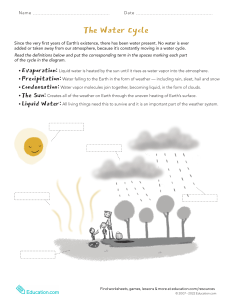
reactions that an organic compound undergoes in order to learn about its structure. Earth Science Reviewer: What is Science? (Latin: “Scientia – Which is to know”) An organized body of knowledge about the natural universe and the processes by which that knowledge is acquired and tested The Scientific method is a method of testing and evaluation by which hypotheses becomes Theory, and then Scientific Law. Observations and Measurements (Qualitative data) The knowledge that is built by science is always open to question and revision. No scientific idea is ever onceand-for-all “proved”. Why not? Well, science is constantly seeking new evidence, which could reveal problems with our current understandings Ideas that we fully accept today may be rejected or modified in light of new evidence discovered tomorrow. Despite the fact that they are subject to change, scientific ideas are reliable. The ideas that have gained scientific acceptance have done so because they are supported by many lines of evidence. Hypothesis (a possible explanation) Experiments (Testing a hypothesis) Theory (a well-tested explanation) Law (describes a fundamental relationship of nature) Hypothesis – And Educated guess Theory – A Hypothesis that has withstood initial testing and evaluation. It is intended to be further investigated by experiment. If the theory does not withstand further experimentation, it must be modified, replaced, or rejected Scientists use models as tools to advance their theories. Model – It is a miniature representation of a real thing. Branches of Science Biological Sciences Physical Sciences (Observational Sciences) (Measuremental Sciences) Biology Chemistry Zoology Physics Microbology and etc... Astronomy These scientific explanations continually generate expectations that hold true, allowing us to figure out how entities in the natural world are likely to behave (e.g. how likely it is that a child will inhrit a particular genetic disease) and how we can harness that understandin to solve problems (e.g. how electricity, wire, glass, and various compounds can be foashined into a working light bulb). References: 1. Shipmen, James T. et. al. “An Introduction to Physical Science” 2. Glencoc Science: National Geographic. “An Introduction to Physical Science” 3. Young and Freedman “ University Physics with Modern Physics” 4. Brown et. al. “Chemistry: The Central Science” 5. Silverio, Angelina, Exploring Life through Science; Physics: Phoenix Publishing 6. Hewitt, Paul G. Conceptual Physics, 11th Edition, San Francisco: Pearson, 2010. Lesson 1: IN THE BEGINNING (Big Bang Theory) Cosmology –this is a branch of astronomy that involves the origin and evolution of the universe, from the Big Bang to today and on into the future. Meteorology Geology Aims and nature of Science: Science aims to explain and understand Science, as a collective institution, aims to produce more and more accurate natural explanations of how the natural world, what its components are, and how the world got to be the way is is now. 3 Cosmic Stages (through which specific groups of elements were formed) 1. •Formed the light elements (H,, He, and Li) 2. Stellar formation and evolution •formed the elements heavier the Be to Fe 3. Classically, science’s main goal has been building knowledge and understanding, regardless of its potential applications – e.g. investigating the chemical Big Bang Nucleosynthesis Stellar Explosion, or supernova •elements heavier than Fe Big Bang Theory – This explains how the elements were initially formed the formation of different elements involved many nuclear reactions, including fusion, fission, and radioactive decay. Nucleosynthesis – this is the process that created new atomic nuclei from pre-existing nucleons, primarily protons and neutrons. Part of its proof is the amount of Hydrogen and Helium we have in the universe today. - As the Universe coold, protons and neutrons can fuse to form heavier atomic nuclei. E.g., Picture 1.6 History of the Universe Stages of the Big Bang Theory: Singularity – This is a point in space and/or a moment in time where they universe was infinitely hot and dense. Chief nuclear reactions responsible for the relative abundances of light atomic nuclei observed throughtout the universe. Recombination – This refers to the epoch at which charged electrons and protons first became bound to form electrically neutral hydrogen atoms. Annihilation – In physics, reaction in which a particle and its antiparticle collides and disappear, releasing energy. Inflation – this is a theory of exponential expansion of space in the early universe. The inflationary epoch lasted from 10-36 seconds. Redshift – This is the displacement of spectral lines toward longer wavelengths (the red end of the spectrum) in radiation from distant galaxies and celestial objects. *If the starlight is blue, the star does not move. *If the Starlight is red, then the star moves away from us. Cosmic Microwave Background (CMB) - is electromagnetic radiation left over from an early stage of the universe from the big bang cosmology. Picture of protostar The Sun After sufficient mass and density was achieved in the Sun, the temperature rose to one million °C, resulting in thermonuclear fusion. H atom + H atom = He atom + energy Protoplanets Gravitational forces allow the inner planets to accrue and compact solid matter (including light and heavy atoms) Solar radiation blew gases (primarily hydrogen, helium) away from inner planets These gases were collected and condensed into the gas giants (Jupiter, Saturn, Uranus, Neptune) Beyond Neptune, ice and frozen gases form Pluto, Sedna and the Kuiper Belt Objects Left-over debris form comets and asteroids Birth of the Solar System (Picture) Size of the pLanets picture Earth is 4,570,000,000 years old. Geologic Time (A Timeline) (MA)Millions of years ago Lesson 2: The History of the Earth Origin of the Universe: The Universe began about 14.4 Billion years ago. The Big Bang Theory states that in the beginning, the universe was all in one place. All of its matter and energy was squished into an infinitely small point called a Singularity. Then, It exploded. After about 10 Billion years after that, our solar system began to form. Birth of the Solar System: The Nebular Hypothesis is the currently accepted argument about how a solar system can form. We have discovered over two hundred planets orbiting other stars. A large gas cloud(nebula) begins to condense. Most of the mass is in the center, there is turbulence in the outer parts. Pictures from the Hubble Space Telescope show newborn stars emerging from dense, compact pockets of interstellar gas called evaporating gaseous globules Picture Gravitational attraction causes the mass of gas and dust to slowly contract and it begins to rotate The dust and matter slowly falls towards the center 4570 MA – Sun and Accretionary disk formed 4560 MA – Planetisimals accreted; accretion of earth has begun. 4510 MA– Moon has been formed 4470 MA – Earth Accretion core formation, differentiation completed 4400 MA = Oldest mineral Grain 4000 MA – End of heavy bombardment, oldest continental rocks. 3800 MA – Earliest evidence of surface liquid water 3500 MA – Earliest evidence of Life 2500 MA – Major Phase of continent formation completed 2450 – 2200 MA – Buildup of oxygen in atmosphere 2200 MA – Nucleus-bearing cells developed 700 MA – Ice covered Earth? (Dirty Ice Balls or asteroids and comets) 565 MA – Worldwide distribution of multicellular organisms 545 – 530 MA – Evolutionary “Big Bang” 439 MA – Mass Extinction 420 MA – Earliest land animals 364, 250, & 208 MA – Mass Extinction 125 MA – Earliest flowering plants 65 MA – Mass Extinction 5 MA – First Hominids 0.12 MA – First Appearance of our species, Homo sapiens sapiens. Formation of the Moon (Picture) The Giant Impact Hypothesis predicts that around 50 million years after the initial creation of Earth, a planet about the size of Mars collided with Earth. A considerable amount of material was blown off into space, but most fell back onto the Earth Part of the material from the collision remained in orbit around the Earth By the process collision and accretion, this orbiting material coalesced into the Moon The early Moon orbited very close to the Earth Three Major factors that cause the heating and melting of the Earth’s Interior: 1. Collisions – Transfer of kinetic energy into heat 2. Compression 3. Radioactivity or elements Global chemical differential, the heavier elements, including the melted iron, began to sink down into the core of the Earth, while the lighter elements such as oxygen and silica floated up towards the surface. This global chemical differential was completed by about 4.3 billion years ago, and the Earth had developed a inner and outer core, a mantle and crust Note that oxygen (O2) gas is not created by volcanic eruptions Creating the ocean It is hypothesized that water vapor escaping from the interior of the Earth via countless volcanic eruptions created the oceans (this took hundreds of millions of years) Astronomers also hypothesize that comets impacting