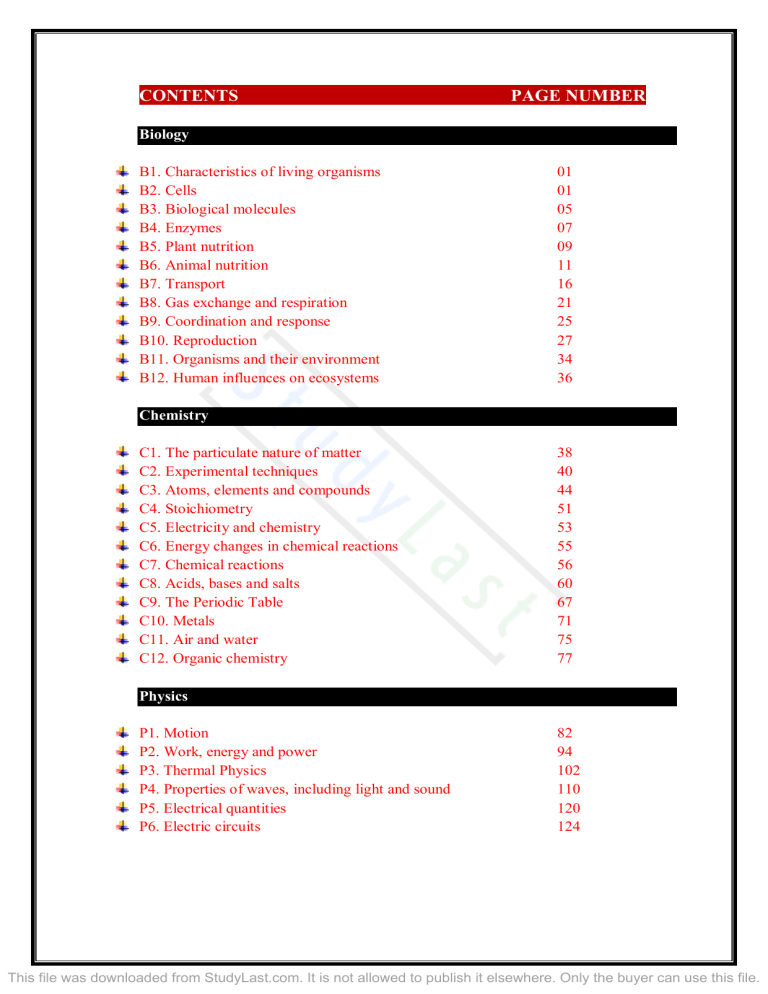
CONTENTS PAGE NUMBER Biology B1. Characteristics of living organisms B2. Cells B3. Biological molecules B4. Enzymes B5. Plant nutrition B6. Animal nutrition B7. Transport B8. Gas exchange and respiration B9. Coordination and response B10. Reproduction B11. Organisms and their environment B12. Human influences on ecosystems 01 01 05 07 09 11 16 21 25 27 34 36 Chemistry C1. The particulate nature of matter C2. Experimental techniques C3. Atoms, elements and compounds C4. Stoichiometry C5. Electricity and chemistry C6. Energy changes in chemical reactions C7. Chemical reactions C8. Acids, bases and salts C9. The Periodic Table C10. Metals C11. Air and water C12. Organic chemistry 38 40 44 51 53 55 56 60 67 71 75 77 Physics P1. Motion P2. Work, energy and power P3. Thermal Physics P4. Properties of waves, including light and sound P5. Electrical quantities P6. Electric circuits 82 94 102 110 120 124 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section Characteristics of living organisms Describing the characteristics of living organisms by defining the following terms Movement: movement refers to an action by an organism causing a change of position or place. Reproduction: reproduction refers to processes that make more of the same kind of organism. Sensitivity: sensitivity refers to the ability to detect and respond to changes in the environment. Growth: growth refers to the permanent increase in size and dry mass by an increase in the number of cells, cell size, or both. Respiration: respiration refers to the chemical reactions in cells that break down nutrient molecules and release energy. Excretion: excretion refers to removal, from organisms, of toxic materials and substances in excess of requirements. These toxic materials are waste products of metabolism and substances taken in excess. Nutrition: nutrition refers to taking in of materials for energy, growth and development. The seven characteristics could be memorized by the term “MRS. GREN” using the first letters of each characteristic mentioned above. Cells Cell structure Stating that living things are made of cells Living organisms are made of cells. Cells are very small; hence, they can only be seen under a microscope. We have two types of cells namely plant and animal cells. Describing and comparing the structure of a plant and animal cell. Note similarities: both contain a cell membrane, cytoplasm and nucleus Note differences: in addition, a plant cell contains a cell wall, chloroplasts, and a sap vacuole Page 1 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section Stating the functions of the structures found in cells 1. Cell wall: This is a non-living thick rigid layer surrounding the cell. It is made of cellulose, and it gives the cell its shape (angular, rectangular or rounded) and prevents it from bursting. It also allows the plant to support itself. The cell wall is permeable i.e. it allows all substances to move into or out of the cell. Only the semi-permeable membrane will allow or dis-allow entry or exit of some substances into the cell. 2. Cell membrane: This is a partially permeable membrane. It controls the movement of substances into and out of the cell. 3. Cytoplasm: This is a jelly like substance made up of mostly water and structures. Metabolic reactions occur in the cytoplasm. 4. Chloroplasts: These are green discs which contain chlorophyll (which is a green pigment that traps sunlight for photosynthesis). 5. Nucleus: Controls all activities of the cell. 6. Sap vacuole: A vacuole is a large room in the center of the cell. It contains cell sap. The cell sap stores dissolved sugars, mineral salts and amino acids. It also controls the movement of water in and out of the cell. Page 2 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section Relating the structure of certain cells to their functions: Type of cell Ciliated cells Where it is found Lining the trachea and bronchi Root hair cells Xylem vessels Near the ends of plant roots In stems, roots and leaves of plants Palisade mesophyll cells Nerve cells Beneath the epidermis of a leaf Red blood cells Sperm and egg cells In the blood of mammals Sperm in testes and egg cells in ovaries Throughout the bodies of animals Function Move mucus upwards to the back of the throat Absorb water and mineral salts Transport water and mineral salts; help in support Photosynthesis Transmit information in the form of electrical impulses Transport oxygen Fuse together to produce a zygote Ciliated Cells: Ciliated cells have cilia whose function is to move the mucus up the trachea and bronchi to the throat. The mucus traps bacteria and dust particles. When it reaches the throat, mucus is swallowed to the stomach where the acid kills the bacteria. Root Hair Cells: Their function is to absorb water and minerals from the soil. They are adapted by 3 ways. One, they have a large surface area for more water intake. Two, they have a large number of mitochondria for respiration to become more active. Three, they have a concentrated vacuole to support the absorption of water by osmosis. Palisade mesophyll cells: Consist of one or two layers of closely-packed, long and cylindrical cells containing chloroplasts. Their function is to facilitate photosynthesis. They contain numerous chloroplasts to allow maximum absorption of light. Leaf structure showing one layer of palisade mesophyll cells Page 3 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section Sperm cells have a tail – to help them swim a head which has digestive enzymes to breakdown the outer membrane of the ovum to allow fertilization to occur a middle piece with numerous mitochondria to provide energy for the sperm to swim (this point is not in the syllabus though) An egg cell Egg cells (ova) possess a yolk which acts as a food store. The spherical shape of ova allows cilia lining the oviducts to easily move the ovum so that it can meet sperm and be fertilized by it. Calculating magnification and size of biological specimens using millimetres as units Magnification = 𝑠𝑖𝑧𝑒 𝑜𝑓 𝑑𝑟𝑎𝑤𝑖𝑛𝑔 𝑠𝑖𝑧𝑒 𝑜𝑓 𝑟𝑒𝑎𝑙 𝑜𝑏𝑗𝑒𝑐𝑡 in mm Movement in and out of cells Defining diffusion Diffusion is the net movement of particles from a region of higher concentration to a region of lower concentration down a concentration gradient, as a result of their random movement. Substances move into and out of cells by diffusion through the cell membrane. Water diffuses through partially permeable membranes by osmosis. Defining osmosis Osmosis is the net movement of water molecules from a region of higher water potential (dilute solution) to a region of lower water potential (concentrated solution), through a partially permeable membrane. Water moves in and out of cells by osmosis through the cell membrane. Water also enters the roots by osmosis. Page 4 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section Investigating and describing the effects of immersing of plant tissues in solutions of different concentrations Water potential is a measure of the tendency of water molecules to move from one region to another. Dilute solutions have a higher water potential than concentrated solutions. Pure water has the highest water potential. Water molecules that dissolve solutes are bound to the solute particles. A dilute solution thus has a lot of unbound water molecules, giving it a higher water potential than a concentrated solution. Immersing plant tissues in concentrated solutions of salts make the tissues smaller (flaccid) due to the movement of water molecules out of the tissues and into the concentrated solution. Immersing plant tissues in pure water can make the tissues bigger (turgid) due to movement of water molecules from the pure water and into the tissues. Cell walls in plant cells prevent them from bursting. Absence of cell walls in animal cells can result in bursting due to too much water uptake by cells. Small dots are water and large dots are solute particles. Biological molecules Listing the chemical elements that make up: 1. Carbohydrates: made up of carbon, hydrogen and oxygen only. 2. Fats: made up of carbon, hydrogen and oxygen (less oxygen than in carbohydrates). 3. Proteins: made up of carbon, hydrogen, oxygen and nitrogen. They sometimes contain sulphur and phosphorus. Large molecules are made from smaller molecules: e.g. 1. Starch and glycogen are made from glucose 2. Proteins are made from amino acids 3. Fats and oils are made from fatty acids and glycerol Page 5 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section Food tests Test for starch 1. The test for starch is called the Iodine test. Iodine is added to the sample and the colour change (if any) is observed. 2. Procedure: Add a few drops of iodine solution (which is brown) to the sample. If the sample contains starch, it will turn blue-black in colour. Test for reducing sugars 1. The test for reducing sugars is known as the Benedict’s test. 2. Reducing sugars are glucose, fructose, galactose, maltose and lactose. Sucrose is not a reducing sugar. 3. Procedure: Add Benedict’s solution to the sample and mix the contents thoroughly. Heat the mixture in a boiling water bath for 5 minutes. 4. The colour of the solution changes from blue to green to yellow to orange to brick-red if a reducing sugar is present. Test for proteins 5. Both test tubes on the left contain reducing sugar, however, the one on the right side contains more than the one on the left. 1. The test for proteins is known as the Biuret test. 2. The main reagents are aqueous sodium hydroxide (potassium hydroxide can also be used) and copper (II) sulfate. A ready mixed reagent of the two chemicals called Biuret reagent can also be used. 3. Procedure: First add copper sulfate solution to a solution of the food sample, followed by either sodium or potassium hydroxide solution. 4. If proteins are present, a colour change from blue to purple will be observed. Test for fats and oils 1. The test for fats is known as the ethanol emulsion test. 2. Fats in the sample are dissolved by adding ethanol. Water is then added to the ethanolic mixture. Since fats do not dissolve in water, they form a cloudy white emulsion. Page 6 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section Nutrient Starch Reducing sugars Proteins Fats Test Iodine solution Benedict’s solution Biuret reagent Ethanol and water Original colour Brown Blue Blue ------------ Positive result Blue/black Brick red Purple Cloudy Negative result Brown Blue Blue Clear Water: About 70% of your weight is water. Water is an essential nutrient. The functions of water include: • As a solvent which dissolves reactants of metabolic reactions. • As a component of blood plasma in which red blood cells, nutrients, hormones and other materials are carried in. • It helps in lowering the body temperature in hot conditions in the form of sweat on the skin. The sweat evaporates using heat energy from the body, thus lowering the temperature. Enzymes Enzymes are proteins that function as biological catalysts. Catalysts speed up the rate of chemical reactions without being altered in the reaction. Explaining enzyme action 1. Enzymes are biological catalysts that speed up the rate of chemical reactions without being altered in the reaction. They are made of proteins. 2. They are substrate-specific. Substrates are the reactants that an enzyme acts on e.g. amylase can only digest starch and not cellulose even though they are both polymers of glucose. Enzymes are believed to function using the lock and key hypothesis. 1. An active site is the region on an enzyme molecule that the substrate binds to. It is usually a groove on the surface of the enzyme. Only the correct substrate is able to fit into the active site. 2. Enzyme action begins when the substrate molecule binds to the active site of the enzyme to form an enzyme-substrate complex. 3. The substrate is then converted into product molecules. 4. The product molecules depart from the active site, leaving the enzyme free to catalyse another reaction. Page 7 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section Investigating and describing the effect of changes in temperature and pH on enzyme activity Effect of temperature 1. At low temperatures, the rate of a reaction is very low because substrate particles are moving too slowly to react. Substrate and enzyme molecules have little kinetic energy; hence the frequency of collision is low. 2. As the temperature increases, the rate of enzyme activity increases. Substrate and enzyme molecules gain more kinetic energy; hence the frequency of collisions between substrate molecules and active sites of enzymes increases. 3. Enzyme activity increases up to an optimum temperature, beyond which it starts decreasing. Enzymes which are found in the human body usually work best at about 37 °C. 4. As the temperature increases beyond the optimum temperature, enzyme activity drops because enzymes are made of proteins, which are denatured at high temperatures. 5. At extremely high temperatures, the enzyme is completely denatured and the rate of reaction drops to zero. Page 8 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section 1. Enzyme activity is highest at the optimum pH of the enzyme. Effect of pH 2. As the pH increases or decreases from the optimum, enzyme activity sharply decreases. This is because the shape of the active site is changed as the enzyme is denatured. When this happens, the active site no longer fits the substrate, so the enzyme can no longer catalyse its reaction. 3. At extreme pH levels, the enzyme is completely denatured and the rate of reaction drops to zero. 4. The optimum pH for each enzyme differs. For example, pepsin works best under the acidic conditions in the stomach, while intestinal enzymes work best under alkaline conditions. Plant nutrition Photosynthesis is the process by which plants manufacture carbohydrates from raw materials using energy from light. The word equation for photosynthesis is: carbon dioxide + water → glucose + oxygen, in the presence of light and chlorophyll The balanced chemical equation for photosynthesis is: Chlorophyll converts light energy into chemical energy in molecules. The chemical energy is then used for the synthesis of carbohydrates. The subsequent use and storage of the carbohydrates made in photosynthesis Glucose, the product of photosynthesis is the most important food of the plant. Plants make other nutrients from glucose. Glucose is transported to other parts of the plant as sucrose e.g to the roots, where it can be converted back to glucose for respiration, and part of it into starch for storage (this is the case with potatoes and sweet potatoes). Growing cells make cellulose for cell walls from sucrose. Fruits use the sucrose to make the attractive scent and tasty nectar to attract insects. Importance of photosynthesis Converts light energy from the Sun to chemical energy in the form of glucose, which can then be used by plants and animals. Page 9 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section In the day, the leaf produces more glucose than can be removed. The excess glucose is then converted into starch and stored in the leaf, or converted to sucrose and transported to other parts of the plant via the phloem. At night, starch stored in the leaf is then converted to glucose for respiration. Excess glucose is converted to sucrose before being transported out of the leaf for use by other parts of the plant, or converted to starch for storage in the various storage organs. The stored starch can be used to make cellulose. Photosynthesis ensures that living things have a constant supply of oxygen. Factors affecting photosynthesis The rate of photosynthesis increases as the amount of water, concentration of carbon dioxide, temperature and light intensity increase. Submerged aquatic plants can have reduced photosynthesis due to events like eutrophication which cause growth of algae on the surface of water bodies resulting in reduced light intensity reaching the submerged aquatic plants. Leaf structure You should be able to identify chloroplasts, cuticle, guard cells and stomata, upper and lower epidermis, palisade mesophyll, spongy mesophyll, vascular bundles, xylem and phloem in leaves of a dicotyledonous plant. Features of leaves that enable it to perform its functions Palisade mesophyll cells for photosynthesis: Their function is to facilitate photosynthesis. They contain numerous chloroplasts to allow maximum absorption of light. Their chloroplasts are arranged alongside the cell wall and are Page 10 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section most abundant on the side facing the sunlight. The position of the chloroplasts actually changes to ensure efficient absorption of sunlight. Stomata, spongy mesophyll cells and guardcells for gas exchange: Spongy mesophyll cells have air spaces to allow for fast diffusion of carbon dioxide, which enters the leaf through the stomata, to all photosynthetic cells. Guard cells are bean-shaped, chloroplast-containing cells located in the lower epidermis. They control the opening and closing of the stoma (plural: stomata), the gap between the guard cells. The stomata allow carbon dioxide to diffuse in, oxygen to diffuse out and water vapour to escape. Plants open their stomata during the day for carbon dioxide intake and close their stomata during the night to minimize water loss through transpiration. Guard cells control the opening and closing of stomata through regulation of water potential within themselves. Xylem for transport and support The xylem transports water and mineral salts from the roots to the leaves. A xylem vessel is made up of hollow tubes joined end to end forming a long drain pipe which runs from the roots to the stem. The xylem also adds mechanical support to the plant since its walls are made of strong cellulose and lignin Phloem for transport The function of the phloem is to transport sugars and amino acids from the leaves to other parts of the plant. Describing the importance of nitrate ions for making amino acids and magnesium ions for making chlorophyll • Mg2+ (Magnesium ions): they are important for the production of the green pigment chlorophyll. Lack of it results in lack of photosynthesis and yellowing between the veins of leaves • Nitrates: these are the sources of nitrogen; they are required to make amino acids and proteins. Lack of it results in weak growth and yellowing of the leaves. Both mineral ions are absorbed from the soil. Animal nutrition A balanced diet: is a perfect diet which contains all of the nutrients in reasonable proportions. Page 11 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section Nutrient Source Carbohydrates Fruits, Honey, sugar, Bread, Potatoes, Pasta, sadza/maize meal etc Fats Butter, cooking oil, red meat Proteins Milk, meat, eggs Vitamin C Citrus fruits e.g oranges and lemons (vitamin C is damaged by cooking) Vitamin D Butter, egg yolk, exposure to sunlight Dietary importance Energy resource, essential in respiration to release energy. Also used in creating cellulose, the substance that makes up cell walls of plant cells. Synthesis of membranes. Solvent for fat soluble vitamins. Storage of energy. Insulating material. For growth and repair of worn out tissues. Synthesis of enzymes and hormones. Formation of antibodies. Essential for the formation of collagen, a protein that functions as a cementing layer between cells. Increases immunity. Promotes absorption of calcium from small intestines and its deposition in bones. Mineral salts Calcium Milk, cheese, eggs Making bones and teeth. Normal functioning of muscles and blood clotting process. Iron Red meat especially liver, green leafy vegetables Needed for the formation of the red pigment haemoglobin, which is essential for the transportation of oxygen around the body in red blood cells. Fibre/roughage Fruits and vegetables (cellulose from roughage is not digested/absorbed. Roughage is from cell walls of plant cells.) Provide bulk to undigested matter and promote peristalsis. The process of pushing the food through the gut is called peristalsis, without roughage peristalsis is very slow and weak Water Water, juices. (70% of the body is water) Medium/solvent for various enzymatic reactions. Main component of blood plasma. Main component of sweat. Sweat lowers body temperature in hot conditions by absorbing heat from the body. This leads to the evaporation of the sweat. Page 12 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section How age, gender and activity affect the dietary needs of humans including during pregnancy and whilst breast-feeding. Pregnant Women: The diet of a pregnant woman needs to be very rich in certain nutrients because she is not only feeding herself; she is feeding her baby as well. In order for the fetus to develop well, it needs extra protein, iron, calcium and vitamin D. Proteins are used to develop the tissues of the fetus, iron is used to make haemoglobin, while calcium and vitamin D are used to develop the baby’s bones. Breast-Feeding Women (Lactation): Lactation means the production of breast milk. After pregnancy, the mother breast-feeds the baby for about 6 months or more. Breast milk needs to be high in proteins, calcium, and vitamins to guarantee the healthy growth of the infant. Growing Children (Passing Puberty): At some point, each child gets a growth spurt. This is a very high growth rate that increases the child’s size and mass in a short period of time. A growing child’s diet needs extra proteins to develop cells and enzymes because their metabolic rate is higher. They also need calcium and vitamin D to develop bones, and iron to make haemoglobin. Some other considerations: Men need more energy than women. Teenagers need more energy, proteins and calcium than adults. Blue collar (industrial) workers need more energy than white collar (office) workers. Effects of malnutrition in relation to starvation, obesity, constipation, coronary heart disease and scurvy Malnutrition is eating inadequate proportions of food. It is a consequence of an unbalanced diet. An unbalanced diet is rich in some nutrients and low in others, or even lacking. There are lots of effects of malnutrition such as starvation, obesity or deficiency diseases. Starvation is an effect of malnutrition. In the case of starvation, the body tends to feed on its own self. When the glucose level is decreased in the body, the liver breaks down fats to respire for energy, when the body is out of fats, it starts respiring proteins from the muscles to release energy, eventually the body ends up looking like a skeleton. Starvation is usually present in countries with famines, which are caused by poverty, large populations, low amounts of food, unsuitable climates and lack of money. Obesity is the opposite of starvation. It is eating too much of every nutrient, especially carbohydrates and fats. Obesity doesn’t strike alone; it brings with it several other diseases such as high blood pressure, cardiac diseases, diabetes, stress on joints and bones, as well as other psychological issues like low self-esteem and lack of confidence. To prevent obesity, you have to control your carbohydrates and fats intake and exercise regularly. Page 13 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section Constipation: lack of fibre in the diet causes undigested matter to lack bulk. This results in poor peristalsis, meaning that food stays too long in the large intestine. Since food stays longer, lots of water is absorbed by the large intestine, resulting in dry hard stools. Coronary heart disease: over consumption of carbohydrates and fats can lead to obesity. The excess carbohydrates are converted into fats resulting in fatty deposits in organs and arteries. These fatty deposits may lead to coronary heart diseases. Scurvy is caused by a deficiency of vitamin C. Its symptoms include bleeding gums. Explaining the causes and effects of vitamin D and iron deficiencies Rickets (soft and deformed bones) is the deficiency disease of both vitamin D and calcium. Bones are made of calcium, which vitamin D helps to deposit in the bones. If either calcium or vitamin D is lacking in the diet, rickets is developed. Anaemia is the deficiency disease of iron. The amount of haemoglobin decreases due to iron deficiency. This causes short breath and tiredness. The Alimentary canal Ingestion is the taking of substances, e.g. food and drink, into the body through the mouth. Digestion is the breakdown of large, insoluble food molecules into small, water soluble molecules using mechanical and chemical processes. Mechanical digestion is the breakdown of food into smaller pieces without chemical change to the food molecules. The teeth are responsible for mechanical digestion. Chemical digestion is the breakdown of large, insoluble molecules into small, soluble molecules. Saliva, in the mouth, aids in chemical digestion. Chemical digestion is also found in the alimentary canal. Absorption is the movement of small food molecules and ions through the wall of the intestine into the blood. It takes place in the small intestine. Egestion is the passing out of food that has not been digested or absorbed, as faeces, through the anus. Identifying the main regions of the alimentary canal and associated organs, limited to the mouth, salivary glands, oesophagus, stomach, small intestine, pancreas, liver, gall bladder, large intestine and anus. Page 14 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section The journey of food from the mouth to the anus through the alimentary canal includes 5 steps: 1. Ingestion: Taking in pieces of food into the mouth 2. Digestion: The breakdown of large, insoluble food particles into smaller more soluble ones by chemical and mechanical means. 