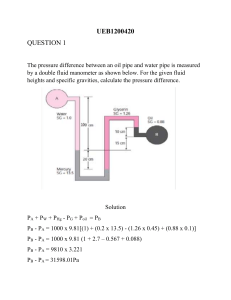
Thermal Science Rub your hands together for 15 seconds. Are your hands warm? Thermal energy What is thermodynamics? • The study of thermodynamics is concerned with ways energy is stored within a body and how energy transformations, which involve heat and work, may take place. • Approaches to studying thermodynamics – Macroscopic (Classical thermodynamics) • study large number of particles (molecules) that make up the substance in question • does not require knowledge of the behavior of individual molecules – Microscopic (Statistical thermodynamics) • concerned within behavior of individual particles (molecules) • study average behavior of large groups of individual particles Applications of Thermodynamics Thermodynamic Systems Thermodynamic System – quantity of matter or a region of space chosen for study Boundary – real or imaginary layer that separates the system from its surroundings Surroundings – physical space outside the system boundary Types of Systems – Closed – Open Closed Systems (fixed masses) Energy, not mass, crosses closed-system boundaries Closed System with Moving Boundary Open Systems (Control Volumes) Mass and Energy Cross Control Volume Boundaries Isolated System • Closed system where no heat or work (energy) may cross the system boundary – typically a collection of a main system (or several systems) and its surroundings is considered an isolated system Properties • • Any characteristic of a system in equilibrium is called a property. Types of properties – Extensive properties - vary directly with the size of the system Examples: total volume, total mass, total energy – Intensive properties - are independent of the size of the system • Examples: temperature, pressure. Extensive properties per unit mass are intensive properties. specific volume, v = Volume/Mass = V/m density, r = Mass/Volume = m/V Gas properties Interactive tool (https://phet.colorado.edu/) State & State Postulate • State Postulate • • • • • The state of a simple compressible system is completely specified by two independent, intensive properties. A system is called a simple compressible system in the absence of electrical, magnetic, gravitational, motion, and surface tension effects. These effects are due to external force fields and are negligible for most engineering problems. Otherwise, an additional property needs to be specified for each effect that is significant. Two properties are independent if one property can be varied while the other one is held constant. • State of a system – system that is not undergoing any change – all properties of system are known & are not changing – if one property changes then the state of the system changes Equilibrium • Thermodynamic equilibrium – “equilibrium” - state of balance – A system is in equilibrium if it maintains thermal (uniform temperature), mechanical (uniform pressure), phase (mass of two phases), and chemical equilibrium – Thermal equilibrium: If the temperature is the same throughout the entire system. – Mechanical equilibrium: If there is no change in pressure at any point of the system with time. – Phase equilibrium: If a system involves two phases and when the mass of each phase reaches an equilibrium level and stays there. – Chemical equilibrium: If the chemical composition of a system does not change with time, that is, no chemical reactions occur. Processes & Paths • Process – when a system changes from one equilibrium state to another one – some special processes: • isobaric process - constant pressure process • isothermal process - constant temperature process • isochoric process - constant volume process • isentropic process - constant entropy • isenthalpic process - constant enthalpy • Path – series of states which a system passes through during a process Terminology Compression Process Quasi-Equilibrium Processes • System remains practically in equilibrium at all times • Easier to analyze (equations of state can apply) • Sufficiently slow process • Properties in one part of the system do not change any faster than those at other parts Thermodynamic Cycle • Cycles – A process (or a series of connected processes) with identical initial and final states Heat and Temperature ➢ Though we all have a feel for temperature (‘like when we are feeling hot’); in the context of TD temperature is technical term with ‘deep meaning’. ➢ Temperature is a measure of the ‘intensity of heat’. ‘Heat flows’ (energy is transferred as heat) from a body at higher temperature to one at lower temperature. (Like pressure is a measure of the intensity of ‘force applied by matter’→ matter (for now a fluid) flows from region of higher pressure to lower