the Earth were a major source of water that contributed to creation of the oceans Remember, that comets are best described as “dirty ice balls” The earliest evidence of surface water on Earth dates back about 3.8 billion years Geologic time the Earth is thought to have had a thin atmosphere composed primarily of helium (He) and hydrogen (H) gases 4570 million of years ago(ma)- sun and accretionary disk formed 4560(ma)- planetesimals accreted; earth acceretion begun 4510 (ma)- moon formed 4470 (ma)- earth accretion core formation and differentiation completed 4400 (ma)- oldest general grain 4000 ma- end of heavy bombardment: oldest continental rocks 3800 (ma)- earliest evidence for liquid water 3500(ma)- earliest evidence for life 2500 (ma)- major phase of continental formation completed 2450-2200 (ma)- buildup of oxygen in atmosphere 2200 (ma)-nucleus-bearing cells developed 700 (ma)- ice covered earth? 565 (ma)- worldwide distribution of multicellular organisms 545-530 (ma)-evolutionary ”big bang” 439 (ma)- mass extinction 420 (ma)- earliest land animals 364 (ma)-mass extinction 250 (ma)- mass extinction 208 (ma)- mass extinction 125 (ma)- earliest flowering plants 65 (ma)- mass extinction 5 (ma)- first hominids 0.12 (ma)- first appearance of our species(homo sapiens) These would include: A billion Year Old Earth Chemical composition of the earth Whole Earth: Crust: Fe+O+Si+Mg = 93% Si+O+Al = 82% Lithosphere: strong, rocky outer shell of the solid Earth including all the crust and the upper part of the mantle to a depth of ~100 km (forms the plates) Asthenosphere: weak,ductile layer of the mantle beneath the lithosphere; deforms to accommodate the motions of the overlying plates Deep Mantle: mantle beneath the asthenosphere (~400 to 2900 km in depth) Outer core: liquid shell composed of mostly iron Inner core: innermost sphere composed primarily of solid iron Continents: Formed from solidified magma that floated up from the Mantle Oceans and Atmosphere: Fluid and gaseous outer layers believed to have been created by out-gassing of gases and fluids from volcanic eruptions (in a process called volatile transfer) The evolving atmosphere Water vapor (H2O) Sulfur dioxide (SO2) Hydrogen sulfide (H2S) Carbon dioxide (CO2) Carbon Monoxide (CO) Ammonia (NH3) Methane (CH4) By 3.5 billion years ago, when the Earth was abillion years old, it had a thick atmosphere composed of CO2, methane, water vapor and other volcanic gases By human standards this early atmosphere was very poisonous It contained almost no oxygen Remember, today our atmosphere is 21% oxygen By 3.5 billion years ago, the Earth also had extensive oceans and seas of salt water, which contained many dissolved elements, such as iron But most important, by 3.5 billion years ago, there was life on Earth The Continents By 2.5 billion years ago, the continents had been formed The density of the continental crust (2.8 gr/cm3) is lighter that the crust found on ocean bottoms (3.2 gr/cm3), so the continents rise above the ocean floor A question that remains unanswered is, when did plate tectonics start? Is Earth Located in Habitable Zone? LESSON 3: Discovery of a new Planet Galactic Neighborhood Galactic environment impacts habitability milky Way galaxy’s edge is a life-favorable spot -Not near active gamma ray source -Not near galactic center with high star density and ionizing radiation -Loneliness in galaxy is helpful for life Spectral class indicates photospheric temperature “HabStars” spectral range -Early F -G - Mid-K - 7000K to 4000K Emit high-frequency UV radiation to trigger atmospheric ozone formation Emits not so much that ionization destroys life Spectral Class of “Sun” Earth rotates around the star, the “Sun” Sun -G2 star -~6,000K Sun is in “Habstar” range! Ozone can form in atmosphere Ionization is not deadly for life All stars change luminosity Stars vary in stability... stars that fluctuate luminosity violently are poor candidates for hosting life The Sun is relatively stable! - Solar variation is ~.1% over 11- year cycle -Slight variations dramatically impact Earth -Little Ice Age – decline in Sun’s luminosity Terrestrial -Silicate rocks -Rocks not accreted to gaseous outer layers Gas Giants = no life - No surface -Enormous Gravity -Satellites are good candidates, however Composition of Earth? Earth is a terrestrial planet, not a gas giant Mass of Planets need to be Just Right for Life Low Mass Bad news for life Lesser gravity – difficult for atmosphere retention Smaller planets lose energy from formation quickly geologically dead Approximately 