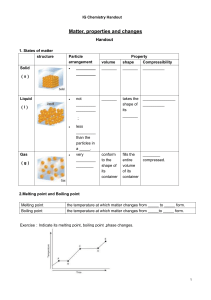
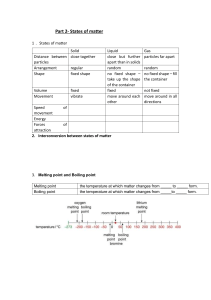
IG Chemistry Handout Matter, properties and changes Handout 1. States of matter structure Solid (s) Liquid Particle arrangement _________ _________ (l) not _________ _________ volume _______ _______ takes the shape of its _______ _______________ __________ conform to the shape of its container fills the entire volume of its container ________ compressed. ; Gas (g) less _________ than the particles in a _____. very _________ ________ Property shape Compressibility _______ __________ 2.Melting point and Boiling point Melting point Boiling point the temperature at which matter changes from _____ to _____ form. the temperature at which matter changes from _____to _____ form. Exercise:Indicate its melting point, boiling point ,phase changes. 1 IG Chemistry Handout 3. Physical change and chemical change Definition Key point Physical change A change which alters a substance without changing its ______ chemical change A process that involves _________________________ _______ See whether there’s any ________ produced. Common types 5.Physical property and chemical property Definition Physical property can be observed without changing the sample’s composition.(___________) chemical property The ability of a substance to combine with or change into one or more other substances (__________). Common properties 2