pressure). ➢ That implies (to reiterate the obvious!) if I connect two bodies− (A)-one weighing 100kg at 10C and the other (B) weighing 1 kg at 500C, then the ‘heat will flow’ from the hotter body to the colder body (i.e. the weight or volume of the body does not matter). Temperature #1 Temperature #2 Heat TEMPERATURE AND THE ZEROTH LAW OF THERMODYNAMICS ➢ Temperature comes in two important ‘technical’ contexts in TD: 1. It is a measure of the average kinetic energy (or velocity) of the constituent entities (say molecules) 2. It is the parameter which determines the distribution of species (say molecules) across various energy states available. ➢ The zeroth law of thermodynamics : If two bodies are in thermal equilibrium with a third body, they are also in thermal equilibrium with each other. ➢ By replacing the third body with a thermometer, the zeroth law can be restated as two bodies are in thermal equilibrium if both have the same temperature reading even if they are not in contact. THE ZEROTH LAW OF THERMODYNAMICS Two bodies reaching thermal equilibrium after being brought into contact in an isolated enclosure Temperature Scales ➢ Any body with at least one measurable property that changes as its temperature changes can be used as a thermometer. ➢ Such a property is called a Thermometric Property. ➢ The particular substance that exhibits changes in the thermometric property is known as a Thermometric Substance. ➢ Temperature scales enable us to use a common basis for temperature measurements. ➢ All temperature scales are based on some easily reproducible states such as the freezing and boiling points of water: the ice point and the steam point. ➢ Ice point: A mixture of ice and water that is in equilibrium with air saturated with vapor at 1 atm pressure (0°C or 32°F). ➢ Steam point: A mixture of liquid water and water vapor (with no air) in equilibrium at 1 atm pressure (100°C or 212°F). Temperature Scales Scale Freezing Boiling point point of water of water Celsius 0°C 100°C Fahrenheit 32°F 212°F Kelvin 273K 373K • Matter is made up of molecules in motion (kinetic energy) • Absolute Zero: occurs when all kinetic energy is removed from an object, 0 K = -273° C Liquid-in-Glass thermometer ➢ Consists of a glass capillary tube connected to a bulb filled with a liquid such as alcohol and sealed at the other end. ➢ Space above the liquid is occupied by the vapor of the liquid or an inert gas. ➢ As temperature increases, the liquid expands in volume and rises in the capillary. ➢ Length L of the liquid in the capillary depends on the temperature. ➢ Liquid is the thermometric substance and L is the thermometric property. Ideal-Gas Temperature Scale ➢ The temperatures on this scale are measured using a Constant-Volume Gas Thermometer ➢ Thermometer is based on the principle that at low pressures, the temperature of a gas is proportional to its pressure at constant volume. ➢ Temperature of a gas of fixed volume varies linearly with pressure at sufficiently low pressures. ➢ ΔT = (V / R) ΔP ➢ Constant-volume gas thermometer is basically a rigid vessel filled with a gas, usually hydrogen or helium, at low pressure. Problem Applications of Thermal Energy http://www.nrel.gov Stress in a fluid Stress: Force per unit area. Normal stress: The normal component of a force acting on a surface per unit area. Shear stress: The tangential component of a force acting on a surface per unit area. Pressure: The normal stress in a fluid at rest. Zero shear stress: A fluid at rest is at a state of zero shear stress. When the walls are removed or a liquid container is tilted, a shear develops as the liquid moves to re-establish a horizontal free surface. The normal stress and shear stress at the surface of a fluid element. For fluids at rest, the shear stress is zero and pressure is the only normal stress. Pascal’s Law ➢ ➢ ➢ ➢ ➢ ➢ In absence of shear stress only 3 components of normal stresses are present. As static fluid has isotropic nature, so these three components have same magnitude for all direction. 