0.3 Earth masses needed to sustain life Mass of Earth Low Stellar Variation? Yes! Earth is located within the expected shell of distance in which liquid water can be on the surface of the planet Pictures of the planet show liquid water covering a large portion of the Earth’s surface Planet Characteristics that Support Life Spectral Class of Star Needed for Life: Habitable Zone Theoretical shell around a star where any planet present would have liquid water on its surface HZ range should not vary over time -Stars increase luminosity as they age - If this happens too quickly (super-massive star), planets are only in window for life for short amount of time -Lowers time to develop life High Mass -Earth is largest by mass and density of terrestrial bodies in the Solar System -Large enough for molten core (heat engine) -Large enough for atmosphere through gravity -Large enough for liquid outer core and metal inner core (magnetic field) Magnetic Fields and Life Planets need protection from solar wind Solar wind- stream of charged particles from stars consisting of electrons and protons Planet must have molten metal interior Does Earth have a Magnetic Field? Yes! Earth has solid metal core with liquid outer core, causing magnetic field Protects the Earth from solar wind Atmosphere and Life Is Earth’s Orbit Suitable? Yes! Earth’s Orbit Almost circular E < .02 Atmosphere – layer of gases that surround a material body of sufficient mass Held by gravity Helps regulate temperature Protects planet from meteors and radiation Composition favors life (oxygen and carbon dioxide) Life-Supporting Rotation Does Earth have an Atmosphere? Yes! Earth’s atmosphere is made up of -Nitrogen (78%) -Oxygen (20.9%) -Argon (.93%) -Carbon Dioxide (.0390%) This composition could support life Atmosphere absorbs/reflects harmful radiation -Visible and Radio reach surface Composition of Planets Four elements vital for life 1. Carbon 2. Hydrogen 3. Oxygen 4. Nitrogen Earth’s Rotation? Element oxygen alone found in Earth’s crust... However, other life elements are found in atmosphere and water Make amino acids (building blocks of protein) Comets and out gassing from volcanoes brought these elements Tectonic Activity of Planet Supply surface with life-sustaining material Supply atmosphere with temperature moderators(CO2) Recycles important chemicals/materials Helps increase environmental complexity Earth is tectonically active Life-Supporting Orbits Stability is critical Eccentricity -Greater e, greater temperature fluctuation -Living organisms can only withstand certain fluctuations -Complex organisms have greater temperature sensitivity Earth’s tilt varies between 21.5 and 24.5 degrees every 41,000 years Day is only 24 hours Moon plays crucial role -Moderates Earth’s climate by stabilizing axial tilt LESSON 4: Earth’s atmosphere Earth’s Composition? Rotation around axis at tilt -Planet should have moderate seasons or biospheric dynamism will disappear -Without tilt, planet would be colder (warm weather could not move pole ward) -Should not be radically tilted because seasons would be extreme -Speed of Rotation -Should be relatively quick so day-night cycle is not too long (temperature differences if long days/nights) Earth’s atmosphere is a layer of gases surrounding the planet. The Earth is surrounded by a blanket of air, which we call the atmosphere. It reaches over 560 kilometers from the surface of the Earth. Atmosphere: Absorbs the energy from the Sun, Recycles water and other chemicals, protects us from high-energy radiation and the frigid vacuum of space. The atmosphere protects and supports life. Earth Has 4 main systems that interact: The Atmosphere The Hydrosphere -All of Earth’s water, whether in the atmosphere, glaciers, oceans, lakes or rivers. The Biosphere The Geosphere itself has been around pretty much as long as the earth has! When the first fish swam in the ocean, your glass of water was part of that ocean. When the Brontosaurus walked through lakes feeding on plants, your glass of water was part of those lakes When kings and princesses, knights and squires took a drink from their wells, your glass of water was part of those wells. And you thought your parents were OLD Evaporation: Evaporation is when the sun heats up water in rivers or lakes or the ocean and turns it into vapor or steam. The water vapor or steam leaves the river, lake or ocean and goes into the air. Do plants Well, sort of.... sweat? Hydrosphere Water on Earth is a unique feature that clearly distinguishes our "Blue Planet" from others in the solar system. Not