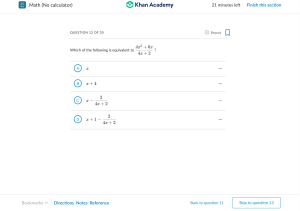
Chemistry Concept of Mole and Chemical Counting Ques 1: A Na2CO3 22g B 150ml H2SO4 22g a. Define analar? b. H2O is a polar compound – Explain. c. Find the percentage composition of the salt which is formed between the two compounds given in the Stem. d. 50 ml more water is added to the beaker-A. Now compare the concentration of the compound A and compound B. Ques 2: To prepare common salt, 39.75 gm of sodium carbonate reacts with 30 gm of Hydrochloric acid. a. b. c. d. What is Limiting reagent? What do you mean by the symbol 23 11𝑋? Explain. Calculate how much common salt will be produced in the stem. How much CaCO3 is required to obtain CO2 gas which is produced in the stem reaction.