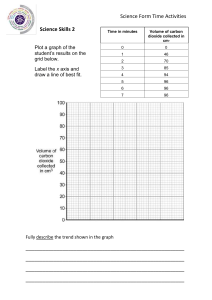
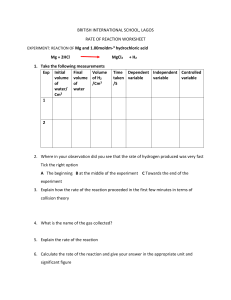
Class 8 Physics Chapter 2. Physical quantities and measurement B. Short/ long Answer Questions Question 1. Define the term density of a substance. Answer 1. Density of a substance is defined as the mass of a unit volume of that substance. Question 2. Name the S.I. unit of density. How it is related to g/cm3? Answer 2. S.I. unit of density is kg/m3. Relationship of kg/m3 with g/cm3 is 1 g/cm3 = 1000 kg/m3 Question 3. The density of brass is 8.4 g/cm3 .What do you mean by this statement? Answer 3. Density of brass is 8.4 g/cm3 it means that a block of brass having volume 1 cm3 contains a mass of 8.4 g. Question 4. Arrange the following substances in order of their increasing density: Iron, Cork, Brass, Water, Mercury. Answer 4. The correct sequence of order is : Cork < Water < Iron < Brass < Mercury Question 5. How does the density of a liquid (or gas) vary with temperature? Answer 5. Most of the liquids increase in volume with the increase in temperature but water shows anomalous behaviour. Water has maximum density at 4 0C . Actually when volume increases density decreases and vice versa. But water when cooled from a high temperature, contracts upto 4 0C because volume decreases and expands when cooled further below 40C . In other words the density of water is maximum at 40C i.e. 1 g/cm3 = 1000 kg/m3 Question 6. A given quantity of a liquid is heated. Which of the following quantity will vary and how? (a) mass (b) volume or (c) density Answer 6. When a given quantity of a liquid is heated (a) Mass : does not change (b) Volume : Changes and increases with rise in temperature. (c) Density : Changes and decreases as density = mass/ volume Question 7. Describe an experiment to determine the density of the material of a coin. Answer 7. Activity 1. Page 22 Figure 2.4 Question 8. Describe an experiment to determine the density of a liquid. Answer 8. Activity 3 . Page 23 Question 9. What is a density bottle? How it is used to find the density of a liquid? Answer 9. Density bottle is a specially designed bottle to measure the density of a liquid. It is a small glass bottle having a glass stopper at its neck. The bottle can store a fixed volume of a liquid. Generally the volume of bottle is 25 mL or 50 mL. The stopper has a narrow hole. When the bottle is filled with the liquid and the stopper is inserted, the excess liquid rises through the hole and drains out. Hence the bottle always contains the same volume of liquid each time when it is filled. Figure 2.6 Question 10. Define the term relative density of a substance. Answer 10. Relative density is the ratio of density of the substance to the density of water. Or It is the ratio of the mass of any volume of the substance to the mass of an equal volume of water. Question 11. What is the unit of relative density? Answer 11. It has no unit as it is the ratio of two similar quantities. Question 12. Distinguish between density and relative density. Answer 12. Density Relative density 1. Density of a substance is defined as 1. Relative density is the ratio of the mass of a unit volume of that density of the substance to the density substance. of water. 3 2.22. S.I. unit of density is kg/m . 2. It has no unit. Question 13. Explain the meaning of the statement ‘Relative density of aluminium is 2.7’. Answer 13. It means that a piece of aluminium of any volume has mass 2.7 times that of an equal volume of water. Question 14. How does the density of a body and that of a liquid determine whether the body will float or sink into that liquid? Answer 14. A body floats on the surface of a liquid if its density is less than the density of liquid. A body sinks on the surface of a liquid if its density is more than the density of liquid. Question 15. A cork piece floats on water surface while an iron nail sinks in it. Explain the reason. Answer 15. A small cork piece will float on water surface because its density is less than the density of water while an iron nail sinks in water because its density is more than the density of water. Question 16. Which of the following will sink or float on water? ( Density of water = 1 g/cm3 ) a) Body A having density 500 kg/m3 b) Body B having density 2520 kg/m3 c) Body C having density 1100 kg/m3 d) Body D having density 0.85 g/cm3 Answer16. Body A, D will float and body B,C will sink Question 17. State the law of floatation? Answer 17. The weight of a floating body is equal to the weight of the liquid displaced by its submerged part ( i.e. buoyant