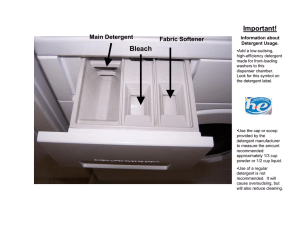
III. Detergents Page 1 Topic III. Detergents Reference Reading Chemistry – A modern view Book 2 pg. 146–158 Objectives 6.3 – recognise that detergents can be made from chemicals derived from petroleum – explain the meaning of the term 'detergent' as a substance which helps to remove dirt by (a) its ability to act as a wetting agent (b) its emulsifying action – explain the emulsifying properties of detergent in terms of it general structure – recognise that soap is another kind of detergent – describe the production of soap by reacting fats or oil which are naturally occurring esters, with an alkali (Equation is NOT required) – compare the cleaning abilities of soaps and petroleum based detergents – explain the following problems associated with the use of detergent (a) health problem e.g. skin allergies (b) pollution on problem caused by the disposal of some non-biodegradable detergents and the resulting ecological disturbance Notes III. Detergent Detergent – a substance which improves the cleaning properties of water. A. Structure of detergent Ionic head (hydrophilic (親水性)) – the polar ionic head attracts polar water and repels non-polar oil. It is soluble in water. Hydrocarbon tail (hydrophobic (疏水性)) – the non-polar hydrocarbon tail attracts oil and repels polar water. It is soluble in oil There are two types of detergents 1. soapless detergent (synthetic detergent) – developed during World War II from petroleum 2. soap (soapy detergent) – invented 2000 years ago from fat or oil. They have different origins and detail structure. III. Detergents Page 2 B. Cleaning properties of detergent 1. As a wetting agent – 2. Detergent is a surfactant (surface active agent). It reduces the surface tension of water and make water spread easier over the dirty area. As an emulsifying (乳化 乳化) 乳化 agent – – – – – Normally, oil does not mix with water. When detergent is added into a mixture of water and oil, the hydrophilic head dissolves in water and the hydrophobic will be dissolved in oil. Upon stirring, the oil layer breaks up and the oil droplets will be enclosed by the detergent anion. Since each droplet is negatively charged, they repel each other and will not join together again. The mixture becomes a oil-water emulsion and detergent is called emulsifying agent. The lather formed during stirring also help to suspend grease and dirt particles. N.B. Only the anionic part of a detergent possesses emulsifying properties, the cationic part (e.g. Na+) only simply dissolves in water. III. Detergents Page 3 C. Preparation of detergent 1. Preparation of soapless detergent (synthetic detergent) – By treating certain hydrocarbon from petroleum with conc. H2SO4(l) and then neutralized by NaOH(aq) or Na2CO3(aq). It is the salt of a strong acid (sulphonic acid). Early soapless detergent was non-biodegradable and caused a lot of pollution. All soapless detergents used nowadays are biodegradable. 2. Preparation of soap (soapy detergent) – By alkaline hydrolysis (saponification of fat or oil) using NaOH(aq). It is the salt of a weak acid (fatty acid / long chain alkanoic acid). Fat or oil is a kind of natural triester formed by condensation between propan-1,2,3-triol (glycerol) and 3 fatty acid molecules. Fat of oil (a triester) can be hydrolysed by heating with NaOH(aq) under reflux to give glycerol and the salt of the fatty acid (soap). The process is called saponification (making of soap). (Equation is NOT required) The fairly soluble soap is precipitated out by adding conc. NaCl(aq). The process is called salting out. The ppt. is filtered, compressed and dried to form a bar of soap. N.B. Both soap and soapless detergent given above are ionic compounds. III. Detergents Page 4 D. Cleaning ability of soap and soapless detergent 1. In soft water and hard water Water containing a lot of mineral is known as hard water and the one containing little mineral is known as soft water. The amount of mineral in water is known as the hardness of water. For example, rainwater is a soft water which contains little mineral while river water, underground water and sea water are hard waters. In Hong Kong, the tap water is from rainwater and Dongjiang and the hardness is not very high. Hard water forms precipitate with soap but no precipitate with soapless detergent. Ca2+(aq) and Mg2+(aq) ions in hard water form gray precipitate known as scum with soap anion. The formation of scum creates two problems 1. Make another kind of dirt in the solution which may stick onto the clothes. 