3. Absorption: Taking the digested food molecules from the alimentary canal and into the bloodstream 4. Assimilation: Movement of digested food molecules into cells where they are used e.g. to release energy or growth etc. 5. Egestion: The elimination of undigested food materials through the anus Digestion Don’t confuse egestion with excretion; excretion is to get rid of waste products of metabolism. Stating the significance of chemical digestion in the alimentary canal in producing small, soluble molecules that can be absorbed The significance of chemical digestion is that it produces small, soluble molecules that can be absorbed. Functions of enzymes in the human alimentary canal 1. Amylase breaks down starch to simpler sugars e.g. maltose 2. Protease breaks down protein to amino acids 3. lipase breaks down fats to fatty acids and glycerol Stating where, in the alimentary canal, amylase, protease and lipase are secreted 1. amylase – for breaking down starch to simpler sugars, e.g. maltose, is secreted in the mouth by salivary glands 2. protease – for digestion of proteins to amino acids, is secreted in the small intestines 3. lipase – for digesting fats into fatty acids and glycerol, is secreted in the small intestines Page 15 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section Stating the functions of the hydrochloric acid in gastric juice The hydrochloric acid in gastric (stomach) juices is for killing bacteria in food and giving an acid pH for enzymes to work well. Transport Transport in plants Stating the functions of xylem and phloem Xylem conducts water and dissolved mineral salts from the roots to the stems and leaves provides mechanical support to the plant Phloem transports manufactured food (sucrose and amino acids) from the leaves to other parts of the plant e.g. the roots and flowers. Transverse section of dicotyledonous stem Transverse section of dicotyledonous root For stems, the xylem is drawn as a hollow tube. Root hair cells as seen under a light microscope, as well as their function Root hairs Root hair cells take in water and minerals from the soil. Xylem vessels then take this water to all parts of the plant. Root Page 16 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section Stating the pathway taken by water through root, stem and leaf Water travels from the root to the stem and then to the leaf. The large surface area of root hairs increases the rate of the absorption of water. The pathway of water through parts of a plant can be investigated using stained water. Transpiration Transpiration is loss of water vapour from plant leaves by evaporation of water at the surfaces of the mesophyll cells followed by diffusion of water vapour through the stomata. Factors which affect transpiration Light intensity – transpiration is greater in light than in darkness Temperature – as temperature increases, the rate of transpiration also increases Humidity of the atmosphere – transpiration is low when the level of humidity is high Explaining the effects of variation of temperature, and humidity on transpiration rate • Humidity: humidity means more water vapour in the air, which means water vapour has a higher concentration in the atmosphere than inside the leaf, so transpiration will be much slower because the diffusion of water vapour outside the leaf will be slow. The higher the humidity the slower the transpiration. [Diffusion is the net movement of particles from a region of higher concentration to a region of lower concentration down a concentration gradient, as a result of their random movement]. • Temperature: when the temperature is high, molecules move faster and evaporate faster, so transpiration rate increases. The higher the temperature the faster the transpiration. Transport in mammals The circulatory system is a system of blood vessels with a pump and valves to ensure oneway flow of blood. Double circulation The heart is a muscular organ which contracts to pump blood around the body. It consists of four chambers – two upper chambers (atria) and two lower chambers (ventricles). Both ventricles have thicker walls than those of the atria. Humans have a double circulation system i.e circulation to the lungs and circulation to the body tissues. There is a low pressure circulation (between the heart and the lungs) and a high pressure circulation (between the heart and the rest of body). Advantages of double circulation Oxygenated and deoxygenated blood are separated Low pressure circulation prevents lung damage High pressure circulation ensures oxygenated blood reaches all parts of the body Page 17 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section Cardiac 1. Muscles of the atria relax allowing blood to enter the heart. Atrioventricular valves (bicuspid and tricuspid valves) open. Semilunar valves closed. Cycle 2. Muscles of the atria contract whilst muscles of the ventricles remain relaxed. Atrioventricular valves open. Semilunar valves closed. Blood is moved from atria to ventricles. 3. Muscles of the atria relax whilst muscles of the ventricles contract. Atrioventricular valves closed. Semilunar valves open. Blood moves out of the heart. Page 18 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section Naming blood vessels Using the diagram above, you should be able to name Blood vessels to and from the heart: vena cava, aorta, pulmonary artery and pulmonary vein. Blood vessels to and from the lungs: pulmonary artery and pulmonary vein. Coronary heart disease Coronary heart disease is caused by the blockage of coronary arteries. Possible risk factors include diet high in fats or cholesterol, stress, smoking, genetic predisposition, age (risk increases with age), and gender (affects males earlier than women). Physical activity and pulse rate. Physical activity and heart rate. A person’s pulse rate and heart rate are the same. Thus, physical activity increases both the pulse and heart rate. Structure and function of blood vessels 1. Arteries Function: The function of arteries is to transport blood away from the heart to the lungs or other parts of the body. Structure: The blood in the arteries (excluding the pulmonary artery) always has a high pressure. The lumen of arteries is very narrow. This adds to the pressure. Arteries have a strong thick wall which is elastic and stretchable to withstand this high pressure. Brief description of characteristics of arteries: • Transport blood away from the heart • Strong but stretchable walls • Have a high blood pressure • Narrow lumen. 2. Veins Function: the function of veins is to transport blood from the body to the heart. The veins always have low blood pressure because by the time the blood reaches the veins after touring the body, it will have lost most of its pressure. This means that blood flows very slowly in the veins. To prevent backflow, veins have valves. Structure: since veins have low blood pressure, they don’t need strong, thick walls like the arteries, instead they have thin and less elastic walls. Their lumen is much wider too. Brief description of characteristics of veins: • carry blood to the heart • have low blood pressure • have thin and less elastic walls • have wide lumen • Valves present Page 19 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section Arteries Blood flows away from the heart Possess thick elastic walls Carry oxygenated blood except pulmonary artery Do not possess valves except aorta Arteries are usually deeper in the flesh than veins Pulse is detactable Have narrow lumen Veins Blood flows towards the heart Possess thin, not very elastic walls Carry deoxygenated blood except pulmonary vein Possess valves to prevent back flow Veins are nearer to the surface of the skin Pulse is usually not detectable Have wider lumen 3. Capillaries Blood capillaries are the smallest blood vessels in our systems. Function: capillaries collect nutrient filled and oxygenated blood from the arteries and bring this blood closer to body cells. Capillaries also link arteries with veins. When arteries come near an organ or a tissue, they divide into arterioles, these arterioles divide further into capillaries that go through the tissue; this is when the exchange of oxygen and food nutrients with carbon dioxide and waste products such as urea takes place by diffusion. Components of blood Structure: Blood capillaries are very well adapted to their function. They are one cell thick to reduce the diffusion distance of materials thus promoting faster diffusion. They also have pores in their walls between the cells, to allow the plasma to get out of the blood and become tissue fluid. Components of blood include red blood cells, white blood cells, platelets and blood plasma Identifying red and white blood cells, as seen under the light microscope, on prepared slides and in diagrams and photomicrographs Diagram showing a red and a white blood cell Microscopic view of one white and many red blood cells Micrograph of red blood cells Page 20 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section Stating the functions of certain components of blood 1. Red blood cells The function of the red blood cells is to transport oxygen from the lungs to the body cells. Red blood cells contain haemoglobin, an iron containing protein which binds reversibly with oxygen. Red blood cells are fully adapted to their function by the following characteristics: Biconcave disc shape gives it large surface area to carry more oxygen Haemoglobin to combine with oxygen No nucleus that takes up space. 2. White Blood Cells: White blood cells are found in blood plasma. White blood cells are part of the Immune System. Their role is to protect the body by killing bacteria which cause disease, also known as pathogens. White blood cells are much bigger than red blood cells, have a nucleus, and are present in fewer amounts. There are two types of white blood cells namely phagocytes and lymphocytes. Phagocytes are important in phagocytosis (the process of killing bacteria by engulfing them, and digesting them using enzymes). Lymphocytes are important in antibody production (Lymphocytes kill bacteria by secreting antibodies which kill the pathogens. Each pathogen is killed by a specific type of antibody). 3. Platelets Platelets prevent bleeding when the skin is cut, and stop bacteria from entering our systems through the wound. Platelets work by causing clotting, when the skin is injured. 4. Blood plasma Blood plasma makes up most of the blood. It is mostly water with some substances dissolved in it. The blood plasma transports blood cells, ions, hormones, soluble nutrients, hormones and carbon dioxide from one place to another. Gas exchange and respiration Gas exchange Naming and identifying the lungs, diaphragm, ribs, intercostal muscles, larynx, trachea, bronchi, bronchioles, alveoli and associated capillaries Air inhaled moves from nostril to larynx to trachea to either of two bronchi to bronchioles and then to alveoli (singular: alveolus). Bronchi form many smaller bronchioles. Bronchi, bronchioles and alveoli are in the lung. Page 21 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section Alveoli and associated blood vessels Listing the features of gas exchange surfaces in animals: These features include Large surface area for faster diffusion Thin surface to make diffusion distance shorter and faster. Both the alveoli and capillaries are one cell thick. Good blood supply and Good ventilation with air Stating the differences in composition between inspired and expired air limited to oxygen, carbon dioxide and water vapour Gas Oxygen Carbon dioxide Water vapour Inspired Air 21% 0.04% Variable Expired Air 16% 4% High Page 22 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section Explaining the differences in composition between inspired and expired air Oxygen decreases in exhaled air because some of it is used by body cells in respiration. Carbon dioxide increases because respiring cells produce carbon dioxide which is then exhaled. Exhaled air contains more water vapour because gas exchange surfaces are made of living cells which must be kept moist; some of this moisture evaporates into the air. Using limewater as a test for carbon dioxide to investigate the differences in composition between inspired and expired air Exhaled air turns lime water more milky because of its high carbon dioxide content. When you inhale, air moves into the mouth from C to D via conical flask A. When you exhale, air moves from the mouth from E to F via conical flask B. The higher carbon dioxide content in exhaled air turns the lime water milky white. Investigating and describing the effects of physical activity on rate and depth of breathing Generally, the rate and depth of breathing increase with physical activity. An increased carbon dioxide concentration in the blood causes an increased rate and depth of breathing. Explaining the role of goblet cells, mucus and ciliated cells in protecting the gas exchange system from pathogens and particles Ciliated cells: Ciliated cells are present in the trachea and bronchi of our respiratory system. Their function is to use their cilia to move mucus up the trachea to the throat. The mucus traps bacteria and dust particles. When it reaches the throat, the mucus is swallowed to the stomach where the stomach acid kills the bacteria. Goblet cells and mucus: The mucus used to trap bacteria and dust particles is secreted by goblet cells which are next to ciliated cells. Page 23 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section Tobacco smoking can cause chronic obstructive pulmonary disease (COPD), lung cancer and coronary heart disease. Describing the effects on the gas exchange system of tobacco smoke and its major toxic components, limited to carbon monoxide, nicotine and tar Effects of Smoking tobacco: • Cilia can’t vibrate anymore, the air inhaled isn’t clean. Goblet cells release more mucus which makes the trachea narrower. • Nicotine increases heart beat rate and blood pressure. • Carbon monoxide combines irreversibly with haemoglobin (in place of oxygen); hence, less oxygen is transported to cells. Smoke particles get trapped inside the lungs. White blood cells try to remove them by secreting chemicals which unfortunately end up doing serious damage to the lungs resulting in chronic obstructive pulmonary disease (COPD). Carbon monoxide combines with haemoglobin in red blood cells. This reduces the amount of oxygen carried by blood to body cells because red blood cells end up carrying carbon monoxide (which binds with haemoglobin much easily and strongly than oxygen). Nicotine makes blood vessels get narrower. This can increase blood pressure, leading to hypertension. Tar contains carcinogens (cancer causing chemicals). Respiration The uses of energy in the body of humans: Energy in the human body is used for muscle contraction, protein synthesis, growth and the maintenance of a constant body temperature. Defining aerobic respiration Aerobic respiration refers to chemical reactions in cells that use oxygen to break down nutrient molecules to release energy. Stating the word and balanced chemical equation for aerobic respiration Word equation for aerobic respiration is Glucose + oxygen → carbon dioxide + water Balanced chemical equation for aerobic respiration is C6H12O6 + 6O2 → 6CO2 + 6H2O Page 24 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section Coordination and response Hormones in humans A hormone is a chemical substance, produced by a gland, and carried by the blood, which alters the activity of one or more specific target organs. Adrenaline is the hormone secreted in ‘fight or flight’ situations. Its effects are to increase breathing and pulse rate, as well as to widen the pupils. The role of the hormone adrenaline in the chemical control of metabolic activity, including increasing the blood glucose concentration and pulse rate. Adrenaline increases your metabolic rate so that you have enough energy for fighting or running away etc. When adrenaline reaches the heart it causes the cardiac muscle to contract and relax rapidly so that oxygen and glucose reach the muscles of the body faster. Adrenaline also makes the liver convert glycogen into glucose and secrete it into the blood to be used in respiration. When adrenaline reaches the diaphragm and the intercostal muscles of the ribs, it makes them contract and relax faster too to increase rate of breathing. These changes cause an increase in the respiration rate so that lots of energy is being released. Giving examples of situations in which adrenaline secretion increases Adrenaline is released in Fight and flight situations. Tropic responses Tropisms are directional growth responses to environmental stimuli. Gravitropism is a response in which parts of a plant grow towards or away from gravity. Phototropism is a response in which parts of a plant grow towards or away from the direction from which light is coming. Since auxin is a chemical ,phototropism and gravitroprism of a shoot are examples of the chemical control of plant growth. Investigating gravitropism and phototropism in shoots and roots Root Shoot A seed is pinned in the dark as shown. The shoot will bend upwards whilst the root bends downwards due to gravitropism. The shoot displays negative gravitropism whilst the root displays positive gravitropism. Explaining the role of auxin in controlling shoot growth [check notes that follow] Page 25 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section Auxin is made only in the tip of a shoot from where it spreads through to the plant. Auxin stimulates cell elongation/ growth. Auxin is only produced by the growing tip of the plant. If this is removed, growth ceases. Diagram A (phototropism): When light shines onto a shoot from all around, the shoot grows straight upwards because auxin is evenly distributed around the tip of the shoot causing equal growth on all sides. However, when light shines onto a shoot from one side, the shoot grows towards the light because auxin at the tip concentrates on the shady side thus causing rapid growth on the shady side than the bright side. This is positive phototropism. Diagram B (gravitropism): If a potted plant is placed on its side in a dark room overnight, the shoot will bend upwards. Since there is no light, the result should be a response to gravity. Auxin collects on the lower side of the stem, causing faster growth there. Therefore, the stem curves upward. The shoot is displaying negative gravitropism. Diagram C (gravitropism): the root and shoot are displaying gravitropism. Tropism can be positive or negative. Positive tropism means growing towards. Negative tropism means growing away from. The root is displaying positive gravitropism. Page 26 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section Reproduction Asexual and sexual reproduction Asexual reproduction: Asexual means not sexual. This means that this kind of reproduction does not involve sex. Asexual reproduction is the production of genetically identical offspring from one parent. It is simply a single organism growing a new organism from itself e.g. in bacteria, fungi and potato plants. Bacteria reproduce by a process called binary fission. In binary fission, one bacterium grows an exact copy of its DNA coil which carries its genetic information. Then the bacterium completely divides with one DNA coil in the parent and one in the daughter bacterium. Each bacterium can undergo binary fission once every 20 minutes making them able to reproduce massive numbers from one parent in very little time. The sporangium of Fungi produce productive structures called spores that can grow into other fungi. At some point, the sporangium will burst open dispersing the spores into the air. If a spore falls on an area of favourable conditions it will germinate and grow into a new identical fungus. A potato plant starts as a lateral bud which grows from a potato tuber under the soil. The bud then forms a plant which will form more potato tubers. These tubers can also form lateral buds which grow into potato plants. Page 27 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section Sexual reproduction: Sexual reproduction is a process involving the fusion of the nuclei of two gametes (sex cells) to form a zygote and the production of offspring that are genetically different from each other. Sexual reproduction in plants Identifying and drawing the sepals, petals, stamens, filaments and anthers, carpels, style, stigma, ovary and ovules, of an insect-pollinated flower Identifying and describing the anthers and stigmas of a wind pollinated flower Methods of Pollination: Pollination is the transfer of pollen grains from the male part of a flower (anther) to the female part of a flower (stigma). There are two methods of pollination, insect pollination and wind pollination. Some flowers pollinate by insects while others pollinate by wind. Functions of certain parts of a flower Sepals – Protect the flower when it is in bud stage. Petals –They are brightly coloured in insect-pollinated plants to attract insects, and form a platform for insects to land on. Anther – Contains pollen grains. Pollen grains in insect-pollinated plants are heavy and sticky. Stigma – Receptor of pollen grains. Secretes a fluid that stimulates germination of pollen grains. Ovary – Each ovary contains one or more ovules. Ovule – Contains female gametes. Page 28 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section Distinguishing between the pollen grains of insect pollinated and wind-pollinated flowers Pollination is the transfer of pollen grains from the anther to the stigma Part of flower Pollen grains Insect pollinated Fairly abundant, large and sticky. Sticky so that they easily attach to insects. Wind pollinated Very abundant, small and light so that wind easily carries them. Agents of pollination are wind and insects. Fertilisation occurs when a pollen nucleus fuses with a nucleus in an ovule. Describing the structural adaptations of insect and wind pollinated flowers Insect pollinated Brightly coloured and large petals to attract insects Strong attractive scents to attract insects Sticky or spiky pollen grains to attach pollen to bodies of insects Contain nectarines (at the base of petals) to attract insects Stigma inside flower such that insects brush past it to reach nectar Anthers inside flower such that insects brush past it to reach nectar Produce fairly large quantities of pollen because some will be eaten or delivered to the wrong flower Wind pollinated Small dull petals/ no petals at all No scent Smooth and light so that it travels easily in air No nectaries Large stigmas which hang outside the flower to capture pollen grains carried by the wind Anthers hang outside the flower to easily distribute pollen grains in air Produce very large quantities of pollen because some of it is blown away and lost Investigating and stating the environmental conditions that affect germination of seeds: limited to the requirement for water, oxygen and a suitable temperature A seed remains dormant until it is put in suitable conditions to start growing. These are: • Water • Air (oxygen) • Suitable temperature Conditions in each test tube Tube A: water; air; suitable temperature Tube B: water; air Tube C: water; air; suitable temperature Tube D: water; suitable temperature Tube E: air; suitable temperature Results: germination occurs