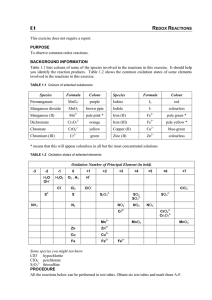
Displacement of Hydrogen
A. Reaction of Metals with Cold Water
a. Place a small quantity of the metals Al, Ca, Cu, Fe, Mg, Pb, Sn, and Zn
into eight separate disposable test tubes.
b. Add about 5 mL distilled water to each tube, observe, and record
data.
c. Write a balanced chemical equation for each of the metals that
react with cold water
2 Na + 2 H2O ⟶ 2 Na+ + 2 OH– + H2
B. Reaction of Metals with Hot Water
a. Heat a beaker of water on a hot plate until it boils
b. Turn off the heat and put the tubes that did not react with cold
water in a hot water bath
c. Leave them there for 5 minutes
d. When one starts to bubble, take it out of the water
e. Record observations
f. write a balanced chemical reaction for the metals that reacted
with hot water
g. Save the hot water for one more reaction with HCl.
{heat}
Mg + 2 H2O ⟶MgO + H2
C. Reaction of Metals with Cold HCl
a. Pour all water out of the tubes that didn't react
b. Add 5 mL of 6 M HCl to each Tube.
c. Leave the tubes alone at room temp for about 5 minutes
d. Observe any reactions and record observations
e. Write out balanced chemical equation
Zn + 2 HCl ⟶ Zn2+ + 2 Cl– + H2
D. Reaction of Metals with Warm HCl
a. put the tubes with HCl and the metals that didn't react and put
them in a beaker of hot water
b. Let the tubes warm for 5 minutes
c. observe and record date
d. Write the balanced equation
Reaction of Metals with Metal Ions
A. Reaction with Silver Ion,
a. Take three small test tubes and add a piece of Cu, Sn, and Pb.
b. Add AgNO3 solution to each tube
c. Make sure all of the metal is fully submerged
d. Wait and observe the reaction
Sn + 2 Ag+ ⟶ Sn2+ + 2 Ag
B. Reaction of Copper Ion, Cu2+
a. Take two clean test tubes and add a piece of Sn and Pb.
b. Cover the metal with Cu(NO3)2 solution.
c. Wait and observe the reaction.
C. Reaction of Lead Ion, Pb2+
a. Take two test tubes and add a piece of Cu and Sn.
b. Cover the metal with Pb(NO3)2 solution.
c. Wait and observe the reaction.