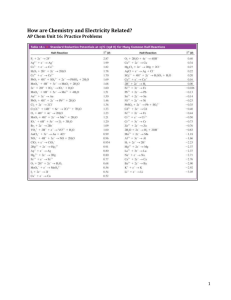
Introduction Electrochemistry is a branch of science that deals with electricity relation to chemical reactions. Electrochemistry presents the conversion of chemicals to electricity, whereas applied commonly in making batteries and fuel cells that produce electric power. During an electrochemical reaction, where electrons were transferred from one substance to another, the term OIL-RIG is invented. OIL-RIG stands for ‘Oxidation Is Losing’ and ‘Reduction Is Gaining,’ where oxidation loses electrons (oxidized) and reduction is the one gaining it (reduced). During 1970’s, galvanic cell was first introduced by Luigi Galvani, an Italian scientist with an experiment of a dissected frog’s lower body. Two wires such as copper wire and zinc wire were attached to the frog’s exposed nerve and leg muscle respectively which results on the muscle contrast that makes the frog’s lower body to create movement [1]. Galvani concluded incorrectly that the electricity was coming from the tissues of the animal. Another Italian scientist, Alessandro Volta, would later establish that the source of the electricity was electron flow between the two metals, and that the frog's body fluids were just a conducting medium. (For this reason, galvanic cells are often referred to as voltaic cells as well). However, Galvani did uncover the electric nature of nerve impulses. His frog experiment is considered the beginning of electrophysiology and the understanding of how bodies convert information between chemical and electrical messaging systems [2]. In the laboratory, its scope was to create a galvanic cell, also known as voltaic cell, in three different experiments such as 1) standard potential galvanic cell, 2) galvanic cell with one unknown electrode and electrolyte solution, and 3) copper concentration solution. A galvanic cell is composed of two half-cell reactions, with two electrodes connected in a multimeter where electrons (𝑒 − ) flow from the anode to the cathode, and electrolyte solutions that are connected by a salt bridge soaked in a strong electrolyte to balance the cell by letting the ions flow (cations going to the cathode and anions going to the anode). The two electrodes in a galvanic cell can either be the same or different, and electrolyte solutions must be different. Standard reactions can be observed with a galvanic cell, and nonstandard reactions can be calculated. To easily determine if a galvanic cell appeared spontaneous or nonspontaneous in standard form, a standard reduction potential table can be used, where the ones placed in the higher part has the higher tendency towards reduction and the ones placed lower has the higher tendency towards oxidation. The standard reduction potential can be applied in 0 0 0 𝐸𝑐𝑒𝑙𝑙 = 𝐸𝑐𝑎𝑡ℎ𝑜𝑑𝑒 − 𝐸𝑎𝑛𝑜𝑑𝑒 Where the higher value will be the cathode and the lower one will serves as the anode. When the 0 calculated 𝐸𝑐𝑒𝑙𝑙 appeared positive, then the reaction is spontaneous, and when appeared negative, then the reaction is nonspontaneous. Note: there can be manipulation that may happen when observed in balance redox reaction between who may be the cathode and the anode. In nonstandard reaction, a reaction can be calculated using 0 𝐸𝑐𝑒𝑙𝑙 = 𝐸𝑐𝑒𝑙𝑙 − 𝑅𝑇 𝑙𝑛𝑄 𝑛𝐹 0 Where 𝐸𝑐𝑒𝑙𝑙 is the measured voltage, 𝐸𝑐𝑒𝑙𝑙 is the standard potential, R is the universal gas 𝐽 constant which is 8.314 , T is the 𝑚𝑜𝑙×𝐾 temperature in Kelvin, n is the number of electrons transferred in the half-reaction, F as 𝐶 Faraday’s constant which is 96485 𝑚𝑜𝑙, and Q is the reaction quotient which can either be [𝑝𝑟𝑜𝑑𝑢𝑐𝑡𝑠] [𝑜𝑥𝑖𝑑𝑎𝑡𝑖𝑜𝑛] 𝑜𝑟 [𝑟𝑒𝑑𝑢𝑐𝑡𝑖𝑜𝑛] in molarity form. [𝑟𝑒𝑎𝑐𝑡𝑎𝑛𝑡𝑠] The experiment aims to present the electrical energy available from the electron transfers in a redox reaction in standard, nonstandard, and concentration cells [3]. Also, the experiment wants to develop an understanding about the limitations and possible errors of the galvanic cell, and calculate cell potentials in either standard, nonstandard, or concentrated cells. Reference: [1] Wells, D. A. (1872). The science of common things: A familiar explanation of the first principles of physical science as cited by Calmes, J., Ansari, A. F., Ross, E. Galvanic Cells. Brilliant.org. Retrieved 14:06 November 7, 2021. https://brilliant.org/wiki/galvanic-cells/ [2] Haas, L.F. J Neurol Neurosurg Psychiatry. 1993 Oct; 56(10): 1084 as cited by Calmes, J., Ansari, A. F., Ross, E. Galvanic Cells. Brilliant.org. Retrieved 14:06 November 7, 2021. https://brilliant.org/wiki/galvanic-cells/ [3] SparkNotes Editors (2005). Galvanic Cells. Retrieved from www.sparknotes.com