𝜎𝑥𝑥 = 𝜎𝑦𝑦 = 𝜎𝑧𝑧 =-p ‘p’ is thermodynamic pressure (-ve sign shows compressive nature). Pressure at a point in a fluid at rest, or in motion, is independent of direction as long as there are no shearing stresses present- Pascal’s Law The pressure applied to a confined fluid increases the pressure throughout by the same amount. Pressure variation in a static fluid Pressure variation in a static fluid ➢ For a fluid at rest, pressure gradient must be balanced by the gravity force ➢ Recall: ∇p is perpendicular everywhere to surface of constant pressure p. ➢ In our customary coordinate z is “upward” and the gravity vector is: ➢ where g = 9.807 m/s2. The pressure gradient vector becomes: Pressure variation in a static fluid ➢ For incompressible fluid: ➢ 𝑝1 − 𝑝2 = ρgh = 𝛾 h ➢ H = 𝑧2 - 𝑧1 = 𝑝1 −𝑝2 𝛾 = ∆𝑝 𝛾 ➢ The quantity, p\γ is a length called the pressure head of the fluid. Hydrostatic pressure distribution ➢ Pressure in a continuously distributed uniform static fluid varies only with vertical distance and is independent of the shape of the container. ➢ The pressure is the same at all points on a given horizontal plane in a fluid. Pressure-Terminology ➢ Absolute pressure: The actual pressure at a given position. It is measured relative to absolute vacuum (i.e., absolute zero pressure). ➢ Gage pressure: The difference between the absolute pressure and the local atmospheric pressure. Most pressure-measuring devices are calibrated to read zero in the atmosphere, and so they indicate gage pressure. ➢ Vacuum pressures: Pressures below atmospheric pressure. The mercury barometer ➢ Atmospheric pressure is measured by a device called a barometer; thus, the atmospheric pressure is often referred to as the barometric pressure. ➢ A frequently used pressure unit is the standard atmosphere, which is defined as the pressure produced by a column of mercury 760 mm in height at 0°C (rHg = 13,595 kg/m3) under standard gravitational acceleration (g = 9.807 m/s2) ➢ Mercury has an extremely small vapor pressure at room temperature (almost vacuum), thus p1 = 0. One can write: The Manometers and Manometry ➢ Science of pressure measurement – Manometry. ➢ The device similar to barometer and used to measure pressure is called Manometer. ➢ Manometers measure a pressure difference by balancing the weight of a fluid column between the two pressures of interest. ➢ Three common types of manometers are Piezometer tube, U-tube Manometer and Inclined tube manometer. Piezometer tube ➢ An elevation change of h1 in a fluid at rest corresponds to Dp/rg. ➢ A device based on this is called a Piezometer tube. ➢ pA = p1 - patm = rgh1 ➢ pAgage = p1gage = rgh1 ➢ pA ∝ h1 ➢ Disadvantages: • • • Can’t measure Gas pressures Can’t measure pressure in vacuum Difficult to measure large pressure. U-tube Manometer ➢ A manometer consists of a Utube containing one or more fluids such as mercury, water, alcohol, or oil. ➢ Heavy fluids such as mercury are used if large pressure differences are anticipated. ➢ Overcomes the disadvantages of Piezometer tube. ➢ It is commonly used to measure small and moderate pressure differences. Manometric calculation 1. 2. 3. 4. 5. 6. Begin writing pressures from any end of manometer. Keep on adding pressure increments on going down through the fluid in same column. Use equation: pdown = pup +𝜸 ∆𝒉 Jump across to other column when two points in same fluid are at equal elevations (Pascal’s Law). Keep on repeating steps 2 and 3 until you reach other end. Solve the system of equations of obtain unknown pressures. Inclined tube manometer ➢ Its difficult to detect small pressure changes in U-tube manometer. ➢ To detect small pressure changes, inclined tube manometer is used. ➢ One leg is inclined at angle θ (variable) with horizontal. ➢ Difference in level as seen in inclined tube is 𝑙2 . ➢ Pressure difference is not due to 𝑙2 but due to its vertical projection i.e. 𝒍𝟐 sinθ. ➢ 𝑝𝐴 - 𝑝𝐵 =𝛾2 𝑙2 sinθ+ 𝛾3 ℎ3 - 𝛾1 ℎ1 ➢ If A and B contain gas (gas pressures are usually neglected), so ➢ 𝑝𝐴 - 𝑝𝐵 =𝛾2 𝑙2 sinθ 𝑝 −𝑝 ➢ or 𝑙2 = 𝐴 𝐵 𝛾2 sinθ Other Pressure Measurement Devices Bourdon tube: Consists of a hollow metal tube bent like a hook whose end is closed and connected to a dial indicator needle. Pressure transducers: Use various techniques to convert the pressure effect to an electrical effect such as a change in voltage, resistance, or capacitance. Pressure transducers are smaller and faster, and they can be more sensitive, reliable, and precise than their mechanical counterparts. Strain-gage pressure transducers: Work by having a diaphragm deflect between two chambers open to the pressure inputs. Piezoelectric transducers: Also called solid-state pressure transducers, work on the principle that an electric potential is generated in a crystalline substance when it is subjected to mechanical pressure. References 1. Thermodynamics An Engineering Approach, Third Edition, Yunus A. Çengel & Michael A. Boles, McGraw-Hill Publication. 2. Basic and Applied Thermodynamics by P. K. Nag, Tata McGraw Hill (2002). 3. Fluid Mechanics: Fundamentals and Applications, 3rd Edition, Yunus A. Cengel, John M. Cimbala, McGraw-Hill, 2014. 4. Fluid Mechanics, Frank M. White, McGraw Hill, 4th Ed. 5. Fundamentals of Fluid Mechanics by Bruce R. Munson, Donald F. Young, Theodore H. Okiishi, Wade W. Huebsch, Wiley, 6th Ed.