only in test tubes A and C Page 29 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section Pyrogallol absorbs oxygen. Sexual reproduction in humans Identifying and naming, on diagrams, parts of the male reproductive system: the testes, scrotum, sperm ducts (vas deferens), prostate gland, urethra and penis [Note that vas deferens are also known as sperm ducts] Page 30 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section Function of the parts of the male reproductive system Testes: a male human has two testes. A testis (singular) produces male gametes (sperms) Scrotum: The scrotum is a sac which holds the testes outside the body Sperm Ducts (Vas deferens): Sperm ducts transfer the sperms from the testis to the urethra Prostate Gland: The prostate gland secretes a fluid for the sperms to swim in, forming a mixture called semen. Urethra: The urethra carries semen and urine out of the body. Penis: The penis transfers semen into the vagina during sexual intercourse. Identifying and naming, on diagrams, parts of the female reproductive system: the ovaries, oviducts, uterus, cervix and vagina Stating the functions of the parts of the female reproductive system Oviducts (fallopian tube): oviducts transfer eggs to the uterus. Fertilisation takes place along the oviducts. Ovaries : ovaries release female gametes (eggs) Uterus: the fetus develops in the uterus Cervix: the cervix is a ring of muscle at the opening of the uterus Vagina: the vagina receives the penis during sexual intercourse Fertilisation Fertilisation is the fusion of the nuclei from a male gamete (sperm) and a female gamete (egg cell/ovum). Comparing Male and Female Gametes: Size: egg cells are much larger than sperms. This is because they need space to store nutrients to feed the embryo before it reaches the uterus. Structure: sperms have a head, a middle piece and long tails which help them swim their way to the egg. The middle part of sperms contains a large number of mitochondria to release Page 31 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section a lot of energy which they use to swim towards egg cells. Egg cells have a spherical structure and are made up of a nucleus, cytoplasm, cell membrane and a layer of jelly. Motility: motile means capable of moving spontaneously as a whole. Sperms are motile whilst egg cells are not. Eggs are unable to move by themselves, they are swept to the uterus by cilia on the walls of the oviduct. Sperms have tails that allow them to swim. Numbers: a woman releases one egg at a time. Sperms, however, are in larger quantities to increase the chance of successful fertilization. The adaptive features of sperm, limited to flagellum and the presence of enzymes The tail (flagellum) beats to propel the sperm towards the egg. The head contains digestive enzymes which break down the outer membrane of the ovum, allowing for fertilization to take place. The middle piece contains numerous mitochondria to provide adequate energy for the sperms to swim to the egg [note that this point is not required by the syllabus]. The adaptive features of egg cells, limited to energy stores and a jelly coating that changes after fertilization The egg contains nutrients or energy stores. They also contain a jelly coating that changes after fertilization. The menstrual cycle The menstrual cycle can be described as “changes in the ovaries and in the lining of the uterus” (knowledge of sex hormones is not required) Changes in the ovaries: A follicle containing an ovum develops in the ovary, as the uterus lining builds up. The follicle then bursts (around day 14), releasing an egg cell from the ovary. The release of the ovum is called ovulation. The follicle turns into a corpus luteum. Changes in the uterus: A woman usually releases one egg every month. The lining of the uterus becomes thick and spongy in preparation for a fertilised egg cell. The thickening of the uterus lining begins before an egg cell is even released, and just after menstruation, from the previous menstrual cycle, ends. If the egg cell is not fertilised, it dies before it reaches the uterus. The unfertilised egg does not sink into the spongy uterus lining, but continues onwards, down through the vagina. The spongy uterus lining then gradually disintegrates and is slowly lost through the vagina. This is called menstruation, or a period. It usually lasts for about five days. After menstruation, the lining of the uterus builds up again, so that it will be ready to receive the next egg, if it is fertilised. Menstruation and the menstrual cycle are two different things. The menstrual cycle, on the other hand, can take about 28 days (the menstrual cycle differs amongst women, some take more and others take less days). A mature ovum (egg cell) is released on day 14 and if not fertilized it can die after 1 or 2 days. Page 32 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section Fertilization results in the formation of a zygote which further forms an embryo in early development. An embryo is a ball of cells that implants into the wall of the uterus. The functions of the umbilical cord, placenta, amniotic sac and amniotic fluid 1. Umbilical cord: The umbilical cord connects the embryo to the placenta. This allows for the exchange of oxygen and dissolved nutrients from the mother’s blood to the child’s blood. It also offers a pathway by which waste products leave the child. 2. Placenta: Provides nutrients and oxygen for the embryo. Removes waste materials such as urea and carbon dioxide. Allows protective antibodies to diffuse from maternal blood into embryonic blood. Provides a barrier preventing maternal blood and embryonic blood from mixing. 3. Amniotic sac: contains amniotic fluid. 4. Amniotic fluid: Absorbs shock, supports and protects the embryo from physical injury Lubricates the vagina during birth to reduce friction Allows the fetus to move freely during development The functions of the placenta and umbilical cord are Oxygen and nutrients in the mother’s blood diffuse across the placenta into the fetus’s blood, and are then carried along the umbilical cord to the fetus. Carbon dioxide and waste materials diffuse from the fetus’s blood to the mother’s blood, and are carried away in the mother’s blood. The placenta provides a barrier to toxins by preventing maternal blood and embryonic blood from mixing. Page 33 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section HIV and AIDS AIDS is a disease caused by the HIV (human immunodeficiency virus). Human immunodeficiency virus (HIV) infection may lead to acquired immune deficiency syndrome (AIDS). The virus can live for years in the body before it starts showing symptoms. AIDS is Acquired Immune Deficiency Syndrome. The disease prevents white blood cells from killing bacteria and viruses, so one or more weak viral or bacterial infections take advantage of the person’s weak immune system to kill the patient. There is no cure for AIDS. HIV is transmitted: By sexual intercourse with an infected person By sharing and reusing contaminated needles during intravenous drug use, getting a tattoo and body piercing By receiving a blood transfusion from an infected donor During pregnancy and childbirth. An infected mother can pass on the virus to her child Spread of HIV can be prevented by: Having protected sexual intercourse. A condom reduces the risk of infection. Abstinence from sex or having sex with only one faithful partner Not sharing objects that could be contaminated with blood or bodily fluids such as hypodermic syringes and razors Proper screening of blood in a blood bank for HIV infection to reduce chances of transmission during blood transfusions Infected mothers should undergo antiretroviral therapies and give birth by caesarean section to minimise risk of transmission to the fetus. Breastfeeding should be avoided after birth. Visiting reliable and hygienic operators for tattoos, piercings or acupuncture where needles are sterilized Organisms and their environment All energy in biological (living systems) is ultimately derived from sunlight. Definition of key terms A food chain shows the transfer of energy from one organism to the next, beginning with a producer. A food web is a network of interconnected food chains. Page 34 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section A producer is an organism that usually uses energy from sunlight to make its own organic nutrients through photosynthesis. A consumer is an organism that gets its energy by feeding on other organisms. A herbivore is an animal that gets its energy by eating plants. A carnivore is an animal that gets its energy by eating other animals. A decomposer is an organism that gets its energy from dead or waste organic matter. An ecosystem is a unit containing all ofthe organisms and their environment, interacting together, in a given area, e.g. a lake A trophic level is the position of an organismin a food chain or food web. The transfer of energy between trophic levels A pyramid of energy represents the total energy in the various trophic levels of a food chain. Producers occupy the first trophic level at the base of the pyramid. This trophic level has the largest amount of energy. The quantity of energy which is available to the next trophic level decreases from one trophic level to another. Explaining why food chains usually have fewer than five trophic levels Since the quantity of energy decreases from one trophic level to another, the number of sustainable trophic levels is limited to five because there isn’t enough energy to support a sixth trophic level. Some energy is lost during transfer between organisms. Part of the food input is wasted as faeces and in respiration. These losses are repeated at every transfer. Constructing simple food chains Tree → caterpillar → small bird → hawk Grass → Grasshopper → Frog → Snake A food web Identifying producers and consumers as trophic levels It is important to understand that producers, primary consumers, secondary consumers, tertiary consumers and quaternary consumers are individually referred to as trophic levels in food webs and food chains. Tree → caterpillar → small bird → hawk The caterpillar is trophic level 2 Page 35 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section Classification of consumers by virtue of their position in a food chain. Consumers obtain their energy by consuming other organisms. They occupy a few trophic levels: Primary consumers feed on primary producers directly. They are called herbivores. Secondary consumers are carnivores that eat herbivores. Tertiary consumers are carnivores that eat other carnivores. Human influences on ecosystems Describing the carbon cycle, limited to photosynthesis, respiration, feeding, decomposition, fossilisation and combustion The carbon cycle is a natural global cycle of the element carbon. It is what maintains a constant level of carbon dioxide in air (0.03%). The cycle goes as follows: • Plants absorb carbon dioxide from air use it for a process called photosynthesis to produce glucose and oxygen 6CO2 + 6H2O → C6H12O6 + 6O2 •The carbon is now stored in plants as glucose. One of two things happen, either the plant gets eaten by animals or humans in a process called feeding, or the plant dies and is decayed by decomposers in a process called decomposition. • If the plant is eaten by animals or humans, glucose in the plant is used by them in a process called respiration to release energy for their body. In addition, fossil fuels and wood can be burnt to release carbon dioxide and energy in a process called combustion. Both combustion and respiration have the same chemical equation as shown below. You do not need to remember this chemical equation. C6H12O6 + 6O2 → 6CO2 + 6H2O Page 36 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Biology section • Respiration is the opposite of photosynthesis. • If plants and animals die, and their remains are buried underground and exposed to certain conditions, fossil fuels are formed by a process called fossilisation. Fossil fuels contain carbon. • Power stations burn carbon-containing fuels like coal (a fossil fuel). This is a combustion reaction. Carbon dioxide produced is released to the air through chimneys of power stations. Discussing the effects of the combustion of fossil fuels and the cutting down of forests on the oxygen and carbon dioxide concentrations in the atmosphere Combustion of fossil fuels Combustion of fossil fuels (coal, oil and natural gas) uses up oxygen and gives out carbon dioxide; hence, it leads to the decrease in oxygen concentrations and the increase in carbon dioxide concentrations in the atmosphere. Cutting down of forests Since green plants absorb carbon dioxide during photosynthesis to form carbohydrates, cutting down trees reduces the number of green plants available for photosynthesis, thus leading to a buildup of carbon dioxide concentration in air. The undesirable effects of deforestation The undesirable effects of deforestation include habitat destruction, extinction of some flora and fauna that depend on that flora, soil erosion, siltation, flooding and an increase of carbon dioxide in the atmosphere. Eutrophication Eutrophication is summarized by the following points Excess nitrates (and other ions) from fertilizers run off into water bodies leading to an increased growth of producers in water bodies e.g. algae. When these producers die, it leads to increased decomposition of producers, followed by increased aerobic respiration by decomposers. A reduction in dissolved oxygen then occurs, leading to the death of aquatic organisms that require dissolved oxygen in water. Page 37 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section The particulate nature of matter 1. Stating the distinguishing properties of solids, liquids and gases 2. Describing the structure of solids, liquids and gases in terms of particle separation, arrangement and types of motion Describing the changes of state in terms of melting, boiling, evaporation, freezing and condensation Explaining changes of state in terms of particle theory and the energy changes involved 1. Melting: Occurs at the melting point. Particles absorb heat and vibrate more vigorously, allowing them to overcome the attractive forces holding them in fixed positions. Page 38 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section 2. Freezing: Occurs at the melting point. Particles release heat and move more slowly. Attractive forces are formed and the particles are forced to be held in a fixed and orderly arrangement. 3. Boiling: Occurs at the boiling point. Particles absorb heat and gain more kinetic energy. The particles move fast enough to completely overcome the forces of attraction acting between them. 4. Evaporation: Occurs below the boiling point. Particles at the surface gain sufficient energy to escape into the surroundings. 5. Condensation: Occurs at the boiling point. Particles release heat and move more slowly. The forces of attraction are then able to hold the particles closely. Describing qualitatively the pressure and temperature of a gas in terms of the motion of its particles A high pressure results in a small volume thus limiting the motion of gas particles. A high temperature results in high kinetic energy of gas particles thus increasing the motion of gas particles. Demonstrating understanding of the terms atom, molecule and ion All matter is made up of tiny particles These particles can be atoms, molecules or ions An atom is the smallest part of an element that can take part in a chemical reaction. An atom has sub atomic particles namely electrons (which are found in electron shells situated around the nucleus), protons and neutrons (both of which are found in the atom‟s nucleus). Page 39 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section A molecule is a group of two or more atoms chemically joined together, e.g. a chlorine molecule has 2 chlorine atoms chemically combined together. A water molecule has 2 hydrogen atoms and one oxygen atom chemically combined together. An ion is a positively or negatively charged species. An atom has an equal number of positively charged protons and negatively charged electrons. This makes an atom to be neutral. Ions arise when atoms lose or gain electrons. When atoms lose electrons, positively charged ions are formed because the total positive charge of the protons would not be cancelled by the total negative charge of the electrons. Similarly, when an atom gains extra electrons, it will not have extra protons to cancel out the negative charge of the added electrons, resulting in a negatively charged ion. A negatively charged ion is called an anion. A positively charged ion is a called a cation. Experimental techniques Measurement Naming and suggesting appropriate apparatus for the measurement of time, temperature, mass and volume, including burettes, pipettes and measuring cylinders Apparatus Used to measure Stop watch Time Thermometer Temperature Electronic balance Mass Measuring cylinder, burette and pipette Volume Criteria of purity Interpreting simple chromatograms Chromatography is a process used to separate and identify two or more substances from a mixture. It is also used to find the number of components in a substance, hence, determining the purity of the substance. A pure substance has one substance in it; an impure substance has two or more. In addition, a pure substance has a definite, sharp, melting point and boiling point. Page 40 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section Interpreting simple chromatograms, including the use of R f values Rf values have no units Substance 1 contains A, B and C. Substance 2 contains B. Substance 3 contains D. Substance 4 contains C. Rf value for each component = = distance moved by substance distance moved by solvent distance from starting point to solvent front distance from starting point to position of each component Rf values for the above 3 situations are 48mm/80mm = 0.6 72mm/120mm = 0.6 120mm/200mm = 0.6 and Page 41 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section Methods of purification 1. Describing and explaining methods of separation and purification by the use of a suitable solvent, filtration, crystallisation, distillation, fractional distillation and paper chromatography 2. Suggesting suitable separation and purification techniques, given information about the substances involved 1. Filtration Filtration is used to separate a mixture of a liquid (or solution) and an insoluble solid. The insoluble solid is collected as the residue (on the filter paper) while the liquid is collected as the filtrate. 2. Evaporation This method is used to evaporate off the solvent from a solution to obtain the dissolved substance. This is only applicable to substances that do not decompose upon heating. 3. Crystallisation Crystallisation can be used to recover a dissolved substance from its solution. This is carried out by heating a solution until it is saturated. The saturated solution is then left to cool, allowing for the substance to crystallise. Page 42 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section 4. Distillation Distillation is used to separate a liquid from a mixture. The substances in the mixture must have large differences in boiling points for the pure liquid to be obtained. 5. Fractional Distillation In cases where a mixture contains liquids that have relatively close boiling points, fractional distillation is used for purification. The fractionating column aids in separating the vapour into individual components, which allow for the collection of pure substances. 6. Separation using a Separating Funnel The separating funnel is used to separate a mixture of liquids that have different densities. The liquid with lower density is found in the top layer while the liquid with higher density is found in the bottom layer. 7. Paper Chromatography This is used in the separation of small quantities of mixtures. The mixture is separated based on i) differences in solubility of its components in a particular solvent and ii) differences in the way the components are attracted to the chromatography paper. The identity of a component in the mixture can be deduced by comparing the Rf value obtained in the chromatogram with existing Rf values of known substances. Page 43 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section Atoms, elements and compounds Physical and chemical changes Identifying physical and chemical changes, and understanding the differences between them A physical change is a change in which no new chemical substance forms; melting and boiling are physical changes. A chemical change is a change in which a new chemical substance forms. Electrolysis is an example of a chemical change. Elements, compounds and mixtures Describing the differences between elements, mixtures and compounds, and between metals and non-metals An element is a substance that cannot be broken down into simpler substances by chemical or physical means. An element is made up of many identical atoms. Atoms of different elements differ. Their differences are caused by the differences in the numbers of sub atomic particles which they contain. The element sodium contains many sodium atoms in it. The element calcium contains many calcium atoms in it. Elements can be combined physically to form mixtures, or chemically to form compounds. Similarly, compounds can also be combined physically and chemically to form mixtures and other compounds respectively. Compound Mixture 1. Component substances cannot be separated by physical means. Chemical methods are required to separate component substances. Component substances can be separated by physical means 2. Its physical and chemical properties are different from those of its constituent substances Its physical and chemical properties are the same as those of its constituent substances 3. Composition by mass is fixed Composition by mass varies 4. Has fixed melting and boiling points Has variable melting and boiling points Page 44 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section Property Metals Non-metals Appearance Lustrous (shiny) Dull State at room temperature Solid (except mercury which is a liquid) About half are solids and the other half are gases. Only one (Bromine) is a liquid Malleable or brittle Malleable (easily shaped by hammering) because they bend without breaking since their atoms can easily slide over each other Brittle (break when hammered) Conduction (thermal and electrical) Good because they possess either free or mobile ions or electrons to carry current or heat. Generally poor (most are insulators) because they do not possess free or mobile ions or electrons to carry current or heat. Melting and boiling points High melting and boiling points Generally low melting and boiling points. Density High density Low density Magnetism Only nickel, iron and cobalt None are magnetic are magnetic Sound produced when struck Sonorous (make a bell or reverberative sound when struck) Make a dull sound Ductile Ductile (Easily drawn into wire) Not ductile Defining the terms solvent, solute, solution and concentration Solute the substance you dissolve in the solvent, to make a solution Solvent the liquid in which a solute is dissolved, to make a solution Solution a mixture obtained when a solute is dissolved in a solvent Concentration tells you how much of one substance is dissolved in another; usually given as gdm-3 or moldm-3. Concentration is also defined as the amount of solute dissolve in a given volume of solvent. Page 45 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section Atomic structure and the Periodic Table Describing the structure of an atom in terms of a central nucleus, containing protons and neutrons, and ‘shells’ of electrons The nucleus is located in the centre of the atom and contains protons and neutrons. In the case of sulfur, the nucleus has 16 protons and 16 neutrons. Electrons are arranged in shells around the nucleus of an atom. The first shell can contain up to 2 electrons and the second and third shells hold a maximum of 8 electrons. Sulfur is represented by the symbol , indicating that it has 16 protons and 16 neutrons. The number of neutrons is calculated by subtracting the atomic number from the nucleon number. Since it is electrically neutral, it has 16 electrons as well. The first electron shell contains 2 electrons, the second shell contains 8 electrons and the third shell contains 6 electrons. The electronic configuration can be written as 2.8.6. 