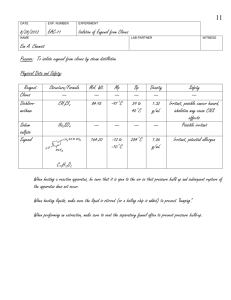
Microbial Transformations of Eugenol and Isoeugenol Thesis
advertisement

MICROBIAL TRANSFORMATIONS OF ORGANIC COMPOUNDS:
EUGENOL AND ISOEUGENOL
THESIS SUBMITTED TO THE
COCHIN
UNIVERSITY OF SCIENCE AND TBCHNOLOGY
IN PARTIAL FULFILMENT OF THE REQUIREMENTS OF THE DEGREE OF
DOCTOR OF PHILOSOPHY
IN
THE FACULTY OF SCIENCE
BY
RAV'KUMAR T.
DEPARTMENT OF APPLIED CHEMISTRV
COCHIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
KOCH. - 682 022
AUGUST 1991
CONTENTS
Chapter
1
INTRODUCTION
1
Chapter
2
HISTORICAL REVIEW
10
2.1
Introduction
11
2.2
Microbial Transformations of Terpenoids
12
2.3
Microbial Transformations of Phenols
19
2.3.1
Phenol and catechol
20
2.3.2
Methyl substituted phenols
24
2.3.3
Carboxy substituted phenols
26
2.3.4
Halogen, nitro and amino substituted
phenols
2.3.5
Tyrosine, tyramine and synephrine
37
44
2.3.6
Methoxy sUbstituted phenols
47
MATERIALS AND METHODS
56
3.1
Chemicals
57
3.2
Source of Microorganisms
62
3.3
Identification of Microorganisms
65
3.4
Growth Studies of Microorganisms
66
3.5
Analytical Methods
69
3.6
Effect of Physico-chemical Factors
on Transformation
72
3.7
Growth Curve
75
3.8
Fermentation Conditions
75
3.9
Isolation and Identification of
Transformation Products
Chapter
3
3.10
Fermentation of Transformation Products
77
79
3.11
Fermentation of Acetates
79
3.12
Mode of Aromatic Ring Cleavage
80
3.13
Immobilisation of Whole Cells
81
3.14
Enzyme Studies
83
RESULTS AND DISCUSSION
MICROBIAL TRANSFORMATIONS OF EUGENOL
87
Introduction
88
Studies with Bacillus sp. CUAC 10
88
4.2.1
Growth studies
89
4.2.2
Effect of physico-chemical factors
on transformation
90
4.2.3
Growth curve
92
4.2.4
Fermentation of eugenol
93
4.2.5
Isolation and identification of
transformation products
93
4.2.6
Fermentation of eugenol acetate
97
4.2.7
Fermentation of transformation product
97
4.2.8
Immobilisation of whole cells
98
4.2.9
Discussion
98
4.3
Studies with Pseudomonas sp. CUAC 20
102
4.3.1
Growth studies
102
4.3.2
Effect of physico-chemical factors
on transformation
104
4.3.3
Growth curve
105
4.3.4
Fermentation of eugenol
106
4.3.5
Isolation and identification of
transformation products
109
Fermentation of transformation
products
III
4.3.7
Fermentation of eugenol acetate
112
4.3.8
Mode of aromatic ring cleavage
112
4.3.9
Immobilisation of whole cells
113
4.3.10
Enzyme studies
113
4.3.11
Discussion
114
4.4
Studies with Pseudomonas aeruginosa
118
Chapter
4
4.1
4
.
;I
~
4.3.6
Chapter
MICROBIAL TRANSFORMATIONS OF ISOEUGENOL
143
5.1
Introduction
144
5.2
Studies with Bacillus sp. CUAC 10
144
5.2.1
Growth studies
145
5.2.2
Effect of physico-chemica1 factors
on transformation
145
5.2.3
Growth curve
147
5.2.4
Fermentation of isoeugeno1
148
5.2.5
Isolation and identification of
transformation products
149
5.2.6
Fermentation of transformation products
154
5.2.7
Fermentation of isoeugeno1 acetate
154
5.2.8
Immobilisation of whole cells
155
5.2.9
Discussion
155
5.3
Studies with Pseudomonas sp. CUAC 30
159
5.3.1
Growth studies
160
5.3.2
Effect of physico-chemica1 factors
on transformation
162
5.3.3
Growth curve
163
5.3.4
Fermentation of isoeugeno1
164
5.3.5
Isolation and identification of
transformation products
165
5.3.6
Fermentation of transformation products
167
5.3.7
Fermentation of isoeugeno1 acetate
168
5.3.8
Mode of aromatic ring cleavage
168
5.3.9
Immobilisation of whole cells
169
5.3.10
Enzyme studies
169
5.3.11
Discussion
170
SUMMARY AND CONCLUSIONS
191
REFERENCES
199
Chapter
5
6
LIST OF TABLES
4.l(a)
Identification tests of CUAC 10
120
4.1(b)
Identification tests of CUAC 20
121
4.2.1
Effect of concentration of eugenol on
growth of CUAC 10
4.2.2
Effect of concentration of sodium chloride
on growth of CUAC 10
4.2.3
122
122
Effect of different nitrogen sources
on growth of CUAC 10
123
4.2.4
Effect of pH on growth of CUAC 10
123
4.2.5
Effect of temperature on growth of CUAC 10
124
4.2.6
Effect of concentration of sodium chloride
on transformation of eugenol
4.2.7
Effect of concentration of glucose on
transformation of eugenol
4.2.8
4.2.9
124
125
Effect of different nitrogen sources on
transformation of eugenol
125
Effect of pH on transformation of eugenol
126
4.2.10
Effect of temperature on transformation
of eugenol
126
4.2.11
Growth curve of CUAC 10
127
4.3.1
Effect of concentration of eugenol on
growth of CUAC 20
4.3.2
Effect of concentration of sodium chloride
on growth of CUAC 20
4.3.3
127
128
Effect of different nitrogen sources
on growth of CUAC 20
128
4.3.4
Effect
129
4.3.5
Effect of temperature on growth of CUAC 20
4.3.6
Effect of concentration of sodium chloride
o~
pH on growth of CUAC 20
on transformation of eugenol
4.3.7
130
Effect of concentration of glucose on
transformation of eugenol
4.3.8
129
131
Effect of different nitrogen sources on
transformation of eugenol
131
4.3.9
Effect of pH on transformation of eugenol
132
4.3.10
Effect of temperature on transformation
of eugenol
132
4.3.11
Growth curve of CUAC 20
133
4.3.12
Time course of formation of products
133
5.1
Identification tests of CUAC 30
174
5.2.1
Effect of concentration of isoeugeno1
on growth of CUAC 10
5.2.2
Effect of concentration of sodium chloride
on transformation of isoeugeno1
5.2.3
176
Effect of pH on transformation of
isoeugeno1
5.2.6
176
Effect of different nitrogen sources
on transformation of isoeugeno1
5.2.5
175
Effect of concentration of glucose
on transformation of isoeugeno1
5.2.4
175
177
Effect of temperature on transformation
of isoeugeno1
177
5.2.7
Growth curve of CUAC 10
178
5.3.1
Effect of concentration of isoeugeno1
on growth of CUAC 30
5.3.2
178
Effect of concentration of sodium chloride
on growth of CUAC 30
179
5.3.3
Effect of different nitrogen sources
on growth of CUAC 30
179
5.3.4
Effect of pH on growth of CUAC 30
180
5.3.5
Effect of temperature on growth of CUAC 30
180
5.3~6
Effect of concentration of sodium chloride
on transformation of isoeugenol
5.3.7
Effect of concentration of glucose on
transformation of isoeugenol
5.3.8
181
182
Effect of different nitrogen sources
on transformation of isoeugenol
182
5.3.9
Effect of pH on transformation of isoeugenol ••
183
5.3.10
Effect of temperature on transformation
of isoeugenol
183
5.3.11
Growth curve of CUAC 30
184
5.3.12
Time course of formation of products
184
LIST OF FIGURES
Growth curve of CUAC 10 in
eugenol medium
134
4.2.2
NMR spectrum of eugenol
135
4.2.3
NMR spectrum of product E
4.2.4
13 C NMR spectrum of product E
4.3.1
Growth curve of CUAC 20
138
4.3.2
NMR spectrum of product E30
139
4.3.3
IR spectrum of product A
140
4.3.4
IR spectrum of product B
141
4.3.5
NMR spectrum of methyl ester of
product C
142
Growth curve of CUAC 10 in
isoeugeno1 medium
185
5.2.2
NMR spectrum of isoeugenol
186
5.2.3
NMR spectrum of product IE
5.2.4
13 C NMR spectrum of product IE
5.2.5
NMR spectrum of product 1E30
189
5.3.1
Growth curve of CUAC 30
190
4.2.1
5.2.1
136
20
20
137
187
20
20
188
CHAPTER
1
INTRODUCTION
INTRODUCTION
The
of
usefulness
microorganisms
synthesis has now been widely recognised.
in
organic
By the judicious
choice of microorganisms or enzymes many desired transformations of compounds can be achieved.
ing
feat ures
specific
means
have
microbi al
conventional
of
of
and
high
also
duction
useful
f
t rans forma t ions
is
that
reactions which had been presumed not
temperature
yield
0
One of the outstand-
pressure
purity
been
of
compounds
in
recent
brought
more
in
reactions
years.
It
in
showing
can
be
of
good
transformations
obtaining
identification
normal
about wi th a
extensively
for
feasible by
under
Microbial
groups
in
also
be
synthesis
products.
functional
and
can
utilised
substances,
specificity
organic
var ious
the
intro-
biologically
stereochemical
unknown
used
for
organic
microbial
decomposition treatment of natural and synthetic wastes for
protecting the environment as well.
A mi crobi al
one
substrate
chemical
to
t rans forma t ion
another
by
a
is
the
convers ion
microorganism.
It
is
0
f
a
reaction catalysed by a particular cellular enzyme
or by an enzyme originally produced within cells.
2
Most of
3
such
enzymes
are
necessary
the
life
processes
of
the
microbes.
enzymes
simply act
of
for
the
normal
functioning
cellular metabolism and
In
as
microbial
catalysts
reproduction
transformations
for chemical
of
these
reactions.
In
addition to their natural substrates, many of these enzymes
c'.an
ut i 1 i se
substrates
reactions
other
st ruct urall y
and
therefore
upon
addition
related
compounds
occasionally
catalyse
of
substrates
foreign
as
unnatural
to
the
reaction medium.
Enzymes
increase
energy
ence
the
as
reaction
normal
between
substrate
involved
rate
catalysts
enzymes
and
regio
isomers.
Only a
catalyse
the
Enzymes
exhibit
conditions
absolutely
activation
striking differ-
ca tal yst s
between
small amount
of
their
which
The most
the
lies
specific
in
the i r
reactions
few structurally related compounds,
conversion
in
do.
transformations
lowering
They catalyse
involving one or only a
almost
by
chemical
specificities.
distinguishing
microbial
ln
a
large
activity
normal
stereo
of enzyme
amount
or
is needed to
of
substrate.
mild
under
chemical
isomers
reaction
catalysts
hardly
function at all,
such as atmospheric pressure,
temperatures
around
pH
These
30°C
and
values
near
neutrality.
unique
4
propert i es
of
enzymes
molecules
are
to
are
be
ext remel y
converted
useful
when
unst abl e
undesired
without
side
reactions.
On
the
occasionally
make
convenient
hand,
other
microbial
A
catalysts.
these
enzymes
hard
microbial
features
unique
to
cell
be
used
contains
as
many
different types of enzymes which may give rise to undesired
products.
As
conducted
with
sations
the
are
the
of
hydroxyl
compounds.
the
types
of
reactions
microorganisms,
possible.
reduction
introduce
for
Thus
carbonyl
groups
Besides,
yeasts
only
are
compounds
into
a
wide
bacteria have
that
some
most
can
be
generali-
suitable
whereas
for
fungi
can
variety
of
organic
the ability
to
oxidise
organic substrate completely to carbon dioxide and water.
Microbial
transformation
is
an
ancient
art.
The
oldest and best known prototype is vinegar production which
has been performed since the dawn of recorded history.
process has
of
been further
fermentation
chemistry.
It
without
was
developed along
knowledge
only much
of
later,
the
traditional
underlying
through
The
lines
bio-
the discovery
of the specific modifications of steroids by microorganisms
5
that
microbial
has
today.
compounds
and
of
olinical
cology
Thousands
different
involving
variety
transformation
natural
chemistry,
also
technological
enzymatic
of
products
used
importance
is
using
of
well
now
with
organic
known.
analysis
biochemistry,
it
transformations
reactions
are
for
produced
process
microbial
of
types
chemicals
are
obtained the significance
and
A
wide
research
in
metabolism
and
pharma-
enzymes.
The
current
microbial
recognised
transformations
or
in
fields
of
benzene)
is
many
biotechnology.
Eugenol
the
ma i n
(1-hydroxy-2-methoxy-4-allyl
const i t uent
of
several
import ant
essent i al
like clove oil, cinnamon oil and pimenta oil.
the
essential
bud of
oil
obtained
the clove tree,
free eugenol
(70-90%),
its main constituents.
possessing
It
a
strong
from
leaf,
i ls
Clove oil is
stem
Eugenia caryophyllata.
or
flower
It contains
eugenol acetate and caryophyllene as
Eugenol is a
odour
of
cloves
faintly yellow liquid
and
a
burning
taste.
is widely used in pharmaceutical preparations, perfumes,
cosmetics and for scenting soaps.
is
the
0
as
a
starting
Isoeugenol
material
for
The main use of eugenol
the production of vanillin.
(1-hydroxy-2-methoxy-4-propenyl
benzene)
occurs
6
in
oil
of
prepared
ylang-ylang,
by
various
nutmeg
methods
potassium hydroxide.
and
champaca.
of
treating
Isoeugenol
It
is
eugenol
also
with
is a clear liquid with an
odour reminiscent of eugenol but more suave and lasting.
is
one
of
serves
the most
equally
well
Vanillin occurs
vanillin
is
on
ingredients
the
in perfume work and
scenting
mild
of
oxidation
sugar.
The
best
vanillin
the
known
plant,
extensively used as a
cosmetics
yields
in nature as a glucoside,
and
is
vanillin
Vanillin
for
Isoeugenol
soaps.
to
useful
Trimethoprim.
plating
It
is
also
which hydrolyses
natural
Vanilla
flavouring
used
Vanillin
industries.
alkaline-air oxidation of lignin,
in
is
source
manufactured
study,
the
isoeugenol
by
the
microbial
were
agent
in
perfumes
manufactured
food
L-dopa
and
by
metal
the
the main component in the
oxidation
of
with
It can also
isoeugenol.
transformations
undertaken
of
plansfolia.
spent sulfite liquors from sulfite pulp mills.
be
and
vanillin.
industry and in the synthesis of drugs like Aldomet,
and
It
a
of
view
In
this
eugenol
and
to
producing
vanillin, an extremely useful compound.
Two microorganisms, one CUAC 20 which can grow on
eugenol and the other CUAC 30 which can grow on isoeugenol,
7
were isolated from the soil.
which
can
mineral
transform
medium
both
eugenol
containing
an
easily
assimilable
organisms
were
then
generic level.
CUAC 30)
single
purified
The
were
carbon
first
identified
as
source.
and
two
from
the
a
carbon
This organism was not capable of growing on eugenol
a
isolated
in
flora.
as
also
isoeugenol
like
isoeugenol
was
and
source
or
glucose
A third microorganism CUAC 10
These
identified
organisms
belonging
to
(CUAC
the
aerial
micro-
upto
the
20
and
Pseudomonas
sp. and the third one (CUAC 10) belonging to the Bacillus sp.
In addition to
which
also
these,
grow
on
a
culture of Pseudomonas aeruginosa,
eugenol,
was
obtained
as
a
gi ft
from
Dr.Kohji Tadasa, Shinshu University, Japan.
The
done
fermentations
in a mi ni
of
eugenol
jar fermentor.
and
isoeugenol were
The t rans forma t ion products
were separated and identified using spectral data, physical
properties
CUAC 10
and
chemical
transformed
reactions.
eugenol
to
The
Bacillus
sp.
bisdehydroeugenol.
Bisdehydroeugenol on oxidation with nitrobenzene and alkali
gives
dehydrodivanillin.
This
strain
CUAC
10
converted
isoeugenol to dehydrodiisoeugenol and bisdehydroisoeugenol.
8
Dehydrodiisoeugenol
oxidation
on
with
nitrobenzene
alkali gives vanillin as the main product. The
CUAC
20
van i 11 ic
were
degraded
ac id
and
obtained
Pseudomonas
eugenol
the
30.
CUAC
were
aromatic
ring resulting
of
further
degraded
acid,
The
of
wi th
vanillin,
same
products
isoeugenol
these
All
Pseudomonas sp.
with
the
transformation
the
cleavage
of
in low yield of the products.
the
The
free extracts of the strains CUAC 20 and CUAC 30 were
prepared
cell
ac id.
fermentation
products
cell
ferulic
protocat echuic
in
sp.
to
and
and
free
t rans forma t ions
of
eugenol/isoeugenol
extracts were studied.
eugenol
and
isoeugenol
by
by
the
The degradation pathways
these
st rains
are
proposed,
specifying the mode of cleavage of the aromatic ring.
transformations
Microbial
eugenol
of
and
isoeugenol were attempted using immobilised microbial cells
also.
If
whole
microbial
cells
without extracting the enzyme,
used
as
systems
a
have
chemicals.
unit
solid
been
Another
production
fermentation
catalyst.
of
broth
the
is
Thus
of
desired
much
immobilised
directly
the immobilised cells can be
increasingly
advantage
are
immobilised
used
this
to
smaller
prepare
method
compound
in
whole
is
the
the
that
volume
case
of
cell
fine
for
of
the
9
continuous method using
immobilised cells than
of conventional batch fermentation methods.
the
studies
using
immobilised
presented in this thesis.
whole
in the case
The results of
cells
are
also
CHAPTER
2
HISTORICAL REVIEW
HISTORICAL REVIEW
2.1 INTRODUCTION
Microbial
transformations
have
been
widely
used
since the early days of mankind for the production of bread,
dairy products and alcoholic beverages.
Louis Pasteur
1
It was in 1862 that
laid a scientific foundation for one of these
early
applications,
namely
the
oxidation
of
alcohol
to
acetic
acid by using a pure culture of Bacterium xylinium. 2
Investigations of the oxidation of glucose to gluconic acid
by
Acetobacter aceti
3
and
of
sorbitol
Acetobacter sp.4 were then reported.
fermenting
yeast,
observed
Dumas
acid
by
to
sorbose
The reducing action of
Saccharomyces cerevisiae
in
from n-propanol
1874. 5
and
A.J.Brown
fructose
by
was
obtained
from mannitol.
first
propionic
2
Numerous
further microbial transformations followed.
However,
have
been
hydrates,
.
stero~ds,
6
simple
11-14
encouraged
the
aliphatic
till
of
of
confined
largely
classification
which
most
the
more
to
reactions
and
aromatic
1950s.
microbial
microbial
In
transformations
involving
compounds
1959,
transformations
fundamental
11
carbo-
7-10
following
by
studies
and
the
Stodola,
15
involving
12
different
ment
classes and types of substrates,
took
place
in
the
microbial
a rapid develop-
transformat ions
of
other
structural classes of compounds.
Todate an enormous amount of work has been done in
this
area
and
several
.
16-23
pub11shed.
excellent
reviews
have
been
To avoid excessive echoing of these reviews
we will limit our survey to the transformations of terpenoids
and
phenolic
compounds
only which
are
more
relevant
to our
investigations.
2.2
MICROBIAL TRANSFORMATIONS OF TERPENOIDS
The
terpenes
and
vast
i nvol ves
suitable
adaptation
substrates
t he
strains
tests
as
maj ori t y
well
breakdown
have
on
of mi crobi al
of
only
t rans forma t ions of
these
been
appropriate
natural
obtained
substrates.
product s
by
Also
as the end products are usually
volatile
compounds
by
the
aeration
However,
microbial
techniques
can
be
specific changes in these compounds.
used
in
used
Ciegler
tedious
the
lost as
fermentation.
to
24
bring
about
and Kieslich
et al. 25 have reviewed the work in this field.
Mayer
and
Neuberg
reduction of citronellal
(1)
were
to
the
first
citronellol
to
report
the
( 2 ) by yeast.
26
13
Some
of
strains
of
citronellal
Acetobacter
to
xylinium
citronellol
and
caused
transformation
citronellic
acid
(~). 27
HO
(1)
(2)
Joglekar
cyclisation
(4)
in
of
addition
acyclic
to
dihydrocitronellol
(6 )
from
Dharlikar
terpenes
citronellol
(~)
citronellal
.
28,29
aerugl.nosa.
(8)
and
(~)
were
able
to
when
they
(2),
citronellic
prove
obtained
menthol
acid
(~),
and 2,8-dihydroxy-2,6-dimethyl octane
by
Linalool
the
action
of
Pseudomonas
(2) was also cyclized to camphor
'
.
30
uSl.ng
a pseu d omona d stral.n.
OH
(4)
the
(5)
(6 )
14
OH
(8 )
(7 )
A strain of Pseudomonas putida degraded camphor to
5-endo-hydroxy
camphor
(9),
5-exo-hydroxy
camphor
(l9.)
and
effect
the
5-oxocamphor (11).31
HO
H
H
OH
(9 )
A strain
of
Serratia
allyl ic
oxidat ion
of
(13)
the
product,
as
major
d-trans-sobrerol
tion
of oC
(~)
and ~
marcescens
d-oC-pi nene
along
(g)
wi th
as minor products.
pinene
was
to
could
d-t rans-verbenol
d-verbenone
32
reported
(14)
and
Allylic oxygenato
be
the
most
characteristic reaction of a str?in of Armillariella mellea.
33
15
~o
Co-oxidation of limonene
ing
a
hydrocarbon,
( 15)
(14)
(13 )
( 12)
with
(~)
in a medium contain-
Corynebacterium
hydrocarboc1astus,
. Id e d carvone (17)'1n goo d quant1t1es.
..
34
Y1e
o
A bacterium isolated from the sewage was found to
degrade
octanoic
(20).35
(-) menthol to menthone
acid
( 19)
and
(~),
3,7-dimethyl-6-hydroxy
3,7-dimethy1-6-oxo-octanoic
acid
16
o
HO
o
( 19)
In
of
1990,
cis-verbeno1
Hansenula
studied
Hunt
(Q)
capsulata
the
l,4-cineole
and
to
and
with
reported
verbenone
Pichia
stereochemistry
( 23)
Borden
of
(~)
.
36
p1nus.
microbial
Bacillus
cereus
the
wi th
Liu
conversion
the
et
yeasts
al.
have
hydroxylations
and
Streptomyces
.
37
gr1seus.
CH 3
I
HO
of
17
The
pregrown
mycelia
of
theobromae was able to transform
the
fungus,
~ -ionone
Lasiodiplodia
(24)
to
p -cyclo-
homogeraniol ( 25 ) as the main product. 38
o
(24)
The
utilised
soil
~-santonin
microorganism
the
(30)
and
his
conversion
and
and
colleagues
of
cichorii
(26) as the sole carbon source producing
1,2-dihydro-~-santonin
Cross
Pseudomonas
in
1965,
gibberellin
gibberellin
A13
A12
(~),
lumisantonin
were
able
(29)
to
by
the
to
(28).39,40
demonstrate
gibberellic
fungus
acid
Gibberella
.. k
. 41
f UJl urOl.
o
o
o
(26)
( 27)
( 28)
18
H
eOOH
( 29)
(30)
eOOH
Dmitrovskii
cleavage
of
of
~
-carotene
.
and
(~)
Pseu d omonas aeruglnosa.
Starikova
have
to vitamin A
42
(32 )
(33)
reported
(~)
by
a
the
strain
19
2.3
MICROBIAL TRANSFORMATIONS OF PHENOLIC COMPOUNDS
Compared
work
has
been
compounds
carbohydrates
done
in
the
may
be
because
which
properties.
to
Our
microbiological
present
and
steroids,
transformation
of
state
transformations
of
their
of
not
much
phenolic
antimicrobial
knowledge
about
aromatic
compounds,
of
the
phenolic compounds in particular, has already been summarised
, a num b er
ln
d etal'1 e d reVlews.
'
43-47
0 f
Because
vi tal
biochemical
microorganisms
Before
an
0-
possible
can
ring,
or
to
in
of
natural
capable
of
cleaving
be
microbial
any
precondition
is
p-dihydroxyphenol
16
aromatic
the
convert
hydroxylation.
lation
a
breakdown
step
are
there
aromatic
have
the
it
to
the
of
these
The enzymes responsible
the
character of
mixed
incorporate
one
oxygen
into
vicinal
the
structure
have
However,
carbon
dioxygenases
atom
can
also
be
for
function
the
oxygen atoms simultaneously.
ring.
of
substrate
or
it
a
many
aromatic
an
must
must
be
structures
by
this
hydroxy-
oxidases which
substrate
used which
is
cycle,
decomposition
that
one
compounds
molecule.
introduce
two
Oxidative cleavage of
the aromatic ring may occur by three different mechanisms:
16
20
a) ortho
the
cleavage - The
C
atoms
ring
which
o-dihydroxyarene
is opened
bear
the
been
the
hydroxy
bond
between
groups
of
producing a cis-2,4-hexadieneoic
Catechol:oxygen-l,2-oxidoreductase
has
at
named
as
the
an
acid.