force on it) Question 18. The density of water is 1 g/cm3. The density of iron is 7.8 g/cm3. The density of mercury is 13.6 g/cm3. Answer the following: a) Will a piece of iron float or sink in water? b) Will a piece of iron float or sink in mercury? Answer a) Iron piece will sink in water as its density is more than that of water. b) Iron piece will float in mercury as its density is less than that of mercury Answer 19. a) Buoyant force is same in each case as the weight of body is same in each case and buoyant force is equal to the weight of liquid displaced by the immersed part of body which balances the weight of body. b) The liquid A has the least density as body immerses the maximum. c) ) The liquid C has the highest density as body immerses the minimum. Question 20. For a floating body, how is its weight related to the buoyant force? Answer 20. When a body floats in a liquid, the weight of the floating body is equal to the weight of the liquid displaced by its immersed part (i.e. buoyant force) . Question 21. Why does a piece of ice float on water? Answer 21. The density of ice is 0.9 g/cm3 is less than density of water i.e. 1 g/cm3. Hence a piece of ice float on water. Question 22. Explain why an iron needle sinks in water, but a ship made of iron floats on water. Answer. As a nail is solid and density of iron is more than that of water. The weight of the nail is more than the buoyant force of water on it. So the iron nail sinks in water. While the ship is hollow and empty from inside hence the empty space contains air. This makes the average density of ship less than that of water. Also it displaces more weight of water than its own weight. Hence a ship floats on water. Question 23. It is easier to swim in sea water than in river water. Explain the reason. Answer. As sea water contains salt so its density is more than the density of river water. Hence in each case the weight of water displaced will be equal to the weight of the man. So ratio of weight of sea water and river water displaced by man is 1:1. Secondly, with smaller portion of man’s body submerged in sea water, the weight of sea water displaced is equal to the total weight of body. While to displace the same weight of river water, a large portion of the body will have to be submerged in water. Therefore it is easier for a man to swim in sea water. Question 24. Icebergs floating on sea water are dangerous for ships. Explain the reason. Answer. Icebergs are very dangerous for ships as the density of ice is less than the density of sea water. The density of ice is 0.9 g/cm3 and the density of sea water is 1.02 g/cm3. Hence an iceberg floats in sea water with its large portion submerged inside the water and only a little portion of it is above the surface of water. Hence a ship can collide with the invisible part of iceberg under the surface of water. Question 25. Explain why it is easier to lift a stone under water than in air. Answer. It is easier to lift the stone under water than in air because when the stone is inside the water an upthrust force is exerted by water is more than air which help to reduce the apparent weight of the stone and make us to feel lighter when we lift the stone. Question 26. What is a submarine? How can it be made to dive in water and come to the surface of water. Answer. Submarine is a water tight boat which can travel under water like a ship. It is provided with water tanks. When submarine has to dive, the tanks are filled with water so that the average density of the submarine becomes more than the density of sea water and hence it sinks. To make the submarine rise to the surface of water, these water tanks are emptied. Hence the average density of the submarine becomes less than the density of sea water, so the submarine rises up to the surface of water. Question 27. A balloon filled with hydrogen rises in air. Explain the reason. Answer. A balloon filled with hydrogen rises in air as the density of hydrogen is less than the density of air. Hence, the buoyant force experienced by the balloon due to air becomes more than the weight of the balloon. Hence the balloon rises up under the influence of net upward force. C. NUMERICALS 1. The density of air is 1.28 g/ l . Express it in: (a) g/cm3 (b) kg/m3 Answer . The density of air is 1.28 g/ l As 1 l = 1000 ml = 1000 cm3 Hence The density of air = 1.28 g / 1 l = 1.28g / 1000 cm3 = 0.00128 g/cm3 a) in g/cm3 = 0.00128 g/cm3 As 1 g/cm3 = 1000 kg/ m3 Hence 0.00128 g/cm3 = 0.00128 × 1000 = 1.28 kg/m3 b) in kg/ m3 = 1.28 kg/m3 2. The dimensions of a hall