1. Describing the build-up of electrons in ‘shells’ and understanding the significance of the noble gas electronic structures and of the outer shell electrons (The ideas of the distribution of electrons in s and p orbitals and in d block elements are not required) 2. Describing the formation of ions by electron loss or gain In terms of the buildup of electrons in shells, electrons occupy the shell closest to the nucleus before filling up other shells further from the nucleus. In drawing the structure of an atom, only shells containing electrons are shown. The first shell should not contain more than 2 electrons. The remaining shells normally contain a maximum of 8 electrons. Atoms usually want to have a configuration which is such that they have full shells only. This configuration is referred to as the noble gas electronic structure. Atoms achieve this noble gas electronic structure by either losing or gaining the number of electrons preventing them from achieving this electronic arrangement. These electrons are lost or gained by the outermost electron shell only. Usually, atoms with electronic configurations whereby the outer shell has 3 or less electrons tend to lose these electrons to achieve a noble gas electronic structure. Those with 4 outermost electrons can either lose or gain these to form noble gas electronic structures, whilst those with more than 4 find it easier to gain the electrons required to reach an octet (from eight) state or noble gas electronic structure. When atoms lose or gain electrons, ions are formed. These ions tend to be more chemically stable than the atoms. Page 46 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section Stating the charges and approximate relative masses of protons, neutrons and electrons Defining and using proton number (atomic number) as the number of protons in the nucleus of an atom Proton number is the number of protons in the atom of an element; it is sometimes called the atomic number . Protons and neutrons are found in the nucleus of an atom. They are collectively known as nucleons. Electrons are found outside the nucleus. They are arranged in shells, also referred to as energy levels, which surround the nucleus. Defining and using nucleon number (mass number) as the total number of protons and neutrons in the nucleus of an atom Nucleon number is the number of protons plus neutrons in an atom of an element; it is sometimes called the mass number. Using proton number and the simple structure of atoms to explain the basis of the Periodic Table, with special reference to the elements of proton numbers 1 to 20 Atoms in the periodic table are arranged according to proton number from lowest to highest. The ascension goes from left to right and from one horizontal period to another e.g period 3 is sodium (proton number 11; electronic configuration 2,8,1) to argon (proton number 18; electronic configuration 2,8,8). Note: a copy of the Periodic Table, as shown in the Appendix, will be provided in Papers 1, 2, 3 and 4. Ions and ionic bonds Formation of Ions An atom is most stable when the valence electron shell is completely filled. Atoms of elements either gain or lose electrons to attain a stable electronic configuration. Non-metals usually gain electrons to form negative ions (anions) while metals usually lose electrons to form positive ions (cations). Page 47 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section The charge of an ion can be found by finding the difference between the number of electrons and the number of protons. Using dot-and-cross diagrams to describe the formation of ionic bonds between Group I and Group VII This type of bonding takes place between oppositely-charged ions. This usually occurs for compounds made from a metal and a non-metal. Ionic bonds are formed by electron transfer, where metal atoms donate electrons to non-metal atoms. The transfer of electrons forms anions and cations. These ions are arranged in an ionic lattice and are held together by strong electrostatic forces of attraction. An example of dot-and-cross diagrams that illustrates the formation of an ionic bond are shown below Sodium (metal) reacts with chlorine (non-metal) to form sodium chloride, NaCl Describing the formation of ionic bonds between metallic and non-metallic elements to include the strong attraction between ions because of their opposite electrical charges Ionic bonding should be defined as guided by the following steps 1. Metal atoms lose electrons to form cations 2. The electrons lost by metal atoms are gained by non-metallic atoms to form anions 3. The oppositely charged ions are held together by strong electrostatic forces of attraction acting between these ions because of their opposite electrical charges. 4. The attractions described in (3) are ionic bonds. It should be noted that an ionic bond can only occur between metals and non-metals only!!! Describing the lattice structure of ionic compounds as a regular arrangement of alternating positive and negative ions, exemplified by the sodium chloride structure The lattice structure of ionic compounds is a regular arrangement of alternating positive and negative ions, eg. the sodium chloride structure Page 48 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section Structure Ionic substances appear as giant lattice structures with a regular arrangement of alternating positive and negative ions. Molecules and covalent bonds Stating that non-metallic elements form simple molecules with covalent bonds between atoms When non metallic elements react with each other, simple molecules are formed. The atoms, of the different elements making up each simple molecule, are joined together by covalent bonds. Weak intermolecular forces (not covalent bonds) hold different molecules together. Describing the formation of single covalent bonds in H2, Cl2, H2O, CH4, NH3 and HCl as the sharing of pairs of electrons leading to the noble gas configuration including the use of dot-and-cross diagrams Covalent bonding is the sharing of a pair of electrons to gain an electronic configuration or structure like that of an inert or noble gas. Covalent bonds occur between non-metals only. In covalent bonds, an atom usually shares the same number of electrons as it needs to form the 2 or 8 valence electrons. E.g. oxygen is 2,6 and carbon is 2,4. Oxygen needs 2 so it shares two electrons forming 2 covalent bonds. Carbon needs 4 and shares 4 forming 4 covalent bonds. One carbon would need to bond with 2 oxygen atoms giving O=C=O The shared electrons appear in pairs Page 49 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section From left to right, top row to bottom, we have simple molecules of HCl; H2O; Cl2; CO2; CH4; NH3 Using and drawing dot-and-cross diagrams to represent the bonding in the more complex covalent molecules such as N2, C2H4, CH3OH, and CO2 Page 50 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section Describing the differences in volatility, solubility and electrical conductivity between ionic and covalent compounds Property Ionic compounds Covalent compounds Volatility Not volatile because of strong ionic bonds holding the particles (ions) together Volatile because of weak intermolecular forces holding the particles (molecules) together Solubility Soluble in water Not soluble in water Electrical conductivity Conduct electricity only in molten or aqueous state Do not conduct electricity (or are poor conductors) Explaining the differences in melting point and boiling point of ionic and covalent compounds in terms of attractive forces Ionic compounds have higher melting and boiling points because ionic bonds have stronger attractive forces holding the particles (ions) together as compared to the weak intermolecular forces holding the particles (molecules) together in covalent bonding. Stoichiometry Using the symbols of the elements and writing the formulae of simple compounds Element Symbol Hydrogen H Helium He Lithium Li Beryllium Be Boron B Carbon C Nitrogen N Elements have symbols. These are usually either one or two letters, and are not necessarily letters extracted from the name of the element e.g. lead is Pb. The first letter is usually a capital letter and the second letter is a small letter. Determining the formula of an ionic compound from the charges on the ions present Determine the formula of an ionic compound made by the ions Al3+ and SO42- Page 51 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section Ions present: Al3+ SO42- Al2 (SO4)3 Therefore, the formula is Al2(SO4)3 Determine the formula of the ionic compound made by the ions Fe 3+ and O2Answer should be Fe2O3 If the charges on the ions are the same eg. An+ and Bn- ions we get AB. Mg2+ & O2give MgO. Similarly Na+ & Cl- give NaCl. Deducing the formula of a simple compound from the relative numbers of atoms present Water has two hydrogen atoms and one oxygen atom; hence, its formula is H2O Deducing the formula of a simple compound from a model or a diagrammatic representation [To be done practically] Constructing and using word equations 1. calcium burns in oxygen to form calcium oxide The word equation is calcium + oxygen → calcium oxide 2. chlorine reacts with potassium bromide to form bromine and potassium chloride The word equation is chlorine + potassium bromide → bromine + potassium chloride Interpreting and balancing simple symbol equations The number of atoms of each element on the left side of the equation should be equal to the number of atoms of that element on the right side of the equation, regardless of the fact that the reactants and products differ. The formulae of the reactants or products should not be changed during balancing; meaning only the numbers in front of the chemical substance can be changed. Balance the following equations: 1. 2. 3. 4. 5. CaCO3 + HNO3 → Ca(NO3)2 + H2O + CO2 Ca+H2O→Ca(OH)2+H2 Pb(NO3)2+NaI→PbI2+NaNO3 Al2(SO4)3+NaOH→Al(OH)3+Na2SO4 Al(OH)3+NaOH→NaAlO2+H2O Answers on next page Page 52 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section 1. 2. 3. 4. 5. CaCO3 + 2HNO3 → Ca(NO3)2 + H2O + CO2 Ca+ 2H2O→Ca(OH)2+H2 Pb(NO3)2+ 2NaI→PbI2+ 2NaNO3 Al2(SO4)3+ 6NaOH→ 2Al(OH)3+ 3Na2SO4 Al(OH)3+NaOH→NaAlO2+ 2H2O Electricity and chemistry Defining electrolysis as the breakdown of an ionic compound when molten or in aqueous solution by the passage of electricity Electrolysis the process of breaking down an ionic compound, when molten or in aqueous solution, by passing electricity through it. The heat in the diagram below is for melting the compound. Aqueous solutions will not have the heat supply as shown below. Using the terms inert electrode, electrolyte, anode and cathode An inert electrode is an electrode which is not changed during electrolysis; all it does is conduct the current. An electrolyte is the liquid through which the current is passed, in electrolysis; the current is carried by ions in the electrolyte. An anode is the positive electrode of an electrolytic cell. A cathode is the negative electrode of an electrolytic cell. Describing electrolysis in terms of the ions present and the reactions at the electrodes, in terms of gain of electrons by cations and loss of electrons by anions to form atoms 1. Describing the electrode products and the observations made, using inert electrodes (platinum or carbon), in the electrolysis of: molten lead(II) bromide concentrated aqueous sodium chloride dilute sulfuric acid Page 53 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section Example Electrolysis of molten PbBr2 Ions present: Pb2+ and Br- only. Reaction at the Anode Br- loses electrons at the anode to form Br atoms. The Br atoms, which are created, bond together to make Br2 gas. This is because elements in group 7 exist as X2 molecules. 2Br-(aq) → Br2(g)+ 2eReaction at the Cathode Pb2+ gains electrons at the cathode to form Pb atoms. Pb2+(aq) + 2e- → Pb(s) Electrolysis of Aqueous Solution Aqueous solutions contain additional H+ and OH- ions from water, totalling 4 ions in the solution: 2 from electrolyte and 2 from water. Only 2 of these ions are selectively discharged. Examples Electrolysis of Concentrated NaCl Ions Present: Na+, H+, OH- and ClReaction at the Anode Cl- ions lose electrons at the anode to form Cl atoms. The Cl atoms formed combine together to make Cl2 molecules. 2Cl- (aq) → Cl2(g) + 2e- Reaction at the Cathode H+ ions gain electrons at the cathode to form H atoms which then combine to make H2(g) (hydrogen gas). Like group 7 elements, hydrogen also exists as X2 molecules. 2H+ (aq) + 2e- → H2(g) Electrolysis of dilute H2SO4 (referred to as the electrolysis of water) Ions Present: H+, OH- and SO42- (note that the source of H+ is both acid and water) Reaction at the anode Page 54 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section OH- ions lose electrons at the anode to become O2 and H2O. 4OH-(aq) → O2 (g) + 2H2O (l) + 4e- (remember this equation) Reaction at the cathode H+ ions gain electrons at the cathode to form H atoms which then combine to form hydrogen gas (H2). 2H+(aq) + 2e- → H2 (g) Predicting the products of the electrolysis of a specified molten binary compound Generally, a molten binary compound only has a single cation and a single anion. The cation is discharged at the negative electrode (cathode). The negative ion is discharged at the positive electrode (anode). Negative ions are discharged by removing electrons from them and positive ions are discharged by adding electrons to them. The number of electrons added or removed is such that no charge remains at the end of the discharging process. Energy changes in chemical reactions Describe the meaning of exothermic and endothermic reactions Chemical reactions that release energy to the surroundings are described as exothermic reactions. In an exothermic reaction the temperature of the surroundings increases. Chemical reactions that absorb energy from the surroundings are described as endothermic reactions. In an endothermic reaction the temperature of the surroundings decreases. Describe bond breaking as an endothermic process and bond formation as an exothermic process A chemical reaction can be divided into 3 steps namely bond breaking, bond rearrangement, and bond formation. Bond breaking requires energy. This energy is required to break bonds; hence, bond breaking is endothermic. Bond formation releases energy, hence, it is exothermic. Heat energy and enthalpy change (ΔH) of reaction When bonds are formed, heat energy is given out. The process is exothermic and ΔH is negative. When bonds are broken, heat energy is absorbed. The process is endothermic and ΔH is positive. Activation energy Activation energy is the minimum energy required to initiate a reaction. It is the energy needed to break the bonds in the reactant particles before new bonds are formed. Page 55 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section Draw and label energy level diagrams for exothermic and endothermic reactions using data provided Interpret energy level diagrams showing exothermic and endothermic reactions and the activation energy of a reaction Chemical reactions Rate of reaction The rate of a reaction is the amount of a reactant used up per unit time The amount of a product produced per unit time. or Describe practical methods for investigating the rate of a reaction which produces a gas For reactions which produce gas, the gas can be collected using a graduated gas syringe. A table of volume vs time is made and used to plot a graph. The time intervals can be every 10 seconds if the reaction is fast, or even minutes or hours if the reaction is slow. Magnesium reacts with hydrochloric acid to form magnesium chloride and hydrogen gas. The rate of reaction can be followed by noting the volume of hydrogen gas collected in the gas syringe over a period of time. When the volume becomes constant (stops changing) it means the reaction has reached completion. Mg (s) + 2HCl (aq)→ MgCl2 (aq) + H2 (g) Page 56 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section The rate changes throughout the course of the reaction. It is greatest at the start, but decreases as the reaction proceeds. Interpret data obtained from experiments concerned with rate of reaction Page 57 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section B A A: In terms of rate, A ˃ B ˃ C ˃ D ˃ E. At point E, gradient is zero meaning that the rate is also zero, so the reaction has stopped/reached completion. The graph/curve shows amount of product formed with time. B: In terms of temperature, curve B shows a reaction carried out at a higher temperature than that in curve A. Notice that curve B‟s mass becomes constant before curve A‟s mass becomes constant. Constant mass/volume indicates the completion of a reaction. C C: In C, the answers are for 1) A (more products formed) 2) C 3) B (larger gradient per unit time meaning higher rate) Suggest suitable apparatus, given information, for experiments, including collection of gases and measurement of rates of reaction Measuring the change in mass of the reaction mixture If the reaction produces a gas, we can use the set up on the left. The mass reading drops as the gaseous product is lost from the conical flask. When the balance reading stops changing, it means the reaction is over. Alternatively, we can use the set up with the gas syringe and take note of the volume of gaseous product collected at fixed intervals of time. We would need a flask, gas syringe and stop watch as some of the apparatus. 1. Describe the effect of concentration, particle size, catalysts and temperature on the rate of reactions 2. Describe and explain the effect of changing concentration in terms of frequency of collisions between reacting particles Page 58 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section Effect of concentration on the rate of a reaction Increase of concentration of either reactant A or B, or even both the reactants, means there are more reactant particles per unit volume. The frequency of collisions between the different reactant particles of reactant A and B increases, resulting in an increase in the rate of the reaction. Effect of temperature on the rate of a reaction High temperatures increase the rate of a reaction by increasing the kinetic energy of the reactant particles. This increases the frequency of collisions between different reactant particles, leading to an increase in the rate of reaction. Effect of particle size or surface area on the rate of a reaction Decreasing the particle size increases the surface area of the particle. This means a larger part of the particle is exposed to the surface and thus also exposed to the other reactant particles. A powdered form of a substance will thus react faster than large lumps of the same substance. Remember: the smaller the particle size, the larger it’s surface area. Page 59 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section Effect of catalysts on the rate of a reaction Catalysts are chemical substances which alter the speed of a reaction without being used up at the end of a reaction. Catalysts can either be enzymes (biological catalysts made up of proteins) or transition metals and their compounds. Transition metals (e.g. Nickel, Iron, Platinum) are good catalysts. Most catalysts catalyse one kind of reaction i.e. they are specific. This is particularly true for biological catalysts (enzymes) Describe and explain the effect of changing temperature in terms of the frequency of collisions between reacting particles and more colliding particles possessing the minimum energy (activation energy) to react Increasing temperature increases the kinetic energy of the particles in a system. This in turn increases the frequency of collisions between reactant particles, leading to an increase in the rate of the reaction. Additionally, only particles with energy equal to or greater than activation energy are able to react to form products. Increasing temperature increases the number of particles with this minimum energy requirement so that when they collide, products are formed. Redox Describe oxidation and reduction in chemical reactions in terms of oxygen loss / gain (Oxidation state limited to its use to name ions, e.g. Iron(II), Iron(III), Copper(II)). Oxidation is the gain or addition of oxygen. Reduction is the loss or removal of oxygen. Iron (III) means the ion of Iron has an oxidation state of 3 i.e. Fe 3+. The chemical formula of Iron (III) oxide is thus Fe2O3. Define and identify an oxidising agent as a substance which oxidises another substance during a redox reaction and a reducing agent as a substance which reduces another substance during a redox reaction For the reaction Fe2O3(s) + 3CO(g) → 2Fe(l) + 3CO2(g) CO is oxidized to CO2 Fe2O3 is reduced to Fe CO is the reducing agent Fe2O3 is the oxidizing agent Acids, bases and salts The characteristic properties of acids and bases 1. Describe neutrality and relative acidity and alkalinity in terms of pH measured using universal indicator. Page 60 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section The pH scale states how acidic or alkaline a solution is by using a scale of numbers. The numbers go from 0 to 14: Universal indicator shows colours which can be used to stand for certain pH values. Green is pH 7. It is neutral. 2. Describe the characteristic properties of acids (exemplified by dilute hydrochloric acid and dilute sulfuric acid) including their effect on litmus paper and their reactions with metals, bases and carbonates Some commonly used acids are hydrochloric acid (HCl) and sulfuric acid (H2SO4). Acids turn blue litmus paper red. Their characteristic reactions are as outlined below. Chemical Properties of Acids Dilute acids reactions with metals Dilute acids react with metals that lie above hydrogen in the reactivity series. The reaction produces salt and hydrogen gas. zinc + dilute hydrochloric acid → zinc chloride + hydrogen Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g) Copper does not react with dilute acids because it is less reactive than hydrogen. Dilute acids reactions with bases. Soluble bases are called alkalis. Acids react with bases to form salt and water. The base could be a metal oxide or hydroxide. sodium hydroxide + dilute hydrochloric acid → sodium chloride + water Page 61 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l) Dilute acids reactions with metal carbonates Acids react with metal carbonates to produce salt, water and carbon dioxide. calcium carbonate + dilute sulfuric acid → calcium sulfate + water + carbon dioxide CaCO3(s) + H2SO4(aq) → CaSO4(aq) + H2O(l) + CO2(g) Describe neutrality and relative acidity and alkalinity in terms of pH (whole numbers only) measured using Universal Indicator A pH indicator displays different colours at different pH values. Universal Indicator is a mixture of pH indicators that gives different colours at different pH values. The table below lists the different colours and the pH range at which they are observed. Summarized as ROYGBV Describe and explain the importance of controlling acidity in soil Plants are sensitive to changes in soil pH. The pH levels can be controlled by adding certain chemicals. For acidic soil, bases such as calcium oxide (quicklime) and calcium hydroxide (slaked lime) can be added to neutralise the excess H+ ions from the acid. This process is known as „liming‟. Care must be taken to avoid adding excess base as this would increase the soil pH. This would make the soil too alkaline for plant growth. Preparation of salts 1. Describe the preparation, separation and purification of salts 2. Suggest a method of making a given salt from suitable starting material, given appropriate information Preparation of salts 1. By reaction of a metal hydroxide and an acid This method is suitable for soluble metal hydroxides called alkalis. The titration method is used in this case. e.g. Page 62 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section base + acid → salt + water e.g. sodium hydroxide + hydrochloric acid → sodium chloride + water sodium hydroxide + sulfuric acid → sodium sulfate + water Usually 25.00 cm3 of acid is placed in a conical flask using a pipette. Alkali is placed in the burette. A few drops of indicator are added to the acid in the conical flask before it is titrated with the alkali from the burette. The indicator changes colour at some point as alkali is added to acid. The change in colour shows the end of the titration. The process is then repeated using the 25.00 cm3 of acid and the same volume of base from the burette as used in the previous experiment, however, no indicator is used this time. Lastly, the solution obtained is crystallised to obtain the crystal salt. 2. By reacting metal with acid This preparation method is suitable for metals like Mg, Al, Zn, Fe (but not K, Na and Ca because these metals react vigorously with acids). In general, excess metal is added to the acid until there is no further reaction (when no more bubbles of hydrogen gas are produced). Excess unreacted metal is then filtered out, and the clear filtrate is crystallized by heating until a saturated solution is formed, and then leaving the saturated solution to cool down and form crystals e.g. zinc + sulfuric acid → zinc sulfate + hydrogen Zn (s) + H2SO4 (aq) → ZnSO4 (aq) + H2(g) Zn is added to dilute H2SO4 until it‟s in excess to ensure no more H2SO4 is present. The mixture is then filtered to separate solid unreacted Zn from ZnSO4 solution. Page 63 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section The filtrate (ZnSO4) is then placed in an evaporating dish to evaporate most of the water resulting in a saturated solution. The saturated solution is then left to cool down so that ZnSO4 crystals begin to form. The crystals are then filtered and dried by squeezing them between filter papers Step 2 Step 1 Step 3 Step 4 3. By reacting metal oxide with acid Nearly all metal oxides react with acids, but most require warming or heating. This method is especially suitable for those metals which do not react with dilute acids e.g. the metal copper. Copper metal has no reaction with dilute acids but copper(II) oxide, if warmed with dilute acids, forms salts. copper (II) oxide + sulfuric acid →copper sulfate + water CuO(s) + H2SO4 (aq) → CuSO4 (aq) + H2O (l) Page 64 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section Excess copper (II) oxide is added to warm sulfuric acid so that all the acid is neutralised. The unreacted oxide is then removed by filtering. The filtrate is a blue solution of copper (II) sulfate. The crystals are obtained by concentrating the solution by evaporation, and then leaving it to cool. The crystals formed can be removed by filtration. Since copper (II) sulfate crystals contain water of crystallisation, it is important not to evaporate the solution to dryness because the water of crystallisation will be lost to form powder. 