(catechol 1,2-oxygenase)
enzyme
responsible
for
ring
the
bond
cleavage.
b) meta
cleavage - The
between
the
ad j acent
C
atoms
cleavage
bearing
unsubst i t uted
a
carbon,
acid.
oxo-2,4-hexadienoic
The
occurs at
hydroxy
produc ing
group
a
and
an
2-hydroxy-6-
splitting
ring
enzyme
(catechol 2,3-
catechol 2,3-oxidoreductase
called
is
ring
oxygenase.
c) p-dihydroxyphenol
atom
bearing
bearing
broken.
a
a
cleavage - The
hydroxy
hydrogen,
This
a
and
bond between the C
an
adjacent
carboxy group or a
produces
hexadieneoic acid
group
ring
from
cis-3,5-heptadienedioic
a
side
C
atom
chain
is
4-hydroxy-6-oxo-cis,cis-2,4-
quinol and
4-hydroxy-2-oxo-cis,
acid
2,5-dihydroxybenzoic
from
acid.
2.3.1
Phenol and catechol
In 1952, Gale isolated a
could transform phenol
(34)
strain of bacteria which
to 1,2-dihydroxybenzene
(35).48
21
Cain and coworkers reported the ring fission of catechol by
o-cleavage, with a bacteria isolated from soil.
49
OH
OH
@
In
bacteria
m-cleavage.
OH
I
Q
~ OOR
( 35 )
(34)
of
:>
>
1959,
which
Dagley
could
and
Stopher
break
50
©JOH
OH
6
COOH
HO
the
isolated
a
strain
aromatic
ring
by
22
In
1965,
Larway
1,4-dihydroxybenzene
the
Ewans
hydroxylated
1,3-
and
(36, 37), with a bacterial strain and
trihydroxybenzene
p-dihydroxyphenol
and
( 38)
formed
was
broken
down
by
cleavage. 51
©tOH
OH
OH
( 36)
QHO
)
OOH
H
OH
(38)
OH
( 37)
The
assimilation
of
phenol
as
a
sole
carbon
energy source by strains of thermophilic bacteria,
stearothermophilus
have
been
reported.
52
Some
and
Bacillus
strains
of
yeasts can oxidise phenol to catechol which is then oxidised
to
cis,
reported
cis-muconic
a
acid
quantitative
(39).53
Smith
conversion
.
. aC1
. d W1t
. h a Nocar d'1a sp. 54
C1s-mucon1C
of
and
Ratledge
catechol
to
have
cis,
In 1990, Gajendiran
23
and
Mahadevan
many
report ed
phenolic
strains
of
thei r
compounds
bacteria
st udi es
by
can
on
the
Rhizobium
a
anaerobically
ut i 1 i sa t i on
sp.
55
of
Certain
degrade o-phenyl-
phenol (40).56
OH
COO~OH
()
I~
(40)
(39)
A Tri chosporon sp.
in
a
medium
until
(0.1%)
20 ppm.
the
containing
57
the
both
preferent iall y
phenol
concentration
(200
of
ut i 1 i sed
ppm)
phenol
and
phenol
glucose
decreased
to
Hoffman and Ute Vogt have reported the studies on
induction
of
Candida
of
some
of
phenol
mal tosa. 58
alkyl
phenols
assimilation
Yi
on
Tin Wang
the
in
has
chemostat
studied
anaerobic
phenol in batch methanogenic cultures.
59
cultures
the effects
biodegradat ion
of
24
2.3.2
Methyl substituted phenols
A strain of
phenol
( 41 )
Anot her
st rain
methylbenzene
to
of
Pseudomonas sp.
oxidised 2,4-dimethyl-
4-hydroxy-3-methylbenzoic
Pseudomonas
(43)61
sp.
met abol i sed
acid
(42).60
I-hydroxy-2-
and I-hydroxy-3-methylbenzene
by m-cleavage.
OH
OH
COOH
(43)
(42 )
The oxidation of thymol
(45) by Pseudomonas putida
affords the hydroxyquinone (46) in about 10% yield.
o
>
(45)
~
(46)
63
25
In 1968 the oxidation of
suspensions
of
a
Hopper and Chapman.
fluorescent
pseudomonad
was
(~)
by cell
reported
by
60
OH
OH
OH
OH
2,4-xylenol
OH
OOH
>
The
same
authors
eOOH
eOOH
eOOH
have
reported
2,5-xylenol (47) and 3,5-xylenol (48).64
the
catabol ism of
26
Achromobacter
An
catechol
( 50) •
65
phenol
to
(49)
sp.
oxidise
2-hydroxy-3-methylmuconic
In 1979 Engelhardt,
and
could
methyl
reported
substituted
phenols
OH
~oo
the
by
3-hydroxy-
semi aldehyde
co-metabol ism of
a
Nocardia
sp.66
(f0
,rtV
;)
#'
H3
OH
eH 3
(50)
(49)
2.3.3
Carboxy substituted phenols
In
1954,
McDonald
and
o-cleavage of 3,4-dihydroxybenzoic
.
bacterium to g1ve
coworkers
acid (51)
reported
by a strain of
(1--2 ) .67
eOOH
eOOH
:>
Q
eOOH
eOOH
OH
(51)
(52)
the
27
Subsequently Tripett et al. reported the m-cleavage
of
the
same
p-dihydroxy
compound
phenol
benzoic acids
68
and
cleavage
Hopper
of
et
al.
2-hydroxy
reported
the
and 3-hydroxy-
(53, 54) to give (55) and (56) respectively.69
eOOH
Jr
HlocAoH
eOOH
@(H
(53 )
eOOH
(56 )
In 1973, a novel microbial synthesis of L-tyrosine
(57)
or
3,4-dihydroxyphenyl-L-alanine
(58)
from
pyruvic
acid, ammonia and phenol or catechol, with Erwinia herbicola,
was reported.
70
28
OH
OH
( 57 )
Some
(58)
strains
of
converted p-hydroxybenzo ic
yeasts
of
ac id (59)
the
genus
Debaromyces
to hydroqu inone
(.~1)? 1
OH
eOOH
:>
OH
OH
(59)
A
strain
p-hydroxybenzoic
hydroxyquinol
of
acid
Trichosporon
via
cutaneum
protocatechuic
metabolises
acid
(52)
and
29
eOOH
OH
)
ring
>
> fission
OH
OH
OH
strain
A
salicylic
and
acid
Arima
have
a
to
Aspergillus
acid
(60).75
niger
2,3-dihydroxybenzoic
isolated
Pseudomonas
"
gentlslc
aCl'd
OH
of
(53)
3-hydroxybenzoic
Using
OH
sp.
a
to
bacteria
transformed
aCl'd • 73
which
2,5-dihydroxybenzoic
salicylic
acid
was
convert
aCl'd • 74
converted
to
Some strains of Streptomyces sp. also
metabolise salicylic acid to gentisic acid.
76
&OH
eOOH
OH
)
( 53)
can
Yano
HO~
( 60)
30
In
Pseudomonas
1989,
sp.
Enge1berts
degraded
4-
et
and
(61,62) by the m-cleavage pathway.
al.
reported
that
5-methy1sa1icy1ic
a
acids
77
COOH
COOH
OH
OH
H3
CH 3
( 61 )
(62)
Nocardia
acid
(63)
opaca
to gentisic acid which
and formy1pyruvates.
anthranilic
metabolises
acid
to
78
The
5~hydroxyanthrani1ic
is degraded
dehydroxy1ation
anthranilic
acid
(64)
to
of
with
.
.
79
coli 1S a very rare react1on.
COOH
COOH
©r~2
(64)
ma1ey1-
5-hydroxyEscherichia
31
Pseudomonas testosteroni oxidises 3- and 4-hydroxybenzoic acids to
3,4-dihydroxybenzoic acid. 80
Lentinus
oxidises
lepideus
3,4-dihydroxycinnamic
acid
A strain of
4-hydroxycinnamic acid
(65)
to
(66).81
CH=CHCOOH
=CHCOOH
OH
OH
OH
(65 )
Salicylic
acid
can
decarboxylation to catechol.
decarboxylate
(67)
82
microbially
undergo
oxidative
The fungus Valsa friesii can
6-methylsalicylic
acid
(67)
to
m-cresol.
83
32
In
enzyme
could
1959,
system
Ribbons
from
and
testosteroni
to
Hayashi
which
isolated
could
a
.:r§:('
HO
eOOH
HO
eOOH
(70)
and
under
In
1978,
84
of
Pseudomonas
acid
85
rn-Y
eOOH
H~eOOH
Hopper
utilisation
have
of
described
a
catabolic
5-hydroxyisophthalic
acid
by a Coryneform sp. through (2!).86
eOOH
OH
HO
(68)
( 69)
Elmorsi
the
which
4-hydroxyphthalic
(68)
for
cell-free
cells
acid
acid.
strain
convert
a
pseudomonad
protocatechuic
(69) to 4,S-dihydroxyphthalic acid.
pathway
prepared
4,5-dihydroxyphthalic
conditions
Nakazawa
Evans
phthalate-grown
decarboxylate
anaerobic
and
;£
o
( 70)
~~HYry
HO
OOH
eOOH :0
( 71)
HO~eOOH
ortho
> fission
33
In
tryptophan
1985,
(l£.)
Showa
can
be
Oenka
produced
acid with Bacillus subtilis.
involvement
metabol i srn
of
of
0-3-
are
87
from
and
to
the
5-hydroxy-L-
5-hydroxyanthranilic
trans-aminase
0-4-hydroxy phenyl
Both
converted
that
Vanden Tweel has studied the
enantioselective
pUtl'd a. 88
Pseu d omonas
glycines
an
reported
0-3- and
in
the
g 1 yc ine (73)
in
0-4-hydroxyphenyl-
corresponding
hydroxyphenyl-
glyoxylates.
CH (NH 2 )COOH
HO
OH
( 73)
The
can
oxidise
enzyme
preparation
melilotic
acid
(74)
from
to
an
Arthrobacter
its
derivative
(74 )
(75 )
sp.
3-hydroxylated
34
Several
(76)
microorganisms
oxidise
(77).90
to 4-hydroxycoumar in
niger converts coumarin (78)
o-coumaric
acid
A strain of Aspergillus
(2.~). 91
to melilotic acid
OH
~CH=CHCO_O_H__~)
OH
(76)
(77)
(78)
Dagley
acetic acid
( 79 )
et
al.
in
1952,
oxidised
. . aC1
.d
to homogent1s1c
o-hydroxyphenyl-
( 80 ) W1t
. h a V1. b r10
. sp. 92
)
(79)
(80)
35
Cooper and Skinner have studied
the catabolism of
3- and 4-hydroxyphenylacetic
acids (~l' 82).93
were
same
catabolised
phenylacetic acid
by
(~~)
the
pathway
with
as the substrate for the
benzene nucleus, by strains of
Both compounds
3,4-dihydroxyfission of the
~.coli.
CH 2 COOH
~COOH
lSloo
H
( 81)
The utilisation of
p-hydroxyphenylacetic
acid
OH
OH
( 82 )
( 83 )
p-hydroxybenzaldehyde
(84)
and
as the sole carbon and energy
source by Pseudomonas solanacearum was studied by Arunakumari
and Mahadevan.
for
the
94
Dugan and Golovlov have proposed a pathway
metabolism
..
homogent1s1c
aC1. d • 95
of
2-hydroxyphenylacetic acid
(~)
via
36
CBO
OH
OH
( 84)
(82)
( 79)
In 1985, David J.Hopper and coworkers reported the
metabolism
pathway
of
for
4-hydroxyacetophenone
its
degradation
with
(~)
an
-->~
H
(85)
OH
OH
proposed
Alcaligenes
)
OH
and
sp.
ring
fission
a
96
37
Chen and his colleagues have studied the degradation
4-hydroxymandelic
of
acid
(86)
with
Rhizobium
leguminosarum to cis,cis-muconic acid (39).97
CH (OH)COOH
(J00H
COOH
OH
(39)
(86)
2.3.4
Halogen, nitro and amino substituted phenols
In
microbial
view of
their
conversion
of
applications
halogen
as
herbicides,
substituted
phenols
the
and
their derivatives has been studied in detail.
In
1968,
Bollag
et
al.
reported
hydroxylation reactions of (87 ) and (88)
the
following
. hm~croorgan~sms.
'
.
98
w~t
38
OH
Cl
(87)
OH
Cl
( 88)
An Achromobacter strain when grown on 2,4-dichlorophenol
(89)
or
2-methyl-4-chlorophenol
3,5-dichlorocatechol
(~)
(90)
oxidised
OH
Cl
(89)
to
or 3-methyl-5-chlorocatechol (92),
.
1 y. 99
respectlve
Cl
it
HO
Cl
Cl
(90)
39
OH
OH
H
Cl
A Nocardia
to
3-chlorogentisate
cleavage.
sp.
(94)
converts
3-chlorosal i cylate
(~)
which then undergoes oxygenative
100
eOOH
eOOH
)
@(
~OH
OH
HO
cl
Bacillus
by a pathway,
brevis
degrades
which does not
~
el
5-chlorosalicylate
follow the general
(95)
schemes
in
40
which
prior
to
ring
fission
thr~
ar..-omatic
nucleus
must
be
substituted with at least two hydroxy groups.lOl
;§rOH
Cl
(95)
In
1974,
Spokes
and
Walker
studied
the
co-meta-
bolism of chlorophenol and chlorobenzoic acid by a Pseudomonas
sp.
and
Bacillus
sp.l02
A
pure
culture
of
Pseudomonas
cepacia completely or partially dehalogenates a wide variety
of
halophenols
(96).103
and
2,4,5-trichlorophenoxy
acetic
acid
In 1989, Haggblom et al. reported that a strain of
Rhodococcus sp. was able to o-methylate chlorophenols to the
corresponding anisoles
(97).104
pentachlorophenol
by
also been reported.
(98)
105
The degradation kinetics of
certain
strains
of
bacteria
have
41
OCH 2 COOH
Cl
Cl
Cl
cl
(96)
Cl
(98)
(97 )
Tewfik and Evans have reported
the
sp.
1
C
herbicide
3,5-dinitro-o-creso1
the degradation of
(99)
by
a
Pseudomonas
106
CH
CH
3
OH
)
')
./
/'
°2
(99 )
N02
3
OH
O2
HO
H
1
ring cleavage
42
Bacillus
The
transformations
and
Pseudomonas
p-Nitrophenol
(101)
' t e pro d uc t s
me d 1a
chrysogenum.
bacteria
have
p-phenetidine
C.B.,
to
by
been
(102)
are
reported.
the
inter-
has
reported
the
hydroquinone
oxidation
by
of
Penicillium
108
Sloane
4-aminophenol
described
p-nitrophenol
f p-n1't rop h
I d egra d
'
107
eno
at1on.
0
Iyayi,
p-nitrophenol
and
of
the
et
al.
has
(103).109
In
degradation
Arthrobacter sp. by the
~
of
reported
1989,
the
metabolism
Lechner
3-aminophenol
-keto adipate pathway.
and
(104)
110
of
Straube
by
an
43
OH
OH
OH
In 1987,
derivatives
(104 )
(103 )
( 37)
of
Faleev and his colleagues prepared
L-tyrosine
(105)
from
fluorophenols
fluoro(106),
pyruvic acid and ammonia using Citrobacter intermedius cells
in 7-12% poly(vinyl alcohol)cryogel as a biocatalyst.
F
OH
lll
44
2.3.5
Tyrosine, tyramine and synephrine
Several microorganisms oxidise suitably derivatised
L-tyrosine
of
to
L-DOPA
(107),
which
'sd '1sease. 112
'
Par k 1nson
reported
from
Singh
that
a
tyrosine
et
of L-DOPA
Vibrio
via
strain
used
Jones
and
produced
in
have
aC1'd
also
from
L-tyrosine or N-formyl-L-tyrosine
microbial
1970,
alcohol
have
acid
(108).113
synthesis
(109)
by a
114
(109)
(108)
(107)
In
a
treatment
homogentisic
al.
'
reported
the
coworkers
'
p- h y d roxyp h eny 1 pyruv1c
pseu d omona d mutant stra1n.
benzyl
is
Robert
from
H.White
prepared
L-tyrosine
with
4-hydroxy-
, 115
E.co 1 1.
45
The
production
of
(110)
was
inhibited
by
the
addition
of
thiamine to the medium.
CH 2 CH(NH 2 )COOH
OH
Lippoldt
degradation
and
of
et
al.
tyrosine,
have
proposed
a
pathway
for
involving p-hydroxyphenylacetic acid
p-hydroxyphenylpyurvic acid with
Candida
ma1tosa.
NH2
I
CH 2 CHCOOH
)
OH
ring fission
the
OH
~(-----
l
OH
<
OH
116
46
Tyramine
responsible
for
oxidase,
the
enzyme
oxidation
the
p-hydroxyphenylacetaldehyde (112)
of
has
from
Sarcina
lutea
tyramine
been
to
isolated
and
'
d 117
crysta 11 1se.
>
OH
OH
( 112 )
A Nocardia
novel
routes - one
sp.
degraded
synephrine
(113)
by
involving monoamine oxidase and the other
involving conversion to
p-hydroxyphenylacetaldehyde
by the
'
synep h r1nase
enzyme. 118
NHCH
I 3
CH OH
IHOH
OH
OH
CHO
OH
two
I
CH OH
COOH
47
2.3.6
Methoxy substituted phenols
In 1919,
Robbins and Lathrop isolated a bacterium
from the soil which could oxidise vanillin (114) to vanillic
acid (115).119
CHO
COOH
>
OH
(115)
(114)
A st rain
of
(116) by ortho cleavage.
Ac inetobacter
sp.
degraded
guaiacol
120
ortho cleavage
48
Washed cell suspensions of Pseudomonas acidovorans
degraded
ferulic
acid
(117)
to
protocatechuic
acid
via
vanl'11'In an d vanl'11'lC aCl'd . 121
CBO
CH=CHCOOH
)
)
)
OH
(117)
Iwahara Shojiro has studied the microbial degradation
ferulic
of
strain
of
whereas
acid
bacteria
different
converted
Rhodotorula
completely.
by
and
Nazareth
Fusarium
solani
strain
transient
intermediate,
ferulic
Fusar i urn
and
,
,122
mlcroorganlsms.
sp.
Mavinkurve
metabolises
acid
vanillic
met abol i sed
have
the
reported
ferulic
4-vinylguaiacol,
123
'
un d ergoes f urt h er d egra d 0tlon.
to
(118)
One
acid
ac id
that
a
to
a
acid
which
then
49
CHO
CH=CHCOOH
OH
OH
OH
OOH
(118)
A wood
can
transform
destroying
vanillic
acid
their aldehydes and alcohols.
fungi,
and
124
Polystictus
isovanillic
COOH
>
>
OH
acid
versicolor
(119)
to
50
In 1983,
for
the
Paul Ander
metabolism
pu 1 veru 1 entum.
of
~
~.
proposed a new pathway
vanillic
acid
by
Sporotrichum
125
eOOH
OH
H
~
;)
OCH3
OCH
---;)
3
~oo
OH
OH
OH
J:
A strain of Acinetobacter lwoffi degrades 4-hydroxy3-methoxymandelic
hydroxylated
to
acid
yield
(120)
to the benzoate which
protocatechuic
strain of Pseudomonas sp.
acid.
126
transforms veratric acid
vanl'11'lC aCl'd an d 'lsovanl'11'lC aCl'd • 127
H(OH)eOOH
eOOH
(121 )
is then
A
mutant
(121)
to
51
DeWulf
Saccharomyces
his
colleagues
cerevisiae
vanillyl
alcohol
reported
the
fluorescens.
and
strain
could
(122).128,129
van i 11 yl
130
ami ne
Under
bolised
vanillyl
vanillyl
alcohol.
In
(123)
growing
to
amine
Under
have
reported
reduce
1988,
conditions
vanillin,
non-growi ng
vanillin
Hisae
metabol i srn
Asai
in
the
that
et
a
to
al.
Pseudomonas
cells
vanillic
condi t ions,
acid
metaand
van i 11 in,
vanillic acid and protocatechuic acid were formed.
OH
(122)
The
(124,
125)
(123)
transformations
and
of
6-bromovanillin
acids have been reported.
131
5(126)
and
to
6-chlorovanillin
the
corresponding
52
CBO
CHO
Cl
Br
OCH 3
OH
OH
(125)
(124 )
Ohta
of
et
al.
dehydrodi con i feryl
have
(126)
reported
alcohol
(127)
the
by
partial
metabolism
Fusar i urn
solan i .132
CHO
>
OCH
OCH3\
3
H3
OH
(127)
CH 2 0H
(
HOec
OCH 3
OH
H3 CC
0
"
CH
3
53
In
bolic
1988,
Habu
pathway
Pseudomonas sp.
of
and
coworkers
reported
dehydrodiconiferyl
the
alcohol
metaby
a
133
CH=CHCOOH
H=CHCH 2 OH
OOH
CH 3
CH==CH
1
)
OCH
OCH 3
H3
H3
CH=CHCH 2 0H
COOH
<
OHC
OH
OH
OH
A glycerol maceration of Russula delica converted
eugenol
to
bisdehydroeugenol
(129)134,135
isoeugenol (130) to dehydrodiisoeugenol (131) .136,137
and
54
OH
(128)
(131 )
(130)
Kohj i
Tadasa
has
reported
that
sp. degraded eugenol to give vanillin,
an d
oo
d s. 138
protocatec h U1C
aCl
on
eugenol
included
more
a
Corynebacter i urn
and vanillic, ferulic
A Pseudomonas
metabolites
sp.
than
when grown
with
the
55
Corynebacterium
sp.,
but
,
l
1soeugeno
, an'1somer
0
this
strain
could
not
degrade
1 139
eugeno.
f
OH
/0"
H2 CH- CH 2
CH 2 CH=CH 2
OCH
3
OH
CH=CHCH OH
2
>
----7
:>
OCH
I
HCHCH 2 0H
OCH
3
OCH
3
3
OH
1
CHO
<
(
OH
OH
OH
In
eugenol was
solani
aC1'd •
123
1986,
Nazareth
transformed
strain,
which
OH
OH
and
Mavinkurve
reported
to 4-vinyl guaiacol with a
degraded
further
to
that
Fusarium
protocatechuic
CHAPTEU
3
MATEnIALS AND METHODS
MATERIALS AND METHODS
3.1
CHEMICALS
Eugenol,
isoeugenol,
eugenol
isoeugenol
dehydrodiisoeugenol,
Ferul ic
ac id
was
Shinshu
University,
acetate,
ferulic
protocatechuic
and
acetate,
acid
obta i ned
as
Japan
bisdehydro
acid,
were
vanillin,
used
a
and
bisdehydro
as
gift
vanillin
isoeugenol,
vanillic
the
from
eugenol,
substrates.
Dr. Koh j i
was
acid
Tadasa,
purchased
from
Sisco Research Laboratories, Bombay.
Isolation of eugenol from clove oil
3.1.1
Eugenol
01.
cove
1
1
by
was
.
treat lng
well
cleaned
250 cc
was
introduced.
extracted
Wl. t h
round
To
hydroxide
solution
5 minutes.
Then
it
bottom flask,
75 ml
added
was
contents
funnel
aqueous
and
of
the
layer
the
and
heated
flask
were
non-phenolic
was
f i 1 tered
a
Into a
of clove
1 N
shaken
on
potassium
thoroughly
water
oil
bath
for
with
The flask was cooled and
poured
portion
through
57
10 ml
aqueous
occasional shaking for 10 minutes.
the
commercially available
potassl. urn h y d rOXl. d e. 140
this
was
from
a
was
into
a
separatory
separated.
f i 1 ter
paper
and
The
the
58
filtered
acid
was
solution
until
The
sulpha te,
8.5 g
acidified
mixture
t rans fer red
ether.
get
the
was
to
a
ether
was
with
dilute
strongly acid
separa tory
extract
was
funnel
dried
to
and
with
hydrochloric
litmus.
This
ext rac t ed
wi t h
anhydrous
f i 1 tered and evaporated under reduced
of
eugenol
as
a
pale
yellow
liquid,
pressure to
boiling
at
141
255°C.
3.1.2
Preparation of eugenol acetate
Into
a
dry
50 ml
RB
flask
1.5 ml
eugenol,
pyridine and 3 ml acetic anhydride were placed.
for
sodium
6
hours,
poured
into
extracted
shaking
intermittently.
ice-cold water,
with
ether
and
stirring
the
4 ml
It was kept
The mixture was
continuously.
ether
extract
was
It
then
was
washed
several times first with 1 N hydrochloric acid and then with
5% sodium bicarbonate solution.
It was dried with anhydrous
sodium sulphate and evaporated under vacuum to get 2.14 g of
eugenol
acetate.
Eugenol
acetate
is
a
pale
yellow
liquid
142
freezing at 30°C.
3.1.3
Preparation of isoeugenol
Isoeugenol was prepared by the reaction of eugenol
.
wlth
.
potasslum
h y d rOXl. d e. 143
In
a
250 ml
RB
flask
10 g
59
eugenol was mixed with 4.5 g KOH
was
then
removed at
in 10 ml of water.
Water
reduced pressure.
Three
80-90°C under
gram diethylene glycol and 1 g triethanolamine were added to
it and the mixture was heated for about 5 minutes at 160°C.
The
me1 t
dilute
the
was
sulphuric
run
acid.
into water and
It
was
extracted
neut ra1ised
with
with
benzene
and
benzene extract was distilled under reduced pressure to
yield
9 g
at 266°C.