a drop of liquid water can be found anywhere else in the solar system. Earth has just the right mass, the right chemical composition, the right atmosphere, and is the right distance from the Sun that permits water to exist mainly as a liquid. Water is the universal solvent and the basis of all life on our Planet. The water Cycle Water, which covers the majority of the Earth’s surface (the hydrosphere), circulates through the crust, oceans, and atmosphere in what is known as the water cycle. Water Cycle—water is in constant motion The Sun provides water cycle’s energy Water on the surface absorbs heat and evaporates, entering the atmosphere Condensation—water vapor changes back into liquid. Clouds of water become heavy and water falls to Earth as precipitation. The cycle repeats itself continuously. The Water Cycle Run and get a glass of water and put it on the table next to you. Take a good long look at the water. Now -- can you guess how old it is? The water in your glass may have fallen from the sky as rain just last week, but the water people perspire (sweat) and plants transpire. Transpiration is the process by which plants lose water out of their leaves. Transpiration gives evaporation a bit of a hand in getting the water vapor back up into the air. Condensation: Water vapor in the air gets cold and changes back into liquid, forming clouds. This is called condensation. You can see the same sort of thing at home... pour a glass of cold water on a hot day and watch what happens. Water forms on the outside of the glass. That water didn't somehow leak through the glass! It actually came from the air. Water vapor in the warm air, turns back into liquid when it touches the cold glass. Precipitation: Precipitation occurs when so much water has condensed that the air cannot hold it anymore. The clouds get heavy and water falls back to the earth in the form of rain, hail, sleet or snow. Collection: When water falls back to earth as precipitation, it may fall back in the oceans, lakes or rivers or it may end up on land. When it ends up on land, it will either soak into the earth and become part of the “ground water” that plants and animals use to drink or it may run over the soil and collect in the oceans, lakes or river where the cycle starts Earth’s atmosphere Earth’s atmosphere is made of a mixture of gases called air. -Nitrogen gas makes up about 78% of Earth’s atmosphere. -The second most abundant gas is oxygen, which makes up 21% of Earth’s atmosphere. - The third Argon (Ar, 0.9%). - Carbon Dioxide (CO2, 0.03%). Composition of the Atmosphere The atmosphere is comprised of a variety of gases: Major Constituents (99%): -Nitrogen (N): 78% -Oxygen (O2): 21% Trace Constituents: -Argon (Ar), about 0.9% -Water vapor (H2O), up to 10000 ppmv -Carbon dioxide (CO2), 350 ppmv -Ozone (O3), near zero at the surface, up to 10 ppmv in the stratosphere -Methane (CH4), 1.7 ppmv and others..... ppmv = “parts per million by volume” Nitrogen cycle Nitrogen is important to protein which is found in the body tissues of all living things. Nitrogen is cycled through the soil and into plants and finally when living things die and decay. Pressure in the atmosphere Atmospheric pressure is the force per unit are exerted into a surface by the weight of air above that surface in the atmosphere of Earth. The gas molecules closest to Earth’s surface are packed together very closely. This means pressure is lower the higher up you go into the atmosphere. Pressure in the atmosphere At sea level, the weight of the column of air above a person is about 9,800 Newtons (2,200 pounds) This is equal to the weight of a small car. Measuring Pressure A barometer is an instrument that measures atmospheric pressure. Long ago, mercury barometers were used Since mercury is a poisonous liquid, aneroid barometers are used today. Layers of Atmosphere The atmosphere has four layers Thermosphere Mesosphere Stratosphere Troposphere Troposphere Lowest and thinnest layer 16 km at equator, 8 km at poles 90% of the atmosphere’s mass Temperature decreases with altitude 6°C per kilometer Top of troposphere averages –50°C -Where weather occurs -Boundary between the troposphere, and the stratosphere is called the tropopause. Stratosphere Extends from 10 km to 50 km above the ground Less dense (less water vapor) Temperature increases with altitude Almost no weather occurrence Contains high level of ozone Ozone layer Upper boundary is called stratopause. Mesosphere Extends to almost 80 km high Gases are less dense. Temperature decreases as altitude increases. Gases