4. By reacting metal carbonate with acid This one is similar to the reaction involving metal oxide and acid, but this time, no heating is required. The carbonate fizzes and gives off carbon dioxide gas. Excess metal carbonate must be added to ensure that all the acid is neutralised The solution is then filtered (to remove unreacted carbonate) and then crystallized (by heating up to saturation point and then leaving the solution to cool down). e.g. Reacting CaCO3 with acids calcium carbonate + sulfuric acid → calcium sulfate + carbon dioxide + water CaCO3 (s) + H2SO4 (aq) → CaSO4 (aq) + CO2 (g) + H2O (l) The same process is used as in the reaction of an acid with a metal; just that carbon dioxide is also produced in this case. Carbon dioxide can be tested by bubbling it into limewater. The limewater will change from colourless to milky white. Revision work Element symbol Sodium Potassium Argon Na K Ar Nucleon or mass number 23 39 40 Number of electrons Number of neutrons Number of protrons 11 19 18 12 20 22 11 19 18 Electronic structure or configuration 2.8.1 2.8.8.1 2.8.8 Page 65 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section Describe and use the following tests to Identify: Aqueous cations: ammonium, calcium, copper (II), iron(II), iron(III) and zinc, by means of aqueous sodium hydroxide and aqueous ammonia as appropriate (formulae of complex ions are not required). Tests for aqueous cations Cation Ammonium (NH4+) Calcium (Ca2+) Copper (Cu2+) Iron (II) (Fe2+) Iron (III) (Fe3+) Zinc (Zn2+) Effect of adding aqueous sodium hydroxide Ammonia produced on warming White ppt, insoluble in excess Light blue ppt, insoluble in excess Green ppt, insoluble in excess Red brown ppt, insoluble in excess White ppt, , soluble in excess giving a colourless solution Effect of adding aqueous ammonia _ no ppt. or very slight white ppt. light blue ppt., soluble in excess, giving a dark blue solution green ppt., insoluble in excess red-brown ppt., insoluble in excess white ppt., soluble in excess, giving a colourless solution Cations: flame tests to identify lithium, sodium, potassium and copper (II) Flame tests Metal ion Lithium (Li+) Sodium (Na+) Potassium (K+) Copper (II) (Cu2+) Flame colour Red Yellow Lilac Blue-green Anions: carbonate (by reaction with dilute acid and then limewater), chloride (by reaction under acidic conditions with aqueous silver nitrate), nitrate (by reduction with aluminium) and sulfate (by reaction under acidic conditions with aqueous barium ions) Page 66 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section Tests for anions Anion Carbonate (CO32-) [in solution] Test Add dilute acid Chloride (Cl-) Add dilute nitric acid, then add aqueous silver nitrate Add aqueous sodium hydroxide, then aluminium foil: warm the mixture carefully Acidify (add acid), then add aqueous barium nitrate [in solution] Nitrate (NO3-) [in solution] Sulfate (SO42-) [in solution] Test result Effervescence, carbon dioxide produced White ppt (precipitate) formed Ammonia produced White ppt formed Gases: ammonia (using damp red litmus paper), carbon dioxide (using limewater), chlorine (using damp litmus paper), hydrogen (using a lighted splint), oxygen (using a glowing splint) Tests for gases Gas Ammonia (NH3) Carbon dioxide (CO2) Chlorine (Cl2) Hydrogen (H2) Oxygen (O2) Test and test result Turns damp red litmus paper blue Turns lime water milky Bleaches damp litmus paper „pops‟ with a lighted splint Relights a glowing splint The Periodic Table Describe the Periodic Table as a method of classifying elements and its use to predict properties of elements A periodic table is used to classify elements by placing them in groups (elements whose atoms have the same outer most electrons are placed in the same group) and periods (elements whose atoms have the same number of shells are placed in the same period). Elements with the same outermost electrons have the same or similar chemical properties e.g. group one elements react similarly with water to form hydroxides. Page 67 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section Periodic trends Describe the change from metallic to non metallic character across a period A trend is a pattern. One trend found in the periodic table is that from left to right, elements gradually change from metals to non-metals. Metals are generally found to the left of the periodic table, and non metals are found to the right. Metalloids are elements that have both the characteristics of metals and non metals. You need not worry about these for now. Describe and explain the relationship between Group number, number of outer shell electrons and metallic/non-metallic character The period number corresponds to the number of electron shells present in an atom of an element. An element in period 2 has 2 electron shells. The relationship between number of shells and period number Element Helium Sodium Magnesium Lithium Electronic configuration 2 2,8,1 2,8,2 2,1 Period Number of shells 1 3 3 2 1 3 3 2 Page 68 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section Group properties 1. Describe lithium, sodium and potassium (elements in Group I) as a collection of relatively soft metals showing a trend in melting point, density and reaction with water 2. Predict the properties of other elements in Group I, given data, where appropriate Elements in Group I are known as alkali metals. The atoms of these elements have one electron each in the outermost shell. These metals are soft and can be cut easily with a knife. These metals show a trend (pattern) in melting point, density and reaction with water. You are expected to be able to predict the behavior of an element in group one when given the behavior of other elements in that group. Moving down the group, the melting and boiling points decrease while the densities increase. Moving down the group, the reaction of these metals with water increases.eg. the element lithium (which is above sodium and potassium in group 1) has a higher melting and boiling point than potassium; lower density than potassium; and reacts less with water as compared to potassium. Describe the halogens, chlorine, bromine and iodine in Group VII, as a collection of diatomic non-metals showing a trend in colour and physical state The group VII elements are known as the halogens. These include the elements chlorine, bromine and iodine. Group VII elements exist as diatomic molecules (e.g. Cl2, Br2, l2). Di means two, hence, diatomic molecules are molecules formed by joining two atoms of the same element together. Group VII is found to the right of the periodic table; hence, elements in this group are non-metals. Group VII elements show a trend (pattern) in colour and physical state. The trend in colour is that the colour darkens down the group (Chlorine is a yellow-green gas, bromine is a reddish-brown liquid and iodine is a black solid). The trend in physical state is that the elements change from gas to liquid and from liquid to solid as we go down Group VII (Chlorine is a gas, bromine is a liquid and iodine is a solid). 1. State the reaction of chlorine, bromine and iodine with other halide ions Halogens at the top of the group are more reactive than those below them. Halogens undergo displacement reactions, where a more reactive halogen displaces a less reactive halogen from its salt. For instance, when chlorine gas is bubbled into sodium bromide solution, bromide ions get displaced. Cl2(g) + 2NaBr(aq) → 2NaCl(aq) + Br2(aq) Generally, chlorine can displace both bromine and iodine. Bromine can displace iodine only. Iodine cannot displace either chlorine or bromine because they are above it in Group VII. Page 69 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section 2. Predict the properties of other elements in Group VII, given data where appropriate To predict the trend, simply remember that halogens are non-metals showing a trend in colour and physical state. An element below Iodine would have to exist as a solid. 3. Identify trends in other groups, given data about the elements concerned To identify the trend in the group, simply make sense of the pattern in the group. Transition elements Describe the transition elements as a collection of metals having high densities, high melting points and forming coloured compounds, and which, as elements and compounds, often act as catalysts Transition elements are a block of metals found between Groups II and III in the Periodic Table. These metals have high melting and boiling points, as well as high densities. Compounds of transition elements are usually coloured. Compounds are formed when two or more elements react together to form one product (known as a compound). One of the elements reacting to form a compound, in this case, would be a transition element. Transition elements and their compounds are good catalysts. Catalysts are substances which speed up the rate of a chemical reaction. They make us obtain the products of the reaction in a much shorter time. Examples of transition metals include iron (Fe), copper (Cu), and vanadium (V). Check where they are located on your periodic table. Noble gases Describe the noble gases, in Group VIII or 0, as being unreactive, monoatomic gases and explain this in terms of electronic structure Elements in Group VIII (or sometimes referred to as Group 0) are known as noble gases. These elements are inert (inert simply means unreactive) non-metals which are found as monoatomic gases. Monoatomic means the atoms of these elements exist as single gaseous atoms. (Remember that the halogens, unlike the noble gases, exist as diatomic molecules). Their lack of reactivity is due to their complete shell of valence or outermost electrons (that is, their electronic structure is such that all the shells are full or contain the maximum possible number of electrons). Due to their unreactive nature, noble gases are often used to provide an inert atmosphere. The following table shows some uses of noble gases. You need to remember these uses. State the uses of the noble gases in providing an inert atmosphere, i.e. argon in lamps, helium for filling balloons Element Helium Argon Application filling balloons Light bulbs Page 70 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section Metals Properties of metals 1 Describe the general physical properties of metals as solids with high melting and boiling points, malleable and good conductors of heat and electricity Metals are solids which are good conductors of electricity and heat. Metals are solids with high melting and boiling points since a lot of energy is required to break the strong electrostatic forces of attraction between the „sea of delocalized electrons‟ and the lattice of positive ions. Metals are malleable which means that metals can be shaped by hammering. The diagram to the left shows metallic bonding. 2 Describe alloys, such as brass, as mixtures of a metal with other elements An alloy is a mixture of a metal and other elements, which may be metals or non-metals.eg. brass is an alloy made by mixing the metals copper and zinc. 3 Explain in terms of their properties why alloys are used instead of pure metals Turning a metal into an alloy changes its properties, and makes it more useful. A pure metal is soft due to the regular arrangement of atoms in the metal lattice. The atoms are arranged in layers which slide past each other easily when a force is applied. In an alloy, however, the regular arrangement of atoms is disrupted by the presence of atoms of different sizes (since an alloy has atoms of different elements in it and these atoms of different elements also differ in size). This prevents the layers of atoms from easily sliding over each other, making the alloy harder than the pure metal. Page 71 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section 4 Identify representations of alloys from diagrams of structure Alloying metals helps to change their properties to make them more suitable for a particular use. For instance, an alloy of iron and chromium has greater resistance to rusting compared to pure iron. Reactivity series Carbon and hydrogen are not metals but are placed due to their characteristic reactions with metals. To remember the above elements in the reactivity series, master the phrase “Please Stop Calling Me A Careless Zulu. I‟m Highly Complex”. Reactivity decreases from potassium to copper. Part of phrase Element Please Potassium (most reactive) Stop Sodium Calling Calcium Me Magnesium A Aluminium Careless Carbon Zulu Zinc I‟m Iron Highly Hydrogen Complex Copper (least reactive) 1 Place in order of reactivity: potassium, sodium, calcium, magnesium, aluminium, (carbon), zinc, iron, (hydrogen) and copper, by reference to the reactions, if any, of the elements with: water or steam Remember that carbon and hydrogen are not metals so they do not react with water or steam. Reactivity of the above metals with water decreases from potassium to iron. From the list, only copper does not react with both water and dilute acids. The metals potassium to iron react with water to form the metal oxide and hydrogen gas. The metals actually displace the hydrogens in a water molecule, and inturn form a bond with the oxygen which had previously been bonded to the displaced hydrogen. The hydrogen is then displaced as hydrogen gas. Metals also displace hydrogen in dilute hydrochloric acid (HCl(aq) ), and inturn bond with the chlorine of the hydrochloric acid to form chlorides (certain salts) dilute hydrochloric acid The metals potassium to iron react with dilute hydrochloric acid to form the metal chloride and hydrogen gas. Copper does not react with either water and dilute hydrochloric acid (remember this!) because if you check the reactivity series above, copper is less reactive than Page 72 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section hydrogen, hence, it cannot displace hydrogen. Thats why if we need to make a salt of copper, we react copper oxide with dilute acids, and not copper with dilute acids (since these two do not react together!!!!). Reactions of metals with either water or dilute hydrochloric acid are displacement reactions where by hydrogen in the water or acid is displaced by a more reactive metal. The reactivity series is like the world heavy weight boxing championships where by the elements are fighting to wear the belt (either oxygen if reacting with water, or chlorine if reacting with dilute acid). These elements need to displace the sitting champion (hydrogen in this case). Only those elements ranked above hydrogen will displace it. Take note that carbon is not a metal so it won‟t displace hydrogen, and also that hydrogen cannot displace itself because if hydrogen displaces hydrogen then the overall change is zero. reduction of their oxides with carbon Metals are usually found in nature as ores, which mainly consist of metal oxides. The extraction of a metal from its ore depends on its reactivity. A more reactive metal usually requires tougher methods of extraction compared to a less reactive metal. Only Zinc, Iron and copper can be extracted from their oxides through heating with carbon. All these metals are below carbon in the reactivity series. 2 Describe the reactivity series in terms of the tendency of a metal to form its positive ion, illustrated by its reaction, if any, with the aqueous ions of other listed metals Reactive metals easily form positive ions by easily losing electrons. When a more reactive metal is placed in a solution containing aqueous ions of a less reactive metal eg. when calcium is placed in a solution containing aqueous ions of copper metal, the calcium metal will form calcium aqueous positive ions by losing its outer two electrons. These electrons are picked up by the aqueous ions of the less reactive metal, and convert those ions to metal atoms. calcium metal + copper nitrate → calcium nitrate + copper metal However, no reaction occurs when a less reactive metal is placed in the salt solution of a more reactive metal. No change is seen when copper metal is placed in magnesium sulfate solution since magnesium is more reactive than copper. Extraction of metals from their ores 1 Describe the use of carbon in the extraction of copper from copper oxide Copper can be extracted from copper oxide by heating copper oxide with carbon. The word equation is: Copper oxide + carbon → copper + carbon monoxide or Copper oxide + carbon → copper + carbon dioxide Page 73 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section 2 Describe and explain the essential reactions in the extraction of iron from hematite in the blast furnace C + O2 → CO2 C + CO2→ 2CO Fe2O3 + 3CO → 2Fe + 3CO2 Extraction of Iron The blast furnace is used to extract iron from its iron ore called haematite, Fe2O3. A mixture of iron ore, coke and limestone, known as the charge, is added at the top of the blast furnace. Hot air is pumped into the blast furnace via ports at the bottom of the furnace. Chemical processes which take place in the blast furnace Oxygen in the air reacts with coke (carbon) to give carbon dioxide. The reaction is exothermic (gives out heat) and gives rise to the high temperatures experienced in the blast furnace. C(s) + O2(g) → CO2(g) The carbon dioxide produced (above) then reacts with more coke to produce carbon monoxide CO2(g) + C(s) → 2CO(g) The carbon monoxide then reacts with iron (III) oxide to produce molten iron 3CO(g) + Fe2O3(s) → 2Fe(l) + 3CO2(g) Page 74 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section 3 Know that aluminium is extracted from the ore bauxite by electrolysis Aluminium is extracted from its ore, bauxite, by electrolysis. It cannot be extracted by heating with carbon because its more reactive than carbon (above carbon in the reactivity series). 4 Relate the method of extraction of a metal from its ore to its position in the reactivity series The extraction of a metal from its ore depends on its reactivity. A more reactive metal usually requires tougher methods of extraction compared to a less reactive metal. Zinc, Iron and copper (all below carbon in the reactivity series) can be extracted from their oxides through heating with carbon. The carbon reduces the oxides to give the metals. Aluminium and other metals above it in the reactivity series form very stable oxides that are not easily reduced by Carbon. They can only be extracted from their ores through electrolysis of their molten oxides. Generally, metals above carbon are extracted by electrolysis and those below are extracted by reduction with carbon. 5 Describe metal ores as a finite resource and hence the need to recycle metals Metal ores are a finite resource i.e. they are limited in the earth‟s crust. Hence, for sustainability, scrap metal should be recycled. Air and water Water 1 Describe a chemical test for water using copper (II) sulfate and cobalt (II) chloride Test for water: Water turns white copper (II) sulfate (CuSO4) blue. Water turns blue Cobalt (II) chloride (CoCl2) pink 2 Describe, in outline, the treatment of the water supply in terms of filtration and chlorination 1. Filtration The water is filtered to remove suspended particles and unsettled floc. Slow sand filters can be used since the water must be passed very slowly through the filters. The filters are constructed using graded layers of sand, with the finest sand at the top and the coarsest sand (along with some gravel) at the bottom. Drains at the base of the filters convey filtered water away for disinfection. 2. Disinfection Disinfection is achieved by adding chlorine or one of its compounds. The chlorine kills harmful organisms because of its strong oxidative properties. Page 75 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section Air 1 State the composition of clean air as being a mixture of 78% nitrogen, 21% oxygen and small quantities of noble gases, water vapour and carbon dioxide Composition of air Clean air is composed of 21% Oxygen, 78% Nitrogen and small quantities of noble gases, water vapour and carbon dioxide. 2 Name the common pollutants in air as being carbon monoxide, sulfur dioxide and oxides of nitrogen Common pollutants in air are carbon monoxide, sulfur dioxide and oxides of nitrogen. Oxides of nitrogen are nitrogen monoxide and nitrogen dioxide. 3 State the adverse effect of these common air pollutants on buildings and on health Oxides of nitrogen and sulfur dioxide lead to acid rain. The acid rain corrodes buildings made of marble and limestone. Sulfur dioxide causes respiratory problems in humans. Oxides of nitrogen irritate lung tissues and the eyes. Carbon monoxide reduces the ability of haemoglobin to carry oxygen. This deprives cells of oxygen, leading to headaches, fatigue or even death. 4 State the conditions required for the rusting of iron (presence of oxygen and water) The conditions required for the rusting of iron are the presence of oxygen and water. In the absence of either one of these two, rusting will not occur. 5 Describe and explain barrier methods of rust prevention, including paint and other coatings Barrier methods of rust prevention are methods which reduce oxygen and water from coming into contact with iron. They place a barrier between iron, and both water and oxygen. Page 76 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section Rusting can be prevented by 1. Painting or covering the metal with a layer of oil. This protects iron from being exposed to oxygen and water. 2. Sacrificial protection. In this method, a more reactive metal is used as the sacrificial metal and corrodes/rusts instead of iron. This is usually done by attaching a block of magnesium or zinc to the iron. 3. Galvanizing Iron (coating of iron with zinc). The zinc coating keeps air and water (in the form of moisture) away. However, if the coating gets damaged, the zinc will still protect the iron by sacrificial protection. Carbon dioxide and methane 1 State the formation of carbon dioxide as follows: Carbon dioxide is a product in the following processes 1. 2. 3. 4. complete combustion of carbon-containing substances respiration the reaction between an acid and a carbonate thermal decomposition of calcium carbonate 2 State that carbon dioxide and methane are greenhouse gases The greenhouse effect which is caused by greenhouse gases leads to global warming. Examples of greenhouse gases are carbon dioxide and methane. 