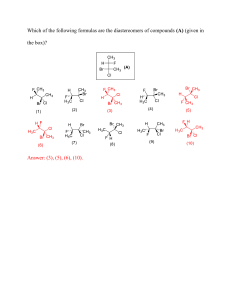
Organofluorine Chemistry Organofluorine Chemistry Synthesis and Applications V. Prakash Reddy Department of Chemistry, Missouri University of Science and Technology, Rolla, MO, United States Elsevier Radarweg 29, PO Box 211, 1000 AE Amsterdam, Netherlands The Boulevard, Langford Lane, Kidlington, Oxford OX5 1GB, United Kingdom 50 Hampshire Street, 5th Floor, Cambridge, MA 02139, United States Copyright © 2020 Elsevier Inc. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without permission in writing from the publisher. Details on how to seek permission, further information about the Publisher’s permissions policies and our arrangements with organizations such as the Copyright Clearance Center and the Copyright Licensing Agency, can be found at our website: www.elsevier.com/permissions. This book and the individual contributions contained in it are protected under copyright by the Publisher (other than as may be noted herein). Notices Knowledge and best practice in this field are constantly changing. As new research and experience broaden our understanding, changes in research methods, professional practices, or medical treatment may become necessary. Practitioners and researchers must always rely on their own experience and knowledge in evaluating and using any information, methods, compounds, or experiments described herein. In using such information or methods they should be mindful of their own safety and the safety of others, including parties for whom they have a professional responsibility. To the fullest extent of the law, neither the Publisher nor the authors, contributors, or editors, assume any liability for any injury and/or damage to persons or property as a matter of products liability, negligence or otherwise, or from any use or operation of any methods, products, instructions, or ideas contained in the material herein. British Library Cataloguing-in-Publication Data A catalogue record for this book is available from the British Library Library of Congress Cataloging-in-Publication Data A catalog record for this book is available from the Library of Congress ISBN: 978-0-12-813286-9 For Information on all Elsevier publications visit our website at https://www.elsevier.com/books-and-journals Publisher: Joe Hayton Acquisitions Editor: Kostas Marinakis Editorial Project Manager: Michelle W. Fisher Production Project Manager: Maria Bernard Cover Designer: Matthew Limbert Typeset by MPS Limited, Chennai, India To My Parents and Teachers Contents Preface 1. 2. xi Nucleophilic reactions in the synthesis of organofluorine compounds 1 1.1 Introduction 2 1.2 Reagents for nucleophilic fluorinations 2 1.3 Nucleophilic deoxyfluorination 3 1.4 Nucleophilic fluorination of pyridines and diazines 8 1.5 Nucleophilic gem-difluorination of carbonyl compounds 10 1.6 Nucleophilic fluoroalkylations 12 1.7 Nucleophilic trifluoromethylthiolation 31 1.8 Trifluoromethoxylations 31 References 35 Electrophilic reactions in the synthesis of organofluorine compounds 43 2.1 Introduction 43 2.2 Reagents for electrophilic fluorination 44 2.3 Enantioselective electrophilic fluorination 48 2.4 Electrophilic fluorination in the synthesis of α-fluorinated amino acids 53 2.5 Electrophilic fluoroalkylation 54 2.6 Electrophilic trifluoromethylthiolation and trifluoromethoxylation 61 2.7 Synthetic methods for trifluoromethylthiolation 63 2.8 Difluoromethylthiolation 68 References 70 vii viii Contents 3. 4. 5. Free-radical reactions in the synthesis of organofluorine compounds 75 3.1 Introduction 75 3.2 Reagents for the free-radical trifluoromethylation 77 3.3 Decarboxylative fluoroalkylation 78 3.4 β-Amino-fluoroalkylation of alkenes 80 3.5 Fluoroalkylation using sodium triflinate (Langlois reagent) 82 3.6 Photoredox-catalyzed S-fluoroalkylation and arylation 94 3.7 Radical fluoroalkylation of enolates 96 References 98 Organotransition metal catalysis in the synthesis of organofluorine compounds 103 4.1 Introduction 104 4.2 Pd-catalyzed fluorination of aryl halides and triflates 105 4.3 Transition metal catalyzed C H fluorination 106 4.4 Au(I)-catalyzed hydrofluorination of alkenes and alkynes 114 4.5 Ni-catalyzed fluoroalkylation of aromatics 116 4.6 Ag(II)-catalyzed oxidative ring-opening fluorination of cyclic amines 121 4.7 Ag(I)-catalyzed decarboxylative fluorination 123 4.8 Cu(I)-mediated dediazoniative difluoromethylation 124 4.9 Fluoroalkylation of arylboronic acids and esters 125 4.10 Cu(I)-catalyzed fluoroalkylation of aryl halides 126 4.11 Ni-catalyzed trifluoromethylthiolation 127 4.12 Pd(II)-catalyzed (amino)trifluoromethoxylation 129 References 131 Pharmaceutical applications of organofluorine compounds 133 5.1 Introduction 134 5.2 Antibacterial pharmaceuticals 141 Contents 6. ix 5.3 Antidiabetic pharmaceuticals 146 5.4 Anti-Alzheimer pharmaceuticals 152 5.5 Anti-HIV pharmaceuticals 163 5.6 Antimalarial pharmaceuticals 165 5.7 Anticancer pharmaceuticals 167 5.8 Antiviral pharmaceuticals 185 5.9 Fluorinated pharmaceuticals for cardiovascular diseases 195 5.10 Antiinflammatory pharmaceuticals 199 5.11 Antidepressants 202 References 204 Synthesis and applications of 18F-labeled compounds 215 6.1 Introduction 216 6.2 Synthetic methods for radiofluorination 220 6.3 Sharpless click reactions for positron emission tomography tracers 225 6.4 Staudinger ligation reactions for positron emission tomography tracers 232 6.5 Radiofluorination via aromatic nucleophilic substitution 235 6.6 Transition metal mediated radiofluorination 243 6.7 Radiofluorination via diaryliodonium salts 250 18 6.8 Enzymatic fluorination reactions for [ F]-labeled positron emission tomography tracers 256 6.9 Positron emission tomography tracers in Alzheimer’s disease 258 6.10 7. 18 F-positron emission tomography tracers in cancer diagnosis 264 References 271 Materials applications of organofluorine compounds 279 7.1 Introduction 280 7.2 Fluorinated surfactants 280 x Contents Index 7.3 Fluoropolymers 286 7.4 Fluorinated π-conjugated polymeric materials in photovoltaic devices 289 7.5 Fluorinated poly(aryl thioethers) in organic electronic materials 298 7.6 Polymer electrolytes 300 7.7 Fluorinated ionomers as proton-exchange membranes in fuel cells 304 7.8 Fluorinated carbon nanoparticles and nonaqueous electrolytes in lithium- and lithium-ion batteries 307 7.9 Fluorinated hyperbranched dendrimers: synthesis and applications 308 7.10 Fluorinated compounds in drug delivery and magnetic resonance imaging 310 7.11 Organofluorine liquid crystal materials 313 7.12 Organofluorine compounds in high-energy materials 313 References 321 329 Preface This book is focused on modern synthetic methods for the incorporation of fluorine and fluoroalkyl moieties into organic compounds, and on the pharmaceutical and materials applications of organofluorine compounds. It is hoped that this book would serve as a text book for the specialized graduate-level courses in organofluorine chemistry as well as a reference book for industrial and academic scientists involved in the drug design, materials chemistry, and organofluorine chemistry. Rapid advances in the efficient synthetic methods of organofluorine compounds contribute to their ever-increasing application in diverse areas, including the design of materials, pharmaceuticals, agrochemicals, and a wide range of consumer goods. Notably, fluoropolymers, which include poly(tetrafluoroethylene), poly(vinylidene fluoride), poly(vinyl fluoride), and Nafion, a perfluorinated ion-exchange membrane, are integral parts of chemical industry. Chapters 1 4 are focused on the synthetic methods for the fluorinations, fluoroalkylations, fluoroalkoxylations, and fluoroalkylthiolations. The synthetic methods are broadly classified to include nucleophilic, electrophilic, free-radical, and organotransition metal catalyzed/mediated reactions. Emphasis is placed on those reactions that are of broad significance in the synthesis of fluorinated pharmaceuticals, positron emission tomography (PET) imaging agents, and materials. Chapter 1, Nucleophilic reactions in the synthesis of organofluorine compounds, outlines a variety of commercially available nucleophilic fluorination reagents, including DAST, DeoxoFluor, XtalFluor, PhenoFluor, and FluoLead. Nucleophilic trifluoromethylations, difluoromethylations, trifluoromethoxylations, and trifluoromethylthiolations have been widely used in the design of pharmaceuticals and materials. Deoxyfluorination of alcohols, phenols, and carboxylic acids can be achieved with a variety of commercially available reagents, including PhenoFluor, PyFluor, XtalFluor, DAST, and related reagents. Nucleophilic fluorinating reagents of broader scope are being continually developed as taskspecific reagents in the synthesis of pharmaceutically interesting compounds. There is emerging interest in the enantioselective nucleophilic fluorinations and trifluoromethylations. Enantioselective trifluoromethylation, in some cases, can be achieved with up to 93% enantioselectivity using cinchonidine-based chital catalysts. Chapter 2, Electrophilic reactions in the synthesis of organofluorine compounds, outlines various electrophilic reagents, such as Selectfluor and NFSI, and electrophilic fluoroalkylations, fluoroalkylthiolations, and fluoroalkoxylations, focusing on electrophilic trifluoromethylation, trifluoromethylthiolations, and difluoromethylthiolation reactions. Electrophilic trifluoromethylation of a wide variety of alkynes, aromatics, amines, and alcohols has found applications in the synthesis of pharmaceuticals. Electrophilic difluoromethylation of alcohols provides access to the corresponding difluoromethoxy compounds, and these reactions can be used in the xi xii Preface late-stage modification of pharmaceuticals. Recent progress in the enantioselective electrophilic fluorination of aldehydes, amides, allylsilanes, and enolsilyl ethers is also outlined, focusing on the reactions that are of broad scope in the design of pharmaceuticals. Chapter 3, Free-radical reactions in the synthesis of organofluorine compounds, covers free-radical reactions in the fluorinations and fluoroalkylations. Commercially available Togni’s or Umemoto’s reagents, originally developed for the electrophilic trifluoromethylations, can be used in the free-radical trifluoromethylation, under photoredox or organometallic catalysis. Free-radical reactions, such as decarboxy-trifluoromethylation and difluoromethylations, mediated by organometallic catalysts, are useful in the late-stage modification of pharmaceuticals. There is emerging interest in the free-radical trifluoromethylations using the cost-effective Langlois reagent, using organometallic catalysts and under photoredox conditions. Organotransition metal catalyzed fluorination and fluoroalkylations are an emerging area that has broad applicability in the synthesis of pharmaceuticals, agrochemicals, materials, and PET tracers. Chapter 4, Organotransition metal catalysis in the synthesis of organofluorine compounds, outlines a variety of transition metal catalyzed reactions, such as Pd(0)-catalyzed fluorination and trifluoromethoxylation of aromatics, Mn(III)-catalyzed mono-fluorinations, Ni(I)-catalyzed fluoroalkylation and trifluoromethylthiolation of aromatics, and Ag(I)-catalyzed decarboxylative fluorination of carboxylic acids. The transition metal catalyzed reactions provide an attractive route for the late-stage modification of pharmaceuticals and in the synthesis of the 18F-labeled PET tracers. Chapter 5, Pharmaceutical applications of organofluorine compounds, and Chapter 6, Synthesis and applications of 18F-labeled compounds, focus on the medical and pharmaceutical applications. Incorporation of fluorine or fluoroalkyl groups as bioisosteres in the lead compounds has emerged as the major focus of drug design efforts. Fluorine-containing pharmaceutical candidates, in general, exhibit enhanced potency, bioavailability, and metabolic stability, as compared to their nonfluorinated analogs. Numerous blockbuster drugs, including the cholesterol-lowering drug atorvastatin (Lipitor) and drugs for the treatment of hepatitis C, such as sofosbuvir (Sovaldi), are fluorine-containing compounds. Furthermore, PET using 18F-labeled compounds afford access to noninvasive monitoring of the disease progression and to follow the effectiveness of the drug candidates. Chapter 5, Pharmaceutical applications of organofluorine compounds, outlines the drug design using organofluorine chemistry, focusing on the recently FDA-approved drugs, and widely prescribed pharmaceuticals, for treating various diseases, including diabetes, cardiovascular diseases, Alzheimer’s disease (AD), various cancers, and bacterial (malaria) and viral infections (HIV). Fluorine-containing compounds play a key role in the design of pharmaceuticals. Structural modification of pharmaceutically interesting compounds through introduction of fluorine, fluoroalkyl, fluoroalkoxy, or fluoroalkylthio moieties enhances their metabolic stability, bioavailability, and potency. In 2018 alone nearly one-third of the FDAapproved drugs are organofluorine compounds. Fluorine-containing pharmaceuticals are used in the treatment of a wide variety of diseases, including diabetes (sitagliptin), malaria (e.g., mefloquine), HIV infections (e.g., bictegravir), antiviral agents (e.g., sofosbuvir, a Preface xiii nucleotide analog inhibitor of the HCV NS5B RNA-polymerase3 inhibitor, for the treatment of hepatitis C), antibacterial agents (e.g., fluoroquinolones and tetracyclines), cancer (e.g., afatinib, dacomitinib, and lorlatinib), cardiovascular diseases (e.g., ezetimibe and atorvastatin), and as inti-inflammatory agents (e.g., celecoxib, a selective COX-2 inhibitor, to treat rheumatoid arthritis). Fluorine-containing compounds have emerging interest as pharmaceutical candidates to treat AD. Although several clinical trials using organofluorine drug candidates (and other drug candidates), as BACE-1 inhibitors, γ-secretase inhibitors, and γ-secretase modulators have not been successful to date, a fluorinated selective BACE-1 inhibitor, CNP540, is currently undergoing clinical trials for its efficacy in preventing AD in individuals susceptible to the development of AD. Chapter 6, Synthesis and applications of 18F-labeled compounds, outlines the recent progress in the synthesis and applications of the 18F-PET tracers in the diagnosis of various diseases, including the AD and cancers. Synthetic methods using the late-stage radiofluorinations have significantly contributed to the advancement of this area. 18F-labeled PET tracers, in combination with magnetic resonance imaging (MRI), PET/MRI, are emerging as alternative to the widely used PET/computed tomography (PET/CT), a technique that requires patients to be exposed to hazardous X-ray radiation, in the diagnosis and monitoring of the disease progression in various cancers, Alzheimer’s disease, and cardiovascular diseases. Furthermore, in some cases, the PET/MRI provides superior imaging of the sites of lesions over that of the PET/CT scans. PET/MRI can be used in probing the blood brain barrier of pharmaceuticals, a key feature for a drug to be active in the neurological disorders. 