3.1.4
isoeugenol
ethanol
placed
as
a
pale
yellow
liquid,
boiling
141
Preparation of bisdehydroeugeno1
In
and
and
precipitate
was
cool ed
a
250 ml
6 g
ferric
shaken
was
RB
4 ml
chloride
in
well.
filtered
recrystallised
flask,
from
The
and
30 ml
mixture
washed
ethanol
eugenol
to
was
with
give
in
of
20 ml
water
cooled
40%
and
ethanol.
1.62 g
of
were
the
It
bisdehydro-
eugenol having a melting point of 106°C.
3.1.5
Preparation of isoeugeno1 acetate
Into a dry 50 ml
RB flask 1.5 ml
isoeugeno1,
pyridine and 3 ml acetic anhydride were placed.
for
6
hours
shaking
occasionally.
The
mixture
into ice-cold water, stirring continuously.
4 ml
It was kept
was
poured
It was filtered
60
and
the
prec i pi ta t e
followed
was
by 5% NaHC0
wi t h
1 N hydroch lor i c
ac id
solution to give 2.31 g of isoeugenol
3
142
acetate melting at 79°C.
3.1.6
washed
Preparation of bisdehydroisoeugeno1 and
dehydrodiisoeugeno1
In a
ethanol
and
250 ml
7 g
RB
ferric
flask,
5 g
chloride
in
placed and shaken well for 5 minutes.
off
under
reduced
was
transferred
The
ether.
sulpha te ,
It
was
melting
to
ether
a
separatory
extract
and
sUbjected
to
under
at
of
water
and
132°C were obtained.
was
were
Ethanol was distilled
funnel
dried
of
and
wi th
cooled.
extracted
anhydrous
It
wi th
sodi urn
con cent ra t ed under reduced pressure.
column
section
158°C
30 ml
in 30 ml
pressure and the mixture was
f i 1 t ered
described
isoeugenol
3.5.3.
1.48 g
chromatography
as
will
be
0.70 g bisdehydroisoeugenol
dehydrodiisoeugenol
melting
at
Both products were recrystallised from
ethanol.
3.1.7
Preparation of vanil1ic acid
Vanillic acid was prepared by fusing vanillin with
potassi urn
hydroxide
conditions. 144
In
and
a
sodi urn
hydroxide
stainless
steel
under
beaker
controlled
of
250 ml
61
capacity equipped
hot
plate,
with
1.8 g
a
of
stirrer
sodium
and
heated
hydroxide
by
an
electric
pellets,
1.8 g
potassium hydroxide pellets and 1 ml water were placed.
mixture was stirred well and heated to 160°C.
was
turned
off
and
1.5 g
of vanillin was
of
The
The hot plate
added
in
portions
at a rate sufficient to maintain the reaction temperature at
about 190°C.
hot
plate
was
stirring.
about
removed
When
150°C,
mixture
with
Stirring was continued for
was
15 ml
and
the mixture
5 more minutes,
allowed
10 ml
then
The
cool
with
the temperature of the mixture came down to
water
cooled
was
to
added
room
and
stirred
temperature
6 N hydrochloric acid keeping
stirring.
to
the
ppt.
formed
was
f i 1 t ered,
well.
and
The
acidified
in an ice bath and
washed
wi t h
wat er
It was recrystallised from water-acetone mixture
and dried.
which yielded, 1.42 g vanillic acid melting at 210°C.
3.1.8
Preparation of protocatechuic acid
Protocatechuic
vanillin
In
a
sodium
stainless
with
a
3.5 g
was
with
stirrer
KOH
pellets
stirred
and
hydroxide
steel
and
was
acid
beaker
heated
and
heated
1 ml
to
and
of
by a
prepared
.
potasslum
250 ml
hot
plate
1 g
Then
1.6 g
fusing
.
145
hydroxlde.
capacity,
water were placed.
160°C.
by
NaOH
equipped
pellets,
The mixture
vanillin
was
62
added
in
about
190°C,
until
the
at
portions
250°C
10 ml
15 ml
ice
to
rate
stirring
to
was
reached
to
the
for
2
hot
temperature at
Heat
was
applied
Temperature was
plate was
stirring.
dissolve
acid.
hours,
mixture
the
the
removed
At
kept
and
about
mixture.
the
150°C,
It
was
room temperature and acidified with
The mixture was
filtered
and
the
cooled
in an
precipitate
was
It was dried and recrystallised from
washed with ice water.
water-acetone
250°C.
with
to
6 N hydro chI or i c
bath
the
cool
added
cool
to maintain
simultaneously.
5 minutes,
allowed
water
allowed
a
temperature
for
mixture
at
to
yield
0.95 g
protocatechuic
acid
melting at 200°C.
3.2
SOURCE OF MICROORGANISMS
As the stock cultures in our laboratory were found
to
be
inactive
on
eugenol,
microorganisms
that
transform
eugenol were isolated from the local soil using the elective
culture
organisms
technique.
used
in
As
this
mentioned
study,
previously,
Pseudomonas
obtained as a gift from Dr.Kohji Tadasa.
one
of
the
aeruginosa
was
63
3.2.1
Screening of soil samples
The
screening
of
the soil samples was done
in the
following mineral salts medium.
NH N0
4
3
1 g
KH 2 P 04
0.9 g
Na HP0
2
4
0.55 g
MgS0 7H O
4
2
0.2 g
CaC1 2H O
2 2
0.1 g
FeS0 7H O
4
2
0.1 g
Tap water
1000 ml
pH
7.2
For
preparing
isoeugenol was added as
To get a
specified).
mineral
the
broth
0.1%
carbon source
solid medium,
2%
(v/v)
eugenol/
(unless otherwise
(w/v)
agar was added
to the above broth.
About
habi ta t
of
clove
tap water
and
and
of
10 ml
2 g
trees
shaken
the
of
soil,
was
collected
suspended
in
It
was
thoroughly.
supernatent
was
from
the
natural
of
st er i le
100 ml
allowed
transferred
to
to
settle
100 ml
of
64
the mineral medium containing eugenol/isoeugenol as the sole
carbon
source.
This was
rotary shaker.
One ml
another
of
100 ml
incubated at
mineral
medium
isoeugenol and incubated at 30°C.
mineral
agar
the
eugenol/
in sterile petri dishes with eugenol-
(EMA)/isoeugenol-mineral
on
containing
After 2 days 1 ml of the
The plat es were i ncubat ed at 30 ° C for
developed
2 days on a
of the broth was then transferred to
the
broth was pour-plated
30°C for
plates
were
agar
(IMA)
2 days.
isolated
medium.
The col oni es
and
purified
streaking repeatedly over fresh EMA/IMA plates.
by
Two strains
of bacteria, one growing on EMA and the other growing on IMA
slants,
were
CUAC 20
and
obtained.
CUAC 30
The
pure
respectively,
strains
were
designated
transferred
to
as
EMA
and IMA slants respectively.
3.2.2
Isolation of the strain CUAC 10
strain
The
contaminant
glucose.
fying
it
in
The
to
a
CUAC 10
mineral
broth was
pH 2.
The
was
medium
extracted
ether
isolated
as
containing
with
extract
ether,
was
a
chance
eugenol
and
after acidi-
concentrated
and
subjected to thin layer chromatography studies, which showed
the
presence
of
one
more
spot
other
than
that
of
eugenol.
65
This
isoeugenol
strain
in
a
CUAC 10
mineral
was
medium
also
able
containing
to
transform
isoeugenol
and
glucose.
But this strain was not able to grow on EMA or IMA
plates.
So
nutrient
it
was
purified
by
streaking
repeatedly
over
agar plates and the pure strain was transferred
to
nutrient agar slants.
3.2.3
Maintenance and preservation of the cultures
The
agar
s 1 ants
CUAC 20
culture
an d
and
CUAC 10
·
nutrl.ent
was
un d
er '
Ol. 1 • 146
agar
Pseudomonas
maintained
aeruginosa
were
on
nutrient
The
cultures
maintained
slants and CUAC 30 was maintained on IMA slants.
were
sterilised
for 15 minutes.
slants
and
by
autoclaving
15 Ibs
EMA
The media
pressure
(l210C)
The cultures were transferred to appropriate
incubated
refrigerator.
at
on
for
They were
one
day
before
transferred
to
storing
fresh
them
slants
in
every
month to keep them viable.
3.3
IDENTIFICATION OF THE MICROORGANISMS
The
gener i c
1 evel
isolated
organisms
based
thei r
characters. 147 ,148
on
were
identified
morpho log i cal
and
to
the
bi ochemi cal
66
3.3.1
Morphological characters
These
tests
spore
staining,
included
staining,
the
cell
morphology,
chromogenesis
and
gram
motility.
Chromogenesis was tested by growing the cultures on nutrient
agar.
3.3.2
Motility
was
tested
by
the
hanging
drop
method.
Biochemical characters
Biochemical
production
nitrate,
of
indole,
hydrogen
glucose,
characterisation
utilisation
sulphide
of
production,
oxidation-fermentation
included
citrate,
gas
test,
tests
for
reduction
production
cytochrome
of
from
oxidase
activity, catalase activity and ability to produce hydrolytic
·
.
149
enzymes name 1 y ge 1 atlnase,
amy 1 ase an d caselnase.
3.4
GROWTH STUDIES OF THE ISOLATED MICROORGANISMS
The
medium
used
for
the
studies
with
the
strain
CUAC 10 was a mineral medium containing 1% (w/v) glucose and
0.1%
(v/v)
eugenol/isoeugenol
The medium used
and
Pseudomonas
0.1 % (v /v)
for
the
otherwise specified).
studies with the strains
aeruginosa
eugenol
(unless
and that
was
used
mineral
for
medium
(CUAC 20)
containing
CUAC 30 was a mi nera 1
67
medium containing 0.1%
the
mineral
3.4.1
(v/v)
medium was
that
isoeugenol.
The composition of
d€',cribed under section
3.2.1.
Preparation of inoculum
A loopful of the culture from the agar slants was
transferred
to
for 24 hours.
100 ml
mineral
broth
and
incubated
at
30°C
The turbidity of the culture was then observed
at 600 nm and the absorbance was adjusted with fresh mineral
broth to give a final
sion,
in
quantities
absorbance of 1.0.
of
2 ml/lOO ml
This cell suspen-
broth,
was
used
as
the
inoculum.
3.4.2
Incubation procedure
To
10 ml
quantity
of
mineral
broth
in
each
test
tube, which was autoclaved at 15 lbs pressure for 15 minutes,
0.2 ml
of
the
incubat ed
at
inoculum
30 ° C
for
was
a
per iod
shaker
(unless
otherwise
tained
for
experiments.
in
all
These
of
24
specified).
All
test
hours
tubes
on
Controls
a
were
were
rot ary
mal.n-
the experiments were done
duplicate.
3.4.3
Measurement of growth
The
The
added.
growth
turbidi ty formed
was
measured
in the
in
ino(~ulated
terms
of
turbidity.
tubes due to the growth
68
of
the
bacteria
was
spectrophotometer
measured
(Hitachi
at
600 nm
Model
in
200-20)
24 hours (unless otherwise stated).
a
at
UV-visible
the
end
of
Growth was expressed as
optical density (OD) values.
3.4.4
Effect of concentration of eugenol/isoeugenol
The
effect
of
concentration of
eugenol/isoeugenol
on the growth of the bacteria was tested by inoculating the
test strain of bacteria in mineral broth adjusted to various
concentrations
0.25%
(v/v).
eugenol/isoeugenol,
of
viz.,
The dispensed medium was inoculated,
0.1
to
incubated
and the growth was measured.
3.4.5
Effect of pH
The
tested
in
substrate
effect
mineral
at
its
of
broth
optimal
pH
on
the
prepared
after
of
bacteria
was
incorporating
the
concentration and adjusting
incubat ing
inoculating
3.4.6
Effect of concentration of sodium chloride
chloride
from
and
for
after
By
broth
incorporat i ng var ious
0
to
10%
(w/v),
it
to
The growth was measured
various levels of pH from 5 to 10.
the
growth
10
concent ra t ions
ml
aliquots
24
hours.
of
sodi urn
of
mineral
69
broth
were
prepared.
The
growth
was
measured
after
inoculating the test strains and incubating it.
3.4.7
Effect of different nitrogen sources
Ammonium nitrate in the mineral broth was replaced
wi th
ammonium
nitrate
and
nitrogen
prepared.
chloride,
sodium
content
ammonium
nitrate
in
the
in
sulphate,
urea,
concentrations
medium
and
10 ml
pot ass i urn
giving
equal
aliquots
were
Cultivation was carried out with the cultures and
the gr~wth was measured after incubation for 24 hours.
3.4.8
Effect of temperature
Ten
optimal
ml
aliquots
conditions
test strains.
were
of the mineral
prepared
These were
and
broth adjusted to
inoculated
with
the
incubated at various temperatures
viz., 15° to 60°C and the growth was measured.
3.5
3.5.1
ANALYTICAL METHODS
Extraction procedure
After
fermentation,
6000 rpm for 15 minutes.
the
broth
was
centri fuged
at
The supernatent was acidified with
70
2 N sulphuric acid
solvent
ether.
anhydrous
The
sodium
pressure.
to pH 2 and exhaustively extracted with
extracts
sulphate
The residue was
and
were
pooled,
concentrated
with
dried
under
reduced
subjected to chromatographic
and
spectrophotometric studies.
3.5.2
Thin layer chromatography
Thin
with
silica
glass
layer
gel
plates
chromatography
plates
wi th
a
of
(TLC)
0.25 mm
spreader.
The
was
thickness
plates
carried
prepared
were
out
on
developed
with the following solvent systems.
i)
Chloroform
ii)
Ethyl acetate: Hexane - 3:7
iii) Benzene: Dioxane: Acetic Acid - 90:25:4
Spots were detected either by exposing the plates
to
iodine
reagents.
3.5.3
vapours
by
spraying
them
with
appropriate
150
Column chromatography
Column
mesh
or
silica
gel
chromatography
(unless
was
otherwise
performed
with
60-120
specified).
The
eluant
71
used was either chloroform : hexane system or ethylacetate :
hexane system,
depending
observed from TLC.
on
the
nature
of the substrate as
As elution progressed, fractions yielding
the same compound as shown by TLC were pooled and distilled
under reduced pressure to get the product.
3.5.4
High pressure liquid chromatography
High
used both for
pressure
440.
chromatography
(HPLC)
was
the detection and estimation of the substrate
and metabolites.
Model
liquid
The instrument used was a Waters Associates
The column used was an analytical reverse phase
The eluant was methanol.
C1S-p-Bondapack.
The UV detector
and refractive index detector were used.
Standard
were prepared and
and
eluted
with
solutions
10 pl
of
each was
methanol.
The
the
substrate
injected
was
observed
drawn
computed.
from
in
the
progress
which
chromatogram,
concentration
of
a
methanol
into HPLC system
of
monitored with the refractive index detector.
heights
in
elution
From the peak
calibration
the
was
curve
substrate
was
72
3.6
EFFECT OF PHYSICOCHEMICAL FACTORS ON TRANSFORMATION
The
temperature,
effect
of
different
physicochemical
nitrogen
factors
sources
and
namely
pH,
concentrations
of sodium chloride and glucose on the rate of transformation
of eugenol/isoeugenol
by the bacterial
For the
studies with
the
mineral
broth
1%
(w/v)
in 250 ml
otherwise
stated)
for
mineral
the
broth
mineral
medium
section 3.2.1.
studies
the
0.1%
used
wi th
strain
(v/v)
was
0.1%
(v/v)
CUAC 30,
isoeugenol
the
same
as
the
aliquots
eugenol
mineral
were
that
(unless
with
50 ml
Pseudomonas aeruginosa,
with
with
the
of
supplemented with
eugenol/ i soeugenol
For
supplemented
studies
supplemented
used.
studied.
50 ml aliquots
Erlenmeyer flasks
were
strains CUAC 20 and
of
strain CUAC 10,
0.1 % (v/v)
gl ucoseand
strains was
and
broth
used.
The
described
under
The medium was autoclaved at 15 Ibs pressure
for 15 minutes before cultivation and preparation of inoculum
was the same as described under section 3.4.1. The inoculated
flasks
were
6,
18,
All
12,
at
30°C
hours
on
incubated
24
experiments
and
36
were
done
kept for all experiments.
in
for
a
specific
rotary
duplicate
shaker
and
periods
of
(220 rpm).
controls
were
73
After
incubation
for
specific
intervals
of
time,
the broth was centrifuged and the supernatent was acidified.
It
was
extracted
with
ether
evaporated to dryness.
The
and
the
residue
ether
was
extract
was
then estimated for
residual eugenol/isoeugenol.
3.6.1
Estimation of eugenol/isoeugenol
Standard
methanol
were
281/260 nm
curves
From
the
prepared
were
were
solutions
measured
then
drawn
absorbance
l!nd
in
a
each
values
of
eugenol/isoeugenol
in
the
absorbance
at
values
spectrophotometer.
for
of
eugenol
the
test
and
Standard
isoeugenol.
solut ions
the
con-
centrations of eugenol/isoeugenol were then estimated.
3.6.2
Effect of concentration of sodium chloride
The effect of concentration of NaCl on transforma-
tion
having
1%
of
eugenol/isoeugenol
various
(w/v).
specific
chloride.
sodium
After
was
chloride
tested
mineral
concentrations,
inoculation the
intervals of time
in
flasks were
viz.,
broths
0
to
incubated for
for each concentration of sodium
The contents of the flasks were then analysed for
residual eugenol/isoeugenol.
74
3.6.3
Effect of concentration of glucose
Fifty ml
aliquots
of
mineral
broth were
prepared
by incorporating various glucose concentrations from 0.2 to
2%
(w/v)
in the case of studies with the strain CUAC 10 and
from 0 to 1%
CUAC 20 and
inoculated
(w/v)
in the case of studies with the strains
CUAC 30.
with
the
The
test
flasks
containing the broth were
strains,
incubated
for
specific
intervals of time and analysed.
3.6.4
Effect of pH
Fi fty
ml
aliquots
of
mineral
and adjusted to pH values ranging
inoculated,
incubated
and
broth were
from 5 to 9.
analysed
for
prepared
These were
residual
eugenol/
isoeugenol.
3.6.5
Effect of temperature
The
flasks
containing
the
mineral
broths
were
inoculated and incubated at different temperatures from 15°C
After specific intervals of time,
the flasks were analysed.
the contents of
75
3.6.6
Effect of different nitrogen sources
Ammonium nitrate in the mineral broth was replaced
wi th
ammoni urn
nitrate
and
nitrogen
out.
chloride,
sodium
content
After
contents of
nitrate
in
the
incubation
the
ammoni urn
flasks
in
sulphate,
concentrations
medium and
for
were
urea,
pot ass i urn
giving
cultivation was
specific
analysed
intervals of
for
equal
carried
time,
the
unreacted eugenol/
isoeugenol.
3.7
GROWTH CURVE
By
i ncorpora t i ng
t he
opt imal
cond i t ions,
mi neral
broths were prepared for the three cultures CUAC 10, CUAC 20
and CUAC 30 and the broths were inoculated with the cultures.
They were incubated and the growth was measured
in terms of
optical
densi ty
A graph was
plot t ed
wi th
at
time
various
interval
intervals of
versus
time.
opt ical
dens i t Y for
each
culture, which formed the growth curve.
3.B
FERMENTATION CONDITIONS
The
fermentor
mineral
fermentation
(Eyela
broth
model
was
M-lOO).
supplemented wi th
carried
out
A volume
of
in
a
1.5
eugenol/i soeugenol
mini
jar
li tres
of
was
used
76
for
fermentation.
strain
CUAC 10,
The
medium.
In
1%
the
case
(w/v)
of
glucose
fermentor
fermentation
was
together
also
with
the
to
the
medium
was
added
the
sterilised at 15 Ibs pressure for 20 minutes.
with
The ferment a-
tion was conducted at pH 7 at 30 D C (unless otherwise stated).
Continuous
aeration at
a
flow
rate of
1 VVM was given with
an air pump and a speed of 220 rpm of the impeller was used.
A loopful
of
the
mineral
inoculated
in
of the culture was transferred to 10 ml
broth
a
and
incubated
100 ml
broth
for
one
contained
in
flask and incubated for one day in a shaker.
transferred
incubated
to
for
10000 rpm for
400 ml
broth
15 hours.
1000 ml
The broth was
20 minutes.
with physiological
in a
day.
an
This
was
Erlenmeyer
This was again
shaking
flask
then centrifuged
and
at
The cells were separated, washed
saline and this was used as the inoculum
for fermentation.
Since eugenol/isoeugenol was strongly antibacterial,
cultivations
which
the
were
done
substrate
by
was
the
added
concentration from 0.01% (v/v).
feeding
in
culture
stages
method
increasing
in
the
77
3.9
ISOLATION AND CHARACTERISATION OF TRANSFORMATION
PRODUCTS
After
6000
rpm
for
supernatent
This
was
ether
fermentation
15
was
minutes
and
acidified
exhaustively
extracts
were
the
broth
was
the
cells
were
with
centrifuged
removed.
2 N sulphuric
extracted
pooled,
with
dried
with
The
acid to
sol.vent
pH
et her.
anhydrous
at
2.
The
sodium
sulphate and filtered.
A small portion of the ether extract
was
thin
layer
which
gave
subjected
solvent
systems,
number of
the
products
The
pressure.
chromatography
an
idea
formed during
extract
ether
graphy.
to
was
then
residue
was
on
concentrated
subjected
separated.
The
pooled
concentrated
and
fractions
the
the
fermentation.
Chloroform-hexane or ethyl
as the eluant dependi ng
about
with
under
nature
and
The rest of
under
column
reduced
chromato-
acetate-hexane was used
nat ure
yielding
to
different
0
f
the
reduced
the mi xt ure
same
product
pressure.
to
be
were
The
separated components were purified by recrystallisation from
appropriate solvents.
The
pure
compounds
were
identified by determining
the physical constants and chromatographic and spectrometric
met hods.
These
incl uded
compar i ng
wi t h
au thent i c
samples
78
the
melting
systems
points,
and
Rf
IR,
UV,
values
NMR
(TLC)
and
in
mass
different
spectra.
solvent
Suitable
derivatives of these compounds were also prepared.
3.9.1
Preparation of acetates of the transformation products
Five
1.5 ml
1 ml
for
mg
of
the
compound
was
dissolved
in
pyridine in a 50 ml clean dry round bottom flask and
ac et i c
6
hundred
anhydr i de was added
hours
poured
shaking
into
filtered.
it.
i nt ermi t tent ly.
water
ice-cold
The flask was kept
The
stirring
mi xt ure
was
then
continuously
and
The precipitate was washed several times with 1 N
hydrochloric
solution.
to
acid
The
followed
product
was
by
then
5%
sodium
recrystallised
bicarbonate
from
dilute
ethanol and the melting point determined.
3.9.2
Preparation of methyl esters of acidic products
I n a 50 m1 r 0 u n d bot tom f 1 ask,
and
2
ml
sulphuric
A reflux
absolute
acid
was
condenser
refluxed for 3 hours.
and 10 ml
of
methanol
were
added
was
pl aced
cautiously
fitted
to
the
700 mg
and
with
flask
0
f
0.2
the a cid
ml
con.
shaking. 151
and
it
was
It was then cooled to room temperature
water was
added.
The
excess of
methanol was
79
di st i lled . of f
flask
was
on
a
water
cooled
and
separatory funnel.
and
washed
wi th
was
then
dried
bath under
the
reduced
contents
pressure.
were
poured
The lower layer of ester was
water
followed
with
by
5%
anhydrous
NaHC0
3
magnesium
The
into
a
separated
solution.
It
sulphate
and
products
were
filtered.
3.10
TRANSFORMATION OF FERMENTATION PRODUCTS
The
studied
by
t rans forma t ions
cultivating
supplemented
with
the
the
of
ferment at ion
cultures
fermentation
in
the
mineral
product.
The
broth
mineral
medium used was the same as that described under section
The
inoculated
30°C.
with
The
a
broth
was
a
to
find
out
rotary
the
After incubation for different
the broth was centrifuged,
extracted
on
shaker
at
turbidity of the broth was periodically observed
spectrophotometer
bacteria.
incubated
3.2.l.
wi th
ether.
growth
of
the
intervals of time,
the supernatent was acidified and
The
ether
extract
was
concentrated
under reduced pressure and subjected to analysis.
3.11
FERMENTATION
OF
ACETATES
OF
EUGENOL
AND
ISOEUGENOL
The acetate of eugenol/isoeugenol was fermented in
the
mineral
medium
described
under
section
3.2.1.
80
For
the
studies
supplemented
with
with
1%
acetate/isoeugenol
CUAC 20
and
mented
with
medium
with
rest
of
the
(w/v)
(v/v)
0.1%
the
CUAC 10
glucose
acetate.
Pseudomonas
0.1%
strain
For
and
eugenol
(v/v)
the
was
eugenol
with
the
strains
medium
was
supple-
for
acetate
procedures
medium
(w/v)
acetate and
isoeugenol
fermentat ion
0.1%
studies
aeruginosa
the
was
CUAC
was
the
30
the
used.
same
The
as
wi th
eugenol/isoeugenol.
3.12
MODE OF AROMATIC RING CLEAVAGE
The
the
procedure
det ermi nat ion
ring. 152
The
agar
slants
days
of
of
of
the
organism
at
mode
was
supplemented
incubation
Ottow
0
and
f
Zolg
cl ea vage
subcul tured
wi th
30°C,
a
was
of
2
Then
tube.
protocatechuate
shaken.
positive
was
A
were
green
ml
for
aroma tic
on
mineral
eugenol/ isoeugenol.