2. Use up the soap and the soap is wasted. a) Methods of softening hard water a) Methods of softening hard water 1. Add washing soda (Na2CO3·10H2O) to precipitate Ca2+(aq) and Mg2+(aq) ions as carbonate and filter before use. Ca2+(aq) + CO32-(aq) → CaCO3(s) Mg2+(aq) + CO32-(aq) → MgCO3(s) 2. Add phosphate (e.g. Na3PO4) to form soluble complex with Ca2+(aq) and Mg2+(aq) ions 3. Use ion exchange resin (e.g. sodium permutit) which remove Ca2+(aq) and Mg2+(aq) ions and replace them with Na+(aq) ions. Calcium ions + Sodium permutit → Sodium ions + Calcium permutit 4. Use soapless detergent which does not form scum with Ca2+(aq) and Mg2+(aq) ions. 2. In acidic medium Soap is a salt of weak acid (fatty acid). If the acidity of the water is too high, the anion will combine with the H+(aq) ion to reform the neutral acid molecule and lose the cleaning properties. RCOO-(aq) + H+(aq) d RCOOH(s) soap anion no ionic head In contrast, soapless detergent is a salt of strong acid. It can work in acidic medium. III. Detergents Page 5 E. Problems of using detergent 1. Health problem Detergent with pH lower than 5 or higher than 9 may cause skin allergy easily. Since soap is a salt of weak acid which hydrolyses in water and give an alkaline solution, it causes skin allergy more easily than soapless detergent. RCOO-(aq) + H2O(l) d RCOOH(aq) + OH-(aq) 2. Pollution a) Early soapless detergent was non-biodegradable due to the side chain on the hydrocarbon tail. This makes layer of thick foam persist in the river. b) Many additives has been added into the detergent (e.g. phosphate). Phosphate is a nutrient for plant. This causes algal bloom (紅潮). The decomposition of dead algae causes rapid use up of dissolved oxygen in water and kill other aquatic lives. F. Advantages and disadvantages of using soap and soapless detergent Soap Soapless detergent Glossary Advantage Non-toxic Biodegradable Disadvantage Not very soluble Expensive (made from fat and oil) Do not work well in hard water Cannot be used in acidic medium May cause skin allergy Very soluble Less expensive (made from petroleum) Work well in hard water Work in acidic medium Can be tailor-made Usually neutral or weakly acidic (causes less allergies) Early ones were non-biodegradable May be toxic detergent ionic head hydrocarbon tail hydrophilic hydrophobic soapless detergent (synthetic detergent) soap (soapy detergent) wetting agent emulsifying agent soft water hard water scum washing soda ion exchange resin biodegradable algal bloom III. Detergents Past Paper Questions Page 6 90 I 5 b i ii iii iv 91 I 1 b ii iii iv vi vii 93 I 1 e i ii 94 I 5 a v 95 I 9 a i iii 97 I 7 b iii 98 I 3 c 90 I 5 b i ii iii iv 5b A student heated a mixture of aqueous sodium hydroxide and ethyl ethanoate for some time using the following set-up: i C (1) (2) (3) (1) Name the type of reaction that took place. Write an appropriate equation for the reaction. What would be observed when the reaction was complete ? Give an industrial application of this type of reaction. alkaline hydrolysis / saponification 1 mark CH3COOCH2CH3 + NaOH → CH3COONa + CH3CH2OH 1 mark OR CH3COOC2H5 + OH- → CH3COO- + C2H5OH (2) only one layer is observed / a homogeneous solution is formed / fruity smell not detected / two layers become miscible 1 mark (3) to make soap / soapy detergents / saponification 1 mark (1) a number of candidates forgot to include water as a product in the equation they wrote for the alkaline hydrolysis of ethyl ethanoate. ii What is the function of the 'cold finger' ? "cold finger" is to prevent the loss of volatile reagents / products involved in the reaction 1 mark OR to condense the reactants/products (or as a condenser) iii State a potential hazard in the set-up shown above. ethyl ethanoate / ethanol / reactants / products may catch fire from the direct flame (or inflammable) 1 mark OR spurting out of chemicals during heating [Do not accept: NaOH is corrosive.] iv The quantity of the products obtained in this experiment was much less than that expected. (1) Give an explanation for this. (2) Draw a labelled diagram of a completely different set-up to illustrate how the quantity of the products can be increased by using the same quantities of reactants. (1) some reactants (or products) vaporized 1 mark OR the "cold finger" is an ineffective / poor condenser (2) 4 1 1 3 III. Detergents Page 7 2 marks Deduct 1 mark for the following mistakes: – no heating – flow of water in wrong direction / not indicated Deduct 2 marks for the following mistakes: – closed system – air condenser used – joint between condenser and flask is not air-tight – use distillation set-up with a slanting condenser Diagrams were poorly drawn. Many candidates had no idea of the experimental set-up for heating substances under reflux and they drew a distillation apparatus instead. C 91 I 1 b ii iii iv vi vii 1b A vegetable oil, X, can undergo reversible hydrolysis in the presence of sulphuric acid as given by the following equation: X + 3H2O CH2 CH CH2 + 3RCOOH (where R respresents alkyl groups) OH OH OH propane-1,2,3-triol ii What is the function of sulphuric acid in this reaction ? as a catalyst 1 mark X can be hydrolysed more effectively by using sodium hydroxide solution instead of sulphuric acid, and the products are propane-1,2,3-triol and Y. iii Name this process. saponification (Do not accept alkaline hydrolysis.) 1 mark (Correct spelling is required.) iv Write the structural formula of Y. O 1 1 1 O - + R C O Na / R C ONa / RCOONa 1 mark O (Do not accept R C O Na ) C O O A common mistake was to give R C O Na as the answer instead of R C O -Na+ . vi C Based on the structural formula of Y, explain why a milky solution is formed. 4 The hydrocarbon group / covalent end of Y dissolves in peanut oil (is hydrophobic). 1 mark 1 mark The carboxylate / ionic end dissolves in water (is hydrophobic). 1 mark After stirring, the oil splits into droplets, due to repulsion of negative charges / carboxylate ions. (Do not accept hydrophilic ends.) 1 mark the droplets cannot coalesce / stick together and milky solution is formed. Many candidates simply mentioned that Y consisted of a hydrophillic head and a hydrophobic tail without making reference to the structure of these parts, i.e. the head being a carboxylate ion and the tail, a hydrocarbon group. Some students thought that the hydrophillic head was a sodium ion. In explaining the formation of the milky solution, many forgot to mention the repulsion of negative charges built up on the surface of the oil droplets as a result of emulsification and hence the droplets, once formed, could not coalesce. vii Name the process leading to the formation of the milky solution and suggest one domestic application of this 2 process. III. Detergents Emulsification / emulsifying action (Do not accept emulsify, emulsion.) 1 mark (Correct spelling is required.) Soap / detergent (cleaning) / removing grease or oil / emulsifier / salad dressing / mustard / cold cream. 1 mark 93 I 1 e i ii 1e i The structure of a typical anionic detergent can be represented by: where and Page 8 4 represents a hydrocarbon tail represents an anionic part attached to the hydrocarbon tail. (1) Using the above representation, draw a diagram to show how the detergent can suspend an oil droplet in water. (2) A table cloth stained with oil can be cleaned using the detergent in water. Explain the cleaning action with reference to your diagram in (1). (1) C ii C Diagram showing a suspended oil droplet 2 mark showing less than 3 detergent particles dissolved in the oil droplet (-1 mark) oil drop not suspended (-1 mark) wrong labelling of oil / water 0 mark no negative charge indicated on detergent particles 0 mark (2) The hydrophobic end / hydrocarbon tail of detergent dissolve in oil and the hydrophilic end / anionic part dissolves in water . 