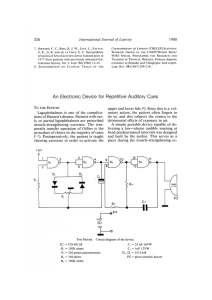
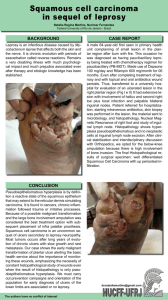
[Downloaded free from http://www.ijpd.in on Wednesday, June 19, 2019, IP: 182.1.209.191] Review Article Childhood Leprosy: A Review Abstract Leprosy in children is of special importance as it is the indicator of transmission in community. It affects both the child and family members psychologically and functionally. In this review, we will discuss regarding epidemiology of childhood leprosy in detail, types of leprosy in children, diagnostic difficulties in children, prevention of disabilities in children, and effect of childhood leprosy on the community. Keywords: Childhood leprosy, community transmission, deformities, diagnosis, treatment Introduction Leprosy is a disease known for its paralyzing deformities and social stigma. It is sad and unfortunate that even though WHO has declared leprosy to be eliminated in 2005, it has remained as endemic disease in various parts of the world specifically developing countries like India.[1] Although leprosy affects all age group, leprosy in children is of special importance as it is an indicator of transmission in the community. In this review, we have discussed regarding epidemiology of childhood leprosy in detail, types of leprosy in children, diagnostic difficulties in children, prevention of disabilities in children and effect of childhood leprosy on community. Epidemiology A total of 135,485 new cases were detected during the year 2016–2017, which gives Annual New Case Detection Rate of 10.17/100,000 population, as against 127,334 cases in 2015–2016. A total of 88,166 leprosy cases are on record as on April 1, 2017, giving a prevalence rate of 0.66/10,000 population, as against 86,028 cases in April 1, 2016.[2] A high proportion (49.57%) of the new leprosy cases detected during 2016-2017 were multibacillary that included (8.7%) of children and grade II deformity was reported in 3.87%. A total of 11792 child cases were recorded indicating the child case rate of 8.7%. Proportion of child This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution‑NonCommercial‑ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non‑commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms. For reprints contact: reprints@medknow.com 112 cases was more than 10% of new case detected in 10 States/Union territories, namely (i) Arunachal Pradesh-10.71%, (ii) Bihar - 13.70%, (iii) Jharkhand - 10.59%, (iv) Maharashtra - 10.18%, (v) Nagaland 11.76%, (vi) Punjab - 17.25%, (vii) Tamil Nadu - 17.64%, (viii) Dadra and Nagar Haveli - 19.79%, (ix) Daman and Diu14.29%, and (x) Lakshadweep-11.11%. Swetalina Pradhan, Bibhu Prasad Nayak1, Gaurav Dash Department of Dermatology, All India Institute of Medical Sciences, Bhubaneswar, 1 Department of Pediatrics, SCB Medical College, Cuttack, Odisha, India Classification of Leprosy Although various types of classifications have been proposed for leprosy, Ridley‑Jopling classification is the most accepted classification as it includes clinical, immunological, and histopathological spectrum of the disease. According to the above classification leprosy is of five types, tuberculoid(TT), borderline tuberculoid (BT), mid-borderline(BB), borderline lepromatous (BL) and lepromatous(LL).[3] For field healthcare workers, WHO has classified leprosy into two broad categories basing upon number of skin lesions: Paucibacillary (PB) and Multibacillary (MB). Paucibacillary (PB) leprosy is defined as five or fewer skin lesions without detectable bacilli on the skin. Multibacillary (MB) leprosy defined as six or more lesions and may be skin smear positive.[4] In majority of studies, the age group that is most commonly affected by the disease among children under 15 years of age was found between 10 and 14 years of age, which can be justified by the disease’s long incubation period of approximately 3–5 years.[5‑12] However, Santos et al.[13] identified a discrete predominance in the Address for correspondence: Dr. Swetalina Pradhan, Department of Dermatology, All India Institute of Medical Sciences, Bhubaneswar, Odisha, India. E‑mail: dr.swetalinapradhan@ gmail.com Access this article online Website: www.ijpd.in DOI: 10.4103/ijpd.IJPD_47_18 Quick Response Code: How to cite this article: Pradhan S, Nayak BP, Dash G. Childhood leprosy: A review. Indian J Paediatr Dermatol 2019;20:112-6. © 2019 Indian Journal of Paediatric Dermatology | Published by Wolters Kluwer - Medknow [Downloaded free from http://www.ijpd.in on Wednesday, June 19, 2019, IP: 182.1.209.191] Pradhan, et al.: Childhood leprosy: A review 5–9‑year‑old age group, in 54.54% (42/77), followed by 10–14‑year‑old age group 44.15%. Although theoretically leprosy should not occur in infants because of long incubation period of leprosy in years, it has been reported in infants as young as 2–3 months of age by Brubaker et al.