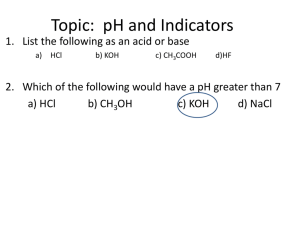
What is Ph? Let's remember the previous topic • What types of reactions in aqueous solutions do you know? • What are indicators? • What indicators do you know? • How can you determine the reaction of aqueous solutions? Answers • • • • • • • • • What types of reactions in aqueous solutions do you know? neutral, alkaline and acidic. What are indicators? substances that can be used to determine the reaction of the environment. What indicators do you know? in solutions: phenolphthalein, litmus, methyl orange. dry: universal indicator paper, litmus paper, methyl orange paper How can you determine the reaction of aqueous solutions? wet and dry. PH is a measure for determining the acidity of aqueous solutions. O ACID 7 ALKALI 14 PH is measured on a range from 0 to 14, with 7 being a neutral pH, values less than 7 indicating acidity, and values greater than 7 indicating alkalinity. pH level is very important for our body. Blood PH=7.4 Acidosis Blood pH drops below 7.4 Alkalosis Blood pH drops below 7.4 In agriculture, soil pH indicates which crop is suitable for growing it. Rice , coffee , banana Acidic soil Grapes , cabbage Alkaline soil How to neutralize the soil? soil acidity level for most plants ranges from 5.5-7.5 pH levels play an important role in the production of toiletries . The pH level is usually adjusted depending on the hair type Why are baby shampoos called “no tears”? Tears What acid is found in our stomach? What happens if the pH level drops? How to help a person? Tooth enamel is the hardest substance in our body . It is insoluble in water and slightly acidic solutions, however, when the pH level in the mouth drops below 5.5, it slowly corrodes . Why Ph level drops below 5.5 ? When we eat food, some food particles remain stuck to the teeth . Dental bacteria present in the mouth produces acid due to the breakdown of sugar and food particles present in the mouth, which is what causes tooth decay . Give some examples of how to avoid tooth decay Methods for determining pH value For definitions pH values of solutions wide use some techniques . 1. And indicators . What indicators do you know ? 2. universal indicator ( representing yourself mixture from several indicators .) 3. device - pH meter Laboratory work (pair work) Topic: Research of soil Answer the questions at home • What pH values are typical for acidic, neutral and alkaline environments? • In what areas of science and production is it necessary to measure the pH values of a solution of a substance? Give some examples. • What is an indicator? How can we use it to determine the nature of the environment? • What are the advantages of a universal indicator? • What is the name of the device for more accurately determining the pH of the environment? Give its advantages.