18F-PET/ MRI imaging of the lung cancers, including adenocarcinoma, squamous cell carcinoma, and small-cell lung carcinoma, is indispensable in monitoring the effectiveness of the various chemotherapeutic agents. Until recently, 2-[18F]-fluoro-2-deoxy-D-glucose is the only FDAapproved 18F-PET imaging agent, for the clinical diagnosis of AD, cancers, and other glucose metabolism linked lesions. Emerging, efficient synthetic methods for the late-stage radiofluorination are now being adapted for the synthesis of various disease-specific 18F-PET agents. For example, FDA-approved 18F-PET imaging agents, florbetapir (Amyvid), florbetaben (Neuraceq), and flutemetamol (Vizamyl), show high specificity for binding to the Aβ plaques and are now widely used in the clinical diagnosis of the AD patients. On the other hand, flortaucipir (AV-1451) shows substantial selectivity in its binding to the neurofibrillary tangles and is useful to distinguish AD from other neurodegenerative diseases, such as behavioral variant frontotemporal dementia, Parkinson’s disease with or without cognitive impairment, and vascular dementia. The latter PET imaging agent is also useful in the diagnosis of the chronic traumatic encephalopathy, also called traumatic brain injury, as demonstrated in the PET scans of the football players with concussion symptoms. Fluciclovine is an 18 F-PET tracer that is used the diagnosis of prostate cancer. Chapter 7, Materials applications of organofluorine compounds, outlines synthesis and applications of a wide range of organofluorine-based materials. Numerous materials, biomaterials, smart materials, liquid crystal displays, solar cells, fuel cells, and numerous consumer goods are fluorine-containing compounds. For example, fluorinated ionomers, such as Nafion-H and fluorinated versions of poly(ether sulfone) and poly(imide) materials, are xiv Preface extensively used as proton-exchange membranes in fuel cells. Fluorinated π-conjugated polymeric materials have found applications in the design of photovoltaic devices. For example, the all-organic solar cells, consisting of fluorinated materials, afford power conversion efficiencies, as high as 13.1%. Furthermore, fluoropolymers have been used as photoresist materials in the 157 nm lithography, as they are transparent at this wave length. Perfluorinated nanomaterials also have medicinal applications. For example, the oxygenenriched fluorinated hydrocarbon and polymeric nanomaterials are being developed for use in the photodynamic therapy of cancer. Fluorinated dendrimer amphiphiles are finding applications as probes for 19F MRI probes and in drug delivery. I am grateful for the continued encouragement and support of the editors and editorial staff, in particular, Dr. Kostas Marinakis and Ms. Michelle Fisher, during the preparation of the manuscript. I appreciate Ms. Swapna Praveen, Sr. Copyrights Coordinator, for her advice and help in getting copyright permissions, and Ms. Maria Bernadette Vidhya Bernard J, Project Manager, for patiently reviewing the manuscript and for incorporating many corrections. I thank Professor G. K. Surya Prakash (University of Southern California) for careful reading of many chapters and for his valuable suggestions and corrections. I also thank Professor Jinbo Hu (Shanghai Institute of Organic Chemistry) for his helpful comments on one of the chapters. I thank all my friends, faculty colleagues, and my graduate students for their encouragement. Some of the cutting-edge advances in the synthesis of organofluorine compounds may have been inadvertently omitted due to the sheer number of the everincreasing publications in this area in the recent years, although every effort is made to include the synthetic methods that are of broad applicability for the design of pharmaceuticals and materials. I hope the readers will find this book useful and appreciate their suggestions and corrections for future editions. 1 Nucleophilic reactions in the synthesis of organofluorine compounds Chapter Outline 1.1 Introduction ..................................................................................................................................... 2 1.2 Reagents for nucleophilic fluorinations ........................................................................................ 2 1.3 Nucleophilic deoxyfluorination...................................................................................................... 3 1.4 Nucleophilic fluorination of pyridines and diazines .................................................................... 8 1.5 Nucleophilic gem-difluorination of carbonyl compounds......................................................... 10 1.6 Nucleophilic fluoroalkylations ..................................................................................................... 12 1.6.1 Nucleophilic difluoromethylation of aldehydes ............................................................... 12 1.6.2 RuppertPrakash reagent (CF3SiMe3) for trifluoromethylation..................................... 13 1.6.2.1 Enantioselective trifluoromethylation......................................................................... 14 1.6.2.2 Synthesis of trifluoromethyl ketones.......................................................................... 16 1.6.2.3 Trifluoromethylation of imines................................................................................... 18 1.6.3 Fluoroacetone hydrates for the nucleophilic fluoroalkylations ...................................... 18 1.6.4 Trifuoromethylations using fluoroform (CHF3)................................................................. 19 1.6.5 Borazine-mediated trifluoromethylation and difluoroalkylation................................... 23 1.6.6 N-Trifluoromethylation of amines ..................................................................................... 26 1.6.7 Tetrakis(dimethylamino)ethylene-mediated fluoroalkylations....................................... 28 1.6.7.1 Trifluoromethylation of acyl chlorides........................................................................ 29 1.6.7.2 Synthesis of gem-(difluoromethyl)thioethers ............................................................. 30 1.7 Nucleophilic trifluoromethylthiolation ....................................................................................... 31 1.8 Trifluoromethoxylations............................................................................................................... 31 1.8.1 Trifluoromethyl benzenesulfonatemediated vicinal (bromo)trifluoromethoxylation.......................................................................................... 33 1.8.2 Trifluoromethyl benzoatemediated trifluoromethoxylation ....................................... 34 References............................................................................................................................................. 35 Organofluorine Chemistry. DOI: https://doi.org/10.1016/B978-0-12-813286-9.00001-8 © 2020 Elsevier Inc. All rights reserved. 1 2 Organofluorine Chemistry 1.1 Introduction Nearly one-third of the pharmaceuticals are fluorinated compounds. Often a single fluorine at the strategic site modulates the pharmacokinetic properties of the pharmaceuticals. 18 F-labeled compounds are used as the state-of-the-art positron emission tomography (PET) tracers in the diagnosis of cancers, cardiovascular diseases, and neurodegenerative diseases. Furthermore, fluorinated compounds are increasingly used in the design of novel materials for a broad range of applications, including photovoltaic solar cells and energy storage devices. Polyfluorinated compounds (fluorous compounds) are used in the 19F nuclear magnetic resonance (19F-NMR) imaging (also called as 19F-magnetic resonance imaging, 19 F-MRI), and as recyclable fluorous catalysts, fluorous solvents, and fluorous stationary phases, in organic synthesis. Early synthetic methods for nucleophilic fluorinations relied on hydrogen fluoride (HF) and its amine complexes [e.g., Olah’s reagent, pyridinium poly (HF; PPHF), and sulfur tetrafluoride (SF4)]. There are currently many safer and more effective fluorinating reagents that have wide functional group tolerance and that would afford high regio- and stereoselectivity. These selective fluorinating agents are invaluable in the synthesis of complex fluorine-containing organic compounds. Nucleophilic fluoroalkylations, especially gem-difluoromethylation and trifluoromethylation, are widely used in the design of functional materials and pharmaceuticals because of the unique and favorable physicochemical and pharmacokinetic properties of these fluoroalkyl compounds. gem-Difluoromethylene (CF2) moiety is isopolar and, to some extent, isosteric with respect to oxygen, and thus the gem-difluoromethyl (CHF2) and difluoromethylene (CF2) moieties serve as bioisosteres of alcohols and ethers, respectively, in the drug design applications. It is also a lipophilic bioisostere of SH and CH3 when attached to the aryl or alkyl moieties. The CF2H is a hydrogen-bond donor as well as acceptor, and the lipophilicity of the compounds is dramatically enhanced when this moiety is introduced adjacent to the ether, sulfoxide, and sulfone moieties.1,2 The trifluoromethyl moiety is bioisosteric with respect to the tert-butyl and isopropyl groups and is used, for example, in the design of γ-secretase inhibitors.3 Many of the nucleophilic reagents for fluorination and fluoroalkylation are now commercially available. This chapter aims to give a comprehensive coverage of the nucleophilic fluorinations and fluoroalkylations that have broad scope in the design of pharmaceuticals, agrochemicals, and materials. 1.2 Reagents for nucleophilic fluorinations A variety of reagents for nucleophilic fluorination are now commercially available. Deoxyfluorination of alcohols and gem-difluoromethylation of carbonyl compounds can be achieved by reagents, such as DAST ((diethylamino)sulfur trifluoride),4 Morpho-DAST (morpholinosulfur trifluoride),4 Deoxo-Fluor [bis(2-methoxyethyl)aminosulfur trifluoride],57 XtalFluor-E [(diethylamino)difluorosulfinium tetrafluorobrate],8 XtalFluor-M (morpholinodifluorosulfinium tetrafluoroborate),8 FluoLead ((4-tert-butyl-2,6-dimethylphenyl)sulfur Chapter 1 • Nucleophilic reactions in the synthesis of organofluorine compounds Cyanuric fluoride 3 Ishikawa's reagent FIGURE 1–1 Selected commercially available reagents for the transformation of the carbonyl compounds to the gem-difluoromethyl and -methylene compounds. trifluoride).9 PhenoFluor [1,3-bis(2,6-diisoproylphenyl)-2,2-difluoro-4-imidazoline]10 and PyFluor (2-pyridinesulfonyl fluoride)11 reagents are useful for selective deoxyfluorination of alcohols in the presence of carbonyl functional groups. Other reagents that are useful in the deoxyfluorinations include Petrov’s reagent (1,1,2,2-tetrafluoroethyl-N,N-dimethylamine), cyanuric fluoride, Ishikawa’s reagent (N,N-diethyl-1,1,2,3,3,3-hexafluoropropylamine), and 3,3-difluoro-1,2-diarylcyclopropenes (Fig. 11).12,13 DAST and Deoxo-Fluor reagents are widely used for the deoxyfluorination of alcohols in the synthesis of numerous biologically and pharmaceutically interesting fluorine-containing compounds.14 Deoxyfluorination reactions using DAST are usually carried out at a low temperature to avoid the decomposition of the reagent. Similarly, carbonyl groups are effectively gem-difluorinated at a relatively low temperature.15 (Trifluoromethyl)trimethylsilane (CF3SiMe3; RuppertPrakash reagent) is widely used for the nucleophilic trifluoromethylation of carbonyl compounds, including aldehydes, ketones, imines, and esters (vide infra).16,17 1.3 Nucleophilic deoxyfluorination Nucleophilic deoxyfluorination of alcohols can be achieved using various commercially available reagents such as DAST, Deoxo-Fluor, Morpho-DAST, XtalFluor-E, XtalFluor-M, PyFluor, and PhenoFluor. Deoxo-Fluor is relatively more thermally stable as compared to DAST and is the preferred reagent over DAST when high temperatures are required for the reactions. 4 Organofluorine Chemistry F iPr OH PhenoFluor/toluene iPr N CsF (3 equiv) N F F iPr iPr 110 ºC 89% PhenoFluor Selected examples: F N O Me N N F N N F N F 58% 34% 78% 93% FIGURE 1–2 PhenoFluor-mediated deoxyfluorination of phenols. Selective fluorinating reagents, especially those that can be used in the stereoselective deoxyfluorination of alcohols, are of great importance for the synthesis of pharmaceutical compounds. Among several such reagents recently developed, PhenoFluor and PyFluor are of broad scope in the deoxyfluorination of alcohols, although DAST is still widely used for deoxyfluorination reactions.18 XtalFluor reagents (XtalFluor-E and XtalFluor-M) show improved selectivity in deoxyfluorination reactions, compared to DAST, as the elimination byproducts are minimized. Similar to that of DAST and Deoxo-Fluor-mediated deoxyfluorination reactions, the XtalFluor reagentmediated deoxyfluorination of alcohols proceed through a SN2 mechanism, with predominant inversion of configuration.14 PhenoFluor was originally discovered by Ritter and coworkers for the deoxyfluorination of phenols.19,20 Phenols as well as heteroaryl phenolic compounds were deoxyfluorinated to their corresponding fluorinated compounds using this reagent. To overcome the moisture instability of this reagent, toluene solutions of this reagent can be used for the deoxyfluorinations (Fig. 12). PhenoFluor can also be used for the deoxyfluorination of primary and secondary alcohols, using slightly different conditions as for the phenols.21 Addition of Hunig’s base, in these reactions, shortens the reaction time, and potassium fluoride (KF) minimizes the elimination products from the aliphatic alcohols. Deoxyfluorinations of secondary alcohols using PhenoFluor proceed in high yields with inversion of configuration.21 Although these deoxyfluorinations proceed at 0 C to room temperature, elimination products are formed as minor byproducts. However, at 80 C in toluene, the elimination reaction is suppressed, and the reaction proceeds in high yields to give the corresponding deoxyfluorination products. A variety of pharmaceutically interesting compounds, such as morphine, galantamine, testosterone, and epi-androsterone, could be stereoselectively transformed into their corresponding fluorinated products with the inversion of configuration in high yields and with high stereoselectivity (Fig. 13). PhenoFluor provides access to the late-stage fluorination of pharmaceuticals and is suitable for the preparation of 18F-labeled compounds for the PET. Chapter 1 • Nucleophilic reactions in the synthesis of organofluorine compounds FIGURE 1–3 PhenoFluor-mediated deoxyfluorination of alcohols. 5 6 Organofluorine Chemistry Doyle and coworkers have developed 2-pyridinesulfonyl fluoride (PyFluor) as a low-cost nucleophilic fluorinating reagent for the fluorination of primary and secondary alcohols. PyFluor is conveniently prepared on a multigram scale via the oxidation of 2-mercaptopyridine with sodium hypochlorite (NaOCl), followed by the halide anion exchange of the resulting 2-pyridylsulfonyl chloride, using potassium bifluoride (KHF2). Deoxyfluorination of alcohols using PyFluor reagent, in the presence of a sterically crowded base, such as 1,8diazabicyclo-[5.4.0]undec-7-ene (DBU), gives the alkyl fluorides in high yields and with high diastereoselectivity.11 Elimination reactions are minimized using the sterically crowded amines, such as DBU or 7-methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene (MTBD). Various biologically interesting fluorinated compounds, including 2-deoxy-2-fluoro-D-glucose and its 18 F-labeled analog, could be synthesized in a one-pot procedure. The 18F-labeled PyFluor reagent, [18F]PyFluor, used in the radiofluorinations was synthesized through the reaction of the 18F-labeled KF with the 2-pyridylsulfonyl chloride, in situ (Fig. 14A).11 The reaction proceeds through the intermediate formation of the 2-pyridylsulfonate ester (1), through SN2 reaction pathway, with the in situ generated DBUHF providing the nucleophilic fluoride anion. Elimination reactions are minimized using the DBU or MTBD reagents, as compared to the other amine-based reagents. Among other related nucleophilic reagents for the deoxyfluorination, N-tosyl-p-chlorobenzene-sulfonimidoyl fluoride (SulfoxFluor) has dramatically high reactivity (1030 min at RT) and similar mechanism as that of the PyFluor, and is also readily synthesized in large scale from the N-Tosyl-p-chlorobenzenesulfonimidoyl chloride (Fig. 14B).22 Sanford and coworkers have developed an operationally simple method for the deoxyfluorination of phenols using the relatively inexpensive reagent combination of sulfuryl fluoride (SO2F2) and tetramethylammonium fluoride (Me4NF). This reagent achieves the deoxyfluorinations under mild conditions, often at room temperature, and in high yields (Fig. 15).23 These deoxyfluorination reactions proceed through the formation of the aryl fluorosulfonate intermediates. This synthetic method was demonstrated to be applicable for the synthesis of pharmaceutically interesting compounds, such as MPPF [20 -methoxyphenyl(N-20 -pyridinyl)-p-fluorobenzamide-ethylpiperazine], a serotonin 1A receptor ligand. DAST and related reagents, such as Deoxo-Fluor and XtalFluor reagents, can be used for the conversion of carboxylic acids to the corresponding acid fluorides.2426 DAST and Deoxo-Fluor are thermally not as stable as XtalFluor and can decompose violently under some circumstances. These reagents, however, are used widely for the deoxyfluorination of alcohols, carboxylic acids, and gem-difluorination of carbonyl compounds. On the other hand, the aminodifluorosulfinium tetrafluoroborate salts—XtalFluor-E and XtalFluor-M— are crystalline salts that show enhanced thermal stability over DAST and Deoxo-Fluor and do not react violently with water, unlike DAST. XtalFluor-E is conveniently synthesized through the reaction of the DAST with BF3Et2O. XtalFluor, in the presence of Et3N3HF, transforms carboxylic acids into the corresponding acid fluorides in high yields (Fig. 16).26 Chapter 1 • Nucleophilic reactions in the synthesis of organofluorine compounds (A) (B) FIGURE 1–4 Deoxyfluorination reactions mediated by PyFluor(A), and SulfoxFluor (B). FIGURE 1–5 Deoxyfluorination of phenols using SO2F2 and Me4NF. 7 8 Organofluorine Chemistry FIGURE 1–6 Deoxyfluorination of carboxylic acids using XtalFluor reagents. Prakash and coworkers have achieved the deoxyfluorination of carboxylic acids to the corresponding acyl fluorides, using a reagent combination of triphenylphosphine (Ph3P), Nbromosuccinimide (NBS), and Et3N3HF, under mild reaction conditions, in high yields. Through this efficient and cost-effective synthetic procedure, pharmaceuticals with a carboxylic acid functional group, such as ibuprofen, naproxen, and ketoprofen, were transformed to the corresponding acid fluorides in high yields. The acid fluorides could be transformed, in situ, to their corresponding amide derivatives in a one-pot procedure.27 The acyl fluorides have numerous synthetic applications, including their conversions to trifluoromethylarenes, ketones, aldehydes, amides, esters, and hydrocarbons (Fig. 17).2733 The above deoxyfluorination reactions may proceed through the transiently formed acyloxyphosphonium salt (2), the identity of which was confirmed by NMR spectroscopy. The latter acyloxyphosphonium salt, 2, is presumably protonated by Et3N3HF to give the dicationic intermediate 3, which upon nucleophilic substitution by the fluoride anion would give the acyl fluoride (Fig. 17).27 1.4 Nucleophilic fluorination of pyridines and diazines Fluorinated heterocycles are ubiquitous in agrochemicals, pharmaceuticals, and materials. Hartwig has developed a broadly applicable synthetic method for the ortho-fluorination of pyridines and diazines using Ag(II)F2.34 These fluorination reactions have a broad scope and a range of pyridines and diazines, including quinolines, pyrazines, pyrimidines, and pyridazines, have been regioselectively fluorinated in moderate to high yields. Pharmaceutically interesting compounds, such as 4, 5, and 6, could be synthesized in moderate to high yields through this aryl-fluorination (Fig. 18). This reaction tolerates both electron-donating and electron-withdrawing substituents, such as ketone, ester, amide, amine, and nitrile moieties, in pyridines and diazines.34 Chapter 1 • Nucleophilic reactions in the synthesis of organofluorine compounds 9 for example D R′ R′ R′ R′ for example K N I FIGURE 1–7 Deoxyfluorination of carboxylic acids to the acid fluorides. A proposed mechanism involves complexation of AgF2 to pyridine nitrogen, followed by intramolecular transfer of fluoride to the ortho-position and then AgF2-mediated rearomatization. The reaction mechanism resembles that of Chichibabin reaction, a reaction involving ortho-amination of pyridines by NaNH2 (Fig. 18).34 10 Organofluorine Chemistry FIGURE 1–8 ortho-Fluorination of pyridines and diazines using AgF2. 