are 10 m × 7 m × 5 m. If the density of air is 1.11 kg/m3, find the mass of air in the hall. Answer. The dimensions of a hall are 10 m × 7 m × 5 m. Hence volume of hall = 10 m × 7 m × 5 m = 350 m3 As density of air = 1.11 kg/m3 Density = mass /volume So mass = volume × density = 350 × 1.11 mass = 388.5 kg 3. The density of aluminium is 2.7 g/cm3 . Express it in kg/m3 . Answer. As 1 g/cm3 = 1000 kg/ m3 So 2.7 g/cm3 = 2.7 × 1000 kg/ m3 = 2700 kg/ m3 4. The density of alcohol is 600 kg/ m3. Express it in g/ cm3 . Answer. . As 1000 kg/m3 = 1 g/ cm3 So 600 kg/m3 = 600 / 1000 = 0.6 g/ cm3 5. A piece of zinc of mass 438.6 g has a volume of 86 cm3. Calculate the density of zinc. Answer. Mass = 438.6 g Volume = 86 cm3 As density = Mass/ Volume So density = 438.6/86 density = 5.1 g / cm3 6. A piece of wood of mass 150 g has a volume of 200 cm3. Find the density of wood in (a) c.g.s. unit (b) S.I. unit Answer. Mass = 150 g Volume = 200cm3 As density = Mass/ Volume So density = 150/200 density = 0.75 g / cm3 (a) In c.g.s. unit density = 0.75 g / cm3 As 1 g/cm3 = 1000 kg/ m3 So 0.75 g /cm3 = 0.75 × 1000 kg/ m3 = 750 kg/ m3 ( b) In S.I. unit density = 750 kg / m3 7. Calculate the volume of wood of mass 6000 kg if the density of wood is 0.8 g /cm3. Answer. Mass = 6000 kg Volume = ? Density = 0.8 g /cm3 As 1 g/cm3 = 1000 kg/ m3 So 0.8 g /cm3 = 0.8 × 1000 kg/ m3 = 800 kg/ m3 As density = Mass/ Volume So volume = Mass/ density = 6000/800 volume = 7.5 m3 8. Calculate the density of solid from the following data: (a) Mass of solid = 72 g (b) Initial volume of water in measuring cylinder = 24 mL (c) Final volume of water when solid is completely immersed in water = 42 mL Answer. Mass of solid = M = 72 g Initial volume of water V1 = 24 mL Final volume of water V2 = 42 mL Hence volume of solid V = V2 – V1 = 42-24 =18 mL As density = Mass/ Volume So density = 72 g/ 18 mL = 72 g / 18 cm3 = 4 g/cm3 So density of solid = 4 g/cm3 9. The mass of an empty density bottle is 21.8 g, when filled completely with water it is 41.8 g and when filled completely with liquid it is 40.6 g. Find: (a) the volume of density bottle (b) the relative density of liquid Answer. Mass of empty bottle = M1 = 21.8 g Mass of bottle + water = M2 = 41.8 g Mass of bottle + liquid = M3 = 40.6 g Mass of water = M2 – M1 = 41.8 – 21.8 = 20 g Mass of liquid = M3 – M1 = 40.6 – 21.8 = 18.8 g (a) As density of water = 1 g /cm3 As density of water = Mass of water/ Volume So volume = Mass/ density = 20 / 1 volume = 20 cm3 So volume of density bottle V = 20 cm3 = 20 mL (b) Relative density of liquid = Mass of liquid / Mass of equal volume of water = M3 – M1 / M2 – M1 = 18.8 / 20 Relative density of liquid = 0.94 10. From the following observations, calculate the density and relative density of a brine solution Mass of empty density bottle = 22 g Mass of bottle + water = 50 g Mass of bottle + brine solution = 54 g Answer. Mass of empty bottle = M1 = 22 g Mass of bottle + water = M2 = 50 g Mass of bottle + brine solution = M3 = 54 g Mass of water = M2 – M1 = 50 – 22 = 28 g Mass of brine solution = M3 – M1 = 54 – 22 = 32 g As density of water = 1 g /cm3 As density of water = Mass of water/ Volume So volume = Mass/ density = 28 / 1 So volume of density bottle V = 28 cm3 = 28 mL (a) Mass of brine solution = 32 g Volume of brine solution = 28 cm3 Hence density of liquid = Mass of brine solution / Volume of brine solution = 32 /28 = 1.14 g/ cm3 So density of liquid = 1.14 g/ cm3 (b) Relative density = Mass of brine solution / Mass of equal volume of water So relative density = M3 – M1 / M2 – M1 = 32 / 28 Relative density of brine solution = 1.14 11.The mass of an empty density bottle is 30 g, it is 75 g when filled completely with water and 65 g when filled completely with a liquid. Find: (a) volume of density bottle (b) density of liquid, and (c) relative density of liquid Answer. . Mass of empty bottle = M1 = 30 g Mass of bottle + water = M2 = 75 g Mass of bottle + liquid = M3 = 65 g Mass of water = M2 – M1 = 75 – 30 = 45 g Mass of liquid = M3 – M1 = 65 – 30 = 35 g (a) As density of water = 1 g /cm3 As density of water = Mass of water/ Volume So volume = Mass/ density = 45 / 1 volume = 45 cm3 So volume of density bottle V = 45 cm3 = 45 mL (b) Mass of liquid = 35 g Volume of liquid = 45 cm3 Hence density of liquid = Mass of brine solution / Volume of brine solution = 35 /45 = 0.77 g/ cm3 So density of liquid = 0.77 g/ cm3 (c) Relative density of liquid = Mass of liquid / Mass of equal volume of water = M3 – M1 / M2 – M1 = 35 / 45 Relative density of liquid = 0.77