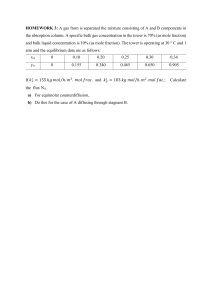
CENG 411 Homework #2 Solutions 3.2. The unimolecular diffusion benzene from the tank is described by (1 − 𝑥! )𝑁! = −𝐷! 𝑐 (#$%) "#! "$ &! %! & è 𝑁! = − '() 𝑙𝑛[1 − 𝑥! (𝑧 = 0)] where 𝑥! (𝑧 = 0) = & is the mole fraction benzene at the surface, and h is the thickness of the stagnant layer. The rate of evaporation is then 𝑚̇! = 𝑁! (𝑀𝑊)! 𝐴 = − %! *(,-)! & '() 𝑙𝑛 51 − (#$%) &! & 6 = 987 𝑙𝑏/𝑑𝑎𝑦. 3.5. a) The equimolar counter-diffusion of Ar (in Xe) is governed by N Ar = −DArXe c dx Ar è dz DArXe P x Ar (L) − x Ar (0) = 0.00159mol / (m2 s) è n& Ar = N Ar A = 5.00 ×10−9 mol / s . Due to RTL equimolar counter-diffusion, n& Xe = −n& Ar = −5.00 ×10−9 mol / s . b) ( N Ar = − v Ar = ) N N Ar N Ar RT 4.95×10−5 m / s 4.95×10−5 m / s = = and v Xe = Xe = − . c) Due to equimolar cAr Py Ar y Ar c Xe y Xe counter-diffusion, the average molar velocity is zero. 3.22. As derived in class, the concentration profile, assuming an infinite medium, is ⎡ ⎢ 2 c(z,t) = c0 ⎢1− π ⎢ ⎣ z 2 Dt ∫ 0 e −ζ 2 ⎤ ⎥ d ζ ⎥ , where c0 is the concentration at the surface (z=0), and the flux ⎥ ⎦ away from the surface is N = −D t ∫ N dt = 2 0 ∂c ∂z = z=0 D c . The total moles per area leaving the surface is πt 0 Dt c = ρ df , where r is the salt density, d is the salt layer thickness, and f is the π 0 π ⎛ ρ df fraction of salt dissolved. è t( f ) = ⎜⎜ D ⎝ 2c0 1/2 ⎞ ⎟⎟ . The surface salt concentration is .265 wt%, and ⎠ assuming a solution density of 1.2 g/cm3, the surface concentration is 0.318 g/cm3. The time to dissolve the salt is as follows: fraction time (s) time (h) 0.1 1953 0.54 0.5 48813 13.56 0.9 158153 43.93 1 195251 54.24 3.25. The flux of water through the air is 𝑁- = 𝑘/,# 𝑐-1 = -. 3 34.6789 ;3<.4<=×<42 4; (,-)$+, & /01 / 3 () 37.=<? ;(687.<@ A) 5∙/01 / -. (4.46@? C)36 ;3<.<7= 4; # / -. <.7×<482 /∙# = = 1.183 2B C4 (#$%) 2',) &* () . The air density is 𝜌 = , so the Reynolds number is 𝑁(D,# = #E7 F G = = 3340 < 5 ∙ 10@ . The flow is thus laminar, and the mass transfer 9 coefficient may be expressed as 𝑘/,# = /; -. 3<.<7= 4 ;H4.6@×<48< I # / 0.332 K <.7×<482 𝑁- = 𝑘/,# 𝑐-1 = -. /∙# (#$%) 2',) &* () 9 : H4.6@×<48< L K /; / I36 ; # # 34.4<9 ;(4.?9 &1K)L = 9 ; = C L = 0.016 J . The water flux through air is then 4.46@? C / # 9 F% : %E ; 0.332 I G J I # 7 J -. /∙#; ?@A :=>< 3 37.=<? ;(687.<@ A) 5∙/01 M CNO = 0.0205 C; ∙J.