After
thick
culture
loopful
of
the
(pH 8.0)
2
in a
toluene and 2.5 ml of 4 mM sodium
added
colour
meta-cleavage.
to
it
within
If
by
shaking
for
prepared
aqueous
1%
incubated
freshly
0.5
the
times
was suspended in 2 ml 0.05 M phosphate buffer
test
adopted
no
18
and
3
it
was
minutes
vigorously
indicated
reaction
occurred,
hours
30°C,
sodium
at
the
and
nitroprusside
a
tube
1 g
of
solution
81
and
0.5 ml
colour
concentrated
wi thin
3
ammonia were
minutes
was
added.
regarded
as
A deep purple
a
positive
test
for the ortho-cleavage of the substrate.
3.13
IMMOBILISATION OF WHOLE CELLS
Immobilisation
entrapment
alginate
in
on
of
calcium
treatment
whole
cells
b ea d s.
alginate
with
(IWC)
calcium
was
done
153,154
chloride
by
Sodium
gave
calcium
alginate beads.
3.13.1
Entrapment in calcium alginate
Hundred ml
distilled water was warmed in a
flask and 4 g sodium alginate was added.
the
start
of
boiling
with
constant
avoid the formation of lumps.
heater
and
allowed
to
cool
It was heated till
stirring
in
order
It was then removed
to
form
250 ml
the
sodium
to
from the
alginate
slurry.
About
homogeneous
20
paste
g
of
with
wet
25
volume was made upto 100 ml.
ml
bacterial
distilled
cells was
water
and
made
then
to
a
the
82
Sodium
alginate
slurry
and
the
bacterial
cell
slurry were mixed in 1:1 ratio and stirred well to obtain a
uniform mix.
It was extruded through a syringe needle into
0.1 M calcium
chloride
were
cured
6 hours.
in
the
solution
same
with
solution
at
stirring.
room
The
beads
temperature
for
The gel beads were washed with distilled water and
stored in bu ffer con ta i ni ng 50 rPM calc i urn chI or ide.
3.13.2
Packing of the column
A glass column of 30 cm
was
used
for
the
packing
of
length and 2.5 cm diameter
the
i mmobil i sed
cell s.
The
column was packed in the order of glass wool at the bottom,
a
perforated
plate,
cell
immobilised
then again a perforated plate.
eugenol
was
continuously
aliginate
beads
and
The mineral broth containing
passed
into
the
column
and
the
eluant was collected periodically.
3.13.3
Activation of immobilised cell beads
About 30 g of immobilised cell beads was packed in
the column.
The mineral broth containing eugenol/isoeugenol
was
into
passed
24 hours.
The
the
broth
column
was
and
eluted
incubated
off
and
at
the
30°C
beads
for
were
83
washed
passing
by
continuously through
freshly
prepared
the column.
saline
solution
This column was then used
for further studies.
3.13.4
Effect of temperature and pH on transformation by IWC
Fifty ml
aliquots of the mineral
the substrate adjusted to different
and
passed
into
and
eluted
and
analysed
at
ml
aliquots
of
Similarly
the
50
optimum
These were
pH
the
were
col umn.
pH values were prepared
These were
different
the
prepared
incubated at different
incubated at
intervals
mineral
and
broth containing
broth
passed
into
of
30 ° C
time.
adjusted
the
to
column.
temperatures from 15°C to
60°C, eluted and analysed.
3.14
ENZYME STUDIES
Cell
free
extracts
(CFE)
of
the
cultures
were
prepared and were used as the source of enzymes.
3.14.1
Preparation of cell free extracts
The
inoculated
ln
organism
400
eugenol/isoeugenol.
ml
It
was
of
was
subcultured
the
two
mineral
incubated
for
times
broth
15-18
and
then
containing
hours
and
84
10,000
centrifuged
at
separated,
washed
(pH 7.0)
cell
for
and the
paste
15
thick
paste
alumina,
cell
was
centrifugation
supernatent
a
two
solution
weights
was
and
at
for
30
used
as
the
O"C.
buffer
The
pestle.
with
walls
rpm
cells were
phosphate
and
ml
cell
The
frozen
of alumina was ground
mortar
10
to
cells
15,000
at
frozen
chilled
diluted
unruptured
0.05 M
with
paste was
wi th
with
20 minutes.
for
twice
together
minutes
rpm
155
buffer
were
minutes
crude
and
removed
4"C.
at
enzyme
The
the
by
The
prepara-
tion.
3.14.2
Effect of pH and temperature on transformation by CFE
Five ml aliquots containing 0.05 ml substrate, 0.5 ml
CFE
4.45
and
different
then
pH
values
analysed.
optimum
pH
0.05
ml
were
M
incubated
S imi lar 1 y
were
phosphate
incubated
5
ml
at
for
buffer
adjusted
3 hours at
al iquots
different
adj ust ed
to
30"C and
to
the
temperatures
for
3 hours and then analysed.
3.14.3
Transformations with CFE
Five
ml
aliquots
containing
0.05
ml
substrate,
0.50 ml CFE and 4.45 ml of 0.05 M phosphate buffer adjusted
to optimum pH were
incubated at the optimum temperature and
periodically analysed by TLC and HPLC.
85
The
formation
of
1-carboxy-oC...-hydroxy-cis,
~-carboxy-cis,
aromatic
cis-muconic
ring
cleavage
acid
products
semialdehyde
and
cis-muconic acid were monitored spectrophoto-
metrically by measuring
.
156 157
respect1vely.
'
the
Thus
absorption at 410 nm and 290 nm
to
a
cuvette
containing
2 ml
of
0.05 M phosphate buffer, 0.1 ml eFE and 0.4 ml of 1 mM sodium
protoca t echua t e
sol ut i on
measured at 410/290 nm.
were
added
and
the
absorpt i on
was
RESULTS AND DISCUSSION
CHAPTER
4
MICROBIAL TRANSFORMATIONS OF EUGENOL
MICROBIAL TRANSFORMATIONS OF EUGENOL
4.1
INTRODUCTION
Eugenol was extracted from clove oil and purified
by column chromatography.
metabolise
culture
eugenol
was
technique.
A strain of bacteria which could
isolated
This
from
strain,
soil
by
designated
enrichment
as
CUAC 20,
showed good growth in a mineral broth containing eugenol as
the
sole
CUAC 10,
carbon
source.
which
fermented
containing
glucose
isolated from
as
belong ing
ively,
from
or
Another
eugenol
any
other
the atmosphere.
to
in
a
nutrient
designated
mineral
broth
as
broth
was
also
The strains were identified
Pseudomonas sp.
their
bacteria,
morphological
and
Bac i 11 us
sp.
and biochemical
respec t-
characters
(Tables 4.1(a) and 4.1(b)).
4.2
STUDIES WITH THE BACILLUS sp. CUAC 10
As this strain was not able to grow in a mineral
medium
studies
containing
were
1.0% (w/v)
eugenol
conducted
glucose
in
as
in
a
addition
(unless otherwise specified).
88
the
sole
mineral
to
carbon
broth
0.1%
source,
containing
(v/v)
eugenol
89
4.2.1
Growth studies
4.2.1.1
Effect of concentration of eugenol
The
results
of
the
studies
of
the
effect
of
concentration of eugenol on the growth of the bacteria are
presented
was
in Table 4.2.1.
increased,
growth
of
0.12%
(v/v)
increase
the
in
there
was
As the concentration of eugenol
a
bacteria.
eugenol
the
corresponding
This
effect
was
concentration.
concentration
of
decrease
much
But
eugenol,
less
with
the
in
the
upto
further
growth
was
retarded.
4.2.1.2
Effect of concentration of sodium chloride
The results,
that
the
On the
bacteria
contrary
had
presented
no
addition
in Table 4.2.2,
affinity
of
NaCl
for
sodium
resulted
indicated
chloride.
in
decreased
in Table 4.2.3,
indicated
that urea favoured maximum growth of the culture.
Ammonium
growth .of the organism.
4.2.1.3
Effect of different nitrogen sources
The results,
salts
also
favoured
presented
good
growth,
but
when
they
replaced by alkali metal nitrates, poor growth resulted.
were
90
4.2.1.4
Effect of pH
Table 4.2.4 shows the results of the effect of pH
on
the
growth
tolerated
of
a wide
the
organism.
range of
pH
Though
from
6.5
to
the
8.5,
bacteria
optimum pH
value was 7.0 to 7.5.
4.2.1.5
Effect of temperature
As shown by the results presented in Table 4.2.5,
the
bacteria
favoured
30 to 45°C.
a
wide
range
of
temperatures
from
It could even tolerate temperatures upto 60°C,
but the optimum temperature was 30 to 37°C.
4.2.2
Effect of physico-chemical factors on transformation
of eugenol
4.2.2.1
Effect of concentration of sodium chloride
The
results
concentration
eugenol
sodium
are presented
chloride
the
of
did
cases
transformed
reaction.
not
tested,
in
of
24
the
studies
chloride
on
the
in Table 4.2.6.
affect
more
hours.
the
of
45
After
per
effect
of
transformation
of
Addition of sodium
transformation
than
the
cent
that
much.
of
In
all
eugenol
was
there was
not
much
91
4.2.2.2
Effect of concentration of glucose
The
that
the
opt imum
When
the
0.5%
(w/v),
When
the
1. 5%
the
rate
first
presented
concent rat ion
concentration
the
rate of
the
18
rate
transformation
4.2.7
of
glucose
was
was
indicated
1%
(w/v).
decreased
to
transformation was also decreased.
of
of
hours was
Table
in
glucose
of
concentration
(w/v),
of
results
glucose
was
transformation
comparatively
increased
so
of
low.
that
increased
eugenol
After
more
during
that
than
to
the
45 per
cent eugenol was fermented in 24 hours.
4.2.2.3
Effect of different nitrogen sources
Ammonium sulphate favoured more transformation of
eugenol,
as
can
be
seen
from
4.2.8.
Table
organism recorded maximum growth with
Though
the
urea as the nitrogen
source, the transformation rate of eugenol was comparatively
less wi th
urea.
The
rate of transformat ion was
the
least
when potassium nitrate was used.
4.2.2.4
Effect of pH
The
formation
of
resul ts
eugenol
of
the
are
effect
of
presented
pH
in
on
the
Table
trans-
4.2.9.
92
The maximum conversion of eugenol was recorded, when the pH
was 7.0.
A pH of
7.5 also
favoured very good conversion.
When the pH was decreased to 6.0,
there was a
considerable
decrease in the conversion rate of eugenol.
4.2.2.5
Effect of temperature
The
the
results
optimum
appreciable
shown in Table 4.2.10 suggested that
temperature
in
decrease
The
upto 45°C.
was
30°C.
the
But
there
transformation
transformation
of
almost as good as that at 30°C.
eugenol
was
of
eugenol
37°C
at
no
was
Decreasing the temperature
to 15°C drastically reduced the transformation.
4.2.3
Growth curve
By
growth of the
of
time
bacteria
Then
the
attained
phase
Thus
to
was
optimal
the
conditions
culture was monitored at different
obtain
in
the
lag
almost
full
at
organism
18
growth
phase
logarithmic
started
the
incorporating
the
of
growth
growth
was
at
and
found
15
to
first
be
a
four
for
The
about
fast
The
hours.
and
started
hours.
lasted
intervals
(Fig.4.2.1).
during
phase
hours
curve
the
it
stationary
3
hours.
growing
one.
93
4.2.4
Fermentation of eugenol
Based
the
effect
of
on
the
various
transformation
of
growth
studies
and
physico-chemical
eugenol,
the
the
parameters
optimal
conditions
fermentation
of eugenol with the Bacillus sp.
obtained.
By
eugenol
was
mineral
medi urn
incorporating
fermented
in
described
a
these
mini
under
jar
on
on
the
for
the
CUAC 10 were
optimal
conditions,
fermentor.
sect ion
with 1.0% (w/v) glucose and 0.1%
studies
3.2.1
158
The
supplemented
(v/v) eugenol was used for
the fermentation.
As
withdrawn
first
was
from
10
the
hours,
not
the
The
45.4%
proceeded,
ferment or
and
format ion
of
cons iderable.
formed.
of
fermentation
By 18
yield
at
the
end
of
ml
samples
analysed.
BDHE
24
about
were
During
bi sdehydroeugenol
hours
concentration of
10
38%
of
the
(BDHE)
BDHE
was
increased to a maximum
hours.
Therea ft er
no
further transformation was recorded.
4.2.5
Isolation
and
identification
of
bisdehydroeugenol
The fermentation broth was centrifuged, acidified
and
extracted
evaporated
with
under
solvent
reduced
ether.
pressure
The
and
ether
extract
subjected
to
was
col umn
94
using
chromatography
system
as
eluant.
chloroform : hexane
The
product,
( 3: 1 )
obtained
as
solvent
whi te
sol id,
was recrystallised from chloroform: hexane mixture.
The
The
very
pure
NMR
similar
to
product
E
had
20
a
melting
point
of
spectrum
(Fig.4.2.3)
of
this
compound was
the
spectrum
eugenol
(Fig.4.2.2).
NMR
of
However, on closer examination it was found that there were
only two aromatic protons corresponding to each -OCH3 group
in the product molecule.
of
number
aromatic
group is three.
spectrum
NMR
oxidative
suggested
coupling
at
weight
elemental
analysis
the
of
coupl i ng
them.
Hence
either
through
takes
place
eugenol,
to
each
the
-OCH
3
position
by
was
also
might
have
to give a
mass
this
dimer.
The
an
The
spectrometry
view.
deduced
been
and
actual
from an anal ys i s
of
Both of the aromatic protons appeared at
between
(~J.
there
supported
indicating
molecul e
that
that
06.75
place
corresponding
determination
spectrum.
NMR
protons
of
case
The absence of one aromatic proton in the
molecular
pos it ion
In the
similar
Knowing
either
at
the
environment
bond
position
that
the
formation
5
or
oxidative
para
and
or
6
no
must
of
have
the
coupling
ortho
coupling
..
of
posltlon,
taken
eugenol
phenols
159
the
95
position
of
(2) ,
structure
biphenyl
coupling
is
may
be
inferred
as
6.
Hence
the
3,3'-diallyl-5,5'-dimethoxy-6,6'-dihydroxy
suggested
for
the
product
obtained
in
this
chloride
gave
process.
OH
(~)
(1)
Eugenol
the
same
values
on
product
with
in different
product
obtained
treatment
wi th
identical
ferric
melting
solvent systems.
by
FeCl
3
points
and
Rf
The structure of the
treatment
had
already
been
established as (~), bisdehydroeugenol. l34 ,l60
The acetyl a ted der i va t i ve of
reacting
it
with
spectrum
of
the
acetic
acetate
anhydride
in
showed
that
(~)
was
prepared
pyridine.
the
by
The
NMR
compound
was
96
diacetylated.
-OCH 3
The diacetate had
protons
& 3.85
at
and
-COCH3
aromat i c
protons
prot ons
S 2.1,
at
at
06.75,
thus confirming the structure of (2).
The
(Fig.4.2.4)
carbon
of
the
product
showed signals for 10 carbons only thus proving
symmetrical
the
and
spectrum
structure
of
the
appeared
and
ppm
39.982
(triplet)
molecule.
at
56.087
respectively
and
The
ppm
the
carbons appeared between 110 ppm and 147 ppm.
shift
place
of
the
is
adjacent
carbon
124.423
to
assignment
the
of
through
ppm
carbon
chemical
which
(singlet)
shifts
of
the
2
has
carbons
Cl - 147.237 ppm (s)
987
"
(s )
- 123.117
"
(d )
C - 131.921
4
"
(s )
- 110.686
"
(d)
C6 - 124.423
"
(s )
115.725
"
(t )
C - 137.659
8
"
(d)
C 9
39.982
"
(t )
C
56.087
"
(q)
C
3
C
OH
-
140.907
2
C
5
7
IO
-
-
taken
it
group.
shown in the figure below:
C
hybrid
that
hydroxyl
all
(quartet)
sp
dimerisation
the
3
The chemical
indicating
bearing
-OCH
are
is
The
as
97
4.2.6
Fermentation of eugenol acetate
Eugenol
medium
acetate
described
1.0% (w/v)
under
the
section
3.2.1
broth
extracted with ether.
found
fermented
glucose and 0.10% (v/v)
fermentation
was
was
that
fermentation.
no
further
mineral
supplemented
eugenol acetate.
centrifuged,
and
yield
BDHE were
of
BDHE
the
with
After
acidified
was
products
47.5%
eugenol was 49.1% at the end of 24 hours.
was
the
and
On analysis of the ether extract, it
eugenol
The
was
in
increase
in
the
and
of
the
that
of
After that there
concentration
of
BDHE
and
only traces of eugenol acetate remained.
4.2.7
Fermentation of transformation product
'k· .
Bisdehydroeugenol
medium
The
containing
Bac i 11 us
BDHE,
as
no
fermenta t ion
sp.
0.1%
CUAC
was
(w/v)
10
broth.
This
BDHE
was
transformation
fermented
and
in
1%
a
(w/v)
found
not
able
product
was
detected
indicated
that
to
mineral
glucose.
ferment
in
the
BHDE was the end
product of fermentation of eugenol with the strain CUAC 10.
98
4.2.8
Immobilisation of whole cells
From the studies on the transformation of eugenol
with immobilised whole cells,
it was found that the optimum
pH was 8.0 and the optimum temperature was the same as that
of
intact
cells,
eugenol.
of
30°C,
for
the
transformation
of
The retention time was 12 hours, when about 42.5%
eugenol
cells were
After
ie.,
10
was
found
act i ve
days
be
converted.
even after
cont inuous
of
to
operation
the
The
use
activity
immobilised
for one week.
was
reduced
to
almost 50% of the initial activity.
4.2.9
Discussion
The organism,
was
identified
morphological
one.
The
addition
strongly
upto
to
belong
the
of
to
eugenol
antibacterial.
0.07%
(v/v)
of
to
The
studies.
growth
of
isolated from the atmospheric flora
the
Bacillus
organism
organism
the
This
eugenol
was
was
medium
effect
growth
affinity
of
of
eugenol
the
for
caused a
bacteria.
sodium
from
fast
by
the
eugenol
was
was quite
negligible
and
(v/v).
was
more
Subsequent
considerable decrease
strain
did
Of
di fferent
the
the
growing
retarded
concentration
The
chloride.
a
since
pronounced from a concentration of 0.10%
addi tion
sp.
not
in
the
show
any
ni trogen
99
5"'G'G. 2
t.< 1'-.,/
5~~17.
sources
the
tested urea had a
bacteria.
This
positive effect on the growth of
might
be
due
to
the
fact
that
urea
served as an additional carbon source for the growth of the
bacteria.
showed
The
good
tolerated
optimal
growth
a
wide
at
pH
range
pH
value
was
7
and
8.0
al so.
6.5
of
to
temperatures
7.5
though
The
from
it
bacteria
30
to
45°C
though the optimum temperature was 30 to 37°C.
The
chemical
studies
factors
on
on
the
the
optimal
conditions.
not
enhance
the
the
effect
of
transformation
Addition
transformation
of
of
(w/v).
tion
the
glucose
eugenol
was
because
of
was
also
the
decreased,
found
poor
to
be
growth
the
easily assimilable carbon source.
of
transformation
glucose was
that
the
inhibi ted
sources
of
eugenol
increased to 2%
products
the
tested
of
though
urea
chloride
The
to
did
opt imal
When the concentra-
transformation
This
bacteria
for
the
rate
of
might
be
want
of
concentration
could be due to
metabolism
of
led
The decrease in the rate
when
(w/v)
transformation
sodium
decreased.
of
physico-
eugenol
eugenol.
concentration of glucose was 1%
of
of
various
of
eugenol.
favoured
an
glucose
Of
the
increase
of
the fact
slightly
nitrogen
in
growth
of the bacteria, the transformation of eugenol was slightly
decreased with urea.
The
maximum conversion was noted when
100
ammonium
sulphate
was
used
as
the
ni trogen
source.
The
optimal pH value was 7.0 to 7.5 and temperature 30 to 37°C.
It was
interesting to note that the transformation was not
much affected when the temperature was increased upto 45°C.
As
growing
mentioned
one
in
the
12 hours
the
when
culture
the
previously
rat e
medium
of
the
tested.
t ransforma t i on
entered
culture
the
was
During
was
logarithmic
eugenol
was
fermented
by
24
hours
fast
the
first
very
low.
Onl y
phase
of
the rate of transformation of eugenol increased.
of
a
and
growth,
About 49%
thereafter
there
was no appreciable reaction.
Thus
conditions
Bacillus
in
the
for
sp.
above
the
led
transformation
the
CUAC
studies
10.
transformation
These
of
of
to
the
eugenol
conditions were
eugenol
in
the
optimal
by
the
incorporated
fermentor
and
the
fermented,
the
yield of bisdehydroeugenol was 45.4%.
When
the
acetate
of
eugenol
was
yield of BDHE was found to increase to 47.5%.
first
The bacteria
hydrolysed eugenol acetate to eugenol which was then
converted
to
BDHE.
It
is possible that when the phenolic
101
compound,
eugenol was produced in situ from its acetate its
concentration
toxic
would
effects
of
it
be
comparatively
would
not
lower
affect
the
and
hence
growth
the
of
the
immobilisation
of
.
161
b acter1.a.
Of
microbial
the
cells,
various
the
methods
entrapping
of
method
was
used
in
this
study.
In the studies for obtaining optimal pH,
a shift of
1
to
comparison
unit
with
that
the
of
alkaline
intact
range
cells
was
and
the
observed
optimum temperature was
Though the yield of BDHE with
less
than
less
and
that
the
with
column
intact
of
cells,
immobilised
in
immobilised cells was
the
retention
cells
could
time
be
was
reused
for more than one week of continuous operation.
The
oxidation
of
BDHE
alkali gives dehydrodivanillin.
with
nitrobenzene
162
CHO
CHO
and
102
4.3
STUDIES WITH THE PSEUDOMONAS sp. CUAC 20
This strain was able to grow in a mineral medium
containing
studies
under
eugenol
were
as
the
conducted
sect ion
3.2.1
in
sole
carbon
the
mineral
supplemented
wi th
source.
medium
All
the
described
0.1 % ( v/v)
eugenol
(unless otherwise stated).
4.3.1
4.3.1.1
Growth studies
Effect of concentration of eugenol
The
eugenol
of
the
the
on
Table 4.3.1
results
of
growth
indicate
of
that
substrate did not
concentration
was
the
effect
the
higher
of
concentration
bacteria,
and
lower
presented
(v/v)
of
in
concentrations
enhance the growth.
0.1%
of
eugenol.
The opt imum
When
the
concentration of eugenol was increased to 0.15% (v/v) there
was
a
slight
decrease
in
growth.
Further
addition
of
eugenol resulted in very poor growth.
4.3.1.2
Effect of concentration of sodium chloride
The
organism
showed
a
slight
increase
in
the
growth with the addition of sodium chloride upto 0.5% (w/v)
103
concentration.
Further
resul ted in a
addition
of
sodium
poor growth of the bacteria,
chloride
as can be seen
from Table 4.3.2.
4.3.1.3
Effect of different nitrogen sources
4.3.3
Table
the
effect
of
the
bac,teria.
more growth.
shows
di fferent
Of all
the
result
ni trogen
the
of
sources
the
studies
on
on the growth of
compounds tested,
urea favoured
The least growth was observed with the alkali
metal nitrates.
4.3.1.4
Effect of pH
The
that
the
tolerate
results
optimum
pH
7.5
pH
with
presented
was
in
7.0.
fairly
Table
The
good
4.3.4
strain
growth.
indicated
could
The
also
growth was
drastically reduced with decrease or increase of pH.
4.3.1.5
Effect of temperature
As
temperature
can
was
be
30°C
growth at 37°C also.
seen
from
though
Table
the
4.3.5
organism
the
optimum
showed
good
Subsequent increase in the temperature
caused a considerable decrease in the growth.
104
4.3.2
Effect of physico-chemical factors on transformation
of eugenol
4.3.2.1
Effect of concentration of sodium chloride
The
concentration
eugenol
are
results
of
of
the
studies
sodium chloride
presented
chloride upto 0.5%
tion
of
in
on
of
the
Table 4.3.6.
the
effect
transformation
of
of
Addition of sodium
(w/v) slightly increased the transforma-
eugenol.
Further
increase
concentration resulted in a decrease
of
sodium
chloride
in the transformation
rate.
4.3.2.2
Effect of concentration of glucose
The
addition
transformation of
The
ef fec t
was
of
eugenol
more
of
rate
during
the
18 hours.
wi th
When 1%
transformation
first
adversely
affected
the
as can be seen from Table 4.3.7.
pronounced
concentration of glucose.
the
glucose
(w/v)
was
But
an
i nc rease
in
the
glucose was added,
considerably
decreased
by 36 hours more than 90%
of eugenol was degraded.
4.3.2.3
Effect of different nitrogen sources
The
results
presented
in
Table
that urea favoured more transformation.
4.3.8
indicated
When urea was used
105
as the nitrogen source about 60% of eugenol was degraded by
18 hours and the reaction was almost over by 24 hours.
The
rate of transformation with the other compounds was almost
the same.
4.3.2.4
Effect of pH
The optimum pH was 7.0.
to
7.5,
the
transformation
considerably
during
can
from
be
seen
remaining
at
the
the
Table
end
of
initial
48
was
eugenol
stages
4.3.9.
of
When the pH was changed
But
hours
of
fermentation
the amount
was
affected
almost
of
the
as
eugenol
same
as
that at pH 7.0.
4.3.2.5
Effect of temperature
The
that
the
results
optimum
presented
temperature
in
was
Table
30°C.
4.3.10
indicated
Decreasing
the
temperature below 30°C or increasing it above 37°C affected
the transformation considerably.