1.5 Nucleophilic gem-difluorination of carbonyl compounds gem-Difluorination of carbonyl compounds can be achieved using nucleophilic fluorinating reagents, such as DAST, Deoxo-Fluor, and XtalFluor. XtalFluor-E and XtalFluor-M reagents, synthesized from the corresponding dialkylaminosulfur trifluorides, are crystalline compounds and are relatively more stable and moisture-sensitive than the conventional fluorinating agents, DAST and Deoxo-Fluor. When used in the presence of Et3N.3HF, these reagents transform aldehydes and ketones into the corresponding gem-difluoro compounds.8 Ester moieties and the N-benzyloxycarbonyl (Cbz) protecting groups are unaffected under the reaction conditions (Fig. 19). XtalFluor reagents can also be used for the transformation of alcohols to the alkyl fluorides, and carboxylic acids to the acid fluorides, under similar reaction conditions (vide supra).8 Chapter 1 • Nucleophilic reactions in the synthesis of organofluorine compounds N SF3 11 F HBF4 .OEt2 N BF 4 S F -HF XtalFluor-E DAST 96% F O XtalFluor-E F Et 3 N.3HF, Et3 N, DCM RT, 24 h 91% O F XtalFluor-E N Cbz O F O Cbz = N Cbz Et3 N.3HF, Et3 N, DCM RT, 24 h 91% F O F 1. 6 N HCl/60 °C/5 h DAST, neat F OH OEt OEt N Cbz 0 °C to RT N Cbz F O 7 O 2. NaHCO3 /THF RT, 24 h N H O 8 9 64% 81% SF3 FluoLead O PPHF (1.7 equiv) DCM, RT, 24 h F F 70% FluoLead, 100 °C, 3 h PhCO2 H PhCF3 FIGURE 1–9 gem-Difluorination of carbonyl compounds and trifluoromethylation of carboxylic acids; Cbz 5 carbobenzyloxy. Peptides consisting of fluorinated proline moieties, such as the 3,3-gem-difluoroproline 9, can act as selective enzyme inhibitors, with favorable pharmacokinetics.35 Toward this goal, the N-carbobenzyloxy-3,3-gem-difluoroproline 9 was synthesized through the DAST-mediated deoxy-gem-difluorination of the 3-prolinone derivative 7, followed by hydrolysis of the ester moiety and deprotection of the N-benzyloxycarbonyl (Cbz) protecting group (Fig. 19). 12 Organofluorine Chemistry 25 °C, 4 h 25 °C, 4 h FIGURE 1–10 gem-Difluorination of carbonyl compounds using sulfuryl fluoride. FluoLead (Fig. 11) has relatively higher thermal stability and is stable up to 100 C. FluoLead achieves transformation of carbonyl compounds to the gem-difluoro compounds at 0 C to room temperature, in high yields.9 Reaction of carboxylic acids with FluoLead at high temperatures (50 C100 C) gives the corresponding trifluoromethyl compounds (Fig. 19). The transformation of the carbonyl compounds to the gem-difluoro compounds can also be achieved using the abundantly available sulfuryl fluoride (SO2F2) as the fluorinating agent. Thus reaction of benzaldehydes and α-ketoesters with sulfonyl fluoride, in the presence of tetrabutylammonium fluoride (TBAF), at room temperature, gives the corresponding gemdifluoro compounds (Fig. 110).36 The 1,3-dithiolanes, hydrazones, or oxime derivatives of the carbonyl compounds could be transformed into their corresponding gem-difluoro compounds, using PPHF, in the presence of an electrophilic reagent such as NBS or nitrosonium tetrafluoroborate (NOBF4).37 Thus reaction of the 1,3-dithiolanes with 1,3-dibromo-5,5-dimethylhydantoin (DBDMH) (or NBS),38 sulfuryl chloride fluoride (SO2ClF),39 nitrosonium tetrafluoroborate (NOBF4),40 or Selectfluor,41 in PPHF, gives the corresponding gem-difluoro compounds. The dithioketals were transformed into the corresponding gem-difluoro compounds by reaction with p-iodotoluene difluoride.42 The gem-difluoro compounds can also be synthesized through the reaction of the hydrazone derivatives of carbonyl compounds with NBS in PPHF,43 or through the reaction of the oximes with NOBF4 in PPHF (Fig. 111).44 1.6 Nucleophilic fluoroalkylations 1.6.1 Nucleophilic difluoromethylation of aldehydes The difluoromethylation of carbonyl compounds could be achieved using CHF2TMS and CsF in a polar solvent such as dimethylformamide (DMF).45,46 Activation of CHF2TMS for difluoromethylation of carbonyl compounds requires somewhat harsher conditions than for the CF3TMS. Difluoromethyl moiety, similar to the trifluoromethyl group, alters the pharmacokinetic properties of the drug candidates. In an attempt to synthesize a wide range of derivatives of the insecticide tebufenpyrad for screening the antiangiogenic potential, the pyrazole Chapter 1 • Nucleophilic reactions in the synthesis of organofluorine compounds 13 FIGURE 1–11 gem-Difluorination of carbonyl compounds. FIGURE 1–12 Difluoromethylation of pyrazole aldehyde in the synthesis of tebufenpyrad analogs, as potential antiangiogenic agents. aldehyde moiety was difluoromethylated using this reagent (Fig. 112). Various difluoromethylated as well as poly-fluoroalkylated derivatives, thus synthesized, exhibited the desired antiangiogenic effect, although their undesired mitochondrial inhibition activity precluded their use as medicinal agents.47 1.6.2 RuppertPrakash reagent (CF3SiMe3) for trifluoromethylation Trifluoromethylation of aldehydes and ketones using the trifluoromethyltrimethylsilane (CF3TMS; RuppertPrakash reagent) is widely used in the synthesis of the α-trifluoromethyl alcohols.16,4850 The CSi bond in CF3TMS is labile and therefore in situ generation of the 14 Organofluorine Chemistry CF3 SiMe3 /Bu4N + F – O R R′ F3 C OSiMe 3 R′ R THF; 1–24 h, RT F3 C OH Aq. HCl + Me 3 SiCl R′ R R/R′ = H. alkyl, aryl Mechanistic outline: F CF3 SiMe3 CF3 Me Si Me F Me R O CF3 O Me Si R′ O R 10 Me Me R′ R F3 C O R R′ R′ CF3 11 F3 C OSiMe 3 CF3 SiMe3 R′ R 12 Commonly used fluoride sources for the activation of CF3TMS: Me N Bu4NF CsF F S N N F F Me Si Me TASF Ph Bu4 N+ Si F Ph Ph TBAT FIGURE 1–13 Trifluoromethylation of carbonyl compounds using CF3TMS. trifluoromethyl anion can be achieved using various anionic reagents, including CsF, TBAF (Bu4NF), tetrabutylammonium difluorotriphenylsilicate, and tris(dimethylamino)sulfonium difluorotrimethylsilicate (TASF) (Fig. 113). The mechanism of the reaction involves formation of a negatively charged penta-coordinated silicon species (10) upon activation by the fluoride anion (or other anionic activators such as tetrabutylammonium acetate). The SiCF3 bond is now elongated and weakened so that the trifluoromethanide anion (CF2 3 ) is readily transferred to the electrophilic carbonyl carbon to give the intermediate 11. The intermediate 11, in turn, acts as a source of the CF2 3 anion and is regenerated continually as the reaction proceeds. Thus catalytic amounts of the anionic activators (e.g., F2) are sufficient to achieve the trifluoromethylations. 1.6.2.1 Enantioselective trifluoromethylation Moderate enantioselectivities of the trifluoromethylation reactions could be achieved using cinchonidine-derived sterically crowded catalysts such as 1315.51,52 Chapter 1 • Nucleophilic reactions in the synthesis of organofluorine compounds 15 Cinchonidine-based chiral catalysts: CF3 O F– Br N O N Br CF3 N CF3 OH OH N N F3C OH N CF3 CF3 13 14 15 OMe Me O O Ar O CF3TMS (2 equiv) Chiral catalyst 15 F3 C Me OMe OTMS O O DCM, –50 °C OMe OMe 16 17 97% (92% ee) CJ-17,493 (NK-1 receptor antagonist) FIGURE 1–14 Enantioselective trifluoromethylation. The cinchonidine catalyst, 15, however, gives 92% enantioselectivity in the trifluoromethylation of the ketone moiety in compound 16, to give 17, an intermediate for the synthesis of the Pfizer’s neurokinin-receptor antagonist, CJ-17,493 (Fig. 114).53 Shibata and coworkers showed that the cinchonidine-derived catalyst 18 gives up to 50% enantioselectivity in the trifluoromethylation of the alkynyl ketone 20, using CF3TIMS (RuppertPrakash reagent) and tetramethylammonium fluoride (Me4NF) as the trifluoromethylating agent, to give compound 21. Chiral resolution of 21, followed by reduction of the nitro moiety, and then reaction with p-nitrophenyl chloroformate gives the anti-HIV drug Efavirenz in 88% overall enantioselectivity. The enantioselectivity could be improved to up to 99% by simple recrystallization (Fig. 115).52 Shibata and coworkers have later shown that a slightly modified cinchonidine-based catalyst 19 exhibited substantially higher enantioselectivity for the trifluoromethylation of compound 20, by up to 93%.54 16 Organofluorine Chemistry H CF3 N Br N Br CF3 CF3 OBu OH N N CF3 F3C 18 CF3 19 O NO2 1. Chiral resolution 2. Fe/AcOH Cl Me3 SiCF3 (2.0 equiv) NO2 Me4 NF, DCM –60 °C 20 OH F 3C 18 or 19 ; 10 mol% Cl 21 88%; 50% ee for catalyst 18 93% ee for catalyst 19 NO2 O F 3C OH Cl Cl O 23 F3C NO2 Cl NH 2 22 + O N H O HO Efavirenz, 88% (anti-HIV drug) FIGURE 1–15 Enantioselective trifluoromethylation for the synthesis of Efavirenz. 1.6.2.2 Synthesis of trifluoromethyl ketones Although Grignard reactions of esters usually give the corresponding tertiary alcohols, because the intermediate ketones are too reactive with the Grignard reagents, the Grignard reactions of ethyl trifluoroacetate (24) almost exclusively give the aryl trifluoromethyl ketones. The tetrahedral intermediate 25 is stable under the reaction conditions, because of the strong electron-withdrawing effect of the trifluoromethyl group, and the trifluoromethyl ketones (26) are formed only during the aqueous workup of the reaction mixture. Therefore tertiary alcohols are not formed as the major products in the trifluoromethylation of esters (Fig. 116).55 Ethyl fluoroacetate, with a single fluorine on the methyl group, also gives the corresponding fluoromethyl ketone as the predominant product. The aryl Grignard reagents Chapter 1 • Nucleophilic reactions in the synthesis of organofluorine compounds 17 FIGURE 1–16 Grignard reaction of ethyl trifluoroacetate to give trifluoromethyl ketones. FIGURE 1–17 Nucleophilic trifluoromethylation of esters using CF3TMS reagent. FIGURE 1–18 Trifluoromethylation of Weinreb amides for the preparation of trifluoromethyl ketones. used in these transformations were formed, in situ, from the corresponding aryl halides and a 1:1 mixture of isopropylmagnesium bromide and LiCl.56 Trifluoromethylation of esters using trifluoromethyltrimethylsilane (CF3TMS) affords the corresponding trifluoromethyl ketones in good yields. However, the scope of this reaction is limited mostly to esters of aromatic carboxylic acids.16 These trifluoromethylations using CF3TMS proceed under relatively mild conditions when carried out in the presence of the relatively weak Lewis acid catalyst, MgCl2 (Fig. 117).57 Trifluoromethylation of Weinreb amides of aliphatic and aromatic carboxylic acids, using CF3TMS and CsF in catalytic amounts, gives the corresponding trifluoromethyl ketones under mild reaction conditions. The intermediate hemiaminal silyl ether is stable under these conditions, and its fluoride anioninduced desilylation, during workup of the reaction, gives the corresponding ketones (Fig. 118).58 Thus, the use of Weinreb 18 Organofluorine Chemistry FIGURE 1–19 Nucleophilic trifluoromethylation of N-alkylimines using CF3TIMS and in situgenerated hydrofluoric acid. amides as substrates in these reactions ensures monotrifluoromethylation, as the intermediate hemiaminal silyl ether decomposes to give the corresponding trifluoromethyl ketone, only after the addition of TBAF. 1.6.2.3 Trifluoromethylation of imines Nucleophilic trifluoromethylation of imines requires the presence of electron-withdrawing substituents on the nitrogen such as N-sulfonyl and N-sulfinyl moieties.5966 Dilman has shown that unactivated N-alkylimines could be trifluoromethylated using CF3TMS and stoichiometric amounts of potassium hydrogen difluoride (KHF2) and trifluoroacetic acid (CF3CO2H).67,68 Under these conditions, solvated HF is generated, which activates the imines toward nucleophilic trifluoromethylation, through N-protonation of the imines. The counter2 ion HF2 2 , in turn, activates the CF3TMS to generate the trifluoromethyl anion (CF3 ) in situ (Fig. 119). 1.6.3 Fluoroacetone hydrates for the nucleophilic fluoroalkylations Hexafluoroacetone (27) under basic conditions, at high temperatures, decomposes to give the trifluoroacetate and trifluoromethyl anion, which reacts with aromatic aldehydes, such as p-anisaldehyde, to give the corresponding α-trifluoromethyl alcohols. The high temperatures required for these reactions are a limiting factor for their applications in the synthesis of fluorinated compounds. However, Colby and coworkers synthesized the amidinate salt 30 (now commercially available) through the reaction of hexafluoroacetone (27) and DBU, as a crystalline, air-stable salt. Compound 27, upon reaction with various aromatic aldehydes and ketones at 2 30 C, gives the corresponding α-trifluoromethyl alcohols in high yields.69 Chapter 1 • Nucleophilic reactions in the synthesis of organofluorine compounds O HO MeO HO OH F3 C NaOH, 100 °C O O F3 C CF3 27 19 CF3 H CF3 CF3 O 28 OMe F3 C O 29 N O N F3 C HO O (DBU) HO OH F3 C CF3 R N CF3 CF3 R R′ 31 78–96% 30 89% HO KOt-Bu/DMF; –30 °C N H Et2 O R′ R, R′ = aryl, H O KOtBu HO OH H O O H CF3 32 R CF3 CF3 HO H R 45–96% 33 34 R = aryl, alkyl O H CF3 O O O HO OH R CF3 O LiBr, Et3 N, THF RT O H F R F F F 35 R′ 36 OH R R′ F F 37 FIGURE 1–20 Trifluoromethylation using hexafluoroacetone as the source of the trifluoromethyl anion, and related fluoroalkylations. Trifluoroacetaldehyde hydrate (32) similarly reacts with aliphatic and aromatic aldehydes in the presence of potassium tert-butoxide (KOtBu) in DMF to give the corresponding α-trifluoromethyl alcohols in good yields.70 Difluoroenolates (36), generated, in situ, from 35, through the base-mediated expulsion of trifluoroacetate anion, undergo aldol reactions with carbonyl compounds, to give the corresponding gem-difluoro compounds, α,α-difluoroβ-hydroxy carbonyl compounds (37) (Fig. 120).71 1.6.4 Trifuoromethylations using fluoroform (CHF3) Prakash and coworkers developed a convenient, direct trifluoromethylation of carbonyl compounds using fluoroform as the source of CF2 3 , generated through potassium 20 Organofluorine Chemistry (A) (B) FIGURE 1–21 Trifluoromethylation of aromatic aldehydes and ketones (A), and preparation of CF3TMS (B), using fluoroform as the source of the nucleophilic CF2 3. hexamethyldisilazide [potassium bis(trimethylsilyl)amide; KHMDS]mediated deprotonation of the fluoroform (HFC-23; trifluoromethane) (Fig. 121).72 Fluoroform is a greenhouse gas, formed as an abundant byproduct in the industrial-scale synthesis of polyvinylidene chloride and poly(tetrafluoroethylene) from chlorodifluoromethane (CHF2Cl). Fluoroform is also a relatively nontoxic compound, and therefore there is a great interest in its use as a trifluoromethylating agent.7382 Interestingly, the stability of the CF2 3 anion is dependent on the counter cations, and the trifluoromethylations proceed under