[14] 59.38% of the cases, with a predominance of ulnar nerve.[9] Similar findings were reported by Singal et al. with 70% of the patients having thickening of nerve, out of which nearly half of the patients had multiple nerve involvement with ulnar nerve being the most common.[11] PB leprosy is commonly found in children as compared to MB because of good immunity than adults.[8] In contrast, two studies found more number of multi-bacillary (60.65% and 91.66%) cases in children.[15,16] In various studies, it has been found that BT leprosy is the most common form found in children followed by BL leprosy, indeterminate, lepromatous leprosy, and pure neuritic leprosy [Figures 1 and 2].[8,15‑17] Diagnosis of Childhood Leprosy Lepra Reaction in Children In general, lepra reaction is rare in children under 15 years of age. Majority of the studies have reported the low frequency of lepra reaction in children varying between 1.36% and 8.33%.[5,8,10,16,17] However, some studies have reported greater percentage than expected varying from 18% to 29.7%.[6,7,11,18,19] In all of these studies, the type 1 reaction was most commonly found, which is expected, given that the most frequent clinical form was BT. Type 1 reaction is associated with neuritis. Severe neuritis can lead to sudden nerve palsy leading to deformities such as foot drop, wrist drop, and clawing. Hence, neuritis should be treated with adequate dose of corticosteroid to bring down inflammation and damage in the nerves. Nerve Involvement in Children Affected with Leprosy Peripheral nerve involvement is the main cause of deformities in children affected with leprosy and associated lepra reaction compounds the problem.[20] Jain et al. found the involvement of peripheral nerves in 186 patients of a total of 306 evaluated children, with more than one thickened nerve in most cases; the ulnar nerve is the most affected.[6] In 2009, Rao reported polyneural involvement in Figure 1: Borderline tuberculoid leprosy: Hypopigmented anesthetic patch covering almost entire face Diagnosis of leprosy is based on the cardinal signs of leprosy. The patient should have anaesthetic skin lesions, enlarged and/or tender peripheral nerve, and slit skin smear positive for acid-fast bacilli. Children usually present with PB leprosy where getting acid‑fast bacilli in slit skin smear not always possible. Hence, diagnosis purely based on clinical examination most of the times. The most challenging part for a physician is to elicit sensory loss to fine touch in children which is impossible and inaccurate. Although pain sensation can be elicited, the child will not cooperate for the same and pain sensation is always last to go among all sensations. One of the easy techniques which can be done is checking for temperature sensation. One can take two test tubes containing warm and cold water. The patient is asked to close eyes and examiner can ask by touching the test tubes on skin to appreciate whether cold or warm. In doubtful cases, one can go for biopsy for confirmation of diagnosis. In a study, 35% of doubtful cases showed findings of Hansen’s disease in histology.[21,22] It has been found in various studies that the skin lesions of leprosy usually undergo self‑healing in children.[23,24] Hence, in doubtful cases, one can wait and keep the child under observation (untreated) for a period to see whether the skin lesion is improving or definite sensory loss is appreciated. However, in endemic areas and with family members being affected with leprosy, it is always wise to start treatment. Treatment of Leprosy in Children In children with PB leprosy, rifampicin and dapsone are given for 6 months, and those with MB leprosy and clofazimine is Figure 2: Borderline lepromatous leprosy: Hypoesthetic hypopigmented macules over trunk Indian Journal of Paediatric Dermatology | Volume 20 | Issue 2 | April-June 2019 113 [Downloaded free from http://www.ijpd.in on Wednesday, June 19, 2019, IP: 182.1.209.191] Pradhan, et al.: Childhood leprosy: A review given in addition to rifampicin and dapsone. The dose of the drugs is different according to the age group [Table 1]. Childhood Disability Due to Leprosy and Prevention of Disability Disability is the most common cause of social stigma in leprosy. Leprosy causes disabilities through damage to peripheral nerves. Disabilities and deformities frequently persist even after successful treatment and affect psychologically and functionally both the children and their family members. It not only affects day‑to‑day activities in children but also can affect their education, interaction with their surrounding though studies pertaining to these aspects in children are lacking. In various studies, it has been found that children affected with leprosy have their initial presentation with deformities which suggests lack of awareness.