1 mark Agitation breaks the oil into small droplets, the repulsion of like charges disperses the oil droplets formed. 1 mark (1) Though the structure of the detergent was given, diagrams for the suspended oil droplet were poorly drawn. The positions of the polar head were sometimes misplaced and the charges were often omitted. Scientists have also developed cationic detergents for special cleaning purposes. The structure of a typical 2 cationic detergent is shown below : Can anionic and cationic detergents be used together ? Explain your answer. No, because the oppositely charged ions attract / stick / combine together / coagulate, 1 mark 1 mark thus reduces the net amount of active species present in the solution / weakens the cleaning effect. (Do not accept react together) OR Yes, because either kind of detergents can form micelles with oil independently / work independently / each kind of detergent work on a particular kind of dirt, (1 mark) (1 mark) therefore will enhance / not affect the cleaning action. For such an open-ended question, candidates were expected to answer 'yes' or 'no' with supportive arguments based on their chemical knowledge. Of those candidates who answered 'no', many wrongly thought that the two detergents of opposite charges would 'react' together or 'neutralize' each other. For those candidates who answered 'yes', their answers should have included either 'the two kinds of detergents can form micelles with oil independently' or 'each kind of detergent works on a particular kind of dirt' and thus the 'cleaning effect of the detergents would be enhanced or would not be affected'. 94 I 5 a v 5a A domestic drain cleaner named 'RAINBOW' contains concentrated sulphuric acid as the active ingredient. A student carried out the following experiment to determine the concentration of sulphuric acid in 'RAINBOW'. III. Detergents 1.0 cm3 of 'RAINBOW' was diluted to 500 cm3 with distilled water. 25.0 cm3 of the diluted solution were measured and transferred to a conical flask. The solution in the flask required 18.2 cm3 of 0.10 M sodium hydroxide solution for complete neutralization. v If 'RAINBOW' is poured into drains blocked with fat, the fat can be removed. Assuming the formula of fat is Page 9 2 H H C OCOR H C OCOR (R represents an alkyl group) H C OCOR H explain how 'RAINBOW' can remove the fat. Pouring 'RAINBOW' / c.H2SO4 to the drain causes hydrolysis of the fat into / c.H2SO4 can react with fat to form glycerol and carboxylic acid to form more soluble products / smaller particles 1 mark which can easily be removed by draining of water. 1 mark OR CH2OCOR CHOCOR + 3H2O CH2OCOR C H2SO4 CH2OH CHOH + 3RCOOH CH2OH OR (1 mark) Dilution of concentrated sulphuric acid liberates large amount of heat (1 mark) which causes softening of the fat and thus it can be easily removed by draining of water. A large number of candidates were unable to recognise that the reaction between RAINBOW and fat was an acid hydrolysis reaction; instead they explained the action of RAINBOW by considering it as a detergent. The other possible explanation for the softening and removal of fat in terms of heat produced by diluting RAINBOW was mentioned by only a few candidates. 95 I 9 a i iii 9a Sodium hydroxide can be used as a raw material in the manufacture of both soapy and soapless detergents. i Briefly describe how a soapy detergent can be prepared from a vegetable oil in a school laboratory. Heat / boil (not warm) vegetable oil with sodium hydroxide solution. 1 + 1 marks 1 mark Add concentrated NaCl solution / salt solution / brine / salt out the soap. 1 mark Separate (filter) the soap from the solution. C Many candidates were weak in describing the experimental procedures for the preparation of soap. Many did not mention the separation of soap from the resulting mixture. iii The structure of a certain soapless detergent is shown below: C (1) What other raw materials, apart from sodium hydroxide, are required in the manufacture of this soapless detergent ? (2) Give ONE advantage and ONE disadvantage of using this soapless detergent for domestic cleaning compared with using a soapy detergent. (1) petroleum (fraction) (Do not accept alkane) 1 mark sulphuric acid / oleum (Do not accept dilute sulphuric acid) 1 mark (2) advantage : the soapless detergent can be used in hard water / acidic solution. (DO NOT accept can be used in sea water.) 