4.3.3
Growth curve
The
studies
were
opt i mal
cond i t ions
incorporated
and
the
obt ai ned
growth
from
of
the
the
above
organism
106
was monitored at different
intervals of time to obtain the
growth curve
The initial
lasted
for about 6 hours.
started
by
stationary
15
hours.
phase
studies
on
various
.
After
for
attaining
about
3
full
growth,
hours,
from
the
21
to
Fermentation of eugenol
incorporating
lon.
The logarithmic phase of growth
lasted
Eugenol
t
lag phase of growth
Then the death phase occurred.
24 hours.
4.3.4
(Fig.4.3.1).
163
was
the
the
fermented
optimal
growth
of
mineral
a
mini
parameters
the
medium
used
jar
obtained
organism
parameters
physico-chemical
The
in
and
on
for
the
the
fermentor
from
the
effect
of
transforma-
fermentation
was
supplemented with 0.11% (v/v) eugenol.
4.3.4.1
Time course of formation of products
During
the
course of
fermentation,
10 ml
samples
of the broth were withdrawn from the fermentor and analysed
for
residual
products.
eugenol
Ferulic
and
acid,
concentration
vanillin
and
of
transformation
vanillic
acid
were
measured by determining the optical density at 310, 347 and
293
nm.,
respectively.
Upto
12
hours
the
transformation
107
rate
was
very
low.
After
that
eugenol started transforming.
almost
After
complete
that
with
there
eugenol
remained.
amounts
from
increased
to
a
than
not
much
Vanillin
12
hours.
The
maximum
considerable
amount
of
By 24 hours the reaction was
more
was
a
of
90%
of
transformation.
reaction
and
formed
in
was
concentration
4.62%
yield
traces
measurable
of
at
of
17
vanillin
hours
and
thereafter decreased to almost zero by the end of 36 hours.
The
other
The
concentration of
of
produc t s
6.25%
yield
at tained a
time
were
at
ferulic
20
maximum of
course
of
also
acid
hours
along
of
wi th
attained a
and
that
yield at
8.9%
formation
formed
of
maximum value
vanillic
about
products
24
is
van ill in.
acid
hours.
given
in
The
Table
4.3.8.
4.3.4.2
Experiments
for
increased
production of
1. Fermentation of eugenol was carried out
with
different
and
2.0
formation
was
was
of
of
volumes
minute (VVM).
aeration
rates
It
air
was
decreased
also
aeration,
per
found
to
0.5
decreased.
in the ferment or
viz.,
volume
that
VVM
0.5,
of
when
the
Even
vanillin
1.0,
medium
the
rate
1.5
per
of
rate
of
trans-
after
24
hours,
108
about 30% eugenol was left unreacted and correspondingly
the
formation of vanillin was also decreased.
flow
rate
was
increased
transformat ion
complete
by
was
21
to
1.5
increased.
to
22
or
2
The
hours,
but
VVM,
When the
the
rate
reaction was
the
of
almost
concentration
of
vanillin was decreased.
2. Fermentation of eugenol was carried out
with aeration at a rate of 1 VVM.
of
glucos~
At 17 hours 0.5% (w/v)
was added to the fermentation broth when the
concentration of vanillin was maximum.
find
out
whether
assimilable
vanillin
samples
from
the
carbon
formed
of
source
was
the
organism
not
intervals.
the
decreased.
It
was
rate
left
were
at
after
transformation
But there was no
for
so
an
then
easily
that
the
Ten ml
wi thdrawn
different
time
the addition of
of
eugenol
was
increase in the concentra-
Even after 24 hours more than 20% of
tion of vanillin.
eugenol was
broth
that
opt
metabolised.
analysed
observed
This was done to
glucose
further
and
of
will
like
fermentat ion
fermentor
the
glucose
in the ferment or
unreacted.
of eugenol remained.
After 48 hours only traces
109
4.3.5
Isolation and identification of products
The
the
cells
fermentation
and
the
broth was
supernatent
centrifuged to
was
acidified
sulphuric acid and extracted with ether.
was
re-extracted
with
5%
aqueous
remove
with
2 N
The ether extract
sodium
bicarbonate
solution to remove strongly acidic products.
The
was
dried
under
ether
with
reduced
anhydrous
with
containing
non-acidic
sodium sulphate
pressure.
chromatography
eluant.
layer
It
was
then
and
(3:1 )
phenyl
hydrazine-H S0 ,
2 4
The
spectrum
aromatic
of
it
the
(3560-3520
(1615-1600
product
cm -1 ),
cm
(Fig.4.3.2)
showed
protons
&6.95-7.55
showed
-1
)
methoxy
indicated
It was
The pure
colouration.
the
presence
cm
(1680-1710
groups.
protons
the
With 2,4-dinitro-
positive
aldehyde
col umn
as
from chloroform: hexane mixture.
product E30 had a melting point of 81°C.
hydroxyl
to
The product was obtained as a white solid.
recrystallised
IR
concentrated
subj ect ed
chloroform : hexane
products
)
and
NMR
spectrum
b 3.95,
aromatic
The
at
-1
of
at
0 9.85.
These spectra completely matched those of vanillin.
The Rf
value
as
at
(TLC)
that
of
and
aldehydic
protons
in different solvent systems was also the same
vanillin.
Thus
t he
product
was
4-hydroxy-3-methoxy benzaldehyde (vanillin).
ident i f i ed
as
110
The
sodium
bicarbonate
layer
was
acidified
with
2 N sulphuric acid to pH 2 and extracted again with ether.
The
ether
layer
was
dried
with
anhydrous
the
presence
of
three
chromatographed
in
acetate : hexane
(3:1)
(A, B
&
C)
were
sulphate
A small portion of
and concentrated under reduced pressure.
it was subjected to thin
sodium
layer chromatography which showed
products.
This
silica gel
a
as
obtained.
the
They
mixture
column
eluant.
were
was
with
Three
then
ethyl
products
recrystallised
from
water-acetone mixture which gave white solids.
The
of
the
spectrum
group,
acid
of
product
was
A melted at 170°C.
prepared
the
ester
methine group,
indicating that
(melting
showed
The methyl ester
point
the
63°C).
presence
of
The
methoxy
aromatic protons and hydroxyl group
it may be 4-hydroxy-3-methoxycinnamic
(ferulic acid).
NMR
acid
The IR spectrum of the product (Fig.4.3. 3)
was identical with that of authentic ferulic acid.
The product B had a melting point of 210°C.
IR spectrum of
that
of
product
the product
authentic
(melting
(Fig.4.3.4
vanillic acid.
point
108°C)
completely matched
The methyl
was
The
ester of the
prepared.
The
NMR
III
spectrum
of
aromatic
and
product
was
the
ester
showed
hydroxyl
the
presence
From
protons.
these
of
methoxy,
results
the
acid
ident i f i ed as 4-hydroxy-3-methoxybenzo i c
(vanillic acid).
The
product
C mel ted
at
200 0 C.
A mixed melting
point with authentic protocatechuic acid was not depressed.
The
NMR
spectrum
(Fig.4.3. 5)
groups
of
showed
and -OCH3
the
signals
group.
of
the
product
aromatic protons,
hydroxyl
acid (protocatechuic acid).
Fermentation of transformation products
acid,
Ferulic
protocatechuic
organism
acid
the
sp.
each
organi srn and
was observed
indicating
the broths were
vanillin,
were
Pseudomonas
supplemented with
wi th
for
ester
Thus the compound was identified
as 3,4-dihydroxybenzoic
4.3.6
methyl
of
found
to
CUAC
20.
these
assimilated
When
an
growth
extracted wi th
be
acid
mineral
compounds were
incubated,
the
vanillic
increase
of
ether
the
in
by
and
the
broths
inoculated
turbid i t y
organi~sm.
When
and anal ysed by TLC,
it was found that these compounds were further metabolised,
indicating that they were not the end-products.
112
4.3.7
Fermentation of eugenol acetate
Eugenol
medium
acid,
the
acetate
described
vanillin,
under
fermented
acid and
fermentation
of
tion
acetate
was
to
almost
The
But
It
completely
difference
maximum
24
by
utilised.
considerable
mineral
Eugenol,
ferulic
eugenol
acetate.
Eugenol
the initial hours of
Only after 15 hours the rate of transforma-
increased.
be
the
protocatechuic acid were
acetate was metabolised slowly during
fermentation.
in
3.2.1.
section
vanillic
products of
was
hours
almost
took two more
used
in
the
concentration
up.
hours
There
amount
of
complete
of
vanillin
for
was
eugenol
eugenol
not
vanillin
was
any
formed.
noticed
at
19 hours.
4.3.8
Mode of aromatic ring cleavage
From
of eugenol
the
final
eugenol,
mechanism
section
the
fermentation
of
transformation
it was found that protocatechuic acid
aromatic
but
compound
in
the
degrada t ion
it was not the end product.
was
3.13.4
ortho-fisaion.
determined
which
by
the
revealed
method
that
PCA
products
(PCA)
was
pa thway
0
f
The ring fission
described
was
under
cleaved
by
113
4.3.9
Immobilisation of whole cells
Culture
cells
18
calcium alginate beads.
the
transformation
hours
was
hours
18
were
immobilised
in
The optimum pH and temperature for
of
eugenol
were obtained as 7.5 and 30 D C,
time
old,
and
the
with
the
immobilised
respectively.
immobilised
cells
The retention
cells were
found
to
be active for more than one week.
4.3.10
Enzyme studies
Cell
free
prepared
and
was
wi th
CFE
at
that
the
optimum
(CFE)
of
the
source
of
enzymes.
used
as
the
d if ferent
pH
values
CFE
vanillin,
extracts
values
were
transformed
vanillic
acid
and
pH
temperatures
8.0
and
eugenol
and
incubation with
CFE was
indicating
ortho-cleavage
the
cis,cis-muconic acid.
reveal ed
temperature
30 D C.
ferulic
acid,
to
prot.ocatechuic
protocatechuic acid.
were
Studies
and
also found to be active on ferulic acid,
acid
culture
CFE
acid.
vanillin,
was
vanillic
Protocatechuate solution on
found to strongly absorb at
of
PCA
to
form
290 nm
~-carbOxy­
114
4.3.11
Discussion
The
identified
organism,
to
belong
morphological
Pseudomonas
isolated
and
biochemical
CUAC
was
strains,
Corynebacterium
sp.
earlier
reported. 13S ,139
pigment-producing
the
Pseudomonas
20
green
sp.
to
from
local
sp.
based
characters.
different
and
strain.
on
This
from
the
Pseudomonas
Pseudomonas
soil,
it
the
strain
bacterial
aeruginosa,
aeruginosa
Moreover
was
was
was
a
reported
to include a few more metabolites besides those produced by
the present strain.
The
optimal
growth
growth
conditions
of
the
studies with
for
the
bacteria
the
growth
organism led
of
increased
the
with
to
strain.
the
the
The
increase
in
After that there
concentration of eugenol upto 0.1% (v/v).
was a decline in growth and the effect was more pronounced
from
0.15%
(v/v)
antibacterial
organism was
chlor ide
sources
property
slightly
upto
tested,
This
onwards.
0.5%
urea
of
might
The
eugenol.
increased by
(w/v).
favoured
Among
be
due
to
the
growth
of
the
the
addition
the
different
bet ter
growth.
of
sodium
ni trogen
It was also
seen that the optimum pH and temperature for the growth of
the organism were 7.0 and 30°C, respectively.
115
Addition
(w/v)
slightly
of
sodium
increased
chloride
the
at
a
rate
transformation
of
which might be due to the increased growth of
at this concentration of NaCl.
an
additional
(w/v).
carbon
Further
in
resulted
eugenol.
a
increase
decrease
Then
the
eugenol
completely.
glucose
was
a
in
This
organism
eugenol,
The inclusion of glucose as
not
of
concentration
the
much
rate
took
might
easily
of
more
be due
assimilable
showed
effect
upto
of
and
a
temperature
of
30°C
transformation
time
to
to
the
of
assimilate
the
reason
that
carbon
source
than
more
were
0.5%
glucose
transformation
eugenol when urea was used as the nitrogen source.
pH
0.5%
the bacteria
had
organism
more
The
eugenol.
source
of
optimal
of
Neutral
parameters
for the increased transformation of eugenol.
The
first
eight
hours
considerable
of
eugenol
almost
rate
was
ie.,
apparently
vanillic
and
transformation
was
increase
complete
maximum,
of
very
in
reacted
by
4.62%
because
low.
the
by
eugenol
Thereafter
transformation
18
hours.
24
hours.
at
17 hours.
vanillin
protocatechuic
of
The
was
acids.
The
yield
After
of
that
further
PCA
was
during
there
and
the
was
a
about
50%
reaction
was
vanillin
was
it
decreased
oxidised
to
subsequently
metabolised by the cleavage of the aromatic ring.
116
The
metabolised
first
present
eugenol
hydrolysed
metabolised.
strain
Pseudomonas
acetate
also.
eugenol
which
to
sp.
Eugenol
then
CUAC
acetate
was
20
was
subsequently
There was not much difference in the yield of
the products.
The
metabolise
isoeugenol
medi urn wi th
found
which
organism
was
for
by
cultivating
as
the
isoeugenol
that
tested
the organism was
its
it
ability
in
the
mineral
sole carbon source.
not
able
to grow
ruled
out
the
possibility
of
intermediate
in
the
degradation
pathway
in
of
It
the
isoeugenol
to
was
broth
being
an
eugenol
to
ferulic acid.
Fermentation
and
cell
products.
vanillin,
free
of
extracts
eugenol
did
not
with
improve
The transformation products
vanillic
acid
and
immobilised
the
cells
yield
viz.,
of
ferulic
protocatechuic
acid
the
acid,
were
all
transformed further when incubated with cell free extracts.
This
showed
that
fermentation.
mechanism
ortho
The
revealed
fission.
they
were
experiment
that
the
Further,
not
the
end
to
find
the
aromatic
the
ring
presence
products
ring
was
of
fission
cleaved
the
of
by
enzyme
117
responsible
catechuic
for
ortho-fission
acid-3,4-oxygenase
protocatechuate
290 nm.
cis,
PCA
to
undergoes
cis-muconic
290 nm.
which
test
for
gave
the
mec h an1sm.
was
confirmed
monitoring
ortho-cleavage
(CMA)
organism,
which
the
to
a
deep
purple
determination
of
by
form
~
at
-carboxy-
strongly
~-keto
in the
aromatic
adding
absorbance
absorbs
colour
proto-
at
adipic
colorimetric
ring
cleavage
164
Prom
proposed
acid
and
the
CMA was metabolised subsequently to
acid,
.
CFE
in
for
the
above
results
the
following
the degradation of eugenol
pathway
by the Pseudomonas
sp. CUAC 20.
CH=CHCOOH
is
CHO
COOH
~
COOH
OOH
118
4.4
STUDIES WITH PSEUDOMONAS AERUGINOSA
The
fermentation
aeruginosa was
under
section 3.2.1
The methods
those
carried out
eugenol
of
in
with
Pseudomonas
the mineral medium described
suppl ement ed
wi th
O. 1 % (v Iv)
eugenol.
of cultivation and incubation were the same as
followed
for
Pseudomonas sp.
CUAC 20.
The fermented
broth was centrifuged, acidified and extracted with solvent
ether.
The ether extract was concentrated and subjected to
thin layer chromatography.
similar
had
to
that
proposedth e
reported
The thin layer chromatogram was
by
following
Tadasa
pathway
and Kayahara.
for
eugenol
139
metabolism
by Pseudomonas aeruginosa.
0
/'\
CH 2 CH-CH 2
CH 2 CH=CH 2
:>
OCH
~
OCH
3
H
3
OH OH
CH 2 6H-br 2
~OCH3
OH
They
)
119
H=CHCOOH
CH=CHCH 2 0H
)
)
OCH:l
OH
COOH
ring
fission
COOH
~(--
CHO
(
OH
OH
Eugenol
was
fermented
with
Pseudomonas
aeruginosa
tion
broth
wit.hdrawn
was
found
maximum
were
that
decrease.
after
and
fermentation was
concentration
1.47%),
and
which
of
in
10 ml
mini
jar
ferment or
samples of
fermenta-
analysed
periodically.
complete by 22
vanillin
the
the
was
at
concentration
16
hours.
hours
was
It
The
(yield
found
to
120
Table 4.1(a): Identification tests for CUAC 10
Tests
Results
Form and arrangement
Long straight rods:
no characteristic
arrangement
Gram reaction
Positive
Spore formation
Positive
Motility
Positive
Chromogenesis in nutrient
agar
Negative
Production of indole
Negative
Hydrogen sulphide production
Negative
Gas production from glucose
Positive
Oxidation-fermentation test
Oxidative
Citrate utilisation
Positive
Nitrate reduction
Negative
Cytochrome oxidase
Negative
Catalase
Positive
Gelatinase
Negative
Amylase
Positive
Caseinase
Negative
121
Table 4.l(b): Identification tests for CUAC 20
Tests
Results
Form and arrangement
Small rods; no characteristic arrangements
Gram reaction
Negative
Spore formation
Negative
Motility
Positive
Chromogenesis in nutrient agar
Negative
Production of indole
Negative
Hydrogen sulphide production
Negative
Gas production from glucose
Positive
Oxidation-fermentation test
Oxidative
Citrate utilisation
Negative
Nitrate reduction
Positive
Cytochrome oxidase
Positive
Catalase
Positive
Gelatinase
Positive
Amylase
Positive
Caseinase
Negative
0.995
Optical density
0.975
0.03
0.954
0.05
0.930
0.07
0.892
0.1
0.863
0.12
0.737
0.15
0.576
0.17
o
0.886
Optical density
0.885
0.5
0.840
1.0
0.754
2
0.651
3
0.542
5
0.404
7
Effect of concentration of sodium chloride on growth
0.991
0.01
Sodium chloride (%, w/v)
Table 4.2.2:
o
Engenol (%, v/v)
Table 4.2.1: Effect of concentration of eugenol on growth
0.213
10
0.353
0.2
I-'
IV
IV
0.877
4
NH Cl
5.5
0.280
optical density
0.544
6.0
0.920
(NH 2 )2 CO
0.816
6.5
0.882
7.0
0.878
7.5
Effect of pH on growth
0.875
(NH 4 )2 S 04
0.831
8.0
3
0.766
8.5
0.836
KN0
Effect of different nitrogen sources on growth
Table 4.2.4:
3
pH
0.886
optical density
4
NH N0
Nitrogen source
Table 4.2.3:
3
0.499
9.0
0.858
NaN0
N
W
I-'
0.891
30
0.882
37
0.845
45
Effect of temperature on growth
0.683
56
0.532
70
100
100
0.5
1.0
hr
100
o
99.16
97.26
98.00
6
95.90
93.65
93.86
12
60.14
57.13
56.20
18
24
53.24
52.06
51. 25
Residual Eugenol (%, v/v)
53.00
51.77
51.04
36
Effect of concentration of sodium chloride on transformation
0.287
15
o
(%, w/v)
Sodium chloride
Table 4.2.6:
Optical density
Temperature (O°C)
Table 4.2.5:
.!:>
N
f-'
125
Table 4.2.7:
Effect of concentration of glucose
on transformation
Residual Eugenol
Glucose
( %, w/v)
0 hr
( %, v/v)
6
12
18
24
36
0.5
100
98.90
96.14
64.10
56.43
.55.37
1.0
100
98.28
94.25
58.39
51.10
51.08
1.5
100
98.80
97.05
65.18
53.30
52.32
2.0
100
98.95
98.60
69.74
58.63
57.85
Table 4.2.8: Effect of different nitrogen sources
on transformation
Nitrogen
0 hr
source
6
Residual Eugenol
12
18
( %, v/v)
24
36
NH N0
4 3
100
96.12
93.80
56.65
51.60
50.80
NH C1
4
100
97.00
94.16
57.90
52.23
51.89
(NH 4 }2S04 100
96.16
93.20
56.13
50.19
48.84
(NH 2 }2CO
100
97.00
94.18
57.95
52.91
52.60
KN0
100
97.63
95.80
58.50
53.86
53.85
100
97.45
95.65
58.07
53.25
52.63
3
NaN0
3
126
Table 4.2.9:
Effect of pH on transformation
Residual Eugenol
pH
o
hr
(%, v/v)
6
12
18
24
36
6.0
100
98.70
98.14
76.05
67.71
66.08
6.5
100
98.81
96.00
60.50
54.95
54.78
7.0
100
97.19
93.14
56.88
51.13
51.00
7.5
100
97.79
95.17
58.12
52.51
51.16
8.0
100
93.08
95.73
59.93
54.65
54.25
Table 4.2.10:
Temperature
( 0 C)
0 hr
Effect of temperature on transformation
6
Residual Eugenol ( %, v/v)
24
12
18
36
15
100
99.10
98.71
89.90
84.75
83.76
30
100
98.00
94.10
56.78
51.14
50.83
37
100
98.11
95.00
57.80
52.01
51.51
45
100
98.25
96.00
58.13
53.70
52.68
56
100
98.40
98.19
60.23
55.18
55.05
o
o
Eugenol
(%, v/v)
Optical
density
0.215
0.02
0.024
4
0.117
6
0.390
9
0.760
12
Growth curve
0.862
15
0.886
18
0.476
0.658
0.07
0.726
0.10
0.713
0.12
0.687
0.15
0.608
0.17
Effect of concentration of eugenol on growth
0.05
0.018
o
Optical density
Table 4.3.1:
2
o
Time (hr)
Table 4.2.11:
0.459
0.20
0.886
21
0.190
0.25
0.881
24
f-'
N
-....J
o
0.730
Optical density
4
NH N0
Nitrogen source
3
0.742
1.0
0.699
1.5
0.638
2
0.550
3
0.459
5
0.726
NH 4 Cl
0.720
(NH 4 )2 S 04
0.748
(NH 2 )2 CO
3
7
0.351
0.716
KN0
Effect of different nitrogen sources on growth
0.743
0.5
Effect of concentration of sodium chloride on growth
Table 4.3.3:
Optical density 0.718
Sodium chloride
(%, w/v)
Table 4.3.2:
3
0.712
NaN0
0.200
10
co
N
I--'
ooe
Optical density
Temperature
Optical density
pH
0.547
0.303
0.731
7.0
0.720
7.5
30
0.729
0.300
0.702
37
0.584
45
0.225
0.437
0.311
9.0
70
0.527
8.5
56
0.676
8.0
Effect of temperature on growth
0.683
6.5
15
Table 4.3.5:
6.0
5.5
Table 4.3.4: Effect of pH on growth
1.0
N
f-'
o
100
100
100
100
0
0.5
1.0
1.5
hr
0.22
1.04
3.99
1.10
2.11
5.02
4.11
6.27
8.95
46.76
47.81
81.47
81.40
84.73
49.18
0.89
2.62
6.43
48.01
82.00
48
36
18
24
12
Residual Eugenol (%, v/v)
Effect of concentration of sodium chloride on transformation
(%, w/v)
NaCl
Table 4.3.6:
0
LV
I-'
131
Table 4.3.7: Effect of concentration of glucose
on transformation
Residual eugenol
Glucose
(%, w/v)
0 hr
0.2
100
0.5
12
(%, v/v)
18
24
36
82.16
48.11
6.46
2.62
0.98
100
83.76
49.40
7.78
3.00
0.99
0.7
100
88.81
52.50
8.80
3.52
1.85
1.0
100
95.05
59.16
14.65
8.48
3.87
Table 4.3.8:
48
Effect of different nitrogen sources
on transformation
Nitrogen
Source
Residual Eugenol
o hr
12
18
24
(%, v/v)
36
48
NH N0
4 3
100
81.87
47.92
6.10
2.44
0.68
NH Cl
4
100
82.19
48.15
6.67
2.81
1.05
(NH 4 )2 S04
100
83.01
48.94
6.96
3.05
1.00
(NH 4 )2 CO
100
80.89
40.67
1. 69
0.18
KN0
100
83.18
48.83
6.86
3.17
loll
100
83.76
49.06
7.00
3.22
1.10
3
NaN0
3
132
Table 4.3.9:
Effect of pH on transformation
Residual Eugenol ( % , v/v)
pH
0 hr
12
18
24
36
48
6.5
100
85.30
60.25
16.48
10.74
7.81
7.0
100
81.90
48.07
6.38
2.58
0.45
7.5
100
84.76
52.40
10.51
4.48
2.60
8.0
100
88.63
63.74
20.00
13.30
10.25
Table 4.3.10:
Effect of temperature on transformation
Residual Eugenol (% , v/v)
Temperature
(
0
C)
0 hr
12
18
24
36
48
15
100
93.78
88.16
82.98
70.48
58.40
30
100
82.16
47.45
6.25
2.40
0.22
37
100
84.35
50.68
11.72
5.06
4.12
45
100
86.98
72.83
49.00
39.01
36.76
56
100
90.07
80.78
66.70
58.71
52.00
0
0
0
Vanillin
Vanillic acid
o hr
Ferulic acid
Product
0.016
o
Optical density
Table 4.3.12:
3
o
Time (hr)
0.128
9
0.240
12
0.387
15
Growth curve
0.593
18
3.3
2.1
4.7
12
7.6
5.0
6.8
18
9.8
3.2
4.4
24
Concentration (mg/l00 ml)
30
6.2
1.4
2.6
0.720
21
Time course of formation of products
0.056
6
Table 4.3.11:
0.718
24
3.8
0.5
1.9
36
O. 709
36
w
w
f-'
134
1.0
----.----,----....,---.,..,
--~---.----.-.-.