[7,18] Kar and Job suggested that children with neural thickening have 6.1 times more chances to develop deformities as compared to those without neural thickening. In their study, out of 275 children under 15 years of age, 10.5% cases had deformities, with the following risk factors: late diagnosis, multiple cutaneous lesions, MB disease, positive slit skin smear, various affected nerves, and reactional state at the time of the diagnosis.[7] The same risk factors were also found in the study by Singal et al. with 12.8% incidence of deformities in the hands, feet, and eyes.[11] Hence, the most important factor to prevent disability in leprosy patients is early detection and adequate treatment of neural impairment. Effect of Childhood Leprosy on Community The main transmission and acquisition pathways for leprosy are the upper airways. Leprosy in children indicates continuing transmission in the community.[14] Source of infection in children could be familial or nonfamilial close contacts.[6] Household contact increases the risk of transmission to 9 folds in children because of early and continuous exposure to the leprosy bacillus.[25] In a study from South India, family history of leprosy was present in 18·2% of the childhood cases and all the affected parents and grandparents who were the household contacts of these children had smear positive, multibacillary leprosy.[15] Community‑based survey of leprosy patients in defined rural and urban areas of Maharashtra state (2007), has found that 19% of the children in the studied rural area and 47% of the children in urban area had history of close contacts with leprosy Age 10‑14 years patients.[26] Hence houshold contact surveys should be an effective way of case finding when the index case is a child. Conversely, children in a household where a new leprosy case has been diagnosed are at increased risk compared with the general population and even compared with adults in the household: the risk to children aged 1–14 years was much higher than in older people.[11] Resistance The emergence of drug resistance is the main cause for concern in leprosy because limited number of drugs are available for treatment. Usually, a combination of more than two drugs, with different mechanisms of action, taken regularly for a sufficient period, will prevent the emergence of drug resistance. Resistance to rifampicin, dapsone, and quinolones is reported due to mutations in the binding sites of these drugs in large number of samples by molecular biological methods.[27,28] Clofazimine and minocycline resistance have not yet been reported. Development of resistance to first‑line drugs is becoming serious threat to the efficacy of existing multidrug therapy (MDT) program. Patients suspected to be rifampicin resistant are also expected to be resistant to dapsone. In the year 1998 WHO, technical advisory committee recommended the following regimen for adults with suspected rifampicin resistance:[29] 1. Daily administration of 50 mg of clofazimine, together with 400 mg ofloxacin and 100 mg of minocycline for 6 months, followed by 2. Daily administration of 50 mg clofazimine, together with 100 mg of minocycline or 400 mg of ofloxacin, for at least an additional 18 months. Newer drug regimens suggested for leprosy in 2009 by the “WHO Report of the Global Programme Managers’ Meeting on Leprosy Control Strategy”[30,31] For rifampicin susceptible MB patients, a fully supervised monthly regimen could include Rifapentine 900 mg (or rifampicin 600 mg), moxifloxacin 400 mg, and clarithromycin 1000 mg (or minocycline 200 mg) for 12 months. For rifampicin‑resistant patients, the intensive phase could include moxifloxacin 400 mg, clofazimine 50 mg, clarithromycin 500 mg, and minocycline 100 mg daily supervised for 6 months. The continuation phase could comprise moxifloxacin 400 mg, clarithromycin 1000 mg, and minocycline 200 mg once monthly, supervised for an additional 18 months. Table 1: WHO multidrug therapy regimen for children PB MB Day‑1 (once a month) Day‑1 (once a month) Rifampicin 450 mg and Dapsone 50 mg Rifampicin 450 mg, clofazimine 150 mg and dapsone 50 mg Day 2‑28 Day 2‑28 Dapsone 50 mg daily Clofazimine 50 mg alternate day and dapsone 50 mg daily Full course‑6 blister packs Full course‑12 blister packs For children younger than 10, the dose must be adjusted according to body weight. PB ‑ Paucibacillary; MB ‑ Multibacillary 114 Indian Journal of Paediatric Dermatology | Volume 20 | Issue 2 | April-June 2019 [Downloaded free from http://www.ijpd.in on Wednesday, June 19, 2019, IP: 182.1.209.191] Pradhan, et al.: Childhood leprosy: A review A single‑dose combination of rifapentine, moxifloxacin, and minocycline killed 99.9% of the viable Mycobacterium leprae and was more bactericidal than a single dose of rifampicin, ofloxacin, and minocycline or rifampicin alone. In the same study, it was also observed that the combination of moxifloxacin‑minocycline was more bactericidal than the combination of ofloxacin‑minocycline.