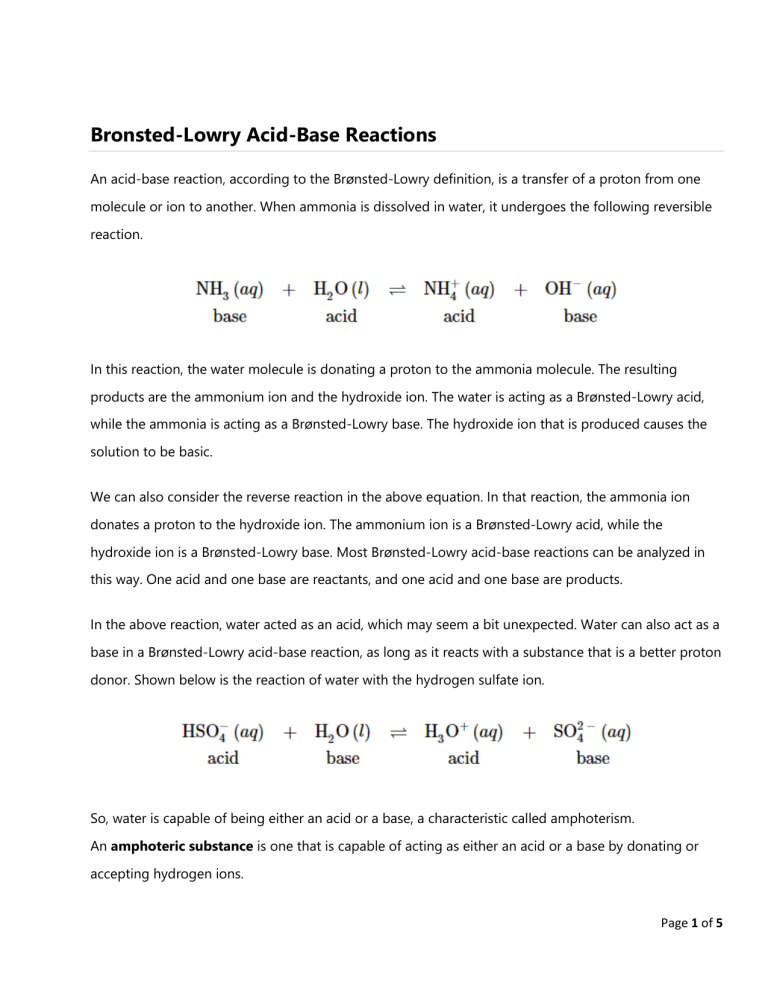
Bronsted-Lowry Acid-Base Reactions An acid-base reaction, according to the Brønsted-Lowry definition, is a transfer of a proton from one molecule or ion to another. When ammonia is dissolved in water, it undergoes the following reversible reaction. In this reaction, the water molecule is donating a proton to the ammonia molecule. The resulting products are the ammonium ion and the hydroxide ion. The water is acting as a Brønsted-Lowry acid, while the ammonia is acting as a Brønsted-Lowry base. The hydroxide ion that is produced causes the solution to be basic. We can also consider the reverse reaction in the above equation. In that reaction, the ammonia ion donates a proton to the hydroxide ion. The ammonium ion is a Brønsted-Lowry acid, while the hydroxide ion is a Brønsted-Lowry base. Most Brønsted-Lowry acid-base reactions can be analyzed in this way. One acid and one base are reactants, and one acid and one base are products. In the above reaction, water acted as an acid, which may seem a bit unexpected. Water can also act as a base in a Brønsted-Lowry acid-base reaction, as long as it reacts with a substance that is a better proton donor. Shown below is the reaction of water with the hydrogen sulfate ion. So, water is capable of being either an acid or a base, a characteristic called amphoterism. An amphoteric substance is one that is capable of acting as either an acid or a base by donating or accepting hydrogen ions. Page 1 of 5 Conjugate Acids and Conjugate Bases When a substance that is acting as a Brønsted-Lowry acid donates its proton, it becomes a base in the reverse reaction. In the reaction above, the hydrogen sulfate ion (HSO4-) donates a proton to water and becomes a sulfate ion (SO42-). The HSO4- and the SO42- are linked to one another by the presence or absence of the H+ ion (a proton). A conjugate acid-base pair is a pair of substances related by the loss or gain of a single hydrogen ion. A conjugate acid is the particle produced when a base accepts a proton. The hydrogen sulfate ion is the conjugate acid of the sulfate ion. A conjugate base is the particle produced when an acid donates a proton. The sulfate ion is the conjugate base of the hydrogen sulfate ion. In the reaction illustrated below, water serves both as acid and base simultaneously. One water molecule serves as an acid and donates a proton. The other water molecule functions as a base by accepting the proton. Figure 1. Bronsted-Lowry Acid-Base Reaction A typical Brønsted-Lowry acid-base reaction contains two conjugate acid-base pairs, as shown below. One conjugate acid-base pair is NHO2/NO2−, while the other pair is HPO42-/PO43-. Summary: • An acid-base reaction, according to the Brønsted-Lowry definition, is a transfer of a proton from one molecule or ion to another. • Every acid has a conjugate base and every base has a conjugate acid. • A conjugate acid-base pair is a pair of substances related by the loss or gain of a single hydrogen ion. • A conjugate acid is the particle produced when a base accepts a proton. • A conjugate base is the particle produced when an acid donates a proton. Page 2 of 5 Lewis Acid-Base Reactions The Lewis classification of acids and bases is broader than the Brønsted-Lowry definition, and encompasses many more substances. Whereas the Brønsted-Lowry and the Arrhenius classifications are based on transfer of protons, Lewis acidity and basicity are based on the sharing of an electron pair. Lewis acids can accept an electron pair, while Lewis bases can donate an electron pair. Lewis acids accept an electron pair. Lewis Acids are electrophilic meaning that they are electron attracting. When bonding with a base the acid uses its lowest unoccupied molecular orbital or LUMO (Figure 2). • Various species can act as Lewis acids. All cations are Lewis acids since they are able to accept electrons. (e.g., Cu2+, Fe2+, Fe3+) • An atom, ion, or molecule with an incomplete octet of electrons can act as an Lewis acid (e.g., BF3, AlF3). • Molecules where