mild conditions using KHMDS [K1 2 N(SiMe3)2] as the base for the deprotonation of the fluoroform.72 The trifluoromethylations of carbonyl compounds, using the reagent combination of fluoroform and NaHMDS (sodium hexamethyldisilazide; sodium bis(trimethylsilyl)amide), or lithium hexamethyldisilazide (LiHMDS), were not successful.83 Furthermore, the Ruppert-Prakash reagent, CF3SiMe3, can be synthesized Chapter 1 • Nucleophilic reactions in the synthesis of organofluorine compounds 21 in high yields through the reaction of fluoroform with chlorotrimethylsilane, in the presence of potassium hexamethyldisilazide (KHMDS) (Fig. 121B). Whereas the earlier reported trifluoromethylations, using fluoroform as the source 2 of the CF2 3 anion, required the use of DMF to stabilize the CF3 anion (as the 7678,81,84,85 2 hemiaminolate salts), the CF3 anion, generated using KHMDS or tBuOK, was found to be stable and reactive in ether solvents, even in the absence of DMF. Using this technique, a variety of aromatic carbonyl compounds could be trifluoromethylated to give the corresponding α-trifluoromethyl alcohols in good yields.72 In subsequent work, Prakash and coworkers have demonstrated the intermediacy of the in situformed CF2 3 anion in nucleophilic trifluoromethylations, through NMR spectroscopy and single-crystal X-ray crystallography at low temperatures.83 Thus the reaction of tris(isopropyl)silyltrifluoromethane [(iPr)3SiCF3; TIPSCF3)] with potassium tert-butoxide in anhydrous tetrahydrofuran (THF) solution, in the presence of 18-crown-6, at 278 C, formed the 19 [K(18-crown-6)]1 CF2 F NMR spectrum of this trifluoromethanide anion shows a 3 . The 19 singlet at δ F 218.71, showing substantial negative charge for the anionic carbon of trifluoromethyl carbanion. The 13C NMR spectrum of the CF2 3 anion showed a quartet with a 1:3:3:1 signal intensity ratio at δ13C 175.0, showing that the three fluorines are equivalent. The 13C NMR spectrum showed an unusually large one-bond CF coupling constant, 1J (CF), of 432.5 Hz, as compared to a one-bond CF coupling constant of 293.3 Hz for the fluoroform (CHF3). As expected, thus-obtained trifluoromethanide anion gave the α-trifluoromethyl alcohols upon reaction with various carbonyl compounds such as benzaldehyde (Fig. 122).83 Grushin and coworkers have subsequently prepared the CF2 3 anion by performing the above reaction of TIPSCF3 with KOtBu in the presence of 2.2.2-cryptand, in anhydrous THF, at 278 C. The latter cryptand is even more effective in coordinating with the K1 ion than is the 18-crown-6 and therefore is expected to give the relatively free trifluoromethyl anion. Reaction of TIPSCF3 with potassium tert-butoxide-2,2,2-cryptand ([cryptand K2.2.2]1 tBuO2) gave the trifluoromethanide anion, which showed a 19F NMR chemical shift (δ19F) of 217.2 ppm, somewhat deshielded as compared to that of [K(18-crown-6)]1CF2 3 . Reaction of thus-generated trifluoromethyl anion with carbonyl compounds, such as benzaldehyde, also gave the expected α-trifluoromethyl alcohols.8688 The trifluoromethyl anion is prone to the loss of the fluoride anion to give the difluoromethyl carbene, because of the thermodynamic force to overcome the repulsion of the nonbonding electron pair on the fluorine with the nonbonding electron pair on the carbon. The CF2 anion, generated from the reaction of fluoroform and dimsyl potassium 3 1 (CH3 SOCH2 2 K ) in DMF, is stabilized as the hemiaminolate salt (38), which reacts with carbonyl compounds to give the corresponding trifluoromethylated alcohols 39.7678,81,84,85 The intermediate 38 also reacts with dialkyl or diaryl disulfides to give the corresponding trifluoromethyl sulfides (RSCF3; 40) (Fig. 123).89 The latter trifluoromethyl thioethers are useful in drug discovery and medicinal chemistry.90 Shibata and coworkers reported the catalytic effect of the monoglyme, triglyme, or tetraglyme in the direct trifluoromethylation of the carbonyl compounds using the reagent –78 °C –78 °C FIGURE 1–22 Direct observation of the trifluoromethyl anion by NMR, through complexation of the K1 by the 18crown-6 and by the cryptand-2.2.2. FIGURE 1–23 Trifluoromethylation of carbonyl compounds using fluoroform, a strong base, and DMF. DMF, Dimethylformamide. Chapter 1 • Nucleophilic reactions in the synthesis of organofluorine compounds O R R′ CHF3 (excess) t-BuOK or KHMDS (2.0 equiv) Monoglyme RT, 6 h (or –40 °C, 12 h) 23 F3 C OH R R′ Selected examples: F3 C OH F3 C OH F3 C OH F3 C OH H 86% 66% 61% 80% FIGURE 1–24 Trifluoromethylation of carbonyl compounds in glyme solvents, using fluoroform as the source of the trifluoromethyl anion. combination of fluoroform and potassium tert-butoxide or KHMDS.74 The glyme solvents, as in the case of 18-crown-6, coordinate with the K1 ions, thus making the CF2 3 anion more nucleophilic. The use of stoichiometric amounts of 18-crown-6 is impracticable in the industrial scale synthesis, and therefore, the use of glyme solvents provides an alternative synthetic strategy. Various aryl aldehydes and ketones are trifluoromethylated, in glyme solvents, to give the corresponding α-trifluoromethyl alcohols in good yields (Fig. 124). A phosphazene superbase (P4-tBu) mediates trifluoromethylation of carbonyl compounds, using fluoroform as the only reagent.91 In this reaction, because of the extended delocalization of the conjugate acid, P4-tBu phosphonium cation, P4-tBu, acts as a strong base to deprotonate the trifluoromethane, and the resulting CF2 3 anion reacts with carbonyl compounds to give the corresponding α-trifluoromethyl alcohols. Due to the steric crowding afforded by the P4-tBu base, the CF2 3 anion is stabilized, even in the absence of the added DMF as a solvent or as a catalyst, and reacts readily with carbonyl compounds to give the corresponding α-trifluoromethyl alcohols (Fig. 125). The CF2 3 anion formed through the superbase P4-tBu- or KHMDS-mediated deprotonation of trifluoromethane reacts with chiral sulfinimines (41) of aromatic and aliphatic aldehydes to give the corresponding trifluoromethylated N-sulfinylamines (42) with high diastereoselectivity.62 Acidification of the resulting sulfinimines in 4M HCl then gives the corresponding α-trifluoromethylammonium salts 43 (Fig. 126). Similarly, high diastereoselectivities were obtained in the trifluoromethylation of chiral sulfinimines using KHMDS and fluoroform. 1.6.5 Borazine-mediated trifluoromethylation and difluoroalkylation The abundantly available hexamethylborazine forms a stable adduct with trifluoromethyl anion and serves as a nucleophilic trifluoromethylating agent toward carbonyl compounds. Geri and Szymczak pioneered the nucleophilic trifluoromethylation reactions using the 24 Organofluorine Chemistry FIGURE 1–25 Trifluoromethylation of carbonyl compounds using the phosphazene superbase P4-tBu and fluoroform. FIGURE 1–26 Diastereoselective trifluoromethylation of N-sulfinylimines. Chapter 1 • Nucleophilic reactions in the synthesis of organofluorine compounds K N B B N N CHF3 KH (or NaH)/DMSO B B O CF3 N B N N 25 Ph Ph HO CF3 Ph B N + B Ph B N N B 72% (Recyclable reagent) CF3 SO2 – Na+ SO 2 Me 3 SiCl Me 3 SiCF3 Langlois reagent 96% Cl 66% I O F3 C CF3 CO2– K+ I O CO 2 Togni’s reagent 78% FIGURE 1–27 Borazine-mediated trifluoromethylation of carbonyl compounds and synthesis of Langlois reagent, Togni’s reagent, and CF3TMS. hexamethylborazinetrifluoromethyl anion adduct as a cost-effective, atom-economical reagent.92 The hexamethylborazinetrifluoromethyl anion adduct could be generated, in situ, from the reaction of hexamethylborazine with the trifluoromethyl anion, which, in turn, is generated through the reaction of fluoroform and a strong base (e.g., NaH). Thus reaction of carbonyl compounds, such as benzophenone, in the presence of borazine, fluoroform, and NaH, gives the α-trifluoromethyl alcohols in high yields, under mild reaction conditions (room temperature, 30 min). The borazine starting material is regenerated during the reaction and is recyclable; hence, borazine-mediated trifluoromethylation is an efficient and cost-effective means of trifluoromethylation of carbonyl compounds. The borazine-mediated trifluoromethylation could also be used in the synthesis of the nucleophilic trifluoromethylating agents, such as CF3TMS (through reaction with trimethylsilyl chloride); free-radical trifluoromethylating agents, such as Langlois reagent (through reaction with sulfur dioxide); and electrophilic trifluoromethylating reagents, such as Togni’s reagent (through reaction with the corresponding iodonium chloride) in high yields (Fig. 127).92 Szymczak and coworkers extended their synthetic method for the difluoromethylation of carbonyl compounds. Thus the reaction of hexamethylborazine with (difluoromethyl) benzene, in the presence of a strong base, such as potassium diisopropylamide KN(iPr)2, gives the borazine-gem-difluorobenzyl adduct, which reacts with carbonyl compounds to give the corresponding gem-(difluoro)benzyl-substituted alcohols in moderate yields. This reagent can also be used in the Pd(0)-catalyzed difluoroalkylation of aryl halides (Fig. 128).93 26 Organofluorine Chemistry Re FIGURE 1–28 Borazine-mediated gem-difluroalkylation of carbonyl compounds and aryl halides. 1.6.6 N-Trifluoromethylation of amines Schoenebeck and coworkers have demonstrated that secondary amines, but not the primary amines, react with (Me4N)SCF3 to give the corresponding thiocarbamoyl fluorides (44).94 This reaction is nearly quantitative in 10 min, and at the end of the reaction, the initially clear, colorless reaction mixture turns into a cloudy solution. Desulfurative fluorination of the latter thiocarbamoyl fluorides (44) with AgF results in the formation of the N-trifluoromethyl derivatives (45). These two reactions were combined in a one-pot two-step method, in effect, to transform secondary amines to the corresponding N-trifluoromethyl amines. The reaction sequence tolerates a variety of functional groups, including nitro, nitrile, amide, sulfonyl, methoxy, and heterocyclic aromatics, and thus serves as an efficient synthetic method for N-trifluoromethyl compounds. Secondary amines consisting of electron-withdrawing as well as electron-donating groups react efficiently under the reaction conditions to give the corresponding N-CF3 compounds. Protected versions of amino acids, such as proline and glycine, could be N-trifluoromethylated in high yields. Synthesis of N-trifluoromethyl analogs of the widely used pharmaceuticals, such as tetracaine (51; an anesthetic), antifungal agent terbinafine (52), drugs for treating neurological disorders, such as amitriptyline (53) and naftifine (54), were synthesized in high yields. This late-stage N-trifluoromethylation of pharmaceutically interesting Chapter 1 • Nucleophilic reactions in the synthesis of organofluorine compounds S R N (Me (Me 4N)SCF 3 H R DCM or MeCN, RT, 10 min R′ N AgF, < 4 h F 44 R Me 4N F S F F 45 88%–99% overall yield for two steps Mechanistic outline: Me 4N N R′ R′ R/R′ = alkyl, aryl F F R 27 F N H R′ F F F S 46 HF Selected examples: O N CF 3 O N Ot Bu CF3 49 48 47 92% N CF3 91% O 2N 50 N CF3 95% 93% CF3 N O O Me OEt N N CF 3 97% 51 ; Tetracaine (anesthetic) analog 95% 52 ; Terbinafine (Lamisil; antifungal) analog CF3 N N CF 3 Me 98% 53; Amitriptyline (Elavil; for mental illness) analog 88% 54 ; Naftifine (Naftin; for mental illness) analog FIGURE 1–29 N-Trifluoromethylation of secondary amines and synthesis of N-CF3 analogs of pharmaceuticals. compounds may be useful in the synthesis of 18F-labeled compounds for PET studies, although the pharmacological effectiveness of the latter N-trifluoromethylated compounds are yet to be evaluated (Fig. 129). 28 Organofluorine Chemistry In these reactions, the trifluoromethylthiolate anion (CF3S2), in equilibrium, forms the thiocarbonyl fluoride (46) by the fluoride anion elimination. Nucleophilic addition of the secondary amines to 46 then gives the corresponding thiocarbamoyl fluorides (44), the substrates for the desulfurative difluorination (using AgF). The in situgenerated thiocarbonyl fluoride (46), formed through the reaction of the CF3SiMe3 with elemental sulfur and KF, also gives high yields of the thiocarbamoyl fluorides (44) of the secondary amines.95 On the other hand, primary amines, under these reaction conditions, give the corresponding isothiocyanates (RNCS). 1.6.7 Tetrakis(dimethylamino)ethylene-mediated fluoroalkylations Pawelke first reported the reductive trifluoromethylation of various silicon and boron halides using tetrakis(dimethylamino)ethylene (TDAE) as the reducing agent.96 Trifluoromethyl iodide (and other perfluoroalkyl halides) forms a strong charge-transfer complex with TDAE and thus serves as a source of the in situformed nucleophilic trifluoromethyl anion. This reaction was later expanded for the synthesis and synthetic applications of various perfluoroalky-trialkylsilanes by Petrov and more extensively by Dolbier and coworkers (Fig. 130).60,97104 The TDAEmediated nucleophilic fluoroalkylation method provides a convenient alternative strategy for the trifluoromethylation and perfluoroalkylation of carbonyl compounds to that using the FIGURE 1–30 TDAE-mediated trifluoromethylation and perfluoroalkylation of aldehydes, ketones, and imines. TDAE, Tetrakis(dimethylamino)ethylene. Chapter 1 • Nucleophilic reactions in the synthesis of organofluorine compounds 29 RuppertPrakash reagent (TMSCF3),16,48,59,66 as the fluoroalkylation reactions can be carried out in a single step without the necessity of isolating the TMSCF3 reagent. As in the case of the TMSCF3, the TDAE-mediated trifluoromethylation of a broad range of aliphatic and aromatic aldehydes, ketones, and N-tosylimines gives the corresponding α-trifluoromethyl alcohols and amines (Fig. 130).60,101,104 1.6.7.1 Trifluoromethylation of acyl chlorides The TDAE-mediated trifluoromethylation of acid chlorides, using excess CF3I, gives the corresponding trifluoromethyl ketones as the initially formed products via nucleophilic substitution reaction. The latter aryl trifluoromethyl ketones (56) rapidly react with the trifluoromethyl anion, generated, in situ, to give the tertiary alkoxides, which undergo esterification with unreacted aroyl chloride (55) to give the tertiary 1,1-bis(trifluoromethyl) benzoate (57) in nearly quantitative yields. Hydrolysis of 57 under mild conditions gives the corresponding 1-aryl-1,1-bis(trifluoromethyl)methanol (58).102 This synthetic strategy, using TDAE as the reductant, provides a better alternative for the preparation of the 1,1-bis(trifluoromethyl)alcohols (58) as compared to the related reactions using CF3TMS, in which case mixtures of the trifluoromethyl ketones and the tertiary alcohols are usually formed (Fig. 131). FIGURE 1–31 TDAE-mediated trifluoromethylation of benzoyl chloride to give the tertiary 1-aryl-1,1-bis (trifluoromethyl)methanols. TDAE, Tetrakis(dimethylamino)ethylene. 30 Organofluorine Chemistry Br N O F F TDAE (1 mol equiv) N RSCN, anhyd. DMF –20 °C to RT, 1–5 h O F SR N N F N N TDAE TDAE N F O F R -[TDAE]2+ Br– (CN – ) S CN [TDAE]2+Br – Ph Examples: SPh N O F F O F S S R F F 43% CF3I (4.2–5.0 mol equiv) TDAE ( 2.2 mol equiv) S N O F 62% 60% R NMe2 S N N RSCF3 R = phenyl, butyl, ethyl, 4-pyridyl ~200% yield based on the disulfide reactant FIGURE 1–32 TDAE-mediated synthesis of gem-difluoro thioethers, as potential anti-HIV-1 agents. TDAE, Tetrakis (dimethylamino)ethylene. 1.6.7.2 Synthesis of gem-(difluoromethyl)thioethers TDAE-mediated sulfanylation of 2-(bromodifluoromethyl)benzoxazole using heteroarylthiocyanates as electrophilic reagents affords the corresponding heteroaryl-CF2SAr compounds, some of which were found to be anti-HIV-1 agents (Fig. 132). This sulfanylation reaction can also be rationalized as proceeding through radical nucleophilic substitution (SRN1).97,105 Reductive trifluoromethylation of dialkyl or diaryl disulfides using excess trifluoromethyl iodide in the presence of TDAE affords the corresponding trifluoromethyl thioethers.101,102 Trifluoromethylation diaryldisulfides, using 18F-labeled CHF218F, in the presence of potassium tert-butoxide in DMF solvent, gives the corresponding 18F-labeled trifluoromethylthiolated aromatics. The 18F-labeled CHF218F is generated, in situ, from the reaction of the [18F] fluoride anion with (difluoromethyl)(mesityl)(phenyl)sulfonium salt. Using this synthetic approach, 18F-labeled trifluoromethylthiolation of aromatics was achieved in high radiochemical yields (Fig. 133; please see Chapter 6: Synthesis and applications of 18F-labeled compounds).106 Chapter 1 • Nucleophilic reactions in the synthesis of organofluorine compounds CHF2 S [ 18F]F ArSSAr CHF218 F 31 ArSCF 218 F KOtBu/DMF Selected examples: SCF218 F 73% RCY NC MeO F 68% RCY FIGURE 1–33 Synthesis of diaryldisulfides. SCF218 F SCF218 F SCF218 F 74% RCY 67% RCY SCF218 F N 45% RCY 18 F-labeled (trifluoromethyl)thio arenes through nucleophilic trifluoromethylation of 1.7 Nucleophilic trifluoromethylthiolation Nucleophilic ring-opening of the cyclic sulfamidates (59), using tetramethylammonium trifluoromethanethiolate (Me4NSCF3), gives the corresponding trifluoromethylthio (SCF3) derivatives (60). Using this synthetic method, S-trifluoromethylated cysteine derivative 62 could be synthesized under mild conditions and further transformed into the corresponding S-trifluoromethyl dipeptides and tripeptides, Gly-Cys (64) and Ser-Phe-Cys (63) (Fig. 134).107 Nucleophilic substitution reactions of thiocyanates and sulfenyl chlorides can be achieved in moderate yields using CF3SiMe3 (Fig. 135). Thus reaction of alkyl thiocyanates, generated, in situ, through the reaction of the alkyl halides with sodium thiocyanate (NaSCN), react with CF3SiMe3 to give the corresponding trifluoromethylthio ethers (an SN2-type of reaction, with cyanide anion as the leaving group).108 Aryl thiocyanates, formed, in situ, through the reaction of aryl diazonium salts with CuSCN, give the corresponding trifluoromethylthioarenes in moderate yields.109 Aryl(alkyl)sulfenyl chlorides, upon reaction with CF3TMS, in the presence of TASF, give the corresponding trifluoromethylthioarenes.110 However, the high toxicity of the latter sulfenyl chlorides limits the applications of these reagents for the trifluoromethylthiolations. 