3 State that increased concentrations of greenhouse gases cause an enhanced greenhouse effect, which may contribute to climate change When greenhouse gases are produced faster than they are removed from the atmosphere (eg by processes like photosynthesis), an accumulation of these gases in the atmosphere results. This accumulation causes of greenhouse gases causes global warming. Global warming refers to an increase in global temperatures due to high levels of greenhouse gases. This global warming may contribute to climate change, cause drought or cause flooding. Organic chemistry Fuels 1 State that coal, natural gas and petroleum are fossil fuels that produce carbon dioxide on combustion Coal, natural gas and petroleum are fossil fuels that produce carbon dioxide on combustion. 2 Name methane as the main constituent of natural gas Natural gas is a mixture of substances. The main constituent of natural gas is methane. Page 77 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section 3 Describe petroleum as a mixture of hydrocarbons and its separation into useful fractions by fractional distillation Petroleum is a mixture of hydrocarbons which can be separated into various useful fractions by fractional distillation. 4 Describe the properties of molecules within a fraction Petroleum consists of hydrocarbons that have different boiling points and condense at different temperatures. Higher parts of the fractionating column have lower temperatures while lower parts of the fractionating column have higher temperatures. Since lighter fractions have lower boiling points, they are tapped off at higher parts of the column. Heavier fractions on the other hand, have higher boiling points and are tapped off at lower parts of the column. Generally, the molecules of fractions at the top have 1. 2. 3. 4. 5. lower boiling point smaller molecules weaker intermolecular/attractive forces greater flammability and lower viscosity…as compared to those at the bottom A simplified diagram of the fractional distillation of petroleum and the fractions collected is shown below. 5 Name the uses of the fractions as follows: Uses of the fractions of petroleum refinery gas: used as „bottled gas‟ for heating and cooking gasoline fraction: used as a fuel (petrol) in cars naphtha fraction: used as a feedstock for making chemicals diesel oil: used as a fuel in diesel engines bitumen: used in making road surfaces Page 78 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section Homologous series 1 Describe the homologous series of alkanes and alkenes as families of compounds with the same general formula and similar chemical properties A homologous series is a family of organic compounds with the same general formula and similar chemical properties. Alkanes and alkenes are two examples of a homologous series. Alkanes 1 Describe alkanes as saturated hydrocarbons whose molecules contain only single covalent bonds Alkanes are referred to as saturated hydrocarbons because their molecules contain only single covalent bonds. Note that a hydrocarbon contains hydrogen and carbon only!!!! Substances made up of larger molecules have greater intermolecular attractive forces acting between their molecules, giving rise to greater melting and boiling points e.g octane, which seems to have the largest molecules, has the highest m.p and b.p 2 Describe the properties of alkanes (exemplified by methane) as being generally unreactive, except in terms of burning alkanes do not react with most chemicals since they are saturated, thus having only C-C and C-H single covalent bonds. Alkanes , however, undergo burning or combustion. 3 Describe the complete combustion of hydrocarbons to give carbon dioxide and water Hydrocarbons undergo complete combustion to give carbon dioxide and water. A lot of heat energy is also given out. Page 79 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section Alkenes 1 Describe alkenes as unsaturated hydrocarbons whose molecules contain one double covalent bond Alkenes are referred to as unsaturated hydrocarbons because their molecules contain one double covalent bond between two Carbon atoms. e.g. ethene 2 State that cracking is a reaction that produces alkenes Alkenes are made from a process called cracking 3 Describe the formation of smaller alkanes, alkenes and hydrogen by the cracking of larger alkane molecules and state the conditions required for cracking Large alkane molecules can be broken down into smaller molecules through cracking. This process requires the following conditions 1. a catalyst (either aluminium oxide or silicon dioxide). 2. High temperatures or heating. Examples of cracking. You do not need to remember the names of the complex molecules. Note that the total number of carbon and hydrogen atoms on the left side is equal to the total number of carbon and hydrogen atoms on the right. This is because matter is neither created nor destroyed; it is simply converted from one form to another. This is known as the law of conservation of matter. Decane (above) has 10 carbon atoms and 22 hydrogen atoms. Pentane, propene and ethene (the products of the cracking of decane) also have a sum of 10 carbon atoms and 22 hydrogen atoms. Page 80 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Chemistry section 4 Recognise saturated and unsaturated hydrocarbons: from molecular structures Two unsaturated structures are shown below. Notice the presence of C to C double bonds. For saturated structures study the structures of the alkanes given on previous pages of these notes. by their reaction with aqueous bromine Aqueous bromine reacts with substances which have a C = C bond (alkenes). The addition of aqueous bromine is thus used in testing whether a substance is a saturated or unsaturated compound. Aqueous bromine is reddish-brown. When added to an unsaturated compound ( alkene) it changes from reddish-brown to colourless. There is no colour change when reddish-brown aqueous bromine is added to a saturated compound (alkane). 5 Describe the formation of poly(ethene) as an example of addition polymerisation of monomer units Just as bricks can be used to make houses in a process called building, monomers can be used to build a polymer in a process called polymerization. Many ethene molecules can be added together to form a polymer called poly(ethene). The word mono means one…..the word poly means many….. The formation of poly(ethene) is an example of addition polymerisation of monomer units Note that there are no C to C double bonds in polythene Page 81 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section Motion Length and time 1 Use and describe the use of rules and measuring cylinders to find a length or a volume Rules are used to measure length. A metre rule is ideal for measuring lengths up to 100 cm. it has an accuracy of up to 0.1cm. When using a metre rule one must be careful to avoid parallax error (an error due to reading from an angle) by making readings at eye level. How to avoid errors by a. correct positioning of the eye b. making sure the object touches the marking of the scale (for measuring tape and metre rule, ensure that the object is in contact with the scale) (a) Precision is how close the measured values are to each other. (b) Accuracy is how close a reading is to the true value of the measurement. The accuracy of a reading can be improved by repeating the measurements. Page 82 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section Volume is measured using burettes, pipettes, measuring cylinders, volumetric flasks and beakers. Measuring cylinders measure a wide range of volumes whilst pipettes and volumetric flasks are designed with one marking only to measure a single specific volume. When using measuring cylinders and burettes, one must be careful to avoid parallax error (an error due to reading from an angle) by making readings at eye level. 2 Use and describe the use of clocks and devices, both analogue and digital, for measuring an interval of time Clocks and stop watches are used for measuring an interval of time. Nowadays digital stopwatches are being used rather than analogue ones because analogue stopwatches are prone to parallax error. However, starting and stopping of stopwatches manually for time interval measurements results in error due to reaction time. 3 Obtain an average value for a small distance and for a short interval of time by measuring multiples (including the period of a pendulum) When asked to make measurements e.g. measuring distance or time, make more than one measurement and calculate the average. Making multiple measurements and calculating their average reduces error and increases accuracy. Motion 1 Define speed and calculate average speed from 𝒕𝒐𝒕𝒂𝒍 𝒅𝒊𝒔𝒕𝒂𝒏𝒄𝒆 𝒕𝒐𝒕𝒂𝒍 𝒕𝒊𝒎𝒆 Speed is the change of distance with time. It is measured in metres/second (m/s) or kilometres/hour (km/h). Average speed is calculated by dividing the total distance travelled by the time taken Page 83 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section Speed = 𝑡𝑜𝑡𝑎𝑙 𝑑𝑖𝑠𝑡𝑎𝑛𝑐𝑒 𝑡𝑜𝑡𝑎𝑙 𝑡𝑖𝑚𝑒 2 Plot and interpret a speed-time graph and a distance-time graph 3 Recognise from the shape of a speed-time graph when a body is: at rest moving with constant (steady) speed moving with changing speed The speed-time graph of a moving object is used to find: (a) Acceleration (using the gradient of graph) (b) Distance travelled (using the area under the graph) An increase in speed is a positive acceleration, a decrease in speed is a negative acceleration / deceleration / retardation. • If acceleration is not constant, the speed/time graph will be curved. • The downwards acceleration of an object is caused by gravity. This happens most when an object is in free fall (falling with nothing holding it up). Objects are slowed down by air resistance 4 Calculate the area under a speed-time graph to work out the distance travelled for motion with constant acceleration 5 Calculate acceleration from the gradient of a speed-time graph 6 Recognise linear motion for which the acceleration is constant (uniform) and calculate the acceleration Page 84 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section 7 Recognise motion for which the acceleration is not constant (or uniform) The area under a speed-time graph gives the distance. Acceleration is calculated from the gradient of a speed-time graph 1 1 Total distance travelled = 2 base × height = 2 4 × 8 = 16m Distance travelled from t = 3 to t = 4 is = area of shaded region = area of triangle part + area of rectangle part = 1 (2 1 × 2) + (6 × 1) = 1 + 6 = 7 Acceleration = 𝑐𝑎𝑛𝑔𝑒 𝑜𝑓 𝑣𝑒𝑙𝑜𝑐𝑖𝑡𝑦 𝑡𝑖𝑚𝑒 Gradient of the graph = 8−0 4−0 = = 2ms-2 For an object moving with constant acceleration, the speed-time graph is a sloping straight line. A constant acceleration means that speed is increasing at a constant rate. For graphs showing non uniform acceleration (two graphs below), acceleration can be calculated for a particular time by drawing a tangent on the curve and calculating its gradient Page 85 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section Summary of some graphs In the diagram to the left, lines a,b and c represent different gradients/steepness. Line "a" is the steepest therefore it has a higher average speed than the rest and "c" has the lowest average speed. Graph-d: The graph is getting less steep, hence, speed is decreasing. Decreasing speed is called deceleration or retardation. Hence, the object is retarding or decelerating. Graph-e: When you throw something up, it goes against gravity because the force of gravity is pulling it down. This constant downward pull of gravity reduces the speed per unit time therefore the gradient of the graph keeps falling; that's why the object has a deceleration graph. Graph-f: Gravity pulls every object downwards therefore the presence of a constant force increases the speed of the falling object. The speed is highest just before reaching the ground. Increasing speed is called acceleration. Page 86 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section Example 1 A model train travels between two stations. The velocity–time graph shows the train's motion. (a) (i) State in which part of the journey A, B or C the train is decelerating. [C] (ii) Explain your answer. [-ve gradient] (iii) What does the graph show about the deceleration? [constant deceleration] (b) (i) What feature of the graph represents the distance travelled between the two stations? [Area under the graph] Example 2 (a) A student walks from home to a library, waits to collect a book and then runs to a friend's house. The distance-time graph for the student is shown. Three sections of the graph are labelled P, Q and R. Complete the sentences with P, Q or R. (i) The student is walking at constant speed in section .............................................(1) (ii) The student is waiting at the library in section ...............................................(1) (iii) The two sections of the graph that take equal amounts of time are........... and .............(1) (b) Use words from the box to complete the sentences. You may use each word once, more than once or not at all. Page 87 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section (i) My answer to (ai) is because the section of the graph is ....... and .......... (2) (ii) My answer to (aii) is because the section of the graph is.....................................(1) (c) How does the graph show that the student's friend lives nearer to the library than the student does? Example 2 Answers ai) P; aii) Q; aiii) Q and R; bi) Sloping straight; bii) Horizontal; c) Less distance travelled in section R than in section P 8 Demonstrate an understanding that acceleration and deceleration are related to changing speed including qualitative analysis of the gradient of a speed-time graph [objective covered above] Mass and weight 1 Distinguish between mass and weight Weight, unlike mass, is a force. Weight and mass are related by the equation Weight = mass × acceleration due to gravity Mass Weight The property of an object that is a measure of The force of gravity acting on an object, measured in Newtons, and given by the formula: its inertia (defined as the resistance of a body to change its state of rest or motion due to its mass), Weight = mass × acceleration due to gravity the amount of matter it contains The more the mass, the heavier the object, therefore, the object is more difficult to move. It's difficult to move a heavy truck than a bicycle. Mass resists the change from rest to motion. However, it is equally difficult to stop the motion of a heavy object than a lighter one. This property of mass by which it can resist the change from rest to motion and motion to rest is called inertia. The greater the mass, the more the inertia. 2 Know that the Earth is the source of a gravitational field The source of the gravitational field is the earth Page 88 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section 3 Describe, and use the concept of, weight as the effect of a gravitational field on a mass Since Weight = mass × acceleration due to gravity, Weight can be described as the effect of a gravitational field on a mass. 4 Recognise that g is the gravitational force acting on a unit mass and is measured in N / kg 5 Recall and use the equation W = mg Weight = mass × acceleration due to gravity W = mg 𝑾 hence, g = 𝒎 Since W is a force known as the gravitational force, g is the gravitational force acting on a unit mass, and is measured in N / kg Density 1 Recall and use the equation ρ = 𝒎 𝑽 𝒎𝒂𝒔𝒔 Density = 𝒗𝒐𝒍𝒖𝒎𝒆 using symbols, ρ = 𝒎 𝑽 The density of 1 kg of iron is the same as the density of 2 kg of iron because density of every material stays the same. Worked Example, (calculating density of regular shaped objects) A gold bar of mass = 5 kg has length = 5 cm, width = 15 cm and height = 10 cm. Find density. Solution: To calculate density we need to measure the volume of the bar; but before we do that, we need to convert it into meters so L = 0.05 m, W = 0.15 m and H = 0.10 m Volume = L x W x H = 0.05 x 0.15 x 0.10m3= 0.00075m3 Density = mass / volume = 5 / 0.00075 = 6667kgm-3 2 Describe an experiment to determine the density of a liquid and of a regularly shaped solid and make the necessary calculation For the density of a liquid find the mass of a certain volume of liquid e.g. 10cm3 divide that mass by the volume to find density Page 89 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section For the density of a regularly shaped solid calculate the volume of the object mathematically e.g. if it‟s a rectangular prism use length × width × height find the mass of the object using a beam balance divide the mass by the mathematically calculated volume to find the density 3 Describe the determination of the density of an irregularly shaped solid by the method of displacement and make the necessary calculation For the density of an irregularly shaped solid eg. a rock find the volume of the object by the method of displacement in a measuring cylinder. This is done by placing a certain volume of water in a measuring cylinder and noting its initial volume. The irregularly shaped object is then placed in the same measuring cylinder where it will displace some of the water to give a new volume reading. The difference between the two volume readings is equal to the volume of the irregularly shaped solid. find the mass of the solid using a beam balance. divide the mass by the volume of the solid to get density A speed time graph The volume of regularly shaped solids can be calculated using mathematical formulas instead of the displacement method. The displacement method (described above) is ideal for irregular solid objects because we cannot calculate their volumes mathematically. Examples of regular shaped objects are spherical objects and rectangular prisms. Volume for rectangular prisms = length × width × height Page 90 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section Effects of forces 1 Describe how forces may change the size, shape and motion of a body A force may produce a change in size and shape of a body; cause acceleration or deceleration or a change in direction depending on the direction of the force. 2 Plot and interpret extension-load graphs and describe the associated experimental procedure 1. set up apparatus i.e hang spring 2. measure initial length of spring 4. measure new length and subtract initial length to calculate extension 3. add 100g mass 5. repeat 6 times, adding 100g masses each time. 6. plot extension against load 3 State Hooke‟s Law and recall and use the expression F = k x, where k is the spring constant Hooke‟s Law states that springs extend in proportion to load, as long as they are under their limit of proportionality. The proportional limit is the region when the graph ceases to be a straight line. Load (N) = spring constant (N/mm) x extension (mm) F=kx 4 Recognise the significance of the term „limit of proportionality‟ for an extension-load graph The limit of proportionality is the point above which the load and extension would no longer be directly proportional. The elastic limit is the point above which the spring will not return to its original shape after being stretched Page 91 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section Hooke's law is only valid for straight line parts of the graph, i.e up to the limit of proportionality where force and extension are directly proportional. Beyond the limit of proportionality, Hooke's law is not valid. The elastic limit is beyond the limit of proportionality. The material, up to the elastic limit, is able to retain its shape e.g. if a spring is stretched, it would be able to go back to its original shape when the force is removed. We say that the material is in an elastic region. If the spring is stretched too much i.e a large force is applied, the spring won‟t be able to return back to its original shape, it will become elongated forever. We say that the spring is now in the plastic region. All materials (copper, iron, rubber etc) behave in the same way as springs; they extend on the application of a force and return back to their original length upon its removal. However, if the force is large, all materials deform permanently (plastic deformation). 5 Understand friction as the force between two surfaces which impedes motion and results in heating Friction is the force between two surfaces which impedes motion and results in heating 6 Recognise air resistance as a form of friction Air resistance is a form of friction because it impedes (opposes) motion. 7 Find the resultant of two or more forces acting along the same line Page 92 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section 8 Recognise that if there is no resultant force on a body it either remains at rest or continues at constant speed in a straight line Newton‟s first law of motion states that a body will continue in its present state of rest or uniform motion unless if acted on by an external force. Example: (a) Complete the sentence. Hooke's law states that a force acting on a material produces an extension which is ............................................................. to the force. (1) (b) A student attaches a load to the end of a spring. (i) Name the type of force acting in the stretched spring. (c) A force–extension graph for rubber is shown. Three regions A, B and C are labelled. Answers: a-(directly) proportional b-(i) tension /weight / gravitational force c-(i) A (ii) B (iii) large extension for small increase in force (i) In which region is Hooke's law obeyed? (ii) In which region is the rubber easiest to extend? (iii) Explain your answer to (ii) (Total 7 marks) Page 93 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section Pressure 1 Relate qualitatively pressure to force and area, using appropriate examples 2 Recall and use the equation p = F / A Pressure = force / area Force is a numerator and area is a denominator. If force is large and area is small then the pressure would be bigger and vice versa. If Force = 5 N and area is 5 m2 then pressure is P = 5/5 = 1 N/m2 or Pascal However, if force = 5 N and area is 0.5 m2 then pressure would be P = 5 / 0.5 = 10 N/m2 That‟s why camels can walk on the desert but a beautiful lady with high and sharp heels cannot. Reason, camel's feet are wide so the area is large and the camel's weight is divided between the four wide legs. However, the beautiful lady's weight is divided only between the two feet whose contact area with the ground is very small due to sharp heels. Example#1: An elephant weighing 40,000 N stands on one foot of area 0.1 m2. What pressure is exerted on the ground? Pressure = Force / Area = 40,000 /0.1 = 400,000 N/m2 Example#2: What is the pressure exerted by a girl weighing 400 N standing on one 'stiletto' heel of area of 0.00001 m2? Pressure = force / area = 400 / 0.00001 = 40000000 N/m2 .This is a hundred times bigger than the elephant's pressure on ground. Example#3: A woman having a weight of 50 N and wearing a pointed heel of 0.1 m2 would apply a pressure of 500 N; whereas, a woman having the same weight, but wearing a heel of 0.5 m2 would apply a pressure of 100 N. So which lady can walk easily on sand? The one with a larger area of heel because a smaller heel would apply more pressure and would dip deeper in sand. Work, energy and power Work 1 Relate (without calculation) work done to the magnitude of a force and distance moved in the direction of the force Work is done whenever a force makes something move. The unit for work is the Joule (J). The work done is always directly proportional to the magnitude of the force and the distance moved in the direction of the force. Page 94 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section 2 Recall and use W = Fd = ΔE Work done = force × distance moved in the direction of the force = change in energy W = Fd = ΔE (a) The diagram shows an energy flow for a motorbike. Fill in the gaps in the diagram. (b) The motorbike travels 2.0 km. The driving force is 700 N. Calculate the work done in joules by this driving force. Work done = ........................................................... J Solution: a) i- Chemical Energy ii- 30 000 J iii- Heat+Sound b) convert 2.0 km into metres as 2000 m. Using Work done = F x d = 700 x 2000 = 14 00 000 J Energy 1 Demonstrate an understanding that work done= energy transferred From the equation W = Fd = ΔE ; work done = energy transferred (remember this) When I push (pushing is applying a force) a table, it will move a certain distance, therefore, work is done. Work done = Force x distance. As the table is moved, the table gains Kinetic energy at the cost of my body‟s chemical energy. Hence, whenever work is done, energy is transferred from one form to another. 