the central atom can have more than 8 valence shell electrons can be electron acceptors, and thus are classified as Lewis acids (e.g., SiBr4, SiF4). • Molecules that have multiple bonds between two atoms of different electronegativities (e.g., CO2, SO2) Lewis Bases donate an electron pair. Lewis Bases are nucleophilic meaning that they “attack” a positive charge with their lone pair. They utilize the highest occupied molecular orbital or HOMO (Figure 2). An atom, ion, or molecule with a lone-pair of electrons can thus be a Lewis base. Each of the following anions can "give up" their electrons to an acid, e.g., OH−, CN−, CH3COO−, :NH3:, H2O:, CO:. Lewis base's HOMO (highest occupied molecular orbital) interacts with the Lewis acid's LUMO (lowest unoccupied molecular orbital) to create bonded molecular orbitals. Both Lewis Acids and Bases contain HOMO and LUMOs but only the HOMO is considered for Bases and only the LUMO is considered for Acids (Figure 22). Figure 2. Homo-Lumo Molecular Orbitals Page 3 of 5 Complex Ion/Coordination Compounds Complex ions are polyatomic ions, which are formed from a central metal ion that has other smaller ions joined around it. While Brønsted theory can't explain this reaction Lewis acid-base theory can help. A Lewis Base is often the ligand of a coordination compound with the metal acting as the Lewis Acid). The aluminum ion is the metal and is a cation with an unfilled valence shell, and it is a Lewis Acid. Water has lone-pair electrons and is an anion, thus it is a Lewis Base. Figure 3. Lewis acid-base reaction: Aluminum ion acts as a Lewis acid and accepts an electron pair from water, which acts as a Lewis base. The reaction of a Lewis acid and a Lewis base will produce a coordinate covalent bond (Figure 11). A coordinate covalent bond is just a type of covalent bond in which one reactant gives it electron pair to another reactant. In this case the Lewis base donates its electrons to the Lewis acid. When they do react this way, the resulting product is called an addition compound, or more commonly an adduct. Boron trifluoride, BF3 acts as a Lewis acid when it combines with a basic ion or molecule that can donate an electron pair. Such a reaction is : BF3 + F- BF4Here, the acid is BF3 and the base is F-. This acid-base reaction allows boron (which is electron-deficient in BF3) to complete its octet. Page 4 of 5 Lewis acidity is the basis for coordination chemistry. This is because coordination chemistry involves metal ions that are Lewis acids, which bond to ligands that are Lewis bases. • Lewis Acid: a species that accepts an electron pair (i.e., an electrophile) and will have vacant orbitals • Lewis Base: a species that donates an electron pair (i.e., a nucleophile) and will have lone-pair electrons Determining the Strength of Metal Ion Lewis Acids There are three determining factors in the Lewis acid strength of a metal ion: 1. The higher positive charge on the metal, the more acidic it is. For example, Al3+ and Fe3+ are good Lewis acids and their salts make acidic solutions in water, but K+and Na+ are not. 2. The smaller the atomic radius of the metal ion, the more acidic it is. Going down the periodic table, the Lewis acidity of metal ions decreases (e.g., Al3+ > Ga3+ > In3+) because the ionic radius increases. 3. For transition metal ions, more electronegative metals tend to make stronger Lewis acids. The electronegativity has maxima at W and Au in the 5d series, so metal ions near in that part of the periodic table are good Lewis acids. Sources: https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Introductory_Chemistry_(CK12)/21%3A_Acids_and_Bases/21.06%3A_Brnsted-Lowry_Acid-Base_Reactions https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Book%3A_Introduction_to_Inorga nic_Chemistry_(Wikibook)/03%3A_AcidBase_Chemistry/3.02%3A_Brnsted_and_Lewis_Acids_and_Bases https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/S upplemental_Modules_(Physical_and_Theoretical_Chemistry)/Acids_and_Bases/Acid/Lewis_Conc ept_of_Acids_and_Bases Page 5 of 5