1.8 Trifluoromethoxylations Trifluoromethoxy substituent, because of its metabolic stability and enhanced lipophilicity, as compared to its methoxy analog, has found applications in the design of pharmaceuticals and agrochemicals. Hansch lipophilicity values (πx) for OCF3, CF3, CH3, OCH3 substituents are 1.04, 0.88, 0.52, 20.02, respectively, showing substantial lipophilicity enhancements afforded by the OCF3 moiety as compared with trifluoromethyl and related nonfluorinated 32 Organofluorine Chemistry FIGURE 1–34 Nucleophilic trifluoromethylthiolation of cyclic sulfamidates to give the S-trifluoromethyl derivatives of cysteine and cysteine-containing peptides. Boc, tert-Butoxycarbonyl; DCM, dichloromethane. RSCN/ TBAF RSCF3 R = alkyl RSCl/ TASF RSCF3 R = alkyl/aryl CF3 TMS N N SCF3 R′ R′ CuSCN, Cs2 CO3 , MeCN R′ = for example OMe, CN, Br, I FIGURE 1–35 Nucleophilic trifluoromethylthiolations of alkylthiocyanates, diazonium salts, and sulfenyl chlorides. TASF, Tris (dimethylamino)sulfonium difluorotrimethylsilicate; TBAF, tetrabutylammonium fluoride. Chapter 1 • Nucleophilic reactions in the synthesis of organofluorine compounds 33 FIGURE 1–36 Selected pharmaceuticals and agrochemicals containing OCF3 moiety. substituents. The higher lipophilicity of a compound reflects in its enhanced cell permeability and bioavailability. Trifluoromethoxyaryl-containing pharmaceuticals include rifluzole, a drug used to treat amyotrophic lateral sclerosis, and sonidegib, an antineoplastic pharmaceutical. Many agrochemicals, including the insecticides indoxacarb and flurprimidol, flucarbazone, a herbicide, and triflumuron, a plant growth regulator, are trifluoromethoxyarenes (Fig. 136). 1.8.1 Trifluoromethyl benzenesulfonatemediated vicinal (bromo) trifluoromethoxylation Trifluoromethyl benzenesulfonate (TFMS), in the presence of AgF, reversibly forms Ag(OCF3) and thereby brings about vicinal (bromo)trifluoromethoxylation of alkenes in the presence of an electrophilic “Br1” source such as 1,3-dibromo-5,5-dimethylhydantoin (DBDMH). Reaction of TFMS with alkenes, in the presence of AgF and DBDMH, gives the corresponding vicinal (bromo)trifluoromethoxylation products in high yields (Fig. 137).111 In the presence of a chiral catalyst, such as (DHQD)2PHAL (dihydroquinidinephthalazine adduct), this reaction affords the corresponding 1-bromo-2-trifluoromethoxy compounds in moderate to high yields and enantioselectivity. 34 Organofluorine Chemistry FIGURE 1–37 Vicinal bromo-trifluoromethoxylation of alkenes using trifluoromethyl benzenesulfonate (TFMS). DBDMH, 1,3-dibromo-5,5-dimethylhydantoin. 1.8.2 Trifluoromethyl benzoatemediated trifluoromethoxylation Hu and coworkers have developed trifluoromethyl benzoate (65) as a convenient, shelfstable, nucleophilic trifluoromethoxylation reagent. This reagent, as in the case of the trifluoromethyl benzenesulfonate (TFMS), forms AgOCF3, in reversible equilibrium, in the presence of AgF, and can be used in the enantioselective α-bromo-trifluoromethoxylation of styrene derivatives 66 and nucleophilic trifluoromethoxylation of alkyl halides (e.g., 68 and 70).112 Trifluoromethyl benzoate (65) reacts with the aryne intermediates, in the presence of an electrophilic “Br1” source (e.g., phenylethynyl bromide 73), to give the ortho-bromo(trifluoromethoxy)arenes (74).112 The latter aryne intermediates were generated, in situ, through Chapter 1 • Nucleophilic reactions in the synthesis of organofluorine compounds 35 cis FIGURE 1–38 Trifluoromethoxylation of aromatics, alkenes, and alkyl halides, using trifluoromethyl benzoate as the source of the nucleophilic trifluoromethoxide anion. the reaction of ortho-(trimethylsilyl)aryl triflates (72) with KF, complexed to a crown ether such as cis-dicyclohexano-18-crown-6. Use of the crown ethers facilitates the stabilization of the in situformed KOCF3 toward its decomposition and enhances its nucleophilicity (Fig. 138).112 References 1. Sessler, C. D.; Rahm, M.; Becker, S.; Goldberg, J. M.; Wang, F.; Lippard, S. J. CF2H, a Hydrogen Bond Donor. J. Am. Chem. Soc. 2017, 139, 93259332. 2. Zafrani, Y.; Sod-Moriah, G.; Yeffet, D.; Berliner, A.; Amir, D.; Marciano, D.; Elias, S.; Katalan, S.; Ashkenazi, N.; Madmon, M.; Gershonov, E.; Saphier, S. CF2H, a Functional Group-Dependent Hydrogen-Bond 36 Organofluorine Chemistry Donor: Is it a More or Less Lipophilic Bioisostere of OH, SH, and CH3? J. Med. Chem. 2019, 62, 56285637. 3. Mukherjee, P.; Pettersson, M.; Dutra, J. K.; Xie, L.; am Ende, C. W. Trifluoromethyl Oxetanes: Synthesis and Evaluation as a tert-Butyl Isostere. ChemMedChem 2017, 12, 15741577. 4. Messina, P. A.; Mange, K. C.; Middleton, W. J. Aminosulfur Trifluorides: Relative Thermal Stability. J. Fluorine Chem. 1989, 42, 137143. 5. Lal, G. S.; Pez, G. P.; Pesaresi, R. J.; Prozonic, F. M.; Cheng, H. Bis(2-Methoxyethyl)Aminosulfur Trifluoride: A New Broad-Spectrum Deoxofluorinating Agent with Enhanced Thermal Stability. J. Org. Chem. 1999, 64, 70487054. 6. Singh, R. P.; Shreeve, J. M. Nucleophilic Fluorination of Amino Alcohols and Diols Using Deoxofluor. J. Fluorine Chem. 2002, 116, 2326. 7. Singh, R. P.; Meshri, D. T.; Shreeve, J. M. DAST and Deoxofluor Mediated Nucleophilic Fluorination Reactions of Organic Compounds. Adv. Org. Synth. 2006, 2, 291326. 8. L’Heureux, A.; Beaulieu, F.; Bennett, C.; Bill, D. R.; Clayton, S.; La Flamme, F.; Mirmehrabi, M.; Tadayon, S.; Tovell, D.; Couturier, M. Aminodifluorosulfinium Salts: Selective Fluorination Reagents with Enhanced Thermal Stability and Ease of Handling. J. Org. Chem. 2010, 75, 34013411. 9. Umemoto, T.; Singh, R. P.; Xu, Y.; Saito, N. Discovery of 4-tert-Butyl-2,6-Dimethylphenylsulfur Trifluoride as a Deoxofluorinating Agent with High Thermal Stability as Well as Unusual Resistance to Aqueous Hydrolysis, and Its Diverse Fluorination Capabilities Including Deoxofluoro-Arylsulfinylation with High Stereoselectivity. J. Am. Chem. Soc. 2010, 132, 1819918205. 10. Neumann, C. N.; Ritter, T. Facile C-F Bond Formation Through a Concerted Nucleophilic Aromatic Substitution Mediated by the PhenoFluor Reagent. Acc. Chem. Res. 2017, 50, 28222833. 11. Nielsen, M. K.; Ugaz, C. R.; Li, W.; Doyle, A. G. PyFluor: A Low-Cost, Stable, and Selective Deoxyfluorination Reagent. J. Am. Chem. Soc. 2015, 137, 95719574. 12. Li, L.; Ni, C.; Wang, F.; Hu, J. Deoxyfluorination of Alcohols with 3,3-Difluoro-1,2-Diarylcyclopropenes. Nat. Commun. 2016, 7 13320 pp. 13. Schumacher, D. P.; Clark, J. E.; Murphy, B. L.; Fischer, P. A. An Efficient Synthesis of Florfenicol. J. Org. Chem. 1990, 55, 52915294. 14. Remete, A. M.; Nonn, M.; Fustero, S.; Fulop, F.; Kiss, L. Synthesis of Fluorinated Amino Acid Derivatives Through Late-Stage Deoxy-Fluorinations. Tetrahedron 2018, 74, 63676418. 15. Doebelin, C.; He, Y.; Kamenecka, T. M. Multigram-Scale Synthesis of Enantiopure 3,3-Difluoroproline. Tetrahedron Lett. 2016, 57, 56585660. 16. Prakash, G. K. S.; Mandal, M. Nucleophilic Trifluoromethylation Tamed. J. Fluorine Chem. 2001, 112, 123131. 17. Shibata, N.; Mizuta, S.; Kawai, H. Recent Advances in Enantioselective Trifluoromethylation Reactions. Tetrahedron: Asymmetry 2008, 19, 26332644. 18. Duan, J. J. W.; Lu, Z.; Jiang, B.; Stachura, S.; Weigelt, C. A.; Sack, J. S.; Khan, J.; Ruzanov, M.; Galella, M. A.; Wu, D.-R.; Yarde, M.; Shen, D.-R.; Shuster, D. J.; Borowski, V.; Xie, J. H.; Zhang, L.; Vanteru, S.; Gupta, A. K.; Mathur, A.; Zhao, Q.; Foster, W.; Salter-Cid, L. M.; Carter, P. H.; Dhar, T. G. M. StructureBased Discovery of Phenyl (3-Phenylpyrrolidin-3-yl)Sulfones as Selective, Orally Active RORγt Inverse Agonists. ACS Med. Chem. Lett. 2019, 10, 367373. 19. Tang, P.; Wang, W.; Ritter, T. Deoxyfluorination of Phenols. J. Am. Chem. Soc. 2011, 133, 1148211484. 20. Fujimoto, T.; Becker, F.; Ritter, T. PhenoFluor: Practical Synthesis, New Formulation, and Deoxyfluorination of Heteroaromatics. Org. Process Res. Dev. 2014, 18, 10411044. Chapter 1 • Nucleophilic reactions in the synthesis of organofluorine compounds 37 21. Sladojevich, F.; Arlow, S. I.; Tang, P.; Ritter, T. Late-Stage Deoxyfluorination of Alcohols with PhenoFluor. J. Am. Chem. Soc. 2013, 135, 24702473. 22. Guo, J.; Kuang, C.; Rong, J.; Li, L.; Ni, C.; Hu, J. Rapid Deoxyfluorination of Alcohols with N-Tosyl-4Chlorobenzenesulfonimidoyl Fluoride (SulfoxFluor) at Room Temperature. Chem. Eur. J. 2019, 25, 72597264. 23. Schimler, S. D.; Cismesia, M. A.; Hanley, P. S.; Froese, R. D. J.; Jansma, M. J.; Bland, D. C.; Sanford, M. S. Nucleophilic Deoxyfluorination of Phenols via Aryl Fluorosulfonate Intermediates. J. Am. Chem. Soc. 2017, 139, 14521455. 24. Markovskii, L. N.; Pashinnik, V. E. Applications of Dialkylaminosulfur Trifluorides for the Syntheses of Acid Fluorides. Synthesis 1975, 12, 801802. 25. Mohammadkhani, L.; Heravi, M. M. XtalFluor-E: A Useful and Versatile Reagent in Organic Transformations. J. Fluorine Chem. 2019, 225, 1120. 26. Beaulieu, F.; Beauregard, L.-P.; Courchesne, G.; Couturier, M.; LaFlamme, F.; L’Heureux, A. Aminodifluorosulfinium Tetrafluoroborate Salts as Stable and Crystalline Deoxofluorinating Reagents. Org. Lett. 2009, 11, 50505053. 27. Munoz, S. B.; Dang, H.; Ispizua-Rodriguez, X.; Mathew, T.; Prakash, G. K. S. Direct Access to Acyl Fluorides From Carboxylic Acids Using a Phosphine/Fluoride Deoxyfluorination Reagent System. Org. Lett. 2019, 21, 16591663. 28. Zhang, Y.; Rovis, T. A Unique Catalyst Effects the Rapid Room-Temperature Cross-Coupling of Organozinc Reagents with Carboxylic Acid Fluorides, Chlorides, Anhydrides, and Thioesters. J. Am. Chem. Soc. 2004, 126, 1596415965. 29. Ogiwara, Y.; Sakino, D.; Sakurai, Y.; Sakai, N. Acid Fluorides as Acyl Electrophiles in Suzuki-Miyaura Coupling. Eur. J. Org. Chem. 2017, 2017, 43244327. 30. Ogiwara, Y.; Maegawa, Y.; Sakino, D.; Sakai, N. Palladium-Catalyzed Coupling of Benzoyl Halides with Aryltrifluorosilanes Leading to Diaryl Ketones. Chem. Lett. 2016, 45, 790792. 31. Ogiwara, Y.; Sakurai, Y.; Hattori, H.; Sakai, N. Palladium-Catalyzed Reductive Conversion of Acyl Fluorides via Ligand-Controlled Decarbonylation. Org. Lett. 2018, 20, 42044208. 32. Keaveney, S. T.; Schoenebeck, F. Palladium-Catalyzed Decarbonylative Trifluoromethylation of Acid Fluorides. Angew. Chem., Int. Ed. 2018, 57, 40734077. 33. Braden, R.; Himmler, T. Catalytic Reduction of Aromatic Carboxylic Acid Fluorides to Aldehydes. J. Organomet. Chem. 1989, 367, C12C14. 34. Fier, P. S.; Hartwig, J. F. Selective C-H Fluorination of Pyridines and Diazines Inspired by a Classic Amination Reaction. Science (Washington, DC) 2013, 342, 956960. 35. Jansen, K.; Heirbaut, L.; Verkerk, R.; Cheng, J. D.; Joossens, J.; Cos, P.; Maes, L.; Lambeir, A.-M.; De Meester, I.; Augustyns, K.; Van der Veken, P. Extended Structure-Activity Relationship and Pharmacokinetic Investigation of (4-Quinolinoyl)Glycyl-2-Cyanopyrrolidine Inhibitors of Fibroblast Activation Protein (FAP). J. Med. Chem. 2014, 57, 30533074. 36. Melvin, P. R.; Ferguson, D. M.; Schimler, S. D.; Bland, D. C.; Sanford, M. S. Room Temperature Deoxyfluorination of Benzaldehydes and α-Ketoesters with Sulfuryl Fluoride and Tetramethylammonium Fluoride. Org. Lett. 2019, 21, 13501353. 37. Reddy, V. P.; Perambuduru, M.; Alleti, R. Synthetic Approaches to gem-Difluoromethylene Compounds. Adv. Org. Synth. 2006, 2, 327351. 38. Sondej, S. C.; Katzenellenbogen, J. A. gem-Difluoro Compounds: A Convenient Preparation From Ketones and Aldehydes by Halogen Fluoride Treatment of 1,3-Dithiolanes. J. Org. Chem. 1986, 51, 35083513. 38 Organofluorine Chemistry 39. Prakash, G. K. S.; Hoole, D.; Reddy, V. P.; Olah, G. A. Synthetic Methods and Reactions. 187. Simplified Preparation of α,α-Difluorodiphenylmethanes From Benzophenone 1,3-Dithiolanes with Sulfuryl Chloride and Pyridinium Polyhydrogen Fluoride. Synlett 1993, (9), 691693. 40. York, C.; Prakash, G. K. S.; Olah, G. A. Synthetic Methods and Reactions. 196. Desulfuration Fluorination Using Nitrosonium Tetrafluoroborate and Pyridinium Poly(Hydrogen Fluoride). Tetrahedron 1996, 52, 914. 41. Reddy, V. P.; Alleti, R.; Perambuduru, M. K.; Welz-Biermann, U.; Buchholz, H.; Prakash, G. K. S. gemDifluorination of 2,2-Diaryl-1,3-Dithiolanes by Selectfluor and Pyridinium Polyhydrogen Fluoride. Chem. Commun. (Cambridge, UK) 2005, (5), 654656. 42. Motherwell, W. B.; Wilkinson, J. A. Observations on the Reaction of Dithioketals with para-Iodotoluene Difluoride: A Novel Route to gem-Difluoro Compounds. Synlett 1991, (3), 191192. 43. Prakash, G. K. S.; Reddy, V. P.; Li, X. Y.; Olah, G. A. Synthetic Methods and Reactions. 156. Convenient Transformation of Carbonyl Compounds via their Hydrazones into gem-Difluoro Compounds with NBromosuccinimide/Pyridinium Poly(Hydrogen Fluoride) or Poly-4-Vinylpyridinium Poly(Hydrogen Fluoride). Synlett 1990, (10), 594596. 44. York, C.; Prakash, G. K. S.; Wang, Q.; Olah, G. A. Synthetic Methods and Reactions. 189. Novel Preparation of gem-Difluorides From Ketoximes with Nitrosonium Tetrafluoroborate and Pyridinium Polyhydrogen Fluoride. Synlett 1994, (6), 425426. 45. Zhao, Y.; Huang, W.; Zheng, J.; Hu, J. Efficient and Direct Nucleophilic Difluoromethylation of Carbonyl Compounds and Imines with Me3SiCF2H at Ambient or Low Temperature. Org. Lett. 2011, 13, 53425345. 46. Chen, D.; Ni, C.; Zhao, Y.; Cai, X.; Li, X.; Xiao, P.; Hu, J. Bis(Difluoromethyl)Trimethylsilicate Anion: A Key Intermediate in Nucleophilic Difluoromethylation of Enolizable Ketones with Me3SiCF2H. Angew. Chem., Int. Ed. 2016, 55, 1263212636. 47. Roman, R.; Navarro, A.; Wodka, D.; Alvim-Gaston, M.; Husain, S.; Franklin, N.; Simon-Fuentes, A.; Fustero, S. Synthesis of Fluorinated and Nonfluorinated Tebufenpyrad Analogues for the Study of AntiAngiogenesis MOA. Org. Process Res. Dev. 2014, 18, 10271036. 48. Prakash, G. K. S.; Krishnamurti, R.; Olah, G. A. Synthetic Methods and Reactions. 141. Fluoride-Induced Trifluoromethylation of Carbonyl Compounds with Trifluoromethyltrimethylsilane (TMS-CF3). A Trifluoromethide Equivalent. J. Am. Chem. Soc. 1989, 111, 393395. 49. Prakash, G. K. S.; Hu, J. Selective Fluoroalkylations with Fluorinated Sulfones, Sulfoxides, and Sulfides. Acc. Chem. Res. 2007, 40, 921930. 50. Ma, J.-A.; Cahard, D. Update 1 of: Asymmetric Fluorination, Trifluoromethylation, and Perfluoroalkylation Reactions. Chem. Rev. (Washington, DC) 2008, 108, PR1PR43 Web Only Content. 51. Okusu, S.; Kawai, H.; Yasuda, Y.; Sugita, Y.; Kitayama, T.; Tokunaga, E.; Shibata, N. Asymmetric Synthesis of Efavirenz via Organocatalyzed Enantioselective Trifluoromethylation. Asian J. Org. Chem. 2014, 3, 449452. 52. Kawai, H.; Tachi, K.; Tokunaga, E.; Shiro, M.; Shibata, N. Cinchona Alkaloid-Catalyzed Asymmetric Trifluoromethylation of Alkynyl Ketones with Trimethylsilyl Trifluoromethane. Org. Lett. 2010, 12, 51045107. 53. Caron, S.; Do, N. M.; Sieser, J. E.; Arpin, P.; Vazquez, E. Process Research and Development of an NK-1 Receptor Antagonist. Enantioselective Trifluoromethyl Addition to a Ketone in the Preparation of a Chiral Isochroman. Org. Process Res. Dev. 2007, 11, 10151024. 54. Okusu, S.; Hirano, K.; Yasuda, Y.; Tanaka, J.; Tokunaga, E.; Fukaya, H.; Shibata, N. Alkynyl Cinchona Catalysts affect Enantioselective Trifluoromethylation for Efavirenz Under Metal-Free Conditions. Org. Lett. 2016, 18, 55685571. Chapter 1 • Nucleophilic reactions in the synthesis of organofluorine compounds 39 55. Creary, X. Reaction of Organometallic Reagents with Ethyl Trifluoroacetate and Diethyl Oxalate. Formation of Trifluoromethyl Ketones and α-Keto Esters via Stable Tetrahedral Adducts. J. Org. Chem. 1987, 52, 50265030. 56. Funabiki, K.; Hayakawa, A.; Inuzuka, T. Convenient, Functional Group-Tolerant, Transition Metal-Free Synthesis of Aryl and Heteroaryl Trifluoromethyl Ketones with the Use of Methyl Trifluoroacetate. Org. Biomol. Chem. 2018, 16, 913918. 