[32,33] These drugs such as ofloxacin, moxifloxacin (quinolone), and minocycline are contradicted in childrens. No alternate regimens are designed for children. Drug Reactions In cases of severe adverse reactions, an alternative multidrug therapy regimen is recommended.[34] In adults, the alternative regimens use ofloxacin (quinolone) and minocycline (tetracycline), which are contraindicated in children under 10 years of age, due to the risk of the early closure of the epiphysis as well as dental and bone alterations, respectively. Relapse Mostly relapse occurs in leprosy due to inadequate treatment. It is an important quality of service indicator in assessing the long term efficacy of MDT. The predisposing factors include the presence of persister bacilli, monotherapy (dapsone), inadequate or irregular therapy, the presence of multiple skin lesions and/or thickened nerves, etc. The majority of relapse cases in a study belonged to the 12–15 years age group. Nearly 80% of the relapses occurred during PB treatment, and 70% had received previous treatment from other institutions. Inadequate treatment of MB as PB might have contributed to higher relapsing rate.[35] In another study,[5] despite completing 6 months of MDT‑PB prescribed according to body weight, relapses occurred in 13 out of 784 children with PB leprosy. Three occurred during the 2‑year surveillance period after completion of treatment, and another five, two, and three occurred 6, 1 2, and 1 8 months after surveillance. Counseling Parent must be counseled about the duration of treatment and to complete treatment without default. In addition, they should be informed regarding the signs and symptoms of lepra reactions and to report to the physicians immediately while encountered with such sign and symptoms. In children having anaesthesia of hands and feet, the parents should be taught how to take care of anaesthetic hands and feet to avoid trophic ulcers. Parents and siblings of the index case must be examined for leprosy. Conclusion In summary, the incidence of childhood leprosy still high in various states of India. Diagnosis of leprosy in children is difficult compared to adults. In case of doubt, it is better to keep the child under observation for few months, however, in endemic areas, it is always wise to treat the cases at the earliest. The parents should be warned regarding the signs of both types of lepra reactions, so that the treatment can be instituted to avoid deformities due to nerve damage in type 1 reaction and systemic complications in type 2 reactions. All the family members should be examined for evidence of leprosy and treated. Declaration of patient consent The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed. Financial support and sponsorship Nil. Conflicts of interest There are no conflicts of interest. References 1. Desikan KV. Elimination of leprosy & possibility of eradication – The Indian scenario. Indian J Med Res 2012;135:3‑5. 2. Available from: www.nlep.nic.in/pdf/Annual%20report_%20 2016-17.pdf. [Last accessed on 2017 Sep 01]. 3. Pardillo FE, Fajardo TT, Abalos RM, Scollard D, Gelber RH. Methods for the classification of leprosy for treatment purposes. Clin Infect Dis 2007;44:1096‑9. 4. World Health Organization. Classification of Leprosy. Geneva: WHO; 2011. 5. Selvasekar A, Geetha J, Nisha K, Manimozhi N, Jesudasan K, Rao PS, et al. Childhood leprosy in an endemic area. Lepr Rev 1999;70:21‑7. 6. Jain S, Reddy RG, Osmani SN, Lockwood DN, Suneetha S. Childhood leprosy in an urban clinic, Hyderabad, India: Clinical presentation and the role of household contacts. Lepr Rev 2002;73:248‑53. 7. Kar BR, Job CK. Visible deformity in childhood leprosy – A 10‑year study. Int J Lepr Other Mycobact Dis 2005;73:243‑8. 8. Nair SP. A clinico‑epidemiological study of pediatric leprosy in the urban leprosy center of a tertiary care institute. Indian J Paediatr Dermatol 2017;18:24. 9. Rao AG. Study of leprosy in children. Indian J Lepr 2009;81:195‑7. 