2 Demonstrate understanding that an object may have energy due to its motion (kinetic energy, K.E.) or its position (potential energy, P.E.) and that energy may be transferred and stored Kinetic energy is movement energy. When an object is moving fast it has more KE. KE can be calculated by the following formula. Kinetic energy = ½ mass x 𝒗𝒆𝒍𝒐𝒄𝒊𝒕𝒚𝟐 Page 95 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section When you ride a bicycle you convert your body‟s chemical energy to kinetic energy. Similarly, an engine converts chemical energy in petrol to kinetic, heat and sound energy. Potential Energy is stored energy. In fact, gravitational, chemical, strain or elastic and nuclear energy are forms of potential energy. Energy can be transferred from one form to another as shown above. It can also be stored e.g. energy from the sun (light energy) can be used by photosynthetic plants to form carbohydrates which possess stored chemical energy. In summary, an object may have energy because of its movement (kinetic energy) or because of its position, for example a book on a shelf has gravitational potential energy - it can fall off the shelf. Energy can be transferred from one form to another for example if the book falls off the shelf its GPE is turned into KE. Energy can be stored, for example, a book on a shelf stores GPE and a glucose molecule stores chemical energy in its bonds. An object can transfer its energy to another object, for example by conducting heat. 3 Give and identify examples of changes in kinetic, gravitational potential, chemical potential, elastic potential (strain), thermal, sound and electrical potential energy that have occurred as a result of an event or process 4 Recall and use the expressions K.E = ½ mv2 and gravitational potential energy, G.P.E = mgh or change in G.P.E = mgΔh Gravitational PE: When you move an object above ground level, the object will store energy in it. This energy is called Gravitational potential energy. This energy depends on how high the object is from the ground. The higher it is above the ground, the more the energy stored. This energy can be calculated from the following formula. Gravitational Potential Energy (J) = Mass (kg) x Gravity (m/s2) x Height (m) GPE = m x g x h Extra information: Another way of looking at the GPE is that in order to raise an object above ground level, we need to provide energy to it. This energy is called GPE, and is actually work done against the gravitational force. This energy is released when the object falls back to ground. Elastic Potential Energy: When you stretch a rubber band, it stores energy in it and returns that energy when it relaxes. Similarly, when you stretch a spring or catapult, they store energy in them. This stored energy is called Elastic Potential Energy or Strain Energy. Elastic Potential Energy or Strain Energy is called stored energy because objects will always return back to their original position and shape after using the energy. When you stretch a rubber cord or spring, your Page 96 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section body‟s chemical energy is converted to kinetic energy to stretch the rubber or spring. Your energy is then stored in the stretched object as elastic potential energy. Chemical Potential Energy: Energy stored by chemicals is called chemical energy. Examples are fossil fuels. Thermal Energy: When food is eaten it reacts with the oxygen we breathe into our lungs and is slowly „burnt‟. As a result, chemical energy stored in food becomes heat energy to warm the body and kinetic energy for muscular movement. Sound energy: A microphone changes sound energy into electrical energy; a loudspeaker does the reverse Electrical energy: A battery changes chemical energy to electrical energy. The electrical energy can be further changed to kinetic energy by an electric motor. A generator can convert kinetic energy to electrical energy. Some Facts which you need to know: 1 –Energy cannot be created nor destroyed; it can only be changed from one form to another. This is called the Law of Conservation of Energy 2 – No energy transfer is 100% efficient. Page 97 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section 5 Recognise that energy is transferred during events and processes, including examples of transfer by forces (mechanical working), by electric currents (electrical working), by heating and by waves Mechanical working is the transfer of kinetic and potential energy. When a crane uses an electric motor to lift a heavy load, energy is transferred to the load by the crane. This is called mechanical working because kinetic energy in the crane is being converted to gravitational potential energy in the load by a force causing movement. Electrical working is the transfer of electrical energy. The potential difference across a circuit component is the work done to drive a unit charge through the circuit component. Electricity can be used to transport energy. The circuit contains devices for transforming energy. In a torch, energy is transferred electrically from the battery to the bulb, where it is transformed into light and heat. Thermal (heat) energy spreads out from hot objects. Waves also transfer energy. 6 Apply the principle of conservation of energy to simple examples The principle of conservation of energy states that energy cannot be created or destroyed, hence, when work is done, energy is changed from one form to another. The most everyday example of this is when we move, our cells turn chemical energy (in glucose) into thermal and kinetic energy. Energy cannot be created or destroyed. The total amount of energy before and after any process/work always stays constant. However, after any process, energy is transformed from one form to another. For example, when 100 J is supplied to a lamp, it transfers 75 J to light and 25 J to heat. This means that the total energy before and after remains as 100 J. The only difference is that before the process, 100 J was in electrical form and after the process 100 J was in light and heat energy form. Page 98 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section Answers: (a) moving (b) Chemical (c) Sound & Heat Power 1 Relate (without calculation) power to work done and time taken, using appropriate examples Power (W) = Work done (J) / Time Taken (s) [symbols of units are given in brackets] 2 Recall and use the equation P = ΔE / t in simple systems, including electrical circuits P = ΔE / t where P is power in watts; ΔE is change in energy in joules; t is time in seconds Energy resources 1 Distinguish between renewable and nonrenewable sources of energy Renewable sources of energy: are inexhaustible, for example solar, hydroelectric, wind etc. Non-renewable sources of energy: are exhaustible for example fossil fuels Page 99 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section 2 Describe how electricity or other useful forms of energy may be obtained from: chemical energy stored in fuel nuclear fission water, including the energy stored heat and light from the Sun (solar cells in waves, in tides, and in water and panels) wind energy behind hydroelectric dams geothermal resources Chemical energy: Fuels can be burnt in thermal power stations to transform the stored chemical energy to thermal energy which makes steam to turns turbines (kinetic energy) which then produce electricity. Advantage: cheap, plentiful, and low-tech. Disadvantage: harmful wastes - produces greenhouse gases and pollutant gases. Hydroelectricity from dams: river and rain water fill up a lake behind a dam. As water rushes down through the dam, it turns turbines which turn generators. Tidal power scheme: a dam is built across a river where it meets the sea. The lake behind the dam fills when the tide comes in and empties when the tide goes out. The flow of water turns the generator. Advantage: no greenhouse gases are produced. Disadvantage: expensive, can‟t be built everywhere, and not as reliable because it depends on the height of the tide which varies daily, monthly and seasonally. Can cause silt to build and can disrupt fish. Wave energy: generators are driven by the up and down motion of the waves at sea. Advantage: does not produce greenhouse gases. Disadvantage: difficult to build Geothermal resources: water is pumped down to hot rocks deep underground and rises as steam which can then be used to turn turbines. Advantage: no carbon dioxide is produced. Disadvantage: deep drilling is difficult and expensive Nuclear fission: uranium atoms are split by shooting neutrons at them. Advantage: produces a lot of energy from using very little resources. Disadvantage: produces radioactive waste Solar cells: are made of materials that can deliver an electrical current when they absorb light energy Solar panels: absorb the energy and use it to heat water. Advantage: does not produce carbon dioxide. Disadvantage: variable amounts of sunshine in some countries. Wind energy: giant windmills called wind turbines, with two or three blades, drive electrical generators. „Wind farms‟ of 20 to 100 turbines provide enough electricity for thousands of homes in the UK and provide a useful „top-up‟ to the National Grid. Advantage: wind turbines provide a clean and renewable source of energy. Disadvantage: wind turbines can be noisy and may be considered unsightly so there is some environmental objection to wind farms, especially as the best sites for setting up turbines are often in coastal or upland areas of great natural beauty. Page 100 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section 3 Give advantages and disadvantages of each method in terms of renewability, cost, reliability, scale and environmental impact Type of energy Renewable or nonrenewable Wind Renewable Form of Energy Conversion Kinetic to Electrical Advantages Disadvantages 1-renewable. 2-no CO2 pollution. 3-very cheap electricity after few years. Water (HEP) Renewable GPE to Electrical Geothermal Resource Renewable GPE to Electrical Solar Heating Systems Renewable Light to Electrical Solar Cells Renewable Light to Electrical Fossil Fuels (natural gas, coal and petroleum) Nonrenewable Chemical to Electrical Nuclear Power Nonrenewable Nuclear Potential Energy to Thermal + Kinetic 1-renewable. 2- no CO2 pollution. 3- very cheap and reliable electricity. 1-renewable. 2- no CO2 pollution. 3- low cost to run 4- reliable 1-renewable. 2- no CO2 pollution. 3-low maintenance 1-renewable. 2- no CO2 pollution. 3- easy to install 4- low maintenance 1- cheap, plentiful, and low-tech. 2- reliable in that they can produce electricity at any time of day and in any season of the year as long as fuel is available. 1-renewable. 2- no CO2 pollution. 3- produces a lot of energy from using very little resources 4-reliable 1-expensive to install. 2-dangerous for birds. 3-requires large spaces of land. 4- not very reliable because the output of a wind turbine changes with the strength of the wind 1- expensive to install. 2-dangerous for wildlife. 3-flooding risk and damage to environment. 1- expensive to install 2-not available everywhere. 3- deep drilling is difficult and expensive 1- expensive to install 2- If there is no Sun it won't work 1-expensive to install. 2- the output of a solar cell changes with the intensity of light falling on it 1- harmful wastes - produces greenhouse gases and pollutant gases. 2- oil spills can cause environmental damage 3- natural gas can cause explosions 1-expensive to install, maintain and decommission. 2-produces radioactive wastes. 4 Understand that the Sun is the source of energy for all our energy resources except geothermal, nuclear and tidal Apart from nuclear, geothermal, hydroelectric or tidal energy, the Sun is the source for all our energy resources. Page 101 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section 5 Understand that the source of tidal energy is mainly the moon The moon is the source of tidal energy. Its gravitational pull causes the level of the ocean‟s surface to rise and fall. Water can be trapped behind a dam at high tides and released at lower tides to drive turbines and generators. 6 Show an understanding that energy is released by nuclear fusion in the Sun Energy is released in the Sun by the process of nuclear fusion. In nuclear fusion, two hydrogen atoms collide and fuse (join up) to form an atom of helium. Nuclear fusion requires very high temperatures and pressures, both of which can be found on the Sun. Thermal Physics Simple kinetic molecular model of matter 1 State the distinguishing properties of solids, liquids and gases Solids: have a fixed shape and volume Liquids: have a fixed volume but change shape depending on their container Gases: do not have a fixed shape or volume. Gases fill up the space or volume of their containers 2 Relate the properties of solids, liquids and gases to the forces and distances between the molecules and to the motion of the molecules Solids Liquids Gases Attractive forces acting between particles Very strong Distances between particles Motion of particles Very close to each other Weaker than solids but stronger than in gases No forces acting Slightly further apart Particles can only vibrate about fixed positions Particles can flow Far apart Move freely about Page 102 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section 3 Describe qualitatively the molecular structure of solids, liquids and gases in terms of the arrangement, separation, and motion of the molecules Solids Liquids Gases Arrangement of particles Particles tightly packed together. Particles are closely packed together but not as tightly as in solids. Particles are spaced far apart from each other. •Liquids and gases do not have a fixed shape because of their weak forces of attraction. Gases can be compressed because there is plenty of space between the particles; solids can‟t because such Motion of particles Particles can only vibrate about fixed positions Particles can move around, but they remain close to each other Particles can move far apart from each other space does not exist. The particles in a solid cannot move because they are held tightly together by the attractive forces, but they can vibrate about fixed positions. 4 Describe qualitatively the pressure of a gas and the temperature of a gas, liquid or solid in terms of the motion of its particles The pressure of a gas is due to the gas particles colliding on the container walls. . The pressure of a gas increases with temperature because the kinetic energy of the gas particles increases, leading to more particles collisions with the walls of the container. An increase in temperature of a gas, liquid or solid results in a corresponding increase in the kinetic energy of the particles. However, solid particles only experience more vibration since they are “locked” in fixed positions. Particles of liquids, on the other hand, experience both vibrational and translational forms of kinetic energy. In summary, heating solids, liquids, or gases, increases the kinetic energy of the particles. 5 Use and describe the use of thermometers to measure temperature on the Celsius scale Liquids expand when they are heated because the particles gain more KE causing them to become further apart and to take up a greater volume. This concept is displayed when the liquid in thermometers expands and contracts when temperature changes. The volume of the liquid taken up in the tube can be used to find the temperature. Units of temperature on the thermometer are 0C (degrees celsius) Page 103 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section 6 State the meaning of melting point and boiling point, and recall the melting and boiling points for water •Melting point is the temperature at which a solid substance changes to liquid (it is numerically equal to the freezing point). •Boiling point is the temperature at which a liquid substance changes to gas. (it is numerically equal to the condensation point). Pure water melts at 0 0C and boils at 100 0C 7 Describe evaporation in terms of the escape of more-energetic molecules from the surface of a liquid Evaporation constantly occurs on the surface of liquids when the high energy particles escape from the liquid, even at low temperatures. Boiling occurs only at the boiling point, and the particles escape from everywhere in the liquid (not just on the surface). Evaporation Boiling Slow process Rapid process Occurs only at the surface of Occurs throughout the liquid the liquid Occurs at all temperatures Occurs at a fixed temperature known as the boiling point of the liquid 8 Relate evaporation to the consequent cooling of the liquid Evaporation causes cooling. Higher energy particles leave the surface of the liquid. If the liquid is in contact with a body, energy is transferred from the body to these particles which then evaporate leading to a drop in the body‟s temperature. 9 Demonstrate an understanding of how temperature, surface area and draught over a surface influence evaporation A few energetic molecules close to the surface of a liquid may escape and become gas molecules. This process occurs at all temperatures and is called evaporation. It happens more rapidly when (i) the temperature is higher, since more molecules in the liquid are moving fast enough to escape from the surface, (ii) the surface area of the liquid is large, giving more molecules a chance to escape because more are near the surface, and Page 104 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section (iii) a wind or draught is blowing over the surface carrying vapour molecules away from the surface, thus stopping them from returning to the liquid and making it easier for more liquid molecules to break free. Matter and thermal properties 1 Describe qualitatively the thermal expansion of solids, liquids and gases at constant pressure When matter is heated it expands and when cooled it contracts. Solids, liquids and gasses expand when they are heated because the atoms vibrate more and this causes them to become further apart, taking up a greater volume. Expansion is highest in gases, then liquids and lowest in solids. 2 Identify and explain some of the everyday applications and consequences of thermal expansion 1. Axles are shrunk by cooling in liquid nitrogen until the gear wheels can be slipped on to them. On regaining normal temperature, the axles expand to give a very tight fit. In the kitchen, a tight metal lid can be removed from a glass jar by immersing the lid in hot water so that it expands. 2. Bimetallic strips are strips made up of two metals which do not expand at the same rate. They have multiple uses e.g. a) Fire alarm: heat from the fire makes the bimetallic strip bend and complete the electrical circuit, so ringing the alarm bell b) A thermostat in an iron 3. Thermal expansion is displayed when the liquid in thermometers expands leading to temperature changes. The volume of the liquid taken up in the tube can be used to find out the temperature. 4. Gaps have to be left on bridges to allow for expansion (rollers allow the bridge to expand) Page 105 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section Thermal processes Conduction 1 Recognise and name typical good and bad thermal conductors Conductors: Metals are the best electrical and thermal conductors because they have free electrons (also known as delocalized electrons). Insulators: Non-metals, liquids and gases are insulators. Materials such as wood, glass, rubber, plastics and fabrics are insulators. 2 Describe experiments to demonstrate the properties of good and bad thermal conductors A simple demonstration of the different conducting powers of various metals is shown below. A match is fixed to one end of each rod using a little melted wax. The other ends of the rods are heated by a burner. When the temperatures of the far ends reach the melting point of wax, the matches drop off. The match on copper falls first, showing that it is the best conductor, followed by aluminium, brass and then iron. Expt 2: the length of melted wax shows which material is the best conductor. Expt 3: water at the top boils without the ice melting. This shows that water is a poor conductor. 3 Explain conduction in solids in terms of molecular vibrations and transfer by electrons In non-metals, conduction happens when heat is supplied to the non metal, causing its atoms to vibrate faster and pass on their vibrations to the adjacent atoms. In metals, conduction Page 106 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section happens in the previous way and in another quicker way as follows; since electrons are free to move, they travel randomly in the metal and collide with atoms, hence, passing on their vibrations. Convection 1 Recognise convection as the main method of energy transfer in fluids Thermal energy (heat) travels through fluids such as liquids and gases mainly by convection. 2 Relate convection in fluids to density changes 3 Interpret and describe experiments designed to illustrate convection in liquids and gases (fluids) 1, 2, 3 and 4 show convection currents. Streams of warm moving fluids are called convection currents. They arise when a fluid is heated because it expands, becomes less dense and is forced upwards by surrounding cooler, denser fluid which moves in under it. Convection can be shown in water by dropping a few crystals of potassium permanganate down a tube to the bottom of a beaker. When the tube is removed and the beaker heated just below the crystals by a small flame, purple streaks of water rise upwards and fan outwards. A laboratory demonstration of convection currents in air can be given using the apparatus on the left. The direction of the convection currents created by the candle is made visible by the smoke from the touch paper. Black marks often appear on the ceiling above a lamp or a radiator. They are caused by dust being carried upwards in air convection currents produced by the hot lamp or radiator. Radiation 1 Recognise radiation as the method of energy transfer that does not require a medium to travel through Page 107 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section Whereas conduction and convection both need matter, radiation can occur in a vacuum; particles of matter are not involved. Thus, radiation does not require a medium to travel through. Radiation is the way in which heat from the Sun reaches us. 2 Identify infra-red radiation as the part of the electromagnetic spectrum often involved in energy transfer by radiation Infra-red radiation is part of the electromagnetic spectrum involved in energy transfer by radiation. Radiation is the flow of heat from one place to another by means of electromagnetic waves. 3 Describe the effect of surface colour (black or white) and texture (dull or shiny) on the emission, absorption and reflection of radiation Dull black surfaces are better absorbers of radiation than white shiny surfaces. White shiny surfaces are better reflectors of radiation than dull black surfaces. If you hold the backs of your hands on either side of a hot copper sheet that has one side polished and the other side blackened, it will be found that the dull black surface is a better emitter of radiation than the shiny one. In general, surfaces that are good absorbers of radiation are good emitters when hot. 4 Interpret and describe experiments to investigate the properties of good and bad emitters and good and bad absorbers of infra-red radiation Expt 1 Expt 2 Page 108 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section Expt 1: boiling water in a metal cube heats all surfaces equally. The thermal radiation detector is placed in turn at the same distance from each surface and the meter readings compared. Expt 2: the metal plates are placed at the same distance from a radiant heater and the temperature readings from the thermometers are compared. Conclusion: Matt black surfaces are the best emitters and absorbers of thermal radiation. Silver surfaces are the worst emitters and absorbers of thermal radiation. Consequences of energy transfer 1 Identify and explain some of the everyday applications and consequences of conduction, convection and radiation Applications Solar panel: the sun‟s thermal radiation is absorbed by a matt black surface and warms up the pipes containing water. Refrigerator: the freezer compartment is located at the top of the refrigerator. It cools down the air which then sinks. Any warm air present rises to the top where it is cooled. This creates a convection current which maintains a cold temperature. Metals are used in cooking pans because they conduct the heat well. A vacuum flask keeps hot liquids hot or cold liquids cold. Transfer by conduction and convection is minimized by making the flask a double-walled glass with a vacuum inbetween the walls. Radiation is reduced by silvering both walls on the vacuum side. Houses in hot areas are painted white; the back of a refrigerator is painted black so that the refrigerator loses heat more quickly; saucepans are polished/shiny to make them poor emitters so that they keep their heat longer. Consequences A metal spoon in a hot drink will warm up because it conducts heat. Convection currents create sea breezes. During the day the land is warmer and acts as a heat source. During the night the sea acts as the heat source. A black saucepan cools better than a white one, and white houses stay cooler than dark ones. Page 109 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section Properties of waves, including light and sound General wave properties 1 Demonstrate understanding that waves transfer energy without transferring matter The motion of ropes and springs and experiments by ripple tanks show that waves transport energy from one place to another without transferring matter. 