---.----,---~
-----.----------
><
.w
OM
tI)
c:: 0.5
Ql
Cl
.-4----+-.--.+--
o
5
10
15
20
25
Time (hr)
Fig.4.2.l:
Growth curve of CUAC 10 in eugenol medium
Fig.4.2.2:
10
3
5
2.5
u~ ----------
I'
Chemical Shift (0 )
NMR spectrum of eugenol
7.5
CH
CH2 CH=CH 2
o
f-'
W
U1
3
CH CH=CH
2
2
10
Fig.4.2.3:
20
Chemical Shift (6)
5
NMR spectrum of product E
7.5
2.5
o
~JiJltvG-)lJL~
CH
I
CH =CHCH
2
2
0'1
W
......
--"
...
" .'
I
I
i
I
1
I
I
1
I
',.
1
I
"
.J..
OH
Fig.4.2.4:
i.A; ,
*
i
I
1
H
'7'
0
I
13 c NMR spectrum of product E20
I
o.dl
OCH 3
CH 2 CH=CH 2
Chemical Shift (ppm)
1-
3
0
* Signals due to the solvent, CDC1 3
,
i
!
I
I
I
CH
CH =CHCH
2
2
j
I
I
I
II
I
1
I
I
I
I
I
'T
''''''T'
I
, ",I.
I
-..J
W
I-'
138
1 . 0 -.. - .....,.
..-..--..,. '--' .--. -.-.,.....-----..,.. . . -.. -.. ---...,. .....--.. -.--...,.---.... ----.-..,..-.- . ---..-,-- ..----.....,...-----,
tI
~
.w
'M
Ul
c:
(j)
Cl
0.5
r-i
1'0
U
I
(
'M
.w
/
//
0..
o
j
I
~
I
I
/
r--
,,/
_.-/./'
,/
/
/
./
I
1._-_-.-' '.~+--.--- .. + _ .. _.. + .-.......-+---+-..--+---+--+----J
+-,._-- +.-
o
5
10
15
Time (hr)
Fig.4.3.1:
Growth curve of CUAC 20
20
25
Fig.4.3.2:
10
OCH
3
Chemical Shift (0)
5
NMR spectrum of product E30
7.5
L~~ __~~l
CHO
2.5
o
1.0
W
I-'
.IJ
8
\..I
ttl
C
ID
E
OM
r
l
4000
I
3
2000
~
CH
I~
I
-1
)
1200
J V\ 1\'
Wave Number (cm
1600
\ . h
IR spectrum of product A
bH
©
Fig.4.3.3:
o
20
I \
I
60r
~ 40
c
u
(J)
~
Bo
CH=CHCOOH
800
400
~
100rF------r------r------~----_.------_r------r_----_T------._----_,------~
0
f-'
.j:>.
60
8
I'll
\..r
o
20
rn
c 40
E
-rl
C
I'll
+.J
+.J
u
Q/
dP
IR spectrum of product B
-1
)
1200
Wave Number (cm
Fig.4.3.4:
1600
j
2000
H3
4000
80
COOH
800
400
100~r-------r-------r------~-------T------~------~------~-------'~----~r-----~
f-'
J:>
f-'
Fig.4.3.5:
10
3
7.5
Chemical Shift (
5
b)
NMR spectrum methyl ester of product C
OH
COOCH
2.5
o
N
I-'
~
CHAPTER
5
MICROBIAL TRANSFORMATIONS OF ISOEUGENOL
MICROBIAL TRANSFORMATIONS OF ISOEUGENOL
5.1
INTRODUCTION
Isoeugenol
column
was
chromatography
eluant.
It
was
purchased
using
also
prepared
with potassium hydroxide.
identified
CUAC 10
medium
strain
during
was
found
containing
of
the
to
metabolise
studies
or
a
carbon
mineral
designated
medium
source.
Pseudomonas
characters
sp.
was
with
hexane
eugenol
by
as
by
the
treatment
Bacillus
isoeugenol
other
isolated
in
a
CUAC
from
Another
which
30
the
sp.
mineral
nutrient.
soil
could
by
the
This strain was able to grow
isoeugenol
It
was
identified
from
its
morphological
the
purified
eugenol,
as
containing
(Table 5.1).
isolated during
on
any
enrichment culture technique.
in
from
transform
isoeugenol
gel
was
One of the cultures isolated and
glucose
bacteria
silica
and
as
the
belonging
as
and
sole
to
biochemical
The strain Pseudomonas sp. CUAC 10
studies
on
eugenol
was
found
not
able
to metabolise isoeugenol.
5.2
STUDIES WITH THE BACILLUS Sp. CUAC 10
This
broth
strain
containing
was
not
isoeugenol
able
as
144
the
to
grow
sole
in
a
mineral
carbon
source.
145
So
the
studies
Baci 11 us
sp.
containing
on
the
CUAC
1%
10
fermentation
were
(w/v)
of
conduct ed
glucose
and
isoeugenol
in
a
0.1%
mineral
(v/v)
by
the
medi urn
isoeugenol
(unless otherwise stated).
5.2.1
Growth studies
the
As
chloride,
effects
of
concentration
different nitrogen sources,
of
sodium
pH and temperature on
the growth of this strain were studied earlier in the case
of
fermentation
However
growth
the
of
presented
increase
retarding
effect
the
in
in
of
eugenol,
of
concentration
organism
was
growth
comparatively
studied
As
Table 5.2.1.
the
these
in
the
less.
not
done
isoeugenol
and
the
but
retarding
here.
on
the
resul ts
are
case of
isoeugenol
organism,
The
of
the
concentration of
of
were
eugenol,
resulted
the
effect
effect
was
pronounced with concentrations above 0.15%
(v/v)
an
in
a
was
more
isoeugeno1
concentration.
5.2.2
Effect of physico-chemical factors on the
transformation of isoeugeno1
5.2.2.1
Effect of concentration of sodium chloride
The
concentration
results
of
sodium
of
the
studies
chloride
on
the
on
the
effect
of
transformation of
146
isoeugenol
sodium
upto
are
presented
chloride
0.5%
had
(w/v).
in
not
But
Table
much
5.2.2.
effect
further
on
The addi t ion
the
addition
of
transformation
of
NaCl
caused
a
decrease in transformation.
5.2.2.2
Effect of concentration of glucose
The
maximum
seen
optimum
conversion
from
the
of
resul ts
concentration
isoeugenol
was
presented
of
glucose
1%
(w/v),
in Table
a
corresponding
isoeugenol.
(w/v)
also,
decrease
in
the
When
the
concentration
there
was
a
slight
as
5.2.3.
concentration of glucose was decreased to 0.5%
was
for
the
can
When
(w/v),
the
there
transformation
was
increased
decrease
in
the
be
of
to
2%
rate
of
transformation of isoeugenol.
5.2.2.3
Effect of different nitrogen sources
Compared
ammon i urn
as
Table
replaced
sulphate
5.2.4
by
to
other
favoured
indicated.
alkal i
comparatively low.
metal
nitrogen
more
sources
conv ers ion
When
ni t ra tes,
the
of
ammoni urn
the
tested
i soeugenol,
sal ts
t rans forma t ion
were
was
147
5.2.2.4
Effect of pH
The
results of the effect of pH on transformation
of isoeugenol,
optimum pH was
to
6.0,
there
of
isoeugenol.
presented in Table 5.2.5,
7.0.
was
When
a
the
sudden
pH
of
decrease
The decrease
in
the
indicated that the
the broth was reduced
in
the
transformation
rate of
transformation
was less when the pH was raised to 8.0.
5.2.2.5
Effect of temperature
presented
As
indicated
that
there was
a
when
the
the
in
Table
optimum
temperature
drastic decrease
temperature
appreciable
was
decrease
in
5 • 2 .6,
was
the
results
30°C.
Though
in the rate of transformation
decreased
the
to
15°C,
there
transformation
was
when
no
the
temperature was raised upto 45°C.
5.2.3
Growth curve
The
different
optimal
that
the
growth
intervals
conditions.
culture
was
of
the
culture
of
time,
The
growth
in
a
lag
after
curve
phase
was
monitored
incorporating
(F ig. 5.2.1)
during
the
at
the
showed
first
148
Then the logarithmic phase of growth started.
four hours.
The culture was in a stationary phase from 18 to 21 hours,
after which was the death phase.
Thus the growth curve was
very similar to that when eugenol was used as the substrate
for fermentation.
5.2.4
Fermentation of isoeugenol
The
studies
factors
chemical
transformation of
for
the
was
growth
the
of
of
different
the
of
isoeugenol
by
the
the
with
Bacillus
sp.
jar fermentor,
165
The
10 ml
broth were wi thdrawn
analysed.
dehydrodiisoeugenol
41%
bisdehydroisoeugenol
(BDHI)
glucose
mineral
During the course of fermentation,
and
(w/v)
parameters
0.11%
fermentat ion
1%
and
and
periodically
about
parameters.
physico-
bacteria
led to the optimal
optimal
supplemented
of
ferment or
hours
effect
isoeugenol
these
isoeugenol.
samples
the
Isoeugenol was fermented in a mini
incorporating
(v/v)
on
fermentation
CUAC 10.
medium
on
were
At
the
(DHDI)
formed.
from
end
and
The
of
the
18
25.5%
yields
of
DHDI and BDHI were increased to 46.5% and 28.7% respectively
at
the
end
of
24
hours.
transformation after that.
There
was
not
any
considerable
149
5.2.5
Isolation and identification of bisdehydroisoeugenol
and dehydrodiisoeugenol
The
supernatent
fermentation
was
broth
acidi fied
and
ether extract was dried with
evaporated under
was
subjected
the
presence
to
thin
of
two
chromatographed
20
and
extracted
wi th
and
the
ether.
The
anhydrous sodium sulphate and
pressure.
layer
spots
over
chloroform: hexane
(IE
centrifuged
A
small
port ion
chromatography,
in
addition
which
to
the
0
fit
showed
spot
of
The rest of the residue from the ether extract
isoeugenol.
was
reduced
was
IE
30
)
a
(3:1)
were
column
as
obtained
the
as
of
silica
eluant.
white
gel
Two
solids
using
products
which
were
recrystallised from chloroform-hexane mixture.
The product
IE
20
a melting point of 133°C.
this
compound
was
the less polar of the two,
The NMR spectrum (r'ig.5.2.3)
significantly
spectrum of isoeugenol
protons
,
corresponding
(Fig.5.2.2).
to
each
molecule was less compared to
of
isoeugenol
was
determined
as
from
the
of
NMR
The number of aromatic
-OCH3
group
isoeugenol
the number of aromatic
to each -OCH3 group is three.
product
different
had
in
(1).
the
product
In the case
protons corresponding
The molecular weight of the
326
by
mass
spectrometry.
150
This
the
together
product
of
analysis
also
revea 1 ed
of
one
that
isoeugenol
aromatic
t here
was
molecules.
appeared
at
and
since the
for -CH=CH- group
CH -C- group
3
oxidative coupling
2,4'-couplings,166
the
to
(b6.3),
in
ox ida t i ve
elemental
7.1,
methoxy
Signals were
CH -C=C- group
3
From these results and
of phenols
structure
an
The
b6.8
(Slo5).
proton
In the NMR spectrum
agreed with this view.
protons
observed
(Slo8)
absence
S 3.9 and hydroxy group at b 5.65.
group at
or
two
aromatic
also
the
mol ecul e
coupling
the
with
( 2) ,
are
2,2 1 -couplings
4-(2,3-dihydro-7-
methoxy-3-methyl-5-propenyl-2-benzofuryl)guaiacol is proposed
for the product IE
20
.
CH=CHCH 3
OH
(1)
(2)
151
Isoeugenol on treatment with ferric chloride gave
two products one of which had the same melting point and Rf
value (TLC) as that of IE
obtained by FeC1
3
20
.
The structure of this product
treatment had already been established as
"
1 • 137,167
(2) , d e h y d ro d 11soeugeno
The
acetylated
derivative
(~),
of
melting
point
114°C, was prepared by reacting it with acetic anhydride in
pyridine.
the
The
compound
NMR
was
-COOCH3 group at
The
-CH
of
the
acetate
The
monoacetylated.
revealed
monoacetate
02.3, two -OCH3 groups absorbing at
that
had
83.8
& 3.9 and aromatic protons at 56.8 to 7.1.
and
in
spectrum
13
Signals were observed
Fig.5.2.4.
3
carbons
carbons
C NMR spectrum of the product IE30 is shown
(17.547
(55.935
and
and
for
18.397 ppm,
55.965
ppm,
quartet) ,
quartet)
carbons (45.628 and 93.806 ppm, doublet)
carbons
appeared
between
108
confirming the structure of (2).
ppm
the two terminal
two
and
-OCH
I
3
I
-CH-CH-
and the sp2 hybrid
and
146
ppm
thus
152
The
The
NMR
product
spectrum
of
1E30
the
had
aromatic
the
product
isoeugenol
-OCH
each
proton
been
an
scopy
the
The
NMR
spectrum
group
Signals
were
position
also
of
30
.
at
that
in
the
group
in
case
of
to
coupling
by
mass
at
the
may
be
structure
biphenyl
6
group
6
or
of
(3 ) ,
is
give
a
spectro-
6.55-6.75,
at
0
5.50.
(cl 6.15)
oxidative
ortho
have
assumption.
at
for -CH=CH- group
the
aroma tic
to
this
protons
hydroxy
Since
ei ther
position
supported
and
one
there might
determined
aromatic
5 ,S'-dimethoxy-6, 6' -dihydroxy
product IE
whereas
3
of
3.80
the
-OCH
absence
(<5 1. 80) .
Thus
molecule.
each
The
observed
place
But the number
corresponding
analysis
6
at
similar to
protons
weight
showed
158°C.
aromatic
coupling
elemental
takes
two
three.
molecular
CH 3 -C=C- group
only
to
of
is
of isoeugenol.
NMR spectrum suggested that
methoxy
the
is
oxidative
and
phenols
number of
group
the
is
point
(Fig. 5.3.5)
corresponding
molecule
3
in
dimer.
The
protons
melting
product
the NMR spectrum (Fig.5.2.2)
of
a
coupling
para
the
and
of
posi t ion,
isoeugenol
3,3'-dipropenylproposed
for
the
153
Isoeugenol on treatment with ferric chloride gave
IE
20
together
point
and
compounds
iron
and/or
Rf
on
(Ill)
wi th
another
value
(TLC)
treatment
species
of
as
wi th
give
isoeugenol
wi th
that
ident i cal
of
one-electron
dimers
2,4 I -coupling. 166
treatment
product
Thus
formed
this
with
FeC1
IE
15
.
Phenolic
oxidants
by
product
3
30
mel t ing
such
as
2,2 1 -coupling
formed
(3),
by
the
bisdehydro-
isoeugenol.
The NMR spect rum of
(3),
prepared
by
reacting
anhydride,
showed that
diacetate
had
o 3. 75
-COOCH3
the
it
(3)
acet yla ted der i vat i ve
with
pyridine
in
group
at
5
2.30,
() 6. 65 - 6 • 90 .
CH=CI-ICII
3
-OCH3
of
acetic
compound was diacetylated.
and a r 0 ma tic pro ton 5 at
CII CH=CH
3
the
group
The
at
l54
5.2.6
Fermentation of isoeugenol acetate
Isoeugenol
medium
1%
described
(w/v)
acetate
under
glucose
fermented
section
0.1%
and
was
3.2.1,
(w/v)
withdrawn
the
periodically
broth
was
was
The
extract
ether
ml
and
extracted
was
samples
to
of
the
the
ether
broth
The
cells
and
isoeugenol,
were
fermentation
after
concentrated
It was found that
wi th
acetate.
After
remove
with
mineral
jar ferment or and as
analysed.
centrifuged
supernatent
analysis.
10
proceeded,
the
supplemented
isoeugenol
fermentation was conducted in the mini
fermentation
in
and
the
acidifying.
subjected
to
DHDI and BDHI were
the products of fermentation of isoeugenol acetate with the
Though
Bacillus Spa CUAC 10.
during
18
the
hours
reaction
initial
more
was
isoeugenol
than
almost
acetate
isoeugenol,
of
hours
60%
of
over
the
rate
transformation
fermentation was very low,
isoeugenol
by
of
24
remained.
bisdehydroisoeugenol
was
hours
fermented.
and
only
The
yields
of
and
isoeugenol
traces
by
The
of
dehydrodiat
the
end
of 24 hours were 49%, 30.4% and 12.3% respectively.
5.2.7
Fermentation of transformation products
Two
containing
1%
100
(w/v)
ml
aliquots
glucose
and
of
0.1%
mineral
(w/v)
medium,
DHDI
and
one
the
155
other containing 1%
inoculated
with
(w/v)
the
glucose and 0.1%
Bacillus
sp.
CUAC
10
(w/v)
BOHI,
and
were
incubated.
At different intervals of time 10 ml samples were withdrawn
from the
flasks
tion
products
which
proved
ascept i call y
were
that
and
detected
OHDI
and
analysed.
in
the
BOHI
were
No t rans f orma-
fermentation
the
end
broths
products
of
fermentation of isoeugenol.
5.2.8
Immobilisation of whole cells
Fifteen
sp.
old
culture
cells
of
the
Bacillus
CUAC 10 were immobilised in calcium alginate beads and
the optimal
with
was
hours
parameters for the transformation of isoeugenol
the immobilised cells were found out.
8.0
and
the
time was 8 hours,
transformed.
opt imal
tempera t ure
30 0 C.
The optimal
The
pH
retention
at which time about 70% of isoeugenol was
The immobilised cells were found to be active
for more than one week.
5.2.9
Discussion
As
mentioned
previously
this
organism
was
isolated from the aerial flora and was identified to belong
to the Bacillus sp.
The growth studies of the strain were
156
conducted
sole
in a
carbon
retarded
by
eugenol,
but
medium containing isoeugenol
source.
the
The
growth
addi t ion of
the
concentration
from 0.15%
mineral
effect
of
was
not
so
in
the
sodium
bacteria
as
in
the
pronounced.
medium
case of
When
was
was
the
increased
the growth was much affected resulting in
a poor growth of the organism.
of
the
isoeugenol,
isoeugenol
(v/v),
of
as the
chloride,
The effects of concentration
different
nitrogen
sources,
pH
and
temperature on the growth of the organism were not studied
here
as
these
studies
were
conducted
in
the
effect
various
case
of
euge.nol.
The
studies
on
the
of
physico-
chemical factors on the transformation of isoeugenol led to
the
optimal
addition of
tion
was
of
1%
parameters.
(w/v).
At
higher
the
in
of
not affect
opt i mal
increase
case
in
eugenol,
the
the transforma-
concent rat ion
of
glucose
concentration
of
glucose
caused a decrease in the transformation of
whereas
resulted
The
An
above 1.5% (w/v)
also
in
sodium chloride did
isoeugenol.
isoeugenol
As
a
decrease
decreasing
concentrations
of
in
the
concentration
rate
glucose,
of
of
glucose
transformation.
the
products
of
metabolism of glucose might have inhibited the transformation
157
of
At
isoeugenol.
decrease
growth
in
of
the
lower
concentrations
transformation
bacteria
for
lack
might
of
be
easily
of
glucose
due
to
the
the
assimilable
poor
carbon
The optimal pH value and temperature were 7.0 and
source.
It
was
interesting
to
note
that
the transformation
of isoeugenol was not much adversely affected at temperatures
upto 45°C.
The
Ouring
the
culture
first
10
attained
hours
of
transformation was very low.
full
growth
by
fermentation,
18
the
hours.
rate
of
But by 24 hours the reaction
was almost over with more than 80% of isoeugenol undergoing
transformation.
46.5%
was
and
not
compounds
The
28.7%
able
yields
respectively.
to
were
final
ferment
the
end
DHDI
of
The
DHOI
fact
and
BDHI
products
and
that
the
proved
of
BOHI
were
culture
that
these
fermentation
of
isoeugenol with the present strain.
When
isoeugenol
acetate
was
fermented
wi th
the
Bacillus sp. CUAC 10, it was first hydrolysed to isoeugenol,
which was then converted to DHDI and BOHI.
with eugenol acetate.,
As in the case
the yields of OHOI and BOHI increased
when isoeugenol acetate was used as the substrate.
158
The
with
st ud ies
on
immobilised whole
the
t rans forma t ion
cells
revealed
and temperature were 8.0 and 30°C.
BOHl
with
the
immobilised
that
with
intact
cells.
cells
But
that
0
f
i soeugenol
the
optimum
pH
The yields of DHDI and
were
the
slightly
immobilised
less
cell
than
system
has the advantage that it can be reused.
Dehydrodiisoeugenol on oxidation with nitrobenzene
and
alkali
gives
with vanillic
pro d uc t s.
with
with
acid
162,168
potassium
nitric
. .
tr1n1tro
vanillin
The
acid
as
the
major
product
and 5-carboxy vanillin
(6)
methyl
on
permanganate
veratro 1 e
CHO
(5)
(4)
gives
ether
gives
of
DHDI
veratric
4,5-dinitro
acid
veratrole
(9) .169
COOH
CHO
HO
OH
(5)
along
as minor
oxidation
(7)
and
and
159
Cousin
and
oxidising ferment
Herissey
had
reported
that
the
of Russula delica converted isoeugenol to
In this study,
DHDI.
(~)
(8 )
(7 )
the Bacillus sp.
CUAC 10 transformed
isoeugenol to dehydrodiisoeugenol and bisdehydroisoeugenol.
5.3
STUDIES WITH THE PSEUDOMONAS Sp. CUAC 30
This
able
to
grow
strain,
in
a
mineral
the sole carbon source.
the
mineral
medium
supplemen ted wi t h
stated).
isolated
0.10%
from
the
local
medium containing
soil,
isoeugenol
was
as
Thus the studies were conducted in
described
(v Iv)
under
i soeugenol
section
3.2.1,
(unl ess ot herwi se
160
5.3.1
Growth studies
As
growth,
the
the
culture
optical
took
density
hours
36
readings
to
were
attain
taken
full
hours
36
after inoculation.
5.3.1.1
Effect of concentration of isoeugenol
The
results
of
the
effect
of
concentration
of
isoeugenol on the growth of the bacteria are given in Table
5 • 3 .1.
The
(v/v).
As
growth
was
addition
opt imum concentrat ion of
the
also
of
concentrat ion
increased
isoeugenol
of
till
i soeugenol
isoeugenol
0.07%
resulted
in
0.07%
increased
(v/v).
a
was
the
Subsequent
poor
growth,
the
effect being more pronounced from 0.12% (v/v) onwards.
5.3.1.2
Effect of sodium chloride
The
increase
when
the medium.
in
the
growth
0.5%
(w /v)
the
0
f
organism
sodi urn
showed
ch lor ide
But further addition of NaCl
growth
Table 5.3.2.
of
of
the
organism,
as
was
c~used
can
be
a
slight
added
to
a decrease
seen
from
161
5.3.1.3
Effect of different nitrogen sources
The
results
presented
in
Table
5.3.3
indicated
that when ammonium chloride was used as the nitrogen source
there was an
increase
in growth.
To a
lesser extent
urea
also favoured an increase in growth of the culture.
5.3.1.4
Effect of pH
As
more growth.
Table
5.3.4
indicated,
neutral
pH
favoured
When the pH was decreased to 5.5, there was a
sudden decrease in the growth of the organism.
Raising the
pH also resulted in poor growth.
5.3.1.5
Effect of temperature
The
that
the
fairly
optimum
good
temperature
bacteria.
results
presented
temperature
growth
caused
at
a
in
was
37°C.
decrease
Table
30°C,
But
in
5.3.5
though
further
the
indicated
there
increase
growth
of
was
in
the
162
5.3.2
Effect of physico-chemical factors on transformation
of isoeugenol
5.3.2.1
Effect of concentration of sodium chloride
The
concentration
results
of
sodium
are
isoeugenol
of
given
di f ference
chlor ide
added
But
tion.
furt her
to
studies
chloride
in
consi derabl e
was
the
on
Table
in
the
the
on
the
the
5.3.6.
upto
add i t i on caused a
of
transformation
There
t rans format i on
medi urn
effect
was
when
1 % (w/v)
decrease
of
no
sod i urn
concentrain
the
rate
of transformation.
5.3.2.2
Effect of concentration of glucose
The
that
when
rate
of
during
results
presented
(w/v)
glucose was
0.2%
transformation
the
initial
concentration
of
of
stages
glucose
in
Table
added
isoeugenol
of
was
5.3.7
to
the
medium,
increased
cultivation.
increased
indicated
to
But
1%
the
slightly
when
(w/v)
the
there
was a decrease in the transformation.
5.3.2.3
was
used
Effect of different nitrogen sources
As
Table
as
the
5.3.8
nitrogen
indicated
source
when
there
potassium
was
an
nitrate
increase
in
163
the
rate
of
favoured
transformation
isoeugenol.
transformation
better
a
of
than
Urea
the
also
ammonium
salts used.
5.3.2.4
Effect of pH
The results of the effect of pH on the transform-
ation of isoeugenol presented in Table 5.3.9 indicated that
the transformation was much affected by change in pH of the
medium.
The optimum pH was 7.0.
5.3.2.5
Effect of temperature
can
be
was
30°C,
As
temperature
transformation
temperature
at
seen
37°C
adversely
from
Table
though
there
also.
But
affected
the
5.3.10
was
the
optimum
good
fairly
change
in
transformation
of
further
isoeugenol.
5.3.3
Growth curve
'The growth of the culture was measured as optical
density
values
conditions
periodically,
(Fig.5.3. 1).
The
incorporating
initial
lag
the
phase of
optimal
growth
164
of
the
phase
cuI ture
of
lasted
growth
started
attained
culture
for
lasted
for
full
by
8 hours.
12
Thus
The
hours.
growth
hours.