57. Cui, B.; Sun, H.; Xu, Y.; Duan, L.; Li, Y.-M. MgCl2-Catalyzed Trifluoromethylation of Carbonyl Compounds Using (Trifluoromethyl)Trimethylsilane as the Trifluoromethylating Agent. Tetrahedron 2017, 73, 67546762. 58. Rudzinski, D. M.; Kelly, C. B.; Leadbeater, N. E. A Weinreb Amide Approach to the Synthesis of Trifluoromethylketones. Chem. Commun. (Cambridge, UK) 2012, 48, 96109612. 59. Prakash, G. K. S.; Mandal, M.; Olah, G. A. Nucleophilic Trifluoromethylation of N-Tosyl Aldimines. Synlett 2001, (1), 7778. 60. Xu, W.; Dolbier, W. R., Jr. Nucleophilic Trifluoromethylation of Imines Using the CF3I/TDAE Reagent. J. Org. Chem. 2005, 70, 47414745. 61. Reddy, V. P.; Perambuduru, M.; Mehta, J. N-Heterocyclic Carbene-Catalyzed Trifluoromethylation of Aromatic N-Tosylaldimines. Top. Catal. 2018, 61, 699703. 62. Punna, N.; Saito, T.; Kosobokov, M.; Tokunaga, E.; Sumii, Y.; Shibata, N. Stereodivergent Trifluoromethylation of N-Sulfinylimines by Fluoroform with Either Organic-Superbase or Organometallic-Base. Chem. Commun. (Cambridge, UK) 2018, 54, 42944297. 63. Prakash, G. K. S.; Mandal, M.; Olah, G. A. Stereoselective Nucleophilic Trifluoromethylation of N-(tertButylsulfinyl)Imines by Using Trimethyl(Trifluoromethyl)Silane. Angew. Chem., Int. Ed. 2001, 40, 589590. 64. Prakash, G. K. S.; Mandal, M. Stereoselective Synthesis of Trifluoromethylated Vicinal Ethylenediamines with α-Amino N-tert-Butanesulfinimines and TMSCF3. J. Am. Chem. Soc. 2002, 124, 65386539. 65. Prakash, G. K. S.; Mandal, M.; Olah, G. A. Asymmetric Synthesis of Trifluoromethylated Allylic Amines Using α,β-Unsaturated N-tert-Butanesulfinimines. Org. Lett. 2001, 3, 28472850. 66. Prakash, G. K. S.; Wang, Y.; Mogi, R.; Hu, J.; Mathew, T.; Olah, G. A. Nucleophilic Perfluoroalkylation of Imines and Carbonyls: Perfluoroalkyl Sulfones as Efficient Perfluoroalkyl-Transfer Motifs. Org. Lett. 2010, 12, 29322935. 67. Kosobokov, M. D.; Dilman, A. D.; Struchkova, M. I.; Belyakov, P. A.; Hu, J. Reactions of sulfur- and Phosphorus-Substituted Fluoroalkylating Silicon Reagents with Imines and Enamines Under Acidic Conditions. J. Org. Chem. 2012, 77, 20802086. 68. Levin, V. V.; Dilman, A. D.; Belyakov, P. A.; Struchkova, M. I.; Tartakovsky, V. A. Nucleophilic Trifluoromethylation of Imines Under Acidic Conditions. Eur. J. Org. Chem. 2008, 2008, 52265230. 69. Riofski, M. V.; Hart, A. D.; Colby, D. A. Amidinate Salt of Hexafluoroacetone Hydrate for the Preparation of Fluorinated Compounds by the Release of Trifluoroacetate. Org. Lett. 2013, 15, 208211. 70. Prakash, G. K. S.; Zhang, Z.; Wang, F.; Munoz, S.; Olah, G. A. Nucleophilic Trifluoromethylation of Carbonyl Compounds: Trifluoroacetaldehyde Hydrate as a Trifluoromethyl Source. J. Org. Chem. 2013, 78, 33003305. 71. Han, C.; Kim, E. H.; Colby, D. A. Cleavage of Carbon-Carbon Bonds Through the Mild Release of Trifluoroacetate: Generation of α,α-Difluoroenolates for Aldol Reactions. J. Am. Chem. Soc. 2011, 133, 58025805. 72. Prakash, G. K. S.; Jog, P. V.; Batamack, P. T. D.; Olah, G. A. Taming of Fluoroform: Direct Nucleophilic Trifluoromethylation of Si, B, S, and C Centers. Science (Washington, DC) 2012, 338, 13241327. 73. Zhang, Y.; Fujiu, M.; Serizawa, H.; Mikami, K. Organocatalysis Approach to Trifluoromethylation with Fluoroform. J. Fluorine Chem. 2013, 156, 367371. 40 Organofluorine Chemistry 74. Saito, T.; Wang, J.; Tokunaga, E.; Tsuzuki, S.; Shibata, N. Direct Nucleophilic Trifluoromethylation of Carbonyl Compounds by Potent Greenhouse Gas, Fluoroform: Improving the Reactivity of Anionoid Trifluoromethyl Species in Glymes. Sci. Rep. 2018, 8, 18. 75. He, L.; Tsui, G. C. Fluoroform-Derived CuCF3 for Trifluoromethylation of Terminal and TMS-Protected Alkynes. Org. Lett. 2016, 18, 28002803. 76. Folleas, B.; Marek, I.; Normant, J.-F.; Saint-Jalmes, L. Fluoroform: An Efficient Precursor for the Trifluoromethylation of Aldehydes. Tetrahedron 1999, 56, 275283. 77. Folleas, B.; Marek, I.; Normant, J.-F.; Saint, J. L. Fluoroform: An Efficient Precursor for the Trifluoromethylation of Aldehydes. Tetrahedron Lett. 1998, 39, 29732976. 78. Zanardi, A.; Novikov, M. A.; Martin, E.; Benet-Buchholz, J.; Grushin, V. V. Direct Cupration of Fluoroform. J. Am. Chem. Soc. 2011, 133, 2090120913. 79. Novak, P.; Lishchynskyi, A.; Grushin, V. V. Fluoroform-Derived CuCF3 for Low-Cost, Simple, Efficient, and Safe Trifluoromethylation of Aryl Boronic Acids in Air. Angew. Chem. Int. Ed. Engl. 2012, 51, 77677770. 80. Mazloomi, Z.; Bansode, A.; Benavente, P.; Lishchynskyi, A.; Urakawa, A.; Grushin, V. V. Continuous Process for Production of CuCF3 via Direct Cupration of Fluoroform. Org. Process Res. Dev. 2014, 18, 10201026. 81. Large, S.; Roques, N.; Langlois, B. R. Nucleophilic Trifluoromethylation of Carbonyl Compounds and Disulfides with Trifluoromethane and Silicon-Containing Bases. J. Org. Chem. 2000, 65, 88488856. 82. Konovalov, A. I.; Benet-Buchholz, J.; Martin, E.; Grushin, V. V. The Critical Effect of the Countercation in the Direct Cupration of Fluoroform with [Cu(OR)2]. Angew. Chem., Int. Ed. 2013, 52, 1163711641. 83. Prakash, G. K. S.; Wang, F.; Zhang, Z.; Haiges, R.; Rahm, M.; Christe, K. O.; Mathew, T.; Olah, G. A. LongLived Trifluoromethanide Anion: A Key Intermediate in Nucleophilic Trifluoromethylations. Angew. Chem., Int. Ed. 2014, 53, 1157511578. 84. Russell, J.; Roques, N. Effective Nucleophilic Trifluoromethylation with Fluoroform and Common Base. Tetrahedron 1998, 54, 1377113782. 85. Shono, T.; Ishifune, M.; Okada, T.; Kashimura, S. Electroorganic Chemistry. 130. A Novel Trifluoromethylation of Aldehydes and Ketones Promoted by an Electrogenerated Base. J. Org. Chem. 1991, 56, 24. 86. Miloserdov, F. M.; Konovalov, A. I.; Martin, E.; Benet-Buchholz, J.; Escudero-Adan, E. C.; Lishchynskyi, A.; Grushin, V. V. The Trifluoromethyl Anion: Evidence for [K(crypt-222)]1 CF2 3 . Helv. Chim. Acta 2017, 100, e1700032. 87. Lishchynskyi, A.; Miloserdov, F. M.; Martin, E.; Benet-Buchholz, J.; Escudero-Adan, E. C.; Konovalov, A. I.; Grushin, V. V. The Trifluoromethyl Anion. Angew. Chem., Int. Ed. 2015, 54, 1528915293. 88. Harlow, R. L.; Benet-Buchholz, J.; Miloserdov, F. M.; Konovalov, A. I.; Marshall, W. J.; Escudero-Adan, E. C.; Martin, E.; Lishchynskyi, A.; Grushin, V. V. On the Structure of [K(crypt-222)]1 CF2 3 . Helv. Chim. Acta 2018, 101, e1800015. 89. Blond, G.; Billard, T.; Langlois, B. R. New Stable Reagents for the Nucleophilic Trifluoromethylation. Part 4. Trifluoromethylation of Disulfides and Diselenides with Hemiaminals of Trifluoroacetaldehyde. Tetrahedron Lett. 2001, 42, 24732475. 90. Landelle, G.; Panossian, A.; Leroux, F. R. Trifluoromethyl Ethers and Thioethers as Tools for Medicinal Chemistry and Drug Discovery. Curr. Top. Med. Chem. (Sharjah, United Arab Emirates) 2014, 14, 941951. 91. Kawai, H.; Yuan, Z.; Tokunaga, E.; Shibata, N. A Sterically Demanding Organo-Superbase Avoids Decomposition of a Naked Trifluoromethyl Carbanion Directly Generated From Fluoroform. Org. Biomol. Chem. 2013, 11, 14461450. Chapter 1 • Nucleophilic reactions in the synthesis of organofluorine compounds 41 92. Geri, J. B.; Szymczak, N. K. Recyclable Trifluoromethylation Reagents From Fluoroform. J. Am. Chem. Soc. 2017, 139, 98119814. 93. Geri, J. B.; Wade Wolfe, M. M.; Szymczak, N. K. The Difluoromethyl Group as a Masked Nucleophile: A Lewis Acid/Base Approach. J. Am. Chem. Soc. 2018, 140, 94049408. 94. Scattolin, T.; Deckers, K.; Schoenebeck, F. Efficient Synthesis of Trifluoromethyl Amines Through a Formal Umpolung Strategy From the Bench-Stable Precursor (Me4N)SCF3. Angew. Chem., Int. Ed. 2017, 56, 221224. 95. Zhen, L.; Fan, H.; Wang, X.; Jiang, L. Synthesis of Thiocarbamoyl Fluorides and Isothiocyanates Using CF3SiMe3 and Elemental Sulfur or AgSCF3 and KBr with Amines. Org. Lett. 2019, 21, 21062110. 96. Pawelke, G. Tetrakis(Dimethylamino)ethylene-trifluoroiodomethane, a Specific Novel Trifluoromethylating Agent. J. Fluorine Chem. 1989, 42, 429433. 97. Burkholder, C.; Dolbier, W. R., Jr.; Medebielle, M. Tetrakis(Dimethylamino)Ethylene as a Useful Reductant of Some Bromodifluoromethyl Heterocycles. Application to the Synthesis of New gemDifluorinated Heteroarylated Compounds. J. Org. Chem. 1998, 63, 53855394. 98. Burkholder, C.; Dolbier, W. R., Jr.; Medebielle, M. Reaction of 2-(Bromodifluoromethyl)Benzoxazole with Tetrakis(Dimethylamino)Ethylene (TDAE) in the Presence of Aldehydes. A Convenient Synthesis of 2-(Difluoromethyl)Benzoxazole Alcohols. Tetrahedron Lett. 1997, 38, 821824. 99. Brauer, D. J.; Pawelke, G. Reactions of Trifluorovinyl-Trifluoromethylboron Derivatives, Structure of (F 2C:CF)(F 3C) 2B NHMe 2 and (HOOC)(F3 C) 2B NHMe2 . J. Organomet. Chem. 2000, 604, 4351. 100. Pooput, C.; Medebielle, M.; Dolbier, W. R., Jr. A New and Efficient Method for the Synthesis of Trifluoromethylthio- and Selenoethers. Org. Lett. 2004, 6, 301303. 101. Pooput, C.; Dolbier, W. R., Jr.; Medebielle, M. Nucleophilic Perfluoroalkylation of Aldehydes, Ketones, Imines, Disulfides, and Diselenides. J. Org. Chem. 2006, 71, 35643568. 102. Medebielle, M.; Dolbier, W. R., Jr. Nucleophilic Difluoromethylation and Trifluoromethylation Using Tetrakis(Dimethylamino)Ethylene (TDAE) Reagent. J. Fluorine Chem. 2008, 129, 930942. 103. Burkholder, C.; Dolbier, W. R., Jr.; Medebielle, M.; Ndedi, A. Tetrakis(Dimethylamino)Ethylene as a Useful Reductant of Some Chlorodifluoromethylated Ketones. A New Approach for the Synthesis of α,α-Difluoro Ketone Derivatives. Tetrahedron Lett. 1998, 39, 88538856. 104. Aiet-Mohand, S.; Takechi, N.; Medebielle, M.; Dolbier, W. R., Jr. Nucleophilic Trifluoromethylation Using Trifluoromethyl Iodide. A New and Simple Alternative for the Trifluoromethylation of Aldehydes and Ketones. Org. Lett. 2001, 3, 42714273. 105. Burkholder, C. R.; Dolbier, W. R.; Medebielle, M. Synthesis and Reactivity of Halogeno-Difluoromethyl Aromatics and Heterocycles. Application to the Synthesis of gem-Difluorinated Bioactive Compounds. J. Fluorine Chem. 2001, 109, 3948. 106. Carbonnel, E.; Besset, T.; Poisson, T.; Labar, D.; Pannecoucke, X.; Jubault, P. 18F-Fluoroform: A 18FTrifluoromethylating Agent for the Synthesis of SCF218F-Aromatic Derivatives. Chem. Commun. (Cambridge, UK) 2017, 53, 57065709. 107. Zeng, J.-L.; Chachignon, H.; Ma, J.-A.; Cahard, D. Nucleophilic Trifluoromethylthiolation of Cyclic Sulfamidates: Access to Chiral β- and γ-SCF3 Amines and α-Amino Esters. Org. Lett. 2017, 19, 19741977. 108. Matheis, C.; Wang, M.; Krause, T.; Goossen, L. J. Metal-Free Trifluoromethylthiolation of Alkyl Electrophiles via a Cascade of Thiocyanation and Nucleophilic Cyanide-CF3 Substitution. Synlett 2015, 26, 16281632. 109. Danoun, G.; Bayarmagnai, B.; Gruenberg, M. F.; Goossen, L. J. Sandmeyer Trifluoromethylthiolation of Arenediazonium Salts with Sodium Thiocyanate and Ruppert-Prakash Reagent. Chem. Sci. 2014, 5, 13121316. 42 Organofluorine Chemistry 110. Movchun, V. N.; Kolomeitsev, A. A.; Yagupolskii, Y. L. Nucleophilic Trifluoromethylation of Organic Substrates Using (Trifluoromethyl)Trimethylsilane in the Presence of a Fluoride Anion. II. A Convenient Route to Aryltrifluoromethyl-Sulfides, -Sulfoxides and -Sulfones. J. Fluorine Chem. 1995, 70, 255257. 111. Guo, S.; Cong, F.; Guo, R.; Wang, L.; Tang, P. Asymmetric Silver-Catalysed Intermolecular Bromotrifluoromethoxylation of Alkenes with a New Trifluoromethoxylation Reagent. Nat. Chem. 2017, 9, 546551. 112. Zhou, M.; Ni, C.; Zeng, Y.; Hu, J. Trifluoromethyl Benzoate: A Versatile Trifluoromethoxylation Reagent. J. Am. Chem. Soc. 2018, 140, 68016805. 2 Electrophilic reactions in the synthesis of organofluorine compounds Chapter Outline 2.1 Introduction ................................................................................................................................... 43 2.2 Reagents for electrophilic fluorination ....................................................................................... 44 2.2.1 Fluorinated bioisosteres of phosphate esters ................................................................... 46 2.3 Enantioselective electrophilic fluorination ................................................................................. 48 2.3.1 Enantioselective α-fluorination of aldehydes ................................................................... 48 2.3.2 Enantioselective α-fluorination of amides ........................................................................ 50 2.3.3 Enantioselective fluorination of allylsilanes and enolsilyl ethers ................................... 50 2.3.4 Enantioselective α-fluorination of ketones and 1,3-dicarbonyl compounds ................. 51 2.4 Electrophilic fluorination in the synthesis of α-fluorinated amino acids................................ 53 2.5 Electrophilic fluoroalkylation ....................................................................................................... 54 2.5.1 Reagents for electrophilic trifluoromethylation............................................................... 55 2.5.2 NHC-catalyzed electrophilic trifluoromethylation............................................................ 57 2.5.3 Electrophilic difluoromethylation ...................................................................................... 57 2.6 Electrophilic trifluoromethylthiolation and trifluoromethoxylation ....................................... 61 2.6.1 Synthetic methods for O-trifluoromethylation................................................................. 61 2.7 Synthetic methods for trifluoromethylthiolation ...................................................................... 63 2.7.1 Reagents for electrophilic trifluoromethylthiolation....................................................... 63 2.7.2 Billard’s reagents ................................................................................................................. 65 2.7.3 Diethylaminosulfur trifluoridemediated trifluoromethylthiolation of silylenol ethers and β-naphthols ........................................................................................ 66 2.8 Difluoromethylthiolation.............................................................................................................. 68 References............................................................................................................................................. 70 2.1 Introduction Electrophilic fluorination, fluoroalkylation, fluoroalkoxylation, and fluoroalkylthiolation reactions play an important role in the synthesis of pharmaceuticals, agrochemicals, fine chemicals, and functional materials. Electrophilic fluorinations of organic compounds can be Organofluorine Chemistry. DOI: https://doi.org/10.1016/B978-0-12-813286-9.00002-X © 2020 Elsevier Inc. All rights reserved. 43 44 Organofluorine Chemistry achieved under relatively mild conditions, using the commercially available electrophilic fluorinating reagents, such as Selectfluor (1-chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2] octane bis(tetrafluoroborate), NFSI (N-fluorobenzenesulfonimide), and N-fluoropyridinium salts (NFPy OTf, NFPy BF4). These reagents are milder alternatives to the use of highly corrosive elemental fluorine or moisture-sensitive xenon difluoride (XeF2).1 However, using microfluidic-based continuous flow reactor techniques, elemental fluorine can be used in the electrophilic fluorination reactions under relatively mild and safe conditions.2,3 Selectfluor-mediated electrophilic fluorination is used in the synthesis of pharmaceuticals, such as fluticasone, a corticosteroid-based drug. Difluoromethylation and trifluoromethylation reactions can be achieved, under mild reaction conditions, using the commercially available Umemoto’s and Togni’s reagents. These reagents can be used, for example, in the trifluoromethylation of enolates, aromatics, alkynes, thiols (S-trifluoromethylation), phosphines (P-trifluoromethylation), and alcohols (O-trifluoromethylation).4,5 Electrophilic trifluoromethylthiolation and difluoromethylthiolation reactions can be achieved using N-fluoroalkyl sulfenamide and N-fluoroalkyl phthalimide reagents.69 The relatively greater lipophilicity-enhancing effect of SCF3 moiety, as compared to that of OCF3 and CF3 substituents, is exploited in the design of pharmaceuticals, such as tiflorex (anorectic) and toltrazuril (antiprotozoal agent). SCF3-containing compounds are also used for veterinary drugs, such as monepantel and toltrazuril. 2.2 Reagents for electrophilic fluorination Some of the commercially available electrophilic fluorinating agents include 1-chloromethyl4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) (1; Selectfluor), 1-fluoro4-hydroxy-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) (2; Accufluor), N-fluoropyridinium triflate (3), N-fluoro-2,4,6-trimethylpyridinium tetrafluoroborate (4), NFSI (5; NFSI). Selectfluor gives better yields and higher selectivity for the α-fluorination, as compared to the other electrophilic fluorinating agents, such as N-fluoropyridinium tetrafluoroborate (NFPy) and NFSI. NFPy reagent is, however, costeffective, although it is less reactive than the Selectfluor. Relative reactivities of some of the electrophilic fluorinating reagents toward enol fluorination, as determined by UVvis kinetic studies, are in the following order: 1 . 