10. Sachdeva S, Amin SS, Khan Z, Sharma PK, Bansal S. Childhood leprosy: Lest we forget. Trop Doct 2011;41:163‑5. 11. Singal A, Sonthalia S, Pandhi D. Childhood leprosy in a tertiary‑care hospital in Delhi, India: A reappraisal in the post‑elimination era. Lepr Rev 2011;82:259‑69. 12. Lana FC, Fabri Ada C, Lopes FN, Carvalho AP, Lanza FM. Deformities due to leprosy in children under fifteen years old as an indicator of quality of the leprosy control programme in Brazilian municipalities. J Trop Med 2013;2013:812793. Indian Journal of Paediatric Dermatology | Volume 20 | Issue 2 | April-June 2019 115 [Downloaded free from http://www.ijpd.in on Wednesday, June 19, 2019, IP: 182.1.209.191] Pradhan, et al.: Childhood leprosy: A review 13. Santos MJ, Ferrari CK, de Toledo OR, de Moraes EV, David FL. Leprosy among children and adolescents under 15 years‑old in a city of Legal Amazon, Brazil. Indian J Lepr 2012;84:265‑9. 14. Brubaker ML, Meyers WM, Bourland J. Leprosy in children one year of age and under. Int J Lepr Other Mycobact Dis 1985;53:517‑23. 15. Palit A, Inamadar AC, Desai SS, Sharma P. Childhood leprosy in the post‑elimination phase: Data from a tertiary health care hospital in the Karnataka state of South India. Lepr Rev 2014;85:85‑92. 16. Jain M, Nayak CS, Chokkar R, Aderao R. Clinical, bacteriological, and histopathological characteristics of children with leprosy: A retrospective, analytical study in dermatology outpatient department of tertiary care centre. Indian J Paediatr Dermatol 2014;15:16. 17. Babu A, Bhat MR, Jayaraman J. Childhood leprosy in the postelimination era: A vision achieved or a concern growing at large. Indian J Paediatr Dermatol 2018;19:26. 18. Rao R, Balachandran C. Multiple grade 2 deformities in a child:tragic effect of leprosy. J Trop Pediatr 2010;56:363-5. 19. Romero‑Montoya IM, Beltrán‑Alzate JC, Ortiz‑Marín DC, Diaz‑Diaz A, Cardona‑Castro N. Leprosy in colombian children and adolescents. Pediatr Infect Dis J 2014;33:321‑2. 20. Nery JA, Bernardes Filho F, Quintanilha J, Machado AM, Oliveira Sde S, Sales AM, et al. Understanding the type 1 reactional state for early diagnosis and treatment: A way to avoid disability in leprosy. An Bras Dermatol 2013;88:787‑92. 21. Jayalakshmi P, Tong M, Sing S, Ganesapillai T. Leprosy in children. Int J Lepr Other Mycobact Dis 1997;65:95‑7. 22. Noussitou FM, Sansarricq H, Walter J. Leprosy in Children. Geneva: WHO; 1976. 23. Lara CB, Nolasco JO. Self‑healing, or abortive, and residual forms of childhood leprosy and their probable significance. Int J Lepr 1956;24:245‑63. 24. Smith WC, Aerts A. Role of contact tracing and prevention strategies in the interruption of leprosy transmission. Lepr Rev 2014;85:2-17. 116 25. Sales AM, Ponce de Leon A, Duppre NC, Hacker MA, et al. Leprosy among patient contacts: A multilevel study of risk factors. PLoS Negl Trop Dis 2011;5:e 1013. 26. Shetty VP, Ghate SD, Wakade AV, Thakar UH, Thakur DV, D’souza E, et al. Clinical, bacteriological, and histopathological characteristics of newly detected children with leprosy: A population based study in a defined rural and urban area of Maharashtra, Western India. Indian J Dermatol Venereol Leprol 2013;79:512‑7. 27. Hasanoor Reja AH, Biswas N, Biswas S, Lavania M, Chaitanya VS, Banerjee S, et al. Report of rpoB mutation in clinically suspected cases of drug resistant leprosy: A study from eastern india. Indian J Dermatol Venereol Leprol 2015;81:15561. 28. Singh SK, Kumar A, Nath G, Singh TB, Mishra MN, Resistance to antileprosy drugs in multi-bacillary leprosy: A cross sectional study from a tertiary care centre in eastern Uttar Pradesh, India. Indian J Dermatol Venereol Leprol 2018;84:275-9. 29. Surveillance of drug resistance in leprosy: 2010. Wkly Epidemiol Rec 2011;86:237. 30. Guidelines for Global Surveillance of Drug Resistance in Leprosy. WHO Regional office of South‑East Asia. SEA‑GLP‑2009.2. New Delhi; 2009. 31. World Health Organization. WHO Report of the Global Programme Managers’ Meeting on Leprosy Control Strategy. SEA‑GLP‑2009.6. New Delhi: World Health Organization; 2009. 32. Grosset J. The new challenges for chemotherapy research: Work shop proceeding of leprosy research at new millennium. Lepr Rev. 2000;71 Suppl:S100-4. 33. Dhople AM. Search for newer antileprosy drugs. Indian J Lepr 2000;72:5‑20. 34. Oliveira MB, Diniz LM. Leprosy among children under 15 years of age: Literature review. An Bras Dermatol 2016;91:196‑203. 35. Sasidharanpillai S, Binitha MP, Riyaz N, Ambooken B, Mariyath OK, George B, et al. Childhood leprosy: A retrospective descriptive study from government medical college, Kozhikode, Kerala, India. Lepr Rev 2014;85:100‑10. Indian Journal of Paediatric Dermatology | Volume 20 | Issue 2 | April-June 2019