6
about
and
the
At
the
logarithmic
30
hours
stationary
culture
was
the
phase
not
a
fast
growing one, compared to Pseudomonas sp. CUAC 20.
5.3.4
Fermentation of isoeugenol
From
the
physico-chemical
and
were
studies
parameters
t rans forma t ion
obtained.
isoeugenol
0
f
on
on
the
the
i soeugenol ,
Incorporating
was
fermented
in
effect
growth
the
mini
optimal
jar
medium was supplemented with 0.10% (v/v)
During
of
the
fermentation
periodically
density
analysed
were
310
nm,
mentioned previously.
had reacted.
were
products.
acid
at
broth
and
transformation
vanillic
course
withdrawn
for
nm
by
and
cuI ture
cond it ions
conditions,
The
isoeugenol.
10
from
residual
samples
ferment or
isoeugenol
and
vanillin
and
acid,
nm
ml
the
determining
293
the
fermentor.
fermentation
Ferulic
measured
347
of
various
opt i mal
these
a
of
of
the
optical
respectively,
as
At 12 hours only about 10% isoeugenol
After that there was a steady increase in the
165
rate
of
transformation
isoeugeno1
remained.
36 hours.
The
and by
The
time
of
6.3%
at
vanillin
24
reaction
course
given in Table 5.3.12.
24 hours only
of
was
formation
less
than
35%
almost
over
by
products
is
of
The yield of vanillin was a maximum
hours.
After
The
decreased.
that
yields
the
of
concentration
ferulic
acid
vanillic acid were maximum (8.2% and 9% respectively)
of
and
at 25
and 28 hours respectively, after which the concentration of
both acids decreased.
5.3.5
Isolation and identification of products
The
the
cells
fermentation
and
the
broth was
supernatent
was
centrifuged to remove
acidified
sulphuric acid and extracted with ether.
was
re-ext racted
wi th
5%
aqueous
with
2 N
The ether extract
sodium
bicarbonate
solution to remove strongly acidic products.
The
was
ether
concentrated
anhydrous
over a
layer
under
sodium
containing
reduced
sulphate.
It
non-acidic
pressure
was
after
then
products
drying
chromatographed
column of silica gel with chloroform: hexane
as the eluant.
recrystallised
wi th
(3:1)
The product, obtained as a white solid, was
from
chloroform-hexane
mixture.
It
had
a
166
melting point of 81°C.
It showed positive colouration with
2,4-dinitrophenyl hydrazine-H So •
2 4
indicated
product
cm -1 ),
(3560-3520
aromatic
ring
the
The
presence
aldehyde
-1
).
of
The
IR
cpmpletely matched those of vanillin.
identified
as
The
hydroxyl
(1680-1710
group
cm
(1615-1600
IR spectrum of
and
the
group
cm-I)
NMR
and
spectra
Thus the product was
4-hydroxy-3-methoxybenzaldehyde (vanillin).
sodium
bicarbonate
layer
was
acidified
with
2 N sulpnuric acid to pH 2 and extracted again with ether.
The
ether
and
concentrated under
of
it
was
revealed
then
layer
was
dried
subjected
the
reduced
to
presence of
subjected
to
"(3:1)
(A, B & C)
obtained
anhydrous
pressure.
thin
layer
three
products.
column
acetate: hexane
were
with
as
sodium
A small
which
port ion
chromatography
eluant.
when
which
The mixture was
chromatography
the
sulphate
with
Three
ethyl
products
recrystallised
from
water-acetone mixture gave white solids.
The
product
ester melted at 63°C.
the
presence
of
A
melted
at
170°C
and
its
methyl
The NMR spectrum of the ester showed
-COOCH3
group,
-OCH3
group,
-CH=CH-group,
-OH group and aromatic protons, which led to the conclusion
168
inoculated
wi th
Pseudomonas
sp.
CUAC
30 and
incubated
an
increase in turbidity was observed indicative of the growth
of the organism.
ether
tha t
and
on
these
these
The fermentation broth was extracted with
anal ys i s
compounds
compounds
of
the
were
were
ether
ext ract
metabo 1 i sed.
intermediates
This
in
the
it
was
showed
found
that
degradation
pathway of isoeugenol.
5.3.7
Fermentation of isoeugenol acetate
Isoeugenol
medi urn
0.10%
described
(w/v)
acetate
under
isoeugenol
tion were isoeugenol,
and
protocatechuic
found
in the
was
fermented
3.2.1,
sect i on
acetate.
in
the
mineral
supplemented
with
The products of ferment a-
ferulic acid, vanillin, vanillic acid
Only
acid.
traces
of
broth after fermentation.
isoeugenol
were
The yields of the
other products were the same as in the case of fermentation
of isoeugenol.
5.3.8
Mode of aromatic ring cleavage
The studies on the fermentation of isoeugenol and
transformation
products
protocatechuic acid was
of
the
isoeugeno]
final
suggested
that
aromatic compound in the
169
metabolic
pathway
metabolised
mechanism
by
of
of
the
isoeugenol.
strain.
aromatic
The
ring
But
PCA
was
experiment
cleavage
to
revealed
further
find
that
the
PCA was
cleaved by meta-fission.
5.3.9
Immobilisation of whole cells
Culture cells,
calcium alginate beads.
24 hours old,
were
immobilised
in
The optimum pH and temperature for
the transformation of isoeugenol with the immobilised whole
cells
was
were
25
7.5
and
30°C
hours when about
transformed
but
there
respectively.
79% of
was
no
The
isoeugenol
increase
in
retention
time
was
found
to be
the
quant it Y
0
f
the products formed.
5.3.10
Enzyme studies
The cell
free extract of the cuI t ure was used as
the source of enzymes.
the
transformation
The optimum pH and temperature for
of
isoeugenol
Isoeugenol,
respectively.
were
ferulic acid,
8.0
and
30°C
vanillin, vanillic
acid and protocatechuic acid were all degraded by the cell
free
extracts.
solution
indicating
it
was
When
found
CFE
to
was
added
strongly
the meta-cleavage of
PCA to
to
protocatechuate
absorb
form
hydroxy-cis,cis-muconic acid semialdehyde.
at
"I
410
-carboxy-
nm
~-
171
increased
to
decrease.
the
the
1% (w/v),
might
(w/v),
Very
increased
when
2%
low
rate
the
concentrations
of
transformation
concentration
the
transformation
of
be due to the reason that
of glucose,
which was
an
isoeugenol.
was
transformation
nitrogen sources tested,
affected.
isoeugenol.
of
was
maximum
isoeugenol
of
used.
conversion
respectively.
The
But
increased
to
This
Of
was
The
optimum
of
isoeugenol
bacteria
was
the
though ammonium chloride
favoured more growth of the organism,
transformation
slightly
easily assimilable carbon source,
different
nitrate
of
to
the products of metabolism
the
urea
glucose
adversely
affected
and
found
of
glucose
transformation was
was
more
pH
when
and
potassium
temperature
7.0
were
very
the rate of
for
and
sensitive
to pH
and
temperature since a change in these parameters affected the
transformation considerably.
The present
it
took
30
hours
transformation
was
very
increased
of
by
concentration of
a
attain
growth.
maXl.mum
isoeugenol
After
low.
and
to
strain was not
36
during
12
hours
hours
the
ferulic
the
fast
the
rate
The
initial
of
reaction was
acid and
growing one as
rate
10
of
hours
transformation
complete.
The
vanillin were maximum at
172
25
and
6.3%
24
hours
vanillin
decreased
respectively,
yielded.
they
as
when
After
were
that
further
peA was
protocatechuic acids.
8.2%
ferulic
the
oxidised
acid
and
concentration
to
vanillic
and
subsequently metabolised
by
the cleavage of the aromatic ring.
This
strain
assimilated
isoeugenol
acetate
also.
Isoeugenol acetate was first hydrolysed to isoeugenol which
was
subsequently
vanillic
acid
metabolised
and
to
ferulic
protocatechuic
acid.
acid,
But
vanillin,
the
bacteria
was not able to assimilate eugenol or eugenol acetate.
The
ferulic
acid,
in
acid,
which
the
was
free
extracts
of
vanillin,
vanillic
acid
proved
that
degradation
determine
peA
cell
the
metabolised
The presence
of
the
peA in the organism,
further
confirmed
protocatechuate
extract.
peA
by
cul ture
and
ring
by
of
intermediates
isoeugenol.
The
test
cleavage mechanism showed
the
enzyme
degraded
protocatechuic
these compounds were
pathway
aromatic
the
meta-cleavage
catalysing
the
of
the
to
that
ring.
meta-cleavage
of
protocatechuic acid 4,5-oxygenase, was
the
solution
then,
strong
was
absorption
added
undergoes
to
at
410 nm when
the
meta-fission
cell
free
to
form
173
acid
Y-carboxy-OC-hydroxy-cis,cis-muconic
(CHMS)
which
absorbs
at
410 nm.
CHMS
semi aldehyde
turns
yellow
spontaneously under alkaline conditions in the colorimetric
test
for
the
determination
of
aromatic
ring
cleavage
following
pathway
isoeugenol
by
170
.
mec h anlsm.
From the above results,
is
proposed
for
the
the
degradation
of
the
Pseudomonas sp. CUAC 30.
CH=CHCH 3
CH=CHCOOH
CHO
:>
>OCH 3
H3
OH
OH
OH
OOH
<
~
COOH
(-)
(
H
H
H
OCH
H
3
174
Table 5.1: Identification tests for the strain CUAC 30
Tests
Form and arrangement
Results
Small rods; no characteristic arrangement
Motility
Positive
Chromogenesis in nutrient agar
Pale green colour
Gram reaction
Negative
Spore formation
Negative
Production of indole
Negative
H S production
2
Negative
Oxidation-fermentation test
Oxidative
Gas production from glucose
Positive
Citrate utilisation
Negative
Nitrate reduction
Negative
Cytochrome oxidase
Positive
Catalase
Positive
Gelatinase
Negative
Amylase
positive
Caseinase
Negative
176
Table 5.2.3:
Effect of glucose on transformation
of isoeugenol
Glucose
( % , w/v)
Residual Ioseugenol
o hr
( %,
v/v)
6
12
18
24
36
0.5
100
97.78
88.65
43.64
33.18
30.19
1.0
100
96.20
84.05
27.43
18.92
18.07
1.5
100
97.00
86.64
32.57
22.76
20.13
2.0
100
98.16
88.72
40.05
29.83
27.61
Table 5.2.4:
Effect of different nitrogen sources on
transformation of isoeugenol
Nitrogen
source
Residual Isoeugenol ( %, v/v)
o hr
6
12
18
24
36
NH N0
4
3
100
96.28
84.00
28.21
19.25
18.12
NH Cl
4
100
96.19
85.17
30.41
22.66
20.50
(NH 4 )2 S 04
100
96.42
84.19
27.12
18.04
16.83
(NH 2 )2 CO
100
97.08
86.37
32.21
25.17
23.45
KN0
100
96.92
87.15
32.14
26.32
24.26
100
97.00
86.76
32.43
25.84
24.07
3
NaN0
3
177
Table 5.2.5:
pH
Effect of pH on transformation of isoeugeno1
hr
0
Residual Isoeugeno1 ( %, v/v)
6
12
18
24
36
6.0
100
97.73
88.50
48.83
42.76
41.22
6.5
100
97.18
85.91
35.42
30.99
29.78
7.0
100
96.40
83.77
28.06
18.92
18.06
7.5
100
96.28
84.18
27.95
22.74
21. 85
8.0
100
96.53
84.37
30.16
26.13
25.44
Table 5.2.6:
Effect of temperature on transformation
of isoeugenol
Residual Isoeugenol ( %, v/v)
Temperature
(
0
C)
o
hr
6
12
18
24
36
15
100
99.00
95.23
84.52
72.57
68.39
30
100
95.98
83.73
28.06
19.14
18.18
37
100
96.52
84.17
28.81
21.96
21.65
45
100
96.64
84.87
31.25
25.41
24.73
56
100
97.43
85.53
36.17
30.94
30.12
0
0
Isoeugenol
( %I v/v)
Optical
density
o
Optical
density
0.022
0.071
0.01
0.186
0.515
9
0.827
12
Growth curve
0.901
15
0.921
18
0.920
21
0.206
0.03
0.329
0.05
0.457
0.07
0.441
0.10
0.394
0.12
0.308
0.15
0.235
0.17
Effect of concentration of isoeugenol on growth
0.038
246
Table 5.3.1:
o
Time (hr)
Table 5.2.7:
0.102
0.20
0.914
24
CD
--J
f-'
4
0.445
Optical
density
NH N0
Nitrogen
source
3
0.452
0.5
0.450
1.0
0.428
2
0.392
3
0.310
5
0.206
7
0.468
NH Cl
4
0.447
(NH 4 )2 S 04
0.462
(NH 2 )2 CO
3
0.438
KN0
Effect of different nitrogen sources on growth
0.444
Optical density
Table 5.3.3:
o
Sodium chloride
(%, w/v)
0.434
3
0.901
10
NaN0
Table 5.3.2: Effect of concentration of sodium chloride on growth
-.J
ID
I-'
0.157
Optical
density
Optical density
Temperature
5.5
pH
0.289
15
0.440
7.0
0.426
7.5
0.400
8.0
37
0.421
0.444
0.354
45
Effect of temperature on growth
0.408
6.5
Effect of pH on growth
30
Table 5.3.5:
0.316
6.0
Table 5.3.4:
0.260
56
0.326
8.5
0.128
70
0.228
9.0
t-'
o
Q:l
1.48
5.15
2.90
6.82
32.76
34.91
66.39
68.42
89.51
91.10
100
100
1.0
2.0
1.13
2.08
31.63
65.57
88.45
100
0.95
2.11
31.82
0.5
48
36
24
66.00
18
12
88.17
hr
100
o
Residual Isoeugenol (%, v/v)
Effect of sodium chloride on transformation of isoeugenol
0
Sodium chloride
(%, w/v)
Table 5.3.6:
f-'
OJ
f-'
182
Table 5.3.7:
Effect of concentration of glucose on
transformation of isoeugenol
Glucose
( %, w/v)
Residual Isoeugenol ( %, v/v)
0 hr
12
18
24
0.2
100
87.46
63.21
0.5
100
88.22
1.0
100
99.26
Table 5.3.8:
36
48
29.87
2.16
0.89
66.17
32.42
2.83
1.15
68.91
35.44
6.75
5.38
Effect of different nitrogen sources on
transformation of isoeugenol
Residual Isoeugenol (%, v/v)
12
18
24
36
Nitrogen
Source
o
NH N0
4 3
100
88.11
65.86
32.02
1.95
0.98
NH Cl
4
100
88.47
66.23
32.71
2.15
1.12
(NH 4 )2 S 04
100
89.24
66.81
33.25
2.83
1. 79
(NH 2 )2 CO
100
87.15
65.14
30.99
1. 74
0.58
KN0
100
87.04
63.42
29.76
0.82
0
100
87.55
64.67
30.90
1.50
0.71
3
NaN0
3
hr
48
183
5.3.9:
Effect of pH on transformation of isoeugeno1
Residual Isoeugeno1 ( % , v/v)
pH
0 hr
12
18
24
36
48
6.5
100
90.97
70.18
40.83
14.50
11. 83
7.0
100
88.32
65.78
32.17
2.06
1.16
7.5
100
88.81
67.00
35.38
5.42
4.09
8.0
100
90.14
70.13
40.00
14.52
11.25
Table 5.3.10:
Effect of temperature on transformation
of isoeugeno1
Residual Isoeugeno1 ( % , v/v)
Temperature
(
0
C)
0 hr
12
18
24
36
48
15
100
96.67
88.39
79.22
61.73
49.68
30
100
88.30
65.84
32.13
1.98
0.83
37
100
88.82
67.19
34.85
5.07
3.74
45
100
90.93
76.45
50.00
33.18
20.00
56
100
94.98
84.32
73.77
55.15
44.62
o
Optical
density
0.092
10
0.297
18
0.364
21
Growth curve
0.425
24
3.2
1.1
0.7
o
o
o
Ferulic acid
Vanillin
Vanillic acid
3.8
3.9
5.8
18
8.3
6.3
7.9
24
Concentration (mg/lOO ml)
7.8
5.4
5.5
30
0.443
30
Time course of formation of products
0.189
15
12
o
Table 5.3.12:
0.037
6
hr
Product
o
Time (hr)
Table 5.3.11:
0.443
36
5.1
1.6
3.3
36
0.431
48
t-'
l>
00
185
1.0
--'-~
--l
.. -.---r--.--'--..,..------r-- _._.,...- - -T·--··--T-----.,..-·---·.......
.-'
---._---
-
....
/ / ----
><
.w
t
• .-1
U)
c:
(l.J
Cl
r-l
0.5
cO
tJ
• .-1
.w
0,
0
/
I
__ / /
/
//
k.::~:: . --... --+-.-..-.-+ ...----__+_ .---+-_.--.-+ --.-- --+.-._.--+-- -.--+------.
o
5
10
15
20
25
Time (hr)
Fig.5.2.1: Growth curve of CUAC 10 in isoeugenol medium
Fig.5.2.2:
10
7.5
Chemical Shi ft
5
NMR spectrum of isoeugenol
OH
3
H3
CH=CHCH
([) )
2.5
o
t-'
(j\
CP
Fig.5.2.3:
10
3
7.5
5
20
Chemical Shi ft
=CHCH 3
NMR spectrum of product IE
CH
(S)
2.5
o
--...)
CD
I-'
I
.Al.'
""r'"
,.
'", r··... ·
Ill:..
. . riP
1'1
I" .~. . .
I
I
100
ra.. •.It.
I
I
bH
0'
I
I
I
I
I
I
!
H3
..LI. IlL
* ".,,'
H3
, /'.ll.lI.
"".l......r
"0
0
I
,
CH=CHCH
3
I
r6i1...L
.,...."
.•. ,'....
50
Chemical Shift (ppm)
* Signals due to the solvent, CDC1
3
13
Fig.5.2.4:
C NMR spectrum of product IE
20
150
I
>Ill
[~.-
I
I
I
I
I
I
------------~-----------CH3
'r'I'~'"
lUll..
I""'f'"'
.,'.10
J.
,,,.
r'
.• ,
0
',1.1. r""
I--'
())
())
3
Fig.5.2.5:
10
CH
7.5
OH
3
5
Chemical Shi f t
H3
CH=CHCH
NMR spectrum of product 1E30
H
CH CH=CH
3
(£> )
2.5
o
f--'
CD
1.0
190
0.50 )... _ .... -.-,- .... --.,. ... -........---..-....-r- ....---
.. -...... -..... _....,. ...... - ....,.- ...... .,. ..
..,.,--~
-~.
--
-
...
-'---l
----
I
>-
-W
.~
(/)
c:
(lJ
Cl
0.25
...-l
10
U
.~
-W
0..
o
\ .
l~-~<'---.
o
8
+-
-+--+--+-- +-+
16
24
Time (hr)
Fig.5.3.l:
Growth curve of CUAC 30
32
+---
40
CHAPTER
6
SUMMARY AND CONCLUSIONS
SUMMARY AND CONCLUSIONS
Microbial
have
been
one of
transformations
the
most
of
organic
compounds
significant developments
in
field of synthetic organic chemistry in recent years.
the
They
display far greater specificities than conventional organic
reactions
and
have
great
commodity
chemicals
is
developing
fields
where
applied.
In
eugenol
and
convert ing
Eugenol
currently
into
one
more
of
both
the
with
product s
a
of
fastest
are
transformations
undertaken
use ful
production
transformations
microbial
are
isoeugenol,
and
The
microbial
work,
isoeugenol
them
compounds,
like
this
potential.
view
of
to
1 ike
vani 11 in.
terpene-related
aromatic
are the main constituents of many essential oils
oil,
clove
Vanillin,
an
cinnamon
important
oil
flavouring
and
oil.
ylang-ylang
agent,
is
manufactured
from eugenol or isoeugenol using chemical methods.
the
Since
laboratory were
isoeugenol,
isolated
were
found
organisms
stock
cultures
to
inactive
be
that
available
on
both
in
our
eugenol
and
transform these substrates were
from
the
aerial
and
soil
flora.
pur if ied
and
i dent i f i ed
upto
the
192
These organisms
gener i c
1 evel
based
193
on
their morphological
three
organisms
and biochemical
isolated,
one
belonged to the Bacillus sp.
and the other two to the Pseudomonas sp.
nutrient
effect
agar
of
and mineral
various
concentration,
different
nitrogen
influence
of
the
growth
substrates
an
to
obtained
growth
obtai n
between
the
the
and
like
the
growth
like
to
obtain
organism
growt h
presence
and
glucose
transformation
studied
curve
rate
of
a
the
of
the
was
and
The
substrate
temperature
source
the
of
respectively.
factors
carbon
and
They were kept on
concentration,
pH
extensively
The
per iodi call y
was
sources,
organism
were
slants,
chloride
additional
of
conditions.
agar
physico-chemical
sodium
Of the
characters.
of
the
on
the
optimal
monitored
correl at ion
organism
and
conditions
the
the transformation rate of the substrate.
After
substrate
progress
samples
of
from
analysis
was
was
made
extracted
by
incorporating
fermented
in
fermentation
the
broth
the
a
with
concentrating
by
under
jar
ether.
reduced
fermentor.
monitored
periodically and
adding
solvent
mini
was
chromatography.
acidic
optimal
After
withdrawing
subjecting them to
fermentation
dilute
The
by
sulphuric
ether
pressure,
The
was
the
broth
acid
extract,
and
after
subjected
to
194
column
chromatography
to
separate
the
products.
The
products were purified by recrystallisation from appropriate
solvents.
their
and
The pure products were identified by analysis of
spectral
also
by
data
such
comparison
as
UV,
of
their
melting points and Rf values
Suitable
derivatives
like
1R,
NMR and
physical
(TLC)
mass
spectra
constants
like
with authentic samples.
acetates
or
methyl
esters
of
these products were also prepared and characterised.
The Bacillus sp. CUAC 10 was not able to grow in a
mineral medium containing eugenol or isoeugenol as the sole
carbon and energy source.
when
an
easi ly
another
ass imi lable
nutrient
converted
However,
was
eugenol
also
to
carbon
added
the organisms did grow
source
to
1 ike
it.
bisdehydroeugenol
glucose
This
(BOHE)
or
strain
in
45.4%
yield.
The
yield of BOHE increased to 47.5% when eugenol
aceta t e
was
used
formed
isoeugenol
as
to
bi sdehydroisoeugenol
respectively.
yields
of
respectively
substrate.
As
these
when
the
subst ra te.
bact er ia
the
products
wi th yields of 46.5%
case
of
and
eugenol
acetate,
to
and
increased
isoeugenol
t rans-
(OH01)
dehydrodiisoeugenol
(BOH1)
in
The
acetate
was
49%
used
and
28.7 %
the
30.4%
as
the
Fifteen hours old culture cells were immobilised
195
in
cal c i urn
alg i na t e
beads
and
t rans forma t ions
of
eugenol
and isoeugenol were performed using these immobilised whole
cells.
Optimum pH
maximum
conversion
immobilised
with
the
of
eugenol
and
Though
the
extent
immobilised
cells
was
less
the
retention
immobilised cells could
one week without
of
than
time
be used
found
out
isoeugenol
cells.
cells,
intact
and temperature were
was
for
with
the
the
transformation
that
less
with
the
and
the
continuously for more than
considerable depreciation
in
the activity
of the cells.
The Pseudomonas sp. CUAC 20 was able to grow in a
mineral
medium
energy source.
The products of
column
utilising
as
the
sole
carbon
and
But it was not able to metabolise isoeugenol
the metabolism of eugenol were isolated by
chromatography
vanillin,
eugenol
vanillic
and
acid
identified
and
as
protocatechuic
ferulic
acid,
acid.
The
protocatechuic acid was subsequently metabolised via orthocleavage of
acid
the aromatic ring
3,4-oxygenase
to
~
by
the enzyme protocatechuic
-carboxy-cis,
cis-muconic
acid
semialdehyde which was then further degraded to p-ketoadipic
acid.
was
The
noted
maximum
at
17
yield of vanillin was only 4.62% which
hours
after
inoculation.
The
maximum
196
yields
of
ferulic
23
were
oxidation.
fermentation
Several
of eugenol
addi t i onal
carbon
and
alginate
beads
immobilised
for
18
source,
cells.
max~mum
the
experiments
that
including
were
t r i ed
for
t he
improved
But the results were not promising.
hours
and
After
acetate and addition of glucose as
production of vanillin.
cells,
respectively.
6.25%
the concentration of all these products decreased due
Culture
hours
acid
time
an
and
vanillic
noted
further
19
and
8.9%,
to
at
acid
old,
were
immobilised
eugenol
was
transformed
The
pH
and
conversion
temperature
of
were
eugenol.
The
in
calcium
with
the
optimised
cell
free
extracts of the culture were prepared and the transformation
of eugenol with the CFE was also studied.
From these data
the degradation pathway of eugenol with the present
was
proposed.
aeruginosa
Eugenol was
reported
then
strain
fermented wi th Pseudomonas
earlier which gave
only 1.47% vanillin
and the results were compared with that of present strain.
The Pseudomonas sp. CUAC 30 was able to assimilate
isoeugenol
mineral
as
medium.
fermentor,
optimisation
the
sole
carbon
Isoeugenol
incorporating
studies.
was
the
The
and
energy
fermented
results
product s
in
source
the mini
obtained
of
in
from
ferment at i on
a
jar
the
were
197
isolated by column chromatography and identified as ferulic
acid,
vanillin,
Prot oca techui c
cleavage of the
vanillic
acid was
was
The
at
yields
acid
(9.0%)
The
concentration
decreased
The
observed
culture.
of
to
of
in
by
acid.
metabol i sed v i a
meta-
the enzyme protocatechuic
calcium
maximum
hours
25
all
yield
after
ferulic
further
Twentyfour
immobilised
24
were maximum at
due
eugenol.
ring
protocatechuic
t -carboxy-oC-hydroxy-cis,cis-muconic
semialdehyde.