3c5 . 4.10 The relative reactivity orders, based on the yields of enolate fluorination of β-ketoesters, are as follows: 1 . 2c5.11 Selectfluor has similar to, but slightly higher reactivity than, the Accufluor. The latter study also revealed that the N-fluoropyridinium tetrafluoroborate reacts about 100 times slower than the Selectfluor (Fig. 21). Selectfluor and NFSI are among the most widely used fluorinating agents in electrophilic fluorinations as well as in photoredox catalyzed CH fluorination reactions.1215 These reagents are also useful in the preparation of 18F-labeled positron emission tomography (PET) imaging agents (see Chapter 6: Synthesis and applications of 18F-labeled compounds). 18 F-labeled Selectfluor bis(triflate) was used for the synthesis of 18F-labeled PET tracers, Chapter 2 • Electrophilic reactions in the synthesis of organofluorine compounds OH N N 2BF4 F F O O N S O O S Me Cl N N 1 N 2BF4 F F 2 Selectfluor CF3 SO3 (or BF4–) Me N F Accufluor NFPy OTf k rel (enol fluorinations) Me BF4 4 3 45 5 NFTMP BF4 NFSI or (NFPy BF4 ) 1 – 0.2 10 –6 10 –4 1.84 – – 0.04 k rel (enolate fluorination of β-ketoesters) 2.72 FIGURE 2–1 Structures of some of the commercially available electrophilic fluorinating reagents (krel is the relative rate constants for enol fluorinations). OSiMe3 Me O Me 18 MeCN, 5–15 min, 80 °C Cl F ~50% RCY N SnMe3 N 18 F 2 OTf 18 F R AgOTf (2 equiv) acetone, RT 20 min R RCY = 14%–18% 18 FIGURE 2–2 Radiofluorination of enolsilanes and arylstannes using [ F]Selectfluor. through the fluorination of enolsilanes or fluoro-destannylation of arylstannes (Fig. 22).16 This fluoro-destannylation is a convenient alternative to the destannylative radiofluorination using [18F]F2.17 A mechanistic study of the Selectfluor-mediated fluorination of enol acetates (6) shows that the reaction proceeds through the formation of an oxygen-stabilized carbocation (7) (Fig. 23).18 Selectfluor is used as an electrophilic fluorinating agent in the synthesis of corticosteroids, such as fluticasone. Several fluorinated corticosteroids, including fluticasone, 46 Organofluorine Chemistry Cl N N O F 2BF4 O O O O O R Selectfluor 6 F R F R H2 O 7 O F R + CH3 CO2 H 8 FIGURE 2–3 Mechanistic outline for the Selectfluor-mediated fluorination of the enol acetates. are approved by the FDA as glucocorticosterone drugs, the topical antiinflammatory agents. Various ester derivatives of fluticasone, such as fluticasone furoate and fluticasone propionate, are used as nasal spray to treat nasal allergic rhinitis.19 Fluticasone furoate, when used as a combination therapy, is also effective in the management of chronic obstructive pulmonary disorders.20,21 Fluorinated corticosteroids, in most cases, have a 6-fluoro substituent, which can be introduced through electrophilic fluorination of the corresponding dienolates. The synthesis of fluticasone propionate or fluticasone furoate involves Selectfluormediated fluorination of the dienol acetate 9 and the ring-opening fluorination of the epoxide as the key steps.22 Thus fluorination of steroidal 3,5-dienol acetate 9 with Selectfluor gave 6-fluoro enone 10 with high stereoselectivity (Fig. 24). The ring-opening hydrofluorination of the epoxide 10, using aqueous hydrogen fluoride (HF) (70% solution), gave compound 11 that could be transformed into the fluticasone furoate or other ester derivatives in a series of steps. The aqueous HF used in the later step also hydrolyzed the acetate ester to the alcohol 11. 2.2.1 Fluorinated bioisosteres of phosphate esters Selectfluor-mediated electrophilic fluorination of the enolate derived from various dibenzylβ-ketophosphonates (12) gives the corresponding α,α-difluoro-β-ketophosphonates (13). The gem-difluoromethylene (CF2) is isoelectronic and, to some extent, isosteric with respect to oxygen (Charton’s steric parameters for OH and CF2H are 0.32 and 0.60, respectively23), and thus these α,α-difluoro-β-ketophosphonates (13) serve as bioisosteric mimetics of the corresponding phosphate esters (15). The α,α-difluoromethylated phosphonate esters are, therefore, useful in the design of the pharmaceutically interesting, hydrolytically stable phosphate esters. Due to the high electrophilicity of the carbonyl group adjacent to Chapter 2 • Electrophilic reactions in the synthesis of organofluorine compounds F F O Me HO Me F OH Me H S O O H F F Fluticasone propionate Fluticasone furoate O O Me O O O H Me Selectfluor H O Me O OH H O O OH HF/H 2 O H MeCN, RT AcO H O F Synthesis: Me H F H S O O Me Me O Fluticasone O Me HO O Me 6 F Me O Me HO H F O S 47 –15 °C to –10 °C 5h F 10 9 O Me HO H Me F OH OH Fluticasone furoate H O F 11 FIGURE 2–4 Electrophilic fluorination of enol acetates by Selectfluor. the gem-difluoromethylene moiety, these β-keto phosphonate esters are in rapid equilibrium with the corresponding hydrate form 14 and thereby can act as the transition state analog inhibitors of proteases. The α-fluorophosphonate esters also serve as hydrolytically stable analogues of the corresponding phosphate esters. For example, the α-monofluorinated D-glucose-1phosphate analogues 20 and 21 serve as transition state analog inhibitors of β-phosphoglucomutase (Fig. 25).24 The monofluorinated, α-fluorophosphonate esters, 21, could be synthesized through sequential electrophilic fluorination of the corresponding α-sulfonyl phosphonates (17), followed by reductive deprotection of the sulfonyl group using tributyltin hydride (Bu3SnH) in the presence of 2,20 -azo-bis(isobutyronitrile) as the free-radical initiator.15,25 gem-Difluoromethylene phosphonate 22, a bioisosteric analogue of N-palmitoylsphingosine-1-phosphate (sphingomyelin), is a hydrolytically sable, noncompetitive inhibitor of sphingomyelinases (Fig. 25).26 48 Organofluorine Chemistry FIGURE 2–5 Synthesis of α,α-difluoro- and α-fluorophosphonate esters as mimetics of the phosphate esters. 2.3 Enantioselective electrophilic fluorination 2.3.1 Enantioselective α-fluorination of aldehydes Asymmetric synthesis of α-fluoro aldehydes can be carried out using Selectfluor or NFSI as the electrophilic reagent, and chiral organocatalysts, such as L-proline or the imidazolidinone catalyst 23.27,28 Although enantioselective fluorination of the α-unbranched aldehydes with chiral proline-derived catalysts gives high yields and enantioselectivities for the α-fluorination, α-fluorination of the α,α-dialkyl (or aryl) aldehydes, under similar conditions, proceeds with poor enantioselectivities. Thus L-proline-catalyzed electrophilic α-fluorination of α-alkyl linear aldehydes, using Selectfluor, as well as NFSI, as the electrophilic fluorinating agent, gives relatively low enantioselectivity [4%25% enantiomeric excess (ee)]. However, NFSI-mediated electrophilic fluorination of aldehydes, in the presence of the chiral imidazolidinone catalyst 23, gives low to moderate enantioselectivities (28%66% ee) for the Chapter 2 • Electrophilic reactions in the synthesis of organofluorine compounds 49 O O NFSI R1 R1 H N R 1 = e.g., iPr, alkyl, benzyl R 2 = e.g., H, Me, alkyl Me Me Ph N H H R2 F Me O R2 40%–97% yields 28%–66% ee for R2 = Me, alkyl 88%–96% ee for R2 = H Organocatalyst (23) FIGURE 2–6 Asymmetric electrophilic α-fluorination of aldehydes. t-Bu Ar NFSI/25 (10 mol%) Ar O R R 3,5-(NO 2) 2C6 H3CO2 H (10 mol%) Toluene; 0 °C 24 t-Bu Ar O F OH R NH2 CO2 Et F t-Bu 27 26 t-Bu Examples: Chiral catalyst 25 F Ph Br OH F 76%; 90% ee 97%; 92% ee 28 F OH F OH F 98%; 92% ee 30 29 Ph Ph O CrO 3 /H 2SO 4 OH F Acetone, RT 4 h OH F F F 69% 31 Analog of flurbiprofen 32 FIGURE 2–7 Enantioselective fluorination of α-branched aldehydes and synthesis of a flurbiprofen analog. α-branched aldehydes and high enantioselectivities of 88%96% for the α-unsubstituted linear aldehydes (Fig. 26).27 The enantioselectivity of the α-fluorination of the α-branched aldehydes is drastically improved when catalyzed by a highly crowded chiral binaphthyl-based primary amine catalyst 25. The intermediate α-fluoro aldehydes 26 were reduced, in situ, to give the corresponding primary alcohols 27. CrO3 oxidation of the compound 31 gives the α-fluorinated version of flurbiprofen, a nonsteroidal antiinflammatory drug (Fig. 27).29 50 Organofluorine Chemistry O O F F O F O N O N O O LDA, NFSI THF, –78 °C O O 34 92% O 33 Me F OH O F O O 35 (GRP5 agonist; lowers blood glucose levels) FIGURE 2–8 α-Fluorination of amides by NFSI in the synthesis of a G Protein-coupled receptor (GPCR) agonist. NFSI, N-Fluorobenzenesulfonimide. 2.3.2 Enantioselective α-fluorination of amides NFSI can be used in the α-fluorination of amide enolates. Thus reaction of the Evans’ chiral N-acyl oxazolidinone 33 with lithium diisopropylamide (LDA), followed by reaction with Nfluorobenzenesulfonimide (NFSI), gives the corresponding α-fluorinated compound 34, which was enantioselectively methylated and hydrolyzed to give the compound 35 (Fig. 28). Compound 35 was found to be a superagonist for the G-protein-coupled receptor, GPR4, which mediates fatty acidinduced glucose-stimulated insulin secretion from the pancreatic beta cells, thereby lowering the blood glucose levels. It was demonstrated that the compound 35 is highly efficacious in lowering the blood glucose levels, has superior in vitro metabolic stability, and forms a stable acylglucuronide metabolite, as compared to its analog that lacks the α-fluorine and methyl groups.30 2.3.3 Enantioselective fluorination of allylsilanes and enolsilyl ethers Shibata and coworkers developed enantioselective fluorination of silylenol ethers and allylsilanes, using NFSI and bis-cinchona alkaloids, such as (DHQ)2PYR [a (2,5-diphenyl)pyrimidine-linked version of dimeric dihydroquinine] or (DHQ)2PHAL (a phthalazine-linked version of dimeric dihydroquinine, used as AD-mix-alpha in the Sharpless asymmetric dihydroxylation) as a chiral catalyst.31 In some cases, high yields and enantioselectivities are obtained using these catalysts (Fig. 29). In these reactions, NFSI reversibly transfers the “F1” to the cinchona alkaloids to form the corresponding N-fluoroammonium salt, which, upon anion metathesis with K2CO3, followed by desilylative fluorination gives the fluorinated compounds, regenerating the cinchona catalysts. The fluorination reactions are relatively Chapter 2 • Electrophilic reactions in the synthesis of organofluorine compounds 51 O Me3Si O NFSI (1.2 equiv) (DHQ)2 PYR (10 mol%) R F N H O H NN N R K2 CO3 /MeCN N O N R = PhCH2 ; 75% yield (94% ee) (DHQ)2PHAL Me3Si O O O R NFSI (1.2 equiv) (DHQ)2 PHAL (10 mol%) F N O R K2 CO3 /MeCN H N R = PhCH2 ; 90% yield (71% ee) R = e.g., PhCH2 , p-Cl, p-Me, or p-OMe-benzyl O N H N N O N (DHQ)2PYR Mechanistic outline: F N(SO2Ph)2 X Me3 SiOK H N F + CO2 H F N R N(SO2Ph)2 K2 CO3 Me3Si H F N X R K+ –N(SO2Ph)2 KCO3 X = O, CH2 FIGURE 2–9 Enantioselective fluorination of allylsilanes and enolsilyl ethers. slower in the absence of the K2CO3, suggesting that the weakly basic sulfonamide anion is ineffective in the desilylation reactions. 2.3.4 Enantioselective α-fluorination of ketones and 1,3-dicarbonyl compounds Use of the cinchona alkaloids as chiral catalysts gives moderate yields and enantioselectivities in the α-fluorination of tetralones (Fig. 210).32 The 2-aryltetralones are potentially useful as pharmaceuticals, such as aromatase inhibitors and hepatitis C virus inhibitors, based on the effectiveness of their isoflavanone analogs. Selectfluor-mediated diastereoselective fluorination of enolates with preexisting chiral centers in the molecule proceeds with high diastereoselectivities. For example, the enolate 52 Organofluorine Chemistry O O 1. 1:1 Cinchonine/Selectfluor (2 equiv; NaH (2.0 equiv)/THF R R F 2. MeCN, 29 h, 0°C Examples: O O O F F F O F Me 16% ee 74% ee O Me 59% ee FIGURE 2–10 Enantioselective fluorination of tetralones. O O O N H O OTBS O KHMDS/Selectfluor O 12 THF, –78°C O N H O OTBS F 12 93%; dr = >20:1 36 37 O TFA/DCM O O N H O F OH 12 38 FIGURE 2–11 Stereoselective electrophilic fluorination of the β-diketone 1 using Selectfluor. DCM, Dichloromethane; KHMDS, potassium hexamethyldisilazide (KN(SiMe3)2); TBS, tert-butyl(dimethyl)silyl; TFA, trifluoroacetic acid. derived from the 1,3-dicarbonyl compound 36 gives the α-fluorinated compound 37 in high yields and diastereoselectivity (diastereomeric ratio . 20:1) (Fig. 211).33 Enantioselective fluorination of β-carbonyl esters, using chiral ((S)-BINAP)Pd(II)-based catalysts, gives access to the chiral α-fluoro carbonyl compounds. The β-amidoester 39 upon electrophilic fluorination, using NFSI, in the presence of the (S)-BINAP-Pd(OTf)2, gave 44% ee of the fluorinated compound 40 (Fig. 212). Chiral enrichment of this compound through chiral HPLC gives 99% ee. The α-fluorinated compound 41 is an intermediate for the synthesis of a selective spleen tyrosine kinase (SYK) inhibitor 43. SYK proteins are nonreceptor kinases, which mediate diverse cellular functions, including cell proliferation, differentiation, and phagocytosis. The SYK inhibitors, therefore, are of pharmaceutical interest for a number of pathologies, including rheumatoid arthritis, B-cell lymphoma, asthma, and nasal rhinitis.34 Through further improvements in the synthetic design, multikilogram scale synthesis of compound 41 could be achieved with high enantiomeric purity. Thus fluorination of (1)-menthol ester 42, (synthesized, in situ, from the ethyl ester 39) in the presence of (S)-BINAP-Pd(OTf)2 (serves as a matched stereochemical combination), gave up to 100% diastereoselectivity for the desired α-fluorinated ester, which was then reduced to the primary alcohol 41 using borane-dimethyl sulfide (BH3-SMe2).34 Chapter 2 • Electrophilic reactions in the synthesis of organofluorine compounds O 1. ((S)-BINAP)Pd(OTf) 2 2,6-lutidine NFSI, EtOH, 0 °C, 18 h O EtO NH O F O F BH 3 -SMe 2 NH EtO 53 O NH HO 2. Chiral HPLC 41 40 39 59% (44% ee; 99% ee after HPLC) O 1. (S)-BINAP-Pd(OTf) 2 2,6-lutidine NFSI, EtOH O F O HO NH Multiple steps NH O 2. BH 3-SMe 2 41 42 68%; up to 100% ee for the first step F HN NH N N N N 43 (SYK inhibitor) FIGURE 2–12 Enantiomeric fluorination of β-amido esters toward the synthesis of a fluorinated SYK inhibitor. SYK, Spleen tyrosine kinase. 2.4 Electrophilic fluorination in the synthesis of α-fluorinated amino acids Fluorinated, noncanonical amino acids, when incorporated into the peptides and proteins, alter their physicochemical and biochemical properties, including pharmacokinetics, metabolic stability, and enhance thermal stabilities. Most of the fluorinated amino acids that have been used in protein engineering and material design are those containing fluoroalkyl(aryl) side chains, such as β-fluoroalanine [(fluoromethyl)glycine; 44], β,β-difluoroalanine [(difluoromethyl)glycine; 45], β,β,β-trifluoroalanine (trifluoromethylglycine; 46), 5,5,5,50 ,50 ,50 -hexafluoroleucine (47), (2-fluorophenyl)alanine (48), and (4-trifluoromethyl)phenylalanine (49) (Fig. 213).3537 Synthesis of α-fluoro amino acids, however, is challenging, as these compounds are too unstable to isolate, because the strong electron-releasing adjacent amino group brings about rapid defluorination and thereby formation of the imines, which are hydrolyzed to the α-keto carboxylic acids. It was, however, possible to synthesize the N-phthalimido-protected α-fluoroglycine and its derivatives.38 The decreased basicity of the amino group in these amino acid derivatives disfavors the elimination of the fluoride anion. Thus bromofluorination of the N-phthalimido-protected dehydroalanine (50), using Olah’s reagent [pyridinium polyhydrogen fluoride (PPHF)] and 1,3-dibromo-5,5-dimethylhydantoin, gave 54 Organofluorine Chemistry O F2 HC O O HF2C OH NH2 44 OH F3C OH NH2 NH2 45 46 F O F 3C O OH OH NH2 CF3 NH2 47 48 O OH NH2 F3 C 49 FIGURE 2–13 Structures of selected fluorinated, noncanonical amino acids. O O O N O DBH, PPHF (9:1 HF-Py) O Me N DCM, RT, 23 h O 50 O O Me F Br 51 FIGURE 2–14 Synthesis of N-protected α-fluoro-β-bromo glycine ester. the β-bromo-α-fluoro alanine derivative (51) (Fig. 214). Deprotection of the phthalimido group, however, results in degradation of the product. An improved strategy for the preparation of the α-fluorinated amino acid derivatives was developed by Molander and coworkers, using the photoredox catalysis and N,N-di(Boc)-protected dehydroalanine esters.14 Photoredox-catalyzed fluoroalkylation of dehydroalanine, using Selectfluor and alkyltrifluoroborates (precursors for the alkyl radicals), in the presence of the mesitylacridinium (MesAcr) organo-photocatalyst, gives α-fluoroninated amino acids in high yields. Various alkyl, alkoxyalkyl, and aminoalkyl α-fluoroalanines could be synthesized using the corresponding alkyltrifluoroborates as the source of the nonstabilized, reactive alkyl radicals (Fig. 215). A possible mechanism of the reaction involves the formation of alkyl radicals from the corresponding alkyltrifluoroborates, catalyzed by the highly oxidizing photo-excited mesitylacridinium salt (Mes Acr1; Ered 5 12.06 V vs SCE). Addition of these alkyl radicals to the dehydroalanine gives the α-amino-radical species (52), which abstracts fluorine atom from the Selectfluor to give the α-fluoro-α-amino acids. The MesAcr1 photocatalyst is regenerated through its redox reaction with the Selectfluor-derived radical cation 53. 