1 mark disadvantage : the soapless detergent is non-biodegradable / may cause water pollution / may cause skin allergies / kill marine lives. (Do not accept pollution only.) 1 mark (1) Majority of candidates gave only one raw material, either petroleum (fraction) or sulphuric acid. Wrong answers included sulphur, sulphite, alkane etc. 97 I 7 b iii 7b The structures of five compounds, I, II, III, IV and V, are shown below: 4 4 III. Detergents Page 10 represents a saturated hydrocarbon chain containing 1 to 6 carbon atoms and In the above structures. represents a saturated hydrocarbon chain containing 12 to 20 carbon atoms. iii Upon heating with sodium hydroxide solution, one of these compounds produces a soapy detergent. (1) What is this compound ? (2) Draw the structure of the soapy detergent produced. (3) Briefly explain the emulsifying action of the detergent when it is used to remove greasy dirt. (1) I 1 mark 6 1 mark (2) (no mark for R–COO–Na, sodium carboxylate is an ionic compound.) 1 mark (3) The hydrocarbon end / tail is hydrophobic / readily soluble in the greasy dirt. 1 mark The COO- (ionic) end is hydrophilic / readily soluble in water. 1 mark Agitation / stirring will cause the grease to break down into droplets. The negative charge on the droplets repels each other and hence oily droplets will become suspended in the aqueous solution. 1 mark C linking the O and Na atoms in the structure of the soapy detergent with a covalent bond (the correct structure is shown below); failure to mention agitation/stirring needed in order to break down the grease into droplets. O - + C O Na 98 I 3 c 3 Consider the following substances: sodium benzoate, sodium chloride, sodium hypochlorite, sodium hydrogencarbonate, sodium hydroxide, sodium sulphite and monosodium glutamate 3c Which substance is commonly used as an active ingredient in oven cleaners ? Briefly explain its action. 2 III. Detergents 90 37 D 37 Clothes stained with grease can be cleaned by detergents because detergents can (1) decrease the surface tension of water. (2) dissolve in both water and grease. (3) emulsify greasy particles. Which of the following combinations is correct ? A. (1) and (2) only B. (1) and (3) only C. (2) and (3) only D. (1), (2) and (3) 90 38 B 91 33 B 91 49 C Page 11 38 Which of the following statements concerning the production of soap from vegetable oils and sodium hydroxide solution is/are correct? (1) Sodium hydroxide acts as a catalyst. (2) Glycerol is formed at the end of the reaction. (3) The reaction between vegetable oils and sodium hydroxide solution is reversible. A. (1) only B. (2) only C. (1) and (3) only D. (2) and (3) only 33 Which of the following statements is/are true for soapless detergents ? (1) All soapless detergents are not biodegradable. (2) Soapless detergents form a scrum with sea water. (3) Soapless detergents are mainly manufactured from products of the petroleum industry. A. (1) only B. (3) only C. (1) and (2) only D. (2) and (3) only 49 In the preparation of soap, sodium chloride is added after the reaction between oil and sodium hydroxide has been completed. Sodium chloride can increase the solubility of soap. III. Detergents 92 23 Directions: Q.22 and Q.23 refer to the making of soap as represented by the following reaction: H2COOC(CH2)16CH3 HCOOC(CH2)16CH3 H2COOC(CH2)16CH3 H2COH + 3NaOH HCOH + 3CH3(CH2)16COONa H2COH animal fat B 93 44 B soap 23 Soap has a hydrophilic head and a hydrophobic tail. Which of the following combinations is correct ? hydrophobic tail hydrophilic head CH3(CH2)16– A. Na+ CH3(CH2)16– B. –COOCH3(CH2)16COOC. Na+ D. CH3(CH2)16COO- Na+ 44 Which of the following statements is INCORRECT ? A. Tin is used for making food can. B. Sulphuric acid is used for making soap. C. Ammonium chloride is used for making dry cells. D. Chlorine is used for sterilizing drinking water. 