(6.3%)
and
subsequent 1 y
aromatic
acid-4,5-oxygenase to
acid
acid
(8.2%)
28
these
old
alginate
and
of
the
vanillic
hours
respectively.
products
subsequently
oxidation,
hours
vanillin
inoculation
acid
and
of
as
in
the
culture
beads
and
case
cells
of
were
transformation
studies were carried out on isoeugenol with the immobilised
cells.
The
cell
free
extract
of
the
strain was
and isoeugenol was fermented with the eFE.
assimilated
isoeugenol
acetate,
prepared
The strain also
and the products and their
yields were almost the same as that wi th isoeugenol.
From
these
with
results,
the
degradation
pathway
of
isoeugenol
the present strain was proposed.
Thus
the
present
work
led
to
the
isolation
and
identification of three new strains of bacteria which could
transform
eugenol,
isoeugenol
and
their
acetates.
198
The
products
isolated,
transformation
purified
parameters
these
of
were
and
The
degradation
proposed
of
for
eugenol
the
these
identified.
optimised
substrates.
of
for
the
pathway
was
The
proposed
of
were
physico-chemical
maximum
confirmed
degradation
compounds
conversion
earlier
and
a
isoeugenol.
of
for
the
pathway
was
Though
the
yields of the products formed by the oxidative coupling of
the
substrates
formed
by
the
were
substantial,
oxidative
that
degradation
of
of
especially vanillin were not satisfactory.
the
the
products
substrates
REFERENCES
REFERENCES
1. Pasteur, L., C.R.Hebd. Seances Acad.Sci., 55, 28 (1862).
2. Brown, A.J., J.Chem.Soc., 49, 172 (1886).
3. Boutroux, L., C.R.Hebd.Seances Acad.Sci., 91, 236 (1880).
4. Bertrand, G., C.R.Hebd.Seances Acad.Sci., 122, 900 (1896).
5. Dumas, J.B., Ann.Chim.Phys., 5, 3 (1874).
6. Spencer,
ations
J.F.T.
of
Gorin,
&
Sugars
and
P.A.G.,
Related
"Microbial
Compounds",
Transform-
in
Progress
in Industrial Microbiology, Vol.7, Heywood and Co., London,
177 (1965).
7. Kallio,
in
R.E.,
Fermentation
Transformation
"Microbial
Advances,
Perlman,
D.
of
Alkanes",
(Ed.),
Academic
Press, New York, 635 (1969).
8. Sebek,
O.K.
& Kieslich,
of Organic Compounds",
Processes,
Perlman,
D.
K.,
"Microbial
Transformation
in Annual Reports on Fermentation
(Ed.),
New York, 649 (1969).
200
Vol.l,
Academic
Press,
201
9. Evans,
W.C.,
Compounds"
"Microbial
Transformation
in Fermentation Advances,
of
Aromatic
Perlman,
D.
(Ed.),
Academic Press, New York, 649 (1969).
10. Stanley, D., Dev.Ind.Microbiol., 25, 53 (1984).
11. Charney,
W.
&
Herzog,
H.L.
(Eds.),
Microbial Transform-
ations of Steroids, Academic Press, New York (1967).
12. Capek, A.,
Hanc, O.
&
Tedra, M.
(Eds.), Microbial Trans-
formations of Steroids, Academic Press, Prague (1966).
13. Ilzuka,
H.
& Naito, A., Microbial Conversion of Steroids
and Alkaloids, University of Tokyo Press (1981).
14. Mahato,
S.B.
&
Mukherjee,
A.,
Phyto.Chem.,
23,
2131
(1984) •
15. Stodola, F.H., Chemical Transformations by Microorganisms,
John wiley & Sons, New York (1958).
16. Kieslich,
K.,
Microbial
Transformation
of
Non-steroid
Cyclic Compounds, John Wiley & Sons (1976).
17. Kieslich, K., Proc.of FEMS Sympos., 13, 311 (1982).
202
18. Jones,
J.B.,
Sih,
Biochemical
C.J.
Systems
Perlman,
&
in
Organic
D.,
Applications
Chemistry,
Wiley,
of
New
York (1976).
19. Klibanov,
20. Sih,
A.M., Science, 219, 722 (1983).
C.J.
&
Chen,
C.S.,
Angew.Chem.lnt.Ed.,
23,
570
(1983) •
21.
Leuenberger,
H.G.W.,
in
Biotransformations,
(Ed.), Vo1.6a, Verlag Chemie, Weinheim,
22. Yamada,
H.
&
Shimizu,
S.,
5,
Kieslich,
K.
(1984).
Angew.Chem.lnt.Ed.,
];2,
622
(1988).
23. Csuk, R.
24. Ciegler,
in
Glanzer, B.I., Chem.Rev., 91, 49
&
A.,
"Microbial
Fermentation
Press, New York,
25. Kieslich,
K.,
Transformations
Advances,
Perlman,
(1991).
of
D.(Ed.),
Terpenes" ,
Academic
689 (1969).
Abraham,
W.R.,
Stumpf,
B.
&
Washausen, P.,
Top.Flavour Res.Proc.lnt.Conf., 405 (1985).
26. Mayer,
P.
&
Neuberg, C., Biochem.Z., 71, 174 (1915).
203
27. Molinari, E., Biochem.Z.,
28. Joglekar,
493
S.S.
&
216, 187 (1928).
Dharlikar,
R.S.,
Current
Sci.,
37,
Appl.Microbiol.,
18,
(1968).
29. Joglekar,
S.S.
&
Dharlikar,
R.S.,
1084 (1969).
30. Mitzutani,
S.,
Hayashi,
T.,
Ueda,
H.
Tatsumi,
&
C.,
J.Agric.Chem.Soc., 45, 368 (1971).
31. Bradshaw, W.H., Conrad, H.E., Corey, E.J., Gunsalus,I.C. &
Lednicer, D., J.Am.Chem.Soc., 81,
32. Wright,
S.J.,
Caunt,
P.,
Appl.Microbiol.Biotechnol.,
5507
Carter,
23,
D.
(1959).
&
Baker,
P.B.,
224 (1986).
33. Draczynska, B., Cagara, C., Siewinski, A., Rymkieziez, A.,
Zabza,
A.
&
Leufuen,
A.,
J.Basic
Microbiol.,
25,
487
(1985) •
34.
Hasegawa Co., Japan Patent,
35. Shukla,
O.P.,
Bartholomus,
Microbiol., 33, 489 (1987).
7, 238, 998 (1972).
R.C.
&
Gunsalus,
I.C., Can.J.
204
36. Hunt,
& Borden,
D.W.A.
J.H.,
J.Chem.Ecol.,
16,
1385
(1990) •
37. Liu,
W.G.,
Goswami,
Sariaslani,
F.S.,
J .0rg.Chem.,
52,
38. Krasnobajew,
v.
A.,
Steffek,
Steffens,
J.J.
R.P.,
Chapman,
Rosazza,
&
R.L.,
J.P.N.,
5700 (1988).
&
Helmlinger,
D.,
Helv.Chim.Acta.,
65,
1590 (1982).
39. Sangodkar,
U.M.X.
&
Mavinkurve,
S.,
J.Biosci.,
4,
79
Indian
J.
(1982) •
40. Naik,
Chem.,
U.,
Paknikar,
21B,
501
S.K.
&
Mavinkurve,
(1982).
41. Cross, B.E. & Nortan, K., Chem.Commun.,
42. Dmitrovskii,
A.A.
&
Starikova,
43. Evans, W.C., J.Gen.Microbiol.,
535
N.A.,
SSSR., 163, 495 (1965): Chem.Abst.,
44.
S.,
63,
(1965).
Dokl.Akad.Nauk.
12276 (1965).
32, 177 (1963).
Dagley, S., Adv.Microb.Physiol.,
6, 01
(1971).
205
45. weide, H., Z.Allg.Mikrobiol.,
46. Buswell,
6, 01
E.,
CRC
Crit.Rev.Biotechnol.,
(1987).
47. WaIter,
263
& Odier,
J.A.
23, 37 (1983).
R.
Knackmuss,
&
H.J.,
Annu.Rev.Microbiol.,
42,
(1988).
48. Gale, G.R., J.Bacteriol., 64, 131
49. Cain,
& Cartwright,
R.B.
N.J.,
(1952).
Biochim.Biophys.Acta,
37, 197 (1960).
50. Dagley, S.
&
51. Larway, P.K.
Stopher, D.A., Biochem.J., 73, 16 (1959).
Evans, W.C. Biochem.J., 95, 52 (1965).
&
52. Golovacheva,
R.S.
&
Oreshkin,
A. E. ,
Mikrobiologiya,
44, 470 (1975).
53.
Itoh,
M.
&
Fujikawa,
N.,
Kaishi,
~,
421
Biotechnol.Lett.,
11,
105
Hakko
Kogaku
(1979) •
54. Smith,
(1989).
M.R.
&
Ratledge,
C.,
206
55. Gajendiran,
& Mahadevan,
N.
A.,
Indian
J.Exp1.Bio1.,
28, 1136 (1990).
56. Sembiring,
T.
Winter,
&
J.,
Appl.Microbiol.Biotechnol.,
31, 89 (1989).
57. Makoto Shoda, Agric.Bio1.Chem., 44, 1841
58. Hoffmann,
K.H.
&
Ute
Vogt,
J.Basic
(1980).
Microbio1.,
27,
441
(1987) •
59. Yi Tin Wang, App1.Environ.Microbio1.,
60. Chapman,
P.J.
&
Hopper,
D.J.,
54, 1277 (1988).
Biochem.J.,
110,
491
(1968) .
61. Ribbons,
D.W., J .Gen.Microb., 44,
62. Cate1ani,
24,
D.,
Fiecchi,
A.
&
221
(1966).
Ga11i,
E.,
Experientia,
113 (1968).
63. Chamberlain, E.M., Biochem.J., 110, 755(1968).
64.
Hopper,
D.J.
&
Chapman, P.J., Biochem.J., 122, 19 (1971).
207
65.
Raymond,
S.H.,
66. Engelhardt,
Biochem.J.,
G.,
Rast,
Microbiol.Lett.,
67. Mc
Donald,
Chem.,
76,
69.
9
Wallnoefer,
&
P.R.,
FEMS
(1979).
R.L.
Ingraham,
&
J.L.,
J.Biol.
(1954).
Dagley,
D.J.,
798
70. Yamada,
S.
Stopher,
&
D.A.,
Biochem.J.,
Y.,
Ivoilov,
72. Katsunori
27,1451
Chapman,
P.J.
&
Dagley,
S.,
Biochem.J.,
(1960).
Biol.Chem.,
71.
377
Stanier,
809
S.,
H.G.
(1970).
(1960).
Hopper,
110,
D.L.,
210,
68. Tripett,
5,
119, 871
Okada,
37,
V.S.,
725
T.,
Ueshima,
&
Konda,
K.,
Agric.
(1973).
Dokl.Akad.Nauk.,
Suzuki
O.
& Masao
249,
Itoh,
474 (1979).
Plant
(1986).
73. Terni, G., J.Ferm.Technol.,
31,65 (1953).
Cell
Physiol.,
208
74. Yano,
K.
&
Arima,
K.,
J.Gen.Appl.Microbiol.,
4,
241
(1958) •
75. Sugiyama,
S.,
Yano,
K.,
Tanaka,
H.,
Arima, K., J.Gen.Appl.Microbiol., 4,
76. Grund,
E.,
Knorr,
C.
223
Eichenlaub,
&
Komagata,
K.
&
(1958).
R.,
Appl.Environ.
Microbiol., 56, 1459 (1990).
77. Engel berts,
K. ,
Microbio1.Lett.,
78. Cain,
R.B.,
Schmidt,
E.
Reineke,
&
W. ,
FEMS
59, 35 (1989).
Antonie Van Leewenhoek J.Microbio1.
Sero1.,
34, 417 (1968).
79. Zeitler,
H.J.,
Hoppe-Seyler's
M.L.,
Pal1eroni,
Z.Physiol.Chem.,
340,
73
(1965).
80. Whee1is,
Microbiol.,
81.
59,
N.J.
&
Stanier,
R.Y.,
302 (1967).
Power, D.M., Can.J.Biochem., 43, 1397 (1965).
82. Walker, N.
&
Evan, W.C.,
Biochem.J.,
52,
23
(1952).
Arch.
209
83. Slater, G.P.,
& Hogge, L.R., Can.J.Microbio1.,
Child, J.J.
19,1169 (1973).
84. Ribbons,
85.
D.W.
&
T.
&
Hayashi,
&
Hopper,
Nakazowa,
Evans,
W.C.,
Biochem.J.,
73,
21
(1959).
Appl.Env.Microbio1.,
38,
379
3,
145
JP 6034,
195
(1978).
86. ElmorsL
E.A.
D.J.,
J.Gen.Microbiol.,
(1979) •
87. Showa
Denko,
(8534,195)
88. Vanden
K.K.,
(C1.C12 P13/22)
Tweel,
De Bont,
Jpn.Kokkai Tokkyo Koho,
J.A.M.,
W.J.J.,
(1985).
Smits,
Appl.Microbio1
J.P.,
Ogg,
Biotechno1.,
R.L.H.
29,
&
224
(1988) •
89. Levy, C.C.
&
90.
Subha Rao,
Moore, K.,
Frost, P., J.Bio1.Chem.,
106,507 (1968).
P.V.
241,997
(1966).
& Towers, G.H.N., Biochem.J.,
210
9l.
Bocks,
92.
Dagley,
S.M.,
S.,
Microbiol.,
93. Ronald
302
Phytochemistry,
Fewster,
8,01
95.
M.E.
127
(1967).
Happold,
&
F.C.,
J.Gen.
(1952).
& Michael A.Skinner, J.Bacteriol., 143,
A.Cooper
(1980).
94. Arunakumari,
22,
6,
32
Dugan,
A.
&
Mahadevan,
A.,
Indian
J.Exp.Biol.,
(1984).
1.N.
&
Golorlev,
E.L.,
Mikrobiologiya,
54,
128
(1985).
96. Hopper,
D.J.,
Jones,
H.G.,
M.E., J.Gen.Microbiol.,
97. Chen,
Y.P.,
Microbiol.,
(1959).
M.J.
&
E.A.
&
Roberts,
(1985).
Glenn,
A.R.,
Arch.
151, 520 (1989).
Briggs, G.G.,
J .Agr.Food Chem.,
Rogoff,
131, 1807
Dilworth,
98. Bollag, J.M.,
99.
Elmorsi,
M.H.
&
16, 829
Wender,
Dawson, J.E.
& Alexander, M.,
(1968).
1.,
J.Bacteriol.,
77,
783
211
100. Hagedorn,
137
S.R.,
Abstr.Annu.Meet.Am.Soc.Microbiol.,
67,
(1980).
101. Crawford,
Olson,
P.E.,
102. Spokes,
Bromley,
R. L • ,
J . W. ,
Perkins,
Appl.Environ.Microbiol.,
J.R.
&
Walker,
N.,
38,
379
P.E.
&
(1979).
Arch.Microbiol.,
96,
125
(1974) •
103. Karns,
Sci.,
J.S.,
28, 03
104. Haggblom,
J.E.,
Bioeng.,
10 7. G bo z d yak,
Reznik,
(1982).
Janke,
Wang,
Mulbry,
W.W.,
Basic
Life
D.
Middledorp,
&
P.J.M.,
Arch.
H.Y.
&
Hickey,
R.F.,
Biotechnol.
(1990).
& Evans, W.C., Biochem.J., 99, 31 (1966).
P • I .,
L.V.
&
152, 06 (1989).
35, 1125
106. Tewfik, M.S.
J.O.
(1984).
M.M.,
Microbiol.,
105. Lin,
Nelson,
&
L i v k e,
V. A.,
Dmitrenko,
Ud 0 d,
G.N.,
V. M.,
By k 0 v a , S • P. ,
Mikrobiol.Zh.,
44,
12
/,12
108. Iyayi, C.B., Microbiol.,
109. Sloane,
N.H.,
Crane,
C.
45,
&
153
(1986).
Mayer,
R.L.,
J.Biol.Chem.,
193,453 (1951).
110. Lechner,
U.
& Straube, G., J.8asic Microbiol.,
28,
629
(1988) •
Ill. Faleev,
N.G.,
Sadovnikova,
Saporovskaya,
974
M.B.
& Belikov,
(Cl. C12Nll/04)
112. Sih,
C.J.,
Foss,
J.Am.Chem.Soc.,
113. Jones,
J.D.,
M.S.,
V.,
Vinogradova,
U.S.S.R.
SU,
N.K.,
1,
351,
Lemberger,
M.,
(1987).
P.,
91,
Smith,
Rosazza,
J.
&
6204 (1969).
B.S.W.
& Evans,
W.C.,
Biochem.J.,
51,11 (1952).
114. Singh, D.V., Mukherjee,
Hakko Hogaku Zasshi,
115. Robert,
(1979).
H.
White,
P.P.,
51, 713
Pal, S.P.
& 8hattacharya,P.K.
(1973).
8iochim.8iophys.Acta,
583,
55
213
116. Lippo1dt,
A.,
Microbiol.,
117. Yamada,
R.
Birnbaum,
&
J.Basic
D. ,
26, 145 (1986).
H.,
Ogata,
Bode,
Uwajima,
T.,
Kumagai,
H.,
Watanabe,
27,
Biochem.Biophys.Res.Commun.,
K. ,
M.
&
350
(1967).
118. Raju,
S.G.
66, 511
&
Vaidyanathan,
C.S.,
J.lndian
Inst.Sci.,
(1986).
119. Robbins,
W.J.
&
Lathrop,
E.C.,
Soil
Sci.,
7,
475
(1919).
120. Vasudevan,
N.
&
Mahadevan,
A.,
J.Biotechnol.,
2.,107
(1989).
121. Toms, A.
122.
Iwahara
& Wood,
Shojiro,
J.M.,
Biochem.J.,
Nippon Nogei
9,337
Kagaku
(1970).
Kaishit
55,
1089
(1981) •
123. Nazareth,
S.
494 (1966).
&
Mavinkurve,
S.,
Can.J.Microbiol.,
32,
214
124. Shimazano,
H.,
Nishimura,
H.
Daigaku Nogakubu Kenkyu Kokuku,
125. Ander,
P.,
Eriksson,
Kawachi,
&
K.E.L.
25,
369
Yu
&
S.,
Miyazaki
(1978).
Hui,
s. ,
Arch.
Microbiol., 136,01 (1983).
126. Isaac,
3833
& Stanley
S.Y.Sze
Omori,
Ishigooka,
biol.Biotechnol.,
29,497
128. De Wulf, 0.,
Thonart,
Paris,
Paquot,
129. De
20,
J.Bacteriol.,
169,
(1987).
127. Toshio
605
Dagley,
A.
&
P.,
M.,
H.
& Minoda,
Y.,
Appl.Micro-
(1988).
Gaingnage,
P.,
Marlier, M.,
Biotechnol.Bioeng.Symp.,
17,
(1986).
Wulf,
165
O.
Thonart,
&
131. Neilson,
Appl.Biochem.Biotechnol.,
(1989).
130. Hisae Asai, Dnozaki,
52,2741
P.,
H.
& Imaseki, H., Agric.Biol.Chem.,
(1988).
A.H.,
Allard, A.S., Hynning, P.A.
Appl.Environ.Microbiol., 54,
2226
(1988).
& Remberger,M.,
215
132. Ohta,
121,
M.,
23
133. Habu,
Higuchi,
T.
Iwahara,
&
S.,
Arch.Microbiol.,
(1979).
Same j i ma ,
N. ,
Gakkaishi,
34,
1026
&
M.
(1988);
Yoshimoto,
T.,
Chem.Abst.,
Mokuzai
110,
170007
(1989) •
134. Cousin,
H.
&
Herissey,
H.,
J.Pharm.Chim.,
28,
H.
&
Herissey,
H.,
Compt.Rend.,
H.
&
Herissey,
H.,
J.Pharm.Chim.,
28,
H.
&
Bull.Soc.Chim.,
3,
49
(1908).
135. Cousin,
146,
1413
(1908).
136. Cousin,
193
(1909) •
137. Cousin,
Herissey,
H.,
1070
(1933).
138. Kohji Tadasa, Agric.Biol.Chem., 41, 925
139. Tadasa,
(1983).
K.
&
Kayahara,
H.,
(1977).
Agric.Biol.Chem.,
47,2630
216
140. Guenther, E., The Essential Oils, Vol.I, 291 (1972).
141. Martha
Windholz
(Ed.),
The
Merck
Index,
IX
Edn.,
Merck & Co. Inc., New Jersey (1976).
142. Buckingham,
Compounds,
J.
v
(Exe.Ed.),
Edn.,
Vol.4,
Dictionary
Chapman
&
of
Hall,
Organic
New
York
(1982) •
143. West, T.F., J.Soc.Chem.Ind., 59, 275 (1940).
144. Irwin
A.Pearl,
Organic
Synthesis,
Collective
Vol.4,
Norman Rabjohn (Ed.), John Wiley & Sons, New York, 974
(1963) •
145. Irwin
A.Pearl,
Horning,
E.C.
Organic
(Ed.),
Synthesis,
John Wiley
&
Collective
Vol.3,
Sons, New York,
745
(1955).
146. Mackie,
G.
&
McCartney,
L.,
Handbook
of
Practical
Bacteriology, McGraw-Hill Book Co., New York (1949).
147. Palleroni,
Manual
N.J.,
"Genus
I
Pseudomonas",
of Systematic Bacteriology,
Vol.I,
in
Bergey's
Krieg,
(Ed.), Wi11iams & Wi1kins, Baltimore, 141 (1984).
N.R.
217
148. Claus,
D.
Bergey's
Sneath,
Berkeley,
&
Manual
of
P.H.A.
R.C.W.,
"Genus
Systematic
(Ed.),
Williams
Bacillus",
Bacteriology,
&
Wilkins,
in
Vol.2,
Baltimore,
1105 (1986).
149. Holding,
Tests",
J.R.
&
A.J.
in
&
Collee,
Methods
Ribbons, D.W.
in
J.G.,
"Routine
Microbiology,
Biochemical
Vol. 6A,
Norris,
(Eds.), Academic Press, London, 1
(1971).
150. Krebs,
Heusser,
K. G. ,
Reagents",
in
Thin
D.
Layer
&
Wimmer,
"Spray
H. ,
Chromatography,
Stahl,
E.
ELBS
and
20,
1059
(Ed.), Springer-verlag, Berlin, 873 (1969).
151. Vogel,
A.I.,
Practical
Organic
Chemistry,
Longman Group Ltd., London, 782 (1973).
152. Ottow,
J.C.G.
&
Zolg,
W.,
Can.J.Microbiol.,
(1974) •
153. Tampion,
Principles
J.
&
and
Tampion,
M.D.,
Applications,
Press, Cambridge (1987).
Immobilised
Cambridge
Cells:
University
218
154. D'Souza,
S.F.
Godbole,
&
S.S.,
Biotechnol.Lett.,
11,
211 (1989).
155. Gunsalus,
I.C.,
organisms",
Colowick,
"Extraction
in
S.P.
Methods
Kaplan,
&
of
Enzymes
in
N.O.
from
Enzymology,
( Eds . ),
MicroVol.I,
Academi c
Press,
New York, 51 (1955).
156. Dagley, S., Geary, P.J. & Wood, J.M., Biochem.J., 109,
559 (1968).
157. Stainer, R.Y.
&
Ingraham, J.L., J.Biol.Chem.,
210, 799
(1954) .
158. Madhavan
Pillai,
Transformations
Clove
Oil",
of
First
P.
&
Ravikumar,
Eugenol,
the
Malaysian
T. ,
Major
"Microbial
Constituent
Int.Conf.Essential
of
Oils,
Kaula Lumpur, December (1988).
159. Taylor,
W. I .
&
Battersby,
A.R.
(Eds.),
Coupling of Phenols, Arnold, London (1967).
160. Erdtman, H., Biochem.Z., 258, 172 (1933).
Oxidative
219
161. Madyastha,
K.M.
&
v.,
Renganathan,
Indian
J.Biochem.
Biophys., 20, 136 (1983).
162. John, C.Pew, J.Am.Chem.Soc., 77, 2831 (1955).
163. Ravikumar,
T.,
Madhavan
Pillai
Fermentation
"Bacterial
of
&
Chandrasekharan,M.,
Eugenol",
Nat.Symp.New
Trends Biotech., Thiruvananthapuram, June (1988).
164. Rothera, A.C.H., J.Physiol.(Lond.), 37, 491 (1908).
165. Ravikumar,
T.
&
Madhavan Pillai,
P.,
Second Malaysian
Int.Conf. Essential Oils and Aroma Chemicals (1990).
166. Stoddart, J.F.
(Ed.),
Comprehensive Organic Chemistry,
Vol.!, Pergamon Press, Oxford (1979).
167. Freudenberg,
K.
&
Richtzenhain,
H.,
Ann.,
552,
126
(1942).
168. Leopold, B., Acta Chem.Scand., 4, 1523 (1950).
169. Erdtman, H., Ann., 503, 283 (1933).
170. Stanier, R.Y.,
Palleroni, N.J.
Microbiol., 43, 159 (1966).
&
Doudoroff, M., J.Gen.