2 Describe what is meant by wave motion as illustrated by vibration in ropes and springs and by experiments using water waves 3 State the meaning of speed, frequency, wavelength and amplitude Wave speed (v) is the distance moved in the direction of travel of the wave by a crest or any point on the wave in 1 second. (units are ms -1) Frequency (f) is the number of waves passing any point per second.(units are Hertz; Hz). If the end of a rope is moved up and down twice in a second, two waves are produced in this time. The frequency of the wave is 2 vibrations per second or 2 hertz Wavelength (λ) is the distance between two consecutive points on a wave in phase (i.e from peak to peak) e.g. from crest to crest or compression to compression (units are metres; m). Amplitude (a) is the height of a crest or the depth of a trough measured from the undisturbed position of what is carrying the wave, such as a rope. Period is the time taken for one oscillation in seconds. Continuous ripples are studied more easily if they are apparently stopped (‘frozen’) by viewing them through a stroboscope. Page 110 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section 4 Distinguish between transverse and longitudinal waves and give suitable examples Transverse waves have oscillations at right-angles to the direction of travel. Longitudinal waves have oscillations in the direction of travel. Transverse waves have crests (peaks) and troughs; whereas longitudinal waves (e.g. sound waves) have compressions and rarefactions. For transverse waves, the oscillations are perpendicular to the direction of travel e.g. light, water and seismic S- waves. A transverse wave can be sent along a rope (or a spring) by fixing one end and moving the other rapidly up and down. For longitudinal waves, the oscillations are in the direction of travel e.g sound and Seismic P- waves from earthquakes. The Cs and Rs below show compressions and rarefactions on the spring. A sound wave, produced for example by a loudspeaker, consists of a train of compressions („squashes‟) and rarefactions („stretches‟) in the air 5 Describe how waves can undergo: reflection at a plane surface refraction due to a change of speed Reflection at plane surface Water cannot pass through surfaces so it bounces back. It bounces back with the same speed, frequency and wavelength. The angle of incidence (i) = the angle of reflection (r) Page 111 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section Refraction due to a change of speed Refraction: when the water waves pass through shallower water they slow down. When waves slow down they change direction. Things to note about refraction: waves slow down when they pass from a less to a more dense material and vice versa when a wave is slowed down, it is refracted towards the normal (i > r) when a wave is sped up, it is refracted away from the normal (i < r) deep water is denser than shallow water angle of incidence ≠ angle of reflection. 6 Recall and use the equation v = f λ Wave speed = frequency × wavelength v=f×λ Question: Calculate the frequency of a wave having wavelength = 1 x 10 -7 m and speed = 3 x 105 km/s. Solution: Apply v = f x λ formula and then plug in the values and calculate. But first convert km/s into m/s. Therefore, v would become 3 x 108 m/s Hence,3 x 108 = f x 1 x 10-7 m so, f = 3 x 1015 Hz Page 112 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section 7 Understand that refraction is caused by a change in speed as a wave moves from one medium to another As demonstrated above, refraction is caused by a change in speed as a wave moves from one medium to another Light Reflection of light 1 Describe the formation of an optical image by a plane mirror and give its characteristics Rays from an object reflect off the mirror into our eyes. The image has these properties: the image is the same size as the object the image is the same distance from the mirror as the object a line joining equivalent points of the image and object meets the mirror at a right angle the image is virtual: no rays actually pass through the image and the image cannot be formed on a screen the image is laterally inverted • Laws of reflection: Angle of incidence = angle of reflection The incident ray, reflected ray and normal are always on the same plane (side of mirror) Looking at the diagram on the left, the image in the mirror is laterally inverted. 2 Recall and use the law angle of incidence i = angle of reflection r recognising these angles are measured to the normal [Objective covered above] Page 113 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section 3 Perform simple constructions, measurements and calculations for reflection by plane mirrors [To be done practically] Refraction of light 1 Interpret and describe an experimental demonstration of the refraction of light As the ray of light enters the block, it slows down and bends towards the normal As the ray of light exits the block, it speeds up and bends away from normal Angle of incidence ≠ angle of refraction Frequency does not change, only speed and wavelength Thin converging lens 1 Describe the action of a thin converging lens on a beam of light A beam of light is composed of light rays. When parallel rays of light pass through a convex (converging) lens, they are focused to a point called the principal focus. Since light can fall on both faces of a lens, we have two focal points, one on each side. The convex lens is represented as a thin line in ray diagrams. 2 Use the terms principal focus and focal length F is the principal focus Distance between C and F (f) is the focal length Page 114 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section 3 Draw ray diagrams for the formation of a real image by a single lens Step 1: Draw the lens with a horizontal axis passing through the middle of it. Step 2: Mark the positions of the principal focuses F on either side, at equal distances from the lens. Mark the position of the object O, an arrow standing on the axis. Step 3: Draw ray 1, a straight line from the top of the arrow and passing undeflected through the middle of the lens. Step 4: Draw ray 2, from the top of the arrow parallel to the axis. As it passes through the lens, it is deflected down through the principal focus. Look for the point where the two rays cross. This is the position of the top of the image I. Examples of ray diagrams Object (O) beyond 2F Used in camera Page 115 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section Object (O) between F and 2F Used in projector Object (O) at 2F 4 Use and describe the use of a single lens as a magnifying glass The previous diagrams gave us real images; however, if the object is between the lens and F, a virtual image is created, instead of a real image. This allows a single convex lens to be used as a magnifying glass. The image is behind the object, virtual, erect (i.e. not inverted), and larger than the object. Page 116 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section Electromagnetic spectrum 1 Describe the main features of the electromagnetic spectrum in order of frequency, from radio waves to gamma radiation (γ) 2 State that all electromagnetic waves travel with the same high speed in a vacuum and approximately the same in air All electromagnetic waves travel with the same high speed in a vacuum and approximately the same in air. 3 State that the speed of electromagnetic waves in a vacuum is 3.0 × 10 8 ms-1 All electromagnetic waves: travel at the speed of light: 3 × 108 ms-1 in a vacuum do not need a medium to travel through i.e. they can travel through a vacuum can transfer energy are produced by particles oscillating or losing energy in some way are transverse waves 4 Describe typical properties and uses of radiations in all the different regions of the electromagnetic spectrum including: radio and television communications (radio waves) satellite television and telephones (microwaves) electrical appliances, remote controllers for televisions and intruder alarms (infra-red) medicine and security (X-rays) Uses: Radio waves are used in radio and television communications. Microwaves are used in satellite television and telephones because they easily pass through the Earth‟s atmosphere as they travel up to a broadcasting satellite in space, after which they are sent back down to subscribers on Earth. Infrared is used in electrical appliances (radiant heaters and grills), intruder alarms and remote controllers for televisions. Page 117 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section Monochromatic light is light, of a single wavelength and colour. It is used in lasers. Ultra violet light causes fluorescence. It is used to sterilize equipment. X-rays are used in medicine (x-ray photography and killing cancer cells) and security. X-rays can penetrate solid materials hence their use in security scanners at airports. In medicine, bones absorb X-rays more strongly than flesh, so bones appear as a shadow in the image formed by X-ray photography. In medicine, X-rays can also be used to kill cancer cells. Gamma rays are used to sterilize food and equipment. They are also used in radiotherapy to destroy cancer cells. 5 Demonstrate an understanding of safety issues regarding the use of microwaves and X-rays Safety issues: X-rays cause cancer due to cell mutations Microwaves can heat and destroy living cells 6 State the dangers of ultraviolet radiation, from the Sun or from tanning lamps UV radiation can cause skin cancer and damage retina Sound 1 Describe the production of sound by vibrating sources Sound is a form of energy that is produced by vibrations. It travels as waves. A Sound wave can only be produced in matter/medium (i.e it is a mechanical wave) and hence, cannot travel through a vacuum. All vibrating sources produce sound. Sources of sound all have some part that vibrates. A guitar has strings, a drum has a stretched skin and the human voice has vocal cords. 2 Describe the longitudinal nature of sound waves Sound waves are longitudinal in nature i.e. particles move parallel to the direction of wave travel. Page 118 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section 3 Describe the transmission of sound waves in air in terms of compressions and rarefactions Sound waves come from a vibrating source for example a loudspeaker. As the loudspeaker cone vibrates, it moves forwards and backwards, which squashes and stretches the air in front. As a result, sound waves are transmitted as a series of compressions (squashes) and rarefactions (stretches) that travel out through the air. It should be noted that the air molecules are merely pushed back and forth causing vibrations which travel to our ears. The individual air molecules do not travel from the loudspeaker to our ears. 4 State that the approximate range of audible frequencies for a healthy human ear is 20 Hz to20 000 Hz Humans can hear frequencies between 20Hz and 20 000Hz. An alarm has alternating high and low pitched sounds. Both should fall within the audible frequency range in order to be detected by humans. 5 Show an understanding that a medium is needed to transmit sound waves Sound cannot travel through a vacuum because there are no molecules or particles in a vacuum to vibrate back and forth. A medium eg. air is required for the transmission of sound. 6 Describe and interpret an experiment to determine the speed of sound in air, including calculation Question: A man shouts close to a high wall. He hears one echo. If the man is 40 m from the wall, how long after the shout will the echo be heard? (Speed of sound in air = 330 m/s.) Step 1: Calculate the distance travelled by the sound. Note that this is twice the distance from the man to the wall (since the sound travels there and back). Distance travelled by sound = 2 × 40 m = 80 m Step 2: Calculate the time taken for the sound to travel this distance. Time taken = 𝑑𝑖𝑠𝑡𝑎𝑛𝑐𝑒 𝑠𝑝𝑒𝑒𝑑 = 80𝑚 330𝑚 /𝑠 = 0.24s So the man hears the echo 0.24 s after his shout. Page 119 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section The diagram shows a „time-offlight‟ method for measuring the speed of sound. The wooden blocks and the two microphones are arranged in a straight line. The bang from the blocks is picked up first by microphone 1, setting off a timer which stops when the bang is picked up by microphone 2. The speed of sound is calculated using the distance between the two microphones and the time taken by the sound to travel between them. The formula speed = used 𝑑𝑖𝑠𝑡𝑎𝑛𝑐𝑒 𝑡𝑖𝑚𝑒 is 7 Recognise that sound travels faster in liquids than gases and faster in solids than in liquids Sound travels fastest in solids followed by liquids and then lastly in gases. 8 Relate the loudness and pitch of sound waves to amplitude and frequency Higher frequency → a higher pitch Larger amplitude → louder sound 9 Describe how the reflection of sound may produce an echo An echo is produced when sound travels from the source and bounces off hard surfaces or walls such that it is reflected back to the source. The more the reflecting surfaces, the more the echoes heard. Electrical quantities Electric charge 1 State that there are positive and negative charges There are 2 types of charges: namely positive and negative charges. 2 State that unlike charges attract and that like charges repel Unlike charges attract and like charges repel. Page 120 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section 3 Describe and interpret simple experiments to show the production and detection of electrostatic charges by friction The production of charges by friction (rubbing) can be explained by supposing that electrons are transferred from one material to the other. For example, when a polythene rod is rubbed with a silk cloth, electrons go from the silk cloth to the polythene rod, leaving the silk cloth positively charged. The polythene rod now has more electrons than protons and becomes negatively charged. Note that it is only electrons which move; the protons remain fixed in the nuclei. 4 State that charging a body involves the addition or removal of electrons Charging a body involves the addition or removal of electrons 5 Distinguish between electrical conductors and insulators and give typical examples Conductors are materials that have free or mobile electrons that travel through them e.g metals (copper, gold, silver etc). Metals are the best electrical conductors because they have free electrons which move from one place to another. These electrons also make them good thermal conductors. Insulators are materials that do not have free or mobile electrons; hence, they do not conduct at all e.g. wood, rubber, air, plastic. Their electrons are firmly held to atoms, meaning they do not move, but they can be transferred by rubbing (as shown above). Current, potential difference and electromotive force (e.m.f.) 1 Demonstrate understanding of current, potential difference, e.m.f. and resistance. 2 State that current is related to the flow of charge Current is the rate of flow of charge through a conductor. Its SI unit is the Ampere (A). Page 121 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section Potential difference, or PD for short, is also known as voltage. Potential difference is the amount of energy the cell gives the electrons it pushes out. It is measured in volts (V) and is measured by a voltmeter (connected in parallel). The electromotive force (e.m.f): When no current is drawn from a battery it is said to be an „open circuit‟ and its terminal p.d. is a maximum. This maximum voltage is termed the electromotive force (e.m.f.) of the battery. Like potential difference, e.m.f. is measured in volts. The p.d. at the terminals of a battery decreases slightly when current is drawn from it. This effect is due to the internal resistance of the battery which transfers some electrical energy to heat as current flows through it. Resistance: The opposition that a conductor offers to the flow of current is called its resistance. A good conductor has a low resistance and a poor conductor has a high resistance. 3 Know and use the formula Q = It Charge (Q) = current (A) x time (s) Q=Ixt units are Q in coulombs (C) I in amps (A) t in seconds (s) 4 Show understanding that a current is a rate of flow of charge and recall and use the equation I = Q / t Current is the rate of flow of charge through a conductor. The SI unit is the Ampere (A). I=Q/t 5 State that current in metals is due to a flow of electrons Current in metals is due to a flow of electrons. 6 State that the potential difference (p.d.) across a circuit component is measured in volts The potential difference (p.d.) across a circuit component is measured in volts 7 Use and describe the use of an ammeter and a voltmeter, both analogue and digital An ammeter measures the current in a circuit. It is connected in series. It can be digital or analogue. Current is measured in Amperes (A) A voltmeter measures the voltage in a circuit. It is connected in parallel. It can be digital or analogue. Voltage is measured in volts (V). Page 122 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section 8 State that the electromotive force (e.m.f) of an electrical source of energy is measured in volts e.m.f. is measured in volts. Resistance 1 State that resistance = p.d. / current and understand qualitatively how changes in p.d. or resistance affect current 2 Recall and use the equation R = V / I Resistance (Ω) = potential difference (V) / current (A) R=V/I @ constant p.d; increase in resistance leads to a decrease in current @ constant resistance; increase in p.d leads to an increase in current 3 Recall and use quantitatively the proportionality between resistance and length, and the inverse proportionality between resistance and cross-sectional area of a wire R=ρ 𝐿 𝐴 R is resistance; ρ resistivity; L is length; and A is cross sectional area Factors affecting resistance: The resistance of a wire (i) (ii) (iii) increases as its length increases, increases as its cross-sectional area decreases, depends on the material. Page 123 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section Electric circuits Circuit diagrams 1 Draw and interpret circuit diagrams containing sources, switches, resistors (fixed and variable), lamps, ammeters, voltmeters and fuses (Symbols for other common circuit components will be provided in questions) Symbols for common circuit components: Notice the two symbols for a lamp. The diagram on the right represents a circuit diagram which shows a battery (source); 3 resistors; 2 ammeters; and 1 voltmeter. V1 is measuring p.d across R1 hence it‟s connected in parallel. A1 measures current from the source hence it‟s connected in series. A2 measures current through the 6 ohm resistor. Series and parallel circuits 1 Understand that the current at every point in a series circuit is the same 2 Calculate the combined resistance of two or more resistors in series 3 Recall and use the fact that the sum of the p.ds across the components in a series circuit is equal to the total p.d across the supply Page 124 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section Current at every point in a series circuit is the same. Hence, I = I1 = I2 I The potential difference across each resistor is different based on their resistance. The sum of the potential differences across the resistors gives the total pd of the cell. V = V1 + V2. Combined resistance: RTotal = R1 + R2 4 State that, for a parallel circuit, the current from the source is larger than the current in each branch The current splits at each branch in a parallel circuit so the total current from the source is always greater than the current in one branch 5 Recall and use the fact that the current from the source is the sum of the currents in the separate branches of a parallel circuit The current from the source is the sum of the currents in the separate branches of a parallel circuit 6 State that the combined resistance of two resistors in parallel is less than that of either resistor by itself The combined resistance of two resistors in parallel is less than that of either resistor by itself 7 Calculate the combined resistance of two resistors in parallel Current: I = I1 + I2 V = V1 = V2 1 𝑅 𝑇𝑜𝑡𝑎𝑙 = 1 𝑅1 + 1 𝑅2 Page 125 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section Total resistance across QW is calculated as follows 1 𝑅 𝑇𝑜𝑡𝑎𝑙 = 1 4.0 + Hence; Rtotal = 1 6.0 12 5 = 3+2 12 = 5 12 = 2.4 Ω If R1 = 3.0 Ω; then total resistance of the circuit = 3.0 + 2.4 Ω = 5.4 Ω Since V = IR; I = V/R = 12.0/ 5.4= 2.22A to 2 decimal places. 8 State the advantages of connecting lamps in parallel in a circuit 1. Lights are connected in parallel to avoid them from becoming dim because in parallel connections, each lamp receives the same voltage (which is equal to the mains supply voltage). Voltages below the mains supply will make the lamps dim. 2. In parallel connections, unlike in series connections, if one bulb fails, the others remain lit. 3. It is easier for current to flow in parallel connections as compared to series connections because the effective resistance is less. Electrical Energy 1 Recall and use the equations P = IV and E = IVt Electrical power (W) = Current (A) × Voltage (V) P=I×V Electrical energy (J) = power (W) × time (s) E = P × t (Since P = I × V) Electrical energy (J) = Voltage (V) × Current (A) × time (s) E=I×V×t Dangers of electricity 1 Identify electrical hazards including: damaged insulation overheating of cables damp conditions •Damaged insulation: when the insulation is damaged, there is risk of contact with the live wire leading to electric shock which can cause serious injury. •Overheating of cables: when too much current flows e.g. because of a short circuit, it may cause a fire because the cables get too hot. •Damp conditions: water can conduct a current, so if electrical equipment is wet someone might get electrocuted. Page 126 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section 2 State that a fuse protects a circuit The purpose of a fuse is to protect a circuit. 3 Explain the use of fuses and choose appropriate fuse ratings A Fuse is a thin piece of wire which overheats and melts (we say the fuse „blows‟) if the current is too high. A fuse prevents overheating and risk of catching fire. A fuse will have a specific current value or rating (e.g. 13A) so when choosing a suitable fuse you must use the one which can have the lowest current value but over the current value of the appliance. Always replace with one of the same value as recommended by the manufacturer of the appliance. Note that the current flowing through resistors rated 5 Ω, 10 Ω and 25 Ω, adds up to 1.7A Total current 1.7A = 1A + 0.5A + 0.2A For (b); effective resistance for parallel connections is less than that of series connections. For parallel connection, 1 𝑡𝑜𝑡𝑎𝑙 𝑟𝑒𝑠𝑖𝑠𝑡𝑎𝑛𝑐𝑒 = 1 5 + 1 10 + 1 20 = 4+2+1 20 = 7 20 total resistance = 20 7 = 2.86 Ω For series connection, total resistance = 5 + 10 + 25 = 40 Ω Page 127 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file. Physics section Diagram “a” shows an electric circuit set up in a lab, and diagram “b” shows the circuit diagram of that very same electric circuit. Page 128 of 128 This file was downloaded from StudyLast.com. It is not allowed to publish it elsewhere. Only the buyer can use this file.