94 24 25 Directions : Q.24 and Q.25 refer to the structural formulae of the following three detergents : D C 24 Which of the above is/are soapless detergent(s) ? A. detergent I only B. detergent III only C. detergents I and II only D. detergents II and III only 25 Which of the following statements concerning these detergents is correct ? A. The hydrocarbon tail of detergent III is hydrophilic. B. Both detergents I and II form scum with seawater. C. Detergent III causes more serious pollution problem than detergent I when discharged into rivers. D. Both detergents II and III are made from fats. Page 12 III. Detergents 96 18 D 18 When a little detergent is added to a drop of water on a piece of woollen cloth as shown above, the drop spreads. Which of the following statements correctly explains this observation ? A. The detergent dissolves readily in water. B. The detergent has a polar end which is hydrophilic. C. The detergent can form an emulsion with water. D. The detergent can reduce the surface tension of water. 96 28 29 A D 97 35 * A 98 15 D Directions : Q.28 and Q.29 refer to the following experiment used to study the causes of hardness of water. A student added some soap solution to four test tubes containing the same volume of different aqueous solutions of the same molarity. He shook the tubes and measured the minimum volume of soap solution needed to form a permanent lather. The results are tabulated below : Minimum volume of soap solution Aqueous solution needed to form a permanent lather / cm3 Sodium chloride 0.6 Calcium chloride 9.3 Potassium chloride 0.9 Magnesium chloride 8.5 28 Which of the following apparatus would be most suitable for measuring the volume of soap solution ? A. 50 cm3 burette B. 50 cm3 measuring cylinder C. 25 cm3 pipette D. 10 cm3 beaker 29 Which of the following substances is/are responsible for the hardness of water ? (1) sodium chloride (2) calcium chloride (3) potassium chloride (4) magnesium chloride A. (1) only B. (2) only C. (1) and (3) only D. (2) and (4) only 35 Dilute ammonia solution is used in domestic glass cleansers because (1) it can saponify grease. (2) it is non-corrosive. (3) it contains ammonium ions which can emulsify grease. Which of the above statements is/are correct ? A. (1) only B. (2) only C. (1) and (3) only D. (2) and (3) only 15 A detergent has the following structure: Page 13 III. Detergents Page 14 Which of the following statements concerning the detergent is correct ? A. Its hydrocarbon chain is hydrophilic. B. It can be manufactured from vegetable oil. C. It is readily degraded by micro-organisms. D. It acts as an emulsifier in the cleaning process. 98 41 A 99 43 C 99 48 * C 41 Which of the following problems are associated with the excessive use of soapless detergents ? (1) They can cause skin allergies. (2) They form foam when discharged into rivers and lakes. (3) They form scum when discharged into the sea. A. (1) and (2) only B. (1) and (3) only C. (2) and (3) only D. (1), (2) and (3) 43 Which of the following statements concerning a soapless detergent are correct ? (1) It can be prepared by heating a cooking oil with sodium hydroxide solution. (2) It acts as a wetting agent by reducing the surface tension of water. (3) It acts as an emulsifying agent in the cleaning process. A. (1) and (2) only B. (1) and (3) only C. (2) and (3) only D. (1), (2) and (3) 48 Local tap water produces a scum with soap. 2000 18 B 18 Some potassium carbonate solution is added to a sample of tap water. The mixture then appears cloudy. Which of the following ions is probably present in the sample ? A. NH4+ B. Mg2+ C. BrD. SO422000 41 C 41 Which of the following statements concerning soaps are correct ? (1) They are esters. (2) They can reduce the surface tension of water. (3) Their aqueous solutions are alkaline. A. (1) and (2) only B. (1) and (3) only C. (2) and (3) only D. (1), (2) and (3) Water containing calcium ions can form an insoluble compound with soap.