in this layer absorb very little UV radiation. Thermosphere Above the mesosphere and extends to almost 600 km high Temperature increases with altitude Readily absorbs solar radiation Temperature can go as high as 1,500°C Reflects radio waves The four layers of theatmosphere include: 1. the troposphere, where we live; 2. the stratosphere, which contains the ozone layer; 3. the mesosphere, where meteors burn; and 4. the thermosphere, where satellites orbit Earth. The exosphere begins at about 500 kilometers above Earth and does not have a specific outer limit. Satellites orbit Earth in the exosphere. The exosphere and ionosphere Communication on Earth depends on satellites. Satellites transmit information used for television shows, radio broadcasts, data and photos used in weather reports, and long distance telephone calls. The heated surface emits infrared light. The majority of Earth’s atmosphere (N2 and O2) are not good greenhouse gas. The small amount of greenhouse gases (H2O, CO2) traps (absorb and re-emit) the infrared radiation, increasing the temperature of the atmosphere... Ozone layer In the 1970s, scientists noticed that the ozone layer in the stratosphere above Antarctica was thinning. Chlorofluorocarbons & the ozone layer A group of chemicals called chlorofluorocarbons (or CFCs) were once commonly used in air conditioners, in aerosol spray cans, and for cleaning machine parts. In the London Agreement of 1991, more than 90 countries banned the production and use of CFCs except for limited medical uses. The ozone layer absorbs the Sun’s high-energy ultraviolet (UV) radiation and protects the Earth. In the stratosphere, the CFCs break down and release chlorine. The chlorine reacts with ozone molecules, which normally block incoming ultraviolet radiation. Acid rain occurs when oxides of sulfur and oxides of nitrogen are emitted into the atmosphere, undergo chemical transformations and are absorbed by water droplets in clouds. Layers of the earth Composition • Composition= what it’s made of Effects of Acid Rain If we could take a chunk out of the Earth, we would see that it is made up of different layers. Earth is made up of 3 main layers – Crust – Mantle – Core Physical Properties and • Physical properties= characteristic that is unique and helps to identify the substance (temp, size, shape, color) Acidification of bodies of water Damage of vegetation Damage to building materials, statues, etc. • Example: Chocolate Chip Cookies -Composition- flour, eggs, sugar, chocolate chips, baking powder, butter -Properties- round, rough, sweet, tan and black, hot, lumpy, CRUST The Greenhouse Effect on Earth Earth’s atmosphere is slightly warmer than what it should be due to direct solar heating because of a mild case of greenhouse effect... The ground is heated by visible and (some) infrared light from the Sun. – Physical Properties: • outermost layer • thinnest layer (5-70km thick) • Surface temperature • 1% of Earth’s mass • where we live • touches the atmosphere – Composition: • consists of loose rocks & soil 2 Types of Crust: • Continental Crust:dry land, granite ess dense Oceanic Crust: ocean floor,Basalt, thinner than cont. crust Mesosphere Bottom Mantle strong, lower part of the mantle layer between asthenosphere and core The Core Outer and Inner • The core is divided into two parts MANTLE –Physical Properties: • thickest layer (2900km thick) • 1600-4000 F • 66% of earth’s mass • flowing – Composition: • Molten rock –Magma – Outer Core: • Liquid iron and nickel that’s spinning – Inner Core: • Solid iron and nickel • Solid because of all the pressure of the rest of the Earth surrounding it. How do we know? CORE – Physical properties: • HOT! 4000-8000 F • Very dense • High pressure • 4000 miles from surface • 33% of Earth’s mass • About the same size as Mars – Composition: • Iron and Nickel (metals) What have you noticed about temperature and pressure? • As you get deeper inside the Earth, temperature Increases • As you get deeper inside the Earth, pressure Increases The 3 main layers of the Earth can be divided further by the way they “act” within the Earth and by their different physical properties. Lithosphere Crust and upper Mantle • outermost layer – includes crust and upper mantle • rigid • divided into pieces or tectonic plates • Rocks and soil Asthenosphere Middle Mantle • composed of solid flowing rock • layer on which pieces of lithosphere move on top (solid rock that flows) – Think of it like caramel • Seismic waves produced by earthquakes travel at different speeds through solid rock and liquids