2.5 Electrophilic fluoroalkylation Trifluoromethyl moiety is one of the most frequently used structural motifs in the drug design. Incorporation of the CF3 moiety into pharmaceutical compounds enhances metabolic stability, bioavailability, and in some cases shows improved enzymesubstrate Chapter 2 • Electrophilic reactions in the synthesis of organofluorine compounds 55 Me R RBF3K (2 equiv) CH2 Boc N Boc Selectfluor (4 equiv) OCH 2 Ph MesAcr (5 mol%) DMF (0.1 M), blue LEDs, RT Dehydroalanine (N,O-protected) OCH 2 Ph Me Me Boc O α-fluoro- α-amino acid (N,O-protected) + Boc O F N N Me BF 4 R = primary or secondary alkyl, α -alkoxymethyl, α-aminomethyl Selected examples: Mes–Acr + Br Ph O S O NH O Boc F N OCH 2 Ph Boc Boc O F N OCH 2 Ph F Boc N OCH 2 Ph Boc O Boc O Boc F N OCH 2 Ph Boc Boc O F N OCH2 Ph Boc O 62% 81% 32% 82% 72% Mechanistic outline: N Cl BF4 R N Mes–Acr + Boc 54 Blue LEDs F N OCH2 Ph Boc O α-fluoro- α-amino acid Cl N N Mes–Acr +* Mes–Acr 53 Cl N N F 2BF 4 Selectfluor R RBF3– K+ 2BF 4 R CH2 Boc N OCH 2Ph Boc O Boc N Boc O Dehydroalanine OCH 2Ph 52 FIGURE 2–15 Selectfluor-mediated synthesis of α-fluoro-α-amino acid derivatives through photoredox catalysis. interactions. Some of the widely prescribed drugs, such as efavirenz, an anti-HIV drug, are trifluoromethylated compounds (Fig. 216). 2.5.1 Reagents for electrophilic trifluoromethylation Umemoto’s and Togni’s reagents are widely used in the trifluoromethylation (or perfluoroalkylation) of a variety of strong as well as weak nucleophiles (Fig. 217).4,5 Due to the 56 Organofluorine Chemistry O F 3C F3C Cl O N H Fluoxetine (antidepressant) N O N H CF 3 N H O Cotoran (herbicide) Efavirenz (anti-HIV) FIGURE 2–16 Structure of selected trifluoromethylated pharmaceuticals and a herbicide. F S CF3 F F F S CF3 BF4– (or –OSO2 CF3 ) Umemoto's reagents – OSO2 CF3 F F S – CF3 OSO2 CF3 Umemoto's second-generation reagents O O I CF 3 O I CF 3 Togni's reagents FIGURE 2–17 Structures of Umemoto’s and Togni’s reagents. broad range of reactions achievable using these reagents, only a cursory coverage of these reagents is provided in this chapter. Umemoto’s second-generation reagents, consisting of 2,8-difluoro- and 2,3,7,8-tetrafluoro-dibenzothiophenium salts, are recyclable reagents and could be synthesized in a onepot process.4 These reagents are also thermally more stable than the first-generation Umemoto’s reagents. As in the case of the Umemoto’s first-generation reagents, these improved-version reagents can be used in the electrophilic trifluoromethylation of a variety of nucleophiles. Trifluoromethylation of 1,3-dicarbonyl enolates, alkynes, thiols, and FriedelCrafts reactions of aromatics, such as indoles, gives the corresponding trifluoromethylated compounds in good yields. Phosphines and arylsulfonyl anions are also trifluoromethylated using Umemoto’s reagents, under mild conditions (Fig. 218).4 Similarly, Togni’s reagents achieve FriedelCrafts trifluoromethylation of aromatics and are effective reagents for the trifluoromethylation of 1,3-dicarbonyl compounds, silylenol ethers, thiols, phosphines, amines, and alcohols (Fig. 219). Togni’s reagents are used in the synthesis of the pharmaceutically interesting compounds, such as S-trifluoromethylated coenzyme A, S-trifluoromethylated nucleoside analogs, and anti-HIV agents.5 Chapter 2 • Electrophilic reactions in the synthesis of organofluorine compounds PhSO2 Na 57 PhSO 2CF3 DMF, RT 70% O H O Me F 3C O F F Me NaH, DMF –50 °C to RT F F S – CF3 OSO2 CF3 O 88% N H CF3 N H 69% DMF, RT Umemoto's second generation reagent H CF3 TsO TsO CuCl,s-collidine DMAC, 30 °C 65% SH Br Et 3 N, DMF, RT SCF3 Br 67% FIGURE 2–18 Electrophilic trifluoromethylations using Umemoto’s reagent. 2.5.2 NHC-catalyzed electrophilic trifluoromethylation N-Heterocyclic carbenes (NHC)-catalyzed electrophilic trifluoromethylations of α-chloro aldehydes, using Togni’s reagent, gives the corresponding α-trifluoromethyl esters.39 A wide range of substituents, including terminal alkyne, olefin, chloro, cyano, azido, ester, and ether substituents, are tolerated in this reaction. Using a chiral NHC, moderate to good enantioselectivities were obtained. The NHC catalysis is rationalized as follows. The aldehyde, in reversible equilibrium with the NHC, forms the enol 58. 1,5-diazabicyclo[4.3.0]non-5-ene (DBN)-mediated dehydrohalogenation of 58 forms the enolate 59, which abstracts the electrophilic “CF31” from the Togni’s reagent to give 60. Methanolysis of 60 then gives the α-trifluoromethyl ester (57), regenerating the NHC catalyst (Fig. 220).39 2.5.3 Electrophilic difluoromethylation The gem-difluoromethylene moiety (CF2) is isopolar and isosteric with carbonyl (CQO) and hydroxymethyl (CH2OH) moieties. The CF2 moiety provides enhanced lipophilicity and metabolic stability to the pharmaceutical candidates, and relatively high thermal and mechanical stability to the materials. Therefore, it is a key structural element in the design of pharmaceuticals, as well as functional materials. Prakash and coworkers have designed an S-(difluoromethyl)diarylsulfonium tetrafluoroborate for the electrophilic 58 Organofluorine Chemistry N H R CF3 O N H R O O OR O OR CF3 RSH RSCF3 CoA-SH CoA-SCF3 O O O or I CF3 O NH I CF3 O N O HO NH Togni’s reagents O N OH SH O HO O OH PH2 SCF3 R P CF3 DBU ROH Zn(NTf 2) 2 N H N R = H (1 equiv of Togni's reagent) R = CF3 (2 equiv of Togni's reagent) ROCF3 N CF3 N Cl O R OH S N N O Anti-HIV agent FIGURE 2–19 Electrophilic trifluoromethylations using Togni’s reagents. difluoromethylation of tertiary amines, phosphines, and sulfonate anions to give their corresponding N-, P-, and O-difluoromethylated products (Fig. 221).40 A related S-(fluoromethyl)diarylsulfonium tetrafluoroborate is effective in the monofluoromethylation of C, S, O, N, and P nucleophiles.41 Chapter 2 • Electrophilic reactions in the synthesis of organofluorine compounds O O R Cl 55 56 R N N O O I CF3 + H NHC (20 mol%) R DBN (2 equiv) MeOH/DCM (1:2), 0 °C, 2 h OMe N CF3 57 Selected examples: MeO 2 C O O 59 NHC CF3 OMe OMe CF3 MeO CF3 N Boc 75% 45% 51% Mechanistic outline: O R O R N N OMe CF3 R H Cl N 57 NHC MeOH OH O R N R R N CF3 R N N Cl N N 58 60 O DBN O OH R N R I N O 56 O I CF3 DBN.HCl N 59 FIGURE 2–20 NHC-catalyzed electrophilic trifluoromethylation of α-halo aldehydes. Shen and coworkers have achieved O-trifluoromethylation of alcohols using difluoromethyl-(4-nitrophenyl)-bis(carbomethoxy) methylide sulfonium ylide (Fig. 222). This sulfonium ylide, as an electrophilic difluoromethylating reagent, was synthesized through the Rh-catalyzed reaction of dimethyl diazomalonate with 4-nitrophenyl(difluoromethyl) thio ether.42 Biologically interesting compounds such as nerol, vitamin-D3, and stigmasterol could be transformed into their difluoromethoxy derivatives in moderate to good yields. 60 Organofluorine Chemistry R1 R2 N R3 CF2H S BF4 R1 R 2 N CF2H R3 BF4 R1 R2 P R3 R O S O R1 R 2 P CF2H R3 BF4 M+ O R O S O O CF2 H FIGURE 2–21 Electrophilic difluoromethylation of tertiary amines, phosphines, and sulfonate anions. CO2 Me MeO 2C S CF2 H ROH S N2 O2 N CO2 Me MeO 2C CF2 H [Rh] ROCF2 H O2 N R = alkyl Selected examples: HF2 CO HO 82% Nerol OH OCF2H 62% Vitamin-D3 Me Me Me H Me Me Me H H H H HO HF2 CO 51% Stigmasterol FIGURE 2–22 Electrophilic O-difluoromethylation of alcohols. H H H Chapter 2 • Electrophilic reactions in the synthesis of organofluorine compounds 61 2.6 Electrophilic trifluoromethylthiolation and trifluoromethoxylation Trifluoromethylthio group (SCF3) is comparable to OCF3 or CF3 groups in terms of enhancing lipophilicity of compounds. Hansch lipophilicity parameters (π) for SCF3 (1.44), OCF3 (1.04), and CF3 (0.88) substituents indicate that SCF3 moiety has relatively greater lipophilicity-enhancing effect as compared to the OCF3 and CF3 substituents.43,44 The Hansch lipophilicity parameter of the SCF3 moiety is comparable to SeCF3 moiety (1.29), a less commonly used substituent in medicinal chemistry.45 The relatively lower electron-withdrawing effect of SCF3 (EN 5 2.44) moiety, as compared to OCF3 (EN 5 3.50) moiety,46 combined with its relatively higher steric crowding, can be used in fine-tuning the stereoelectronic effects of the compounds in drug discovery. Compounds with OCF3 and SCF3 substituents have found applications as pharmaceuticals and agrochemicals (pharmaceuticals: riluzole, sonidegib, tiflorex, toltrazuril; agrochemicals: indoxacarb, flurprimidol, triflumuron, and flucarbazone; veterinary drugs: monepantel and toltrazuril (Figs. 223 and 224).7,43 2.6.1 Synthetic methods for O-trifluoromethylation O-Trifluoromethylation of phenols can be achieved using Umemoto’s reagent 61 or Togni’s reagent.43 Alternatively, the oxidative decarboxylative fluorination of aryloxy(difluoromethyl) carboxylic acids, using xenon difluoride (XeF2), gives the corresponding O-trifluoromethyl phenolic derivatives in moderate yields (Fig. 225).47 F3 CS N H 3C N O Monepantel (Zolvix) (used for the veterinary treatment of gastrointestinal nematodes) N N H H N O F3 CS S NH2 N N H CH3 CH3 Tiflorex (anorectic) SCF3 O Toltrazuril (used for veterinary treatment of coccidiosis) SCF3 H 3C O O O H 3 C CN O N H F F F H 3C N O N SCF3 O CH3 SCF3 analog of riluzole Toltrazuril (antiprotozoal agent) FIGURE 2–23 Selected examples of SCF3-containing pharmaceuticals and veterinary medicines. 62 Organofluorine Chemistry CH 3 H 3C HO O F3 CO F 3CO S N CH 3 O NH2 N N F3CO S O N H O N O N O O Cl N H N N Cl CH3 N CH3 Flucarbazone (herbicide) OCF3 OCH3 OCF3 O N H N Flurprimidol (plant growth regulator) Sonidegib (antineoplastic agent) O OCF3 O O CH3 N N H Riluzole (for the treatment of amyotrophic lateral sclerosis) CH3 O Triflumuron (insecticide) N OCH 3 O Indoxacarb (insecticide) FIGURE 2–24 Selected examples of OCF3-containing pharmaceuticals and agrochemicals. BF 4 OH O CF3 OCF3 61 (iPr) 2 NEt 62 75% O Ar O F F OH XeF2 (1 equiv) CDCl3 Ar O F F F 42%–77% FIGURE 2–25 Electrophilic O-trifluoromethylation of phenols and aryloxy(difluoromethyl)carboxylic acids. The trifluoromethoxy compounds, however, can be more conveniently synthesized through nucleophilic substitutions of alkyl halides, using the in situgenerated silver trifluoromethoxide (CF3OAg) (Fig. 226) (see Chapter 1: Nucleophilic reactions in the synthesis of organofluorine compounds).48 Thus the glycosyl bromide 63, upon reaction with trifluoromethyl benzoate, in the presence of AgF, gives the trifluoromethoxy glycoside 64, with inversion of configuration. Chapter 2 • Electrophilic reactions in the synthesis of organofluorine compounds 63 O OH OH OCF3 O HO HO OH O HO HO AgF, MeCN, RT Br OCF3 OH 84% 63 64 FIGURE 2–26 Trifluoromethoxylation of glycosyl halides through the in situ formed AgOCF3 reagent. O O I N N SCF3 N SCF3 O SCF3 O O O S N SCF3 SCF3 O O 67 66 65 (Haas’ reagent) (Munavalli’s reagent) SO2 CF3 (Shibata’s reagent) (Billard and Langlois’ reagent) 69 (Lu and Shen’s reagent) (Shen’s reagent) SCF3 I O OSCF3 Ar S IPh 70 68 I O N SCF3 O 71 72 (Buchwald’s reagent) (Billard’s reagent) 73 (Lu and Shen’s reagent) FIGURE 2–27 Selected commercially available trifluoromethylthiolating reagents. 2.7 Synthetic methods for trifluoromethylthiolation 2.7.1 Reagents for electrophilic trifluoromethylthiolation A number of electrophilic trifluoromethylthiolation reagents, such as compounds 6573, are commercially available (Fig. 227).8 Because of the pharmaceutical and agrochemical importance of the trifluoromethylthio compounds and the possibility of achieving late-stage trifluoromethylthiolations, there is a resurgence of interest in developing efficient synthetic methods for the trifluoromethylthiolations. Many of the commercially available trifluoromethylthiolating agents, such as Haas's reagent (65),49 Munavalli’s reagent (66),50 Billard and Langlois' reagent (67),51 Shen’s reagent (69),52,53 and Billard’s reagent (72),54 are based on the trifluoromethylsulfenamide moiety. Shibata has developed the hypervalent iodonium ylide reagent 70,55 and Lu and Shen have developed the reagents 68 and 73.52,53 Buchwald and coworkers56 developed the reagent 71, the reagent containing the OSCF3 moiety. Among these reagents, Munavalli's- and Billard's reagents have found relatively wide applications. 64 Organofluorine Chemistry O O N O Li + O N SCF3 O O O N Cl CF3 SCu N SCF3 RNH2 RNHSCF3 or CF3SAg O O O Munavalli's reagent O R H Cl R H Cl O N O K+ CF3 SCl H SCF3 B(OH)2 O R H CuI R SCF3 CuI (10 mol%) SCF3 FIGURE 2–28 Electrophilic trifluoromethylthiolation of carbonyl compounds, amines, alkynes, and arylboronic acids. 2.7.1.1 Munavalli’s reagent The synthesis of the Munavalli’s reagent [N-(trifluoromethylthio)phthalimide] can be achieved by the reaction of potassium phthalimide with trifluoromethylsulfenyl chloride (highly toxic), or through reaction of the N-chlorophthalimide with CF3SCu or CF3SAg (relatively nontoxic), in large quantities. This reagent is moisture- and air-stable and thus is convenient to handle. Munavalli’s reagent serves as a convenient reagent for the electrophilic N-trifluoromethylthiolation of amines, α-trifluoromethylthiolation of carbonyl compounds,7,50,57 and trifluoromethylthiolation of arylboronic acids and terminal alkynes.7 Munavalli’s reagent serves as a source of the electrophilic “CF3S1” in reactions with nucleophilic reagents, such as amines, alkynes, and α-carbanions, derived from the carbonyl compounds. N-(trifluoromethylthio)succinimide can also be used for these trifluoromethylthiolations (Fig. 228).58 2.7.1.2 Asymmetric trifluoromethylthiolation N-Acyl derivatives of the Evan’s chiral oxazolidinones (74) can be diastereoselectively trifluoromethylthiolated at the α-carbon using Munavalli’s reagent as the electrophilic trifluoromethylating reagent at low temperatures (Fig. 229). This reaction proceeds in two steps, involving lithium hexamethyldisilazide (LiHMDS)mediated deprotonation of the acidic α-hydrogen, followed by electrophilic trifluoromethylthiolation by the (N-SCF3)-phthalimide (Munavalli’s reagent) to give 75. These reactions proceed with 87%100% diastereomeric Chapter 2 • Electrophilic reactions in the synthesis of organofluorine compounds O O O O R N 1. LiHMDS (1.2 equiv) R N R O N SCF3 SCF3 2. Munavalli's reagent O (Munavalli's reagent) THF, –78°C, 7 h 74 OH [H] O SCF3 O 65 75 76 R = e.g., Me, benzyl, isopropyl, n-pentyl dr 87:13–100:0 Yields: 60%–85% FIGURE 2–29 Electrophilic α-trifluoromethylthiolation of chiral N-acyl oxazolidinones. selectivity. Reductive cleavage of the oxazolidinone moiety gives the corresponding 2-(trifluoromethylthio)-primary alcohols (76) in high enantiomeric purity (up to 100% ee). Hydrolysis of the oxazolidinone 75 to the corresponding carboxylic acids, on the other hand, resulted in partial racemization at the α-carbon.59 2.7.2 Billard’s reagents The trifluoromethanesulfenamides 77, 78, and 79 (Billard’s reagents) can be used for the trifluoromethylthiolation of amines, thiols, terminal alkynes, Grignard reagents, and α-trifluoromethylthiolation of carbonyl compounds (Fig. 230).9,54,6063 The sulfonamide reagent 79 is more reactive than the first-generation reagents 77 and 78 in these reactions.62 Billard’s reagent 78 was used for the synthesis of N-trifluoromethylthiolated analog of imipramine, an antidepressant.64 2.7.2.1 Synthesis of the Billard’s reagents The synthesis of the Billard’s reagents is achieved through the reaction of primary amines with the reagent combination of CF3TMS and diethylaminosulfur trifluoride (DAST) in the presence of a tertiary amine, such as triethylamine or N,N-(diisopropyl)ethylamine (Hunig’s base, DIEA). Using this convenient synthetic method, a variety of aromatic and aliphatic amines give the corresponding N-trifluoromethylthiolated products 81 (R 5 alkyl/aryl) in good yields.65 A probable mechanism for this reaction sequence involves reaction of the tertiary amine with DAST to give the intermediate ammonium salt 82, which is in equilibrium with compound 83 (not isolated). The latter compound 83 then reacts with the CF32 (generated through the fluoride ioninitiated reaction of CF3TMS) to give the intermediate compound 84. Subsequent steps, involving nucleophilic substitution of the fluoride ion in (84) by the primary amine, followed by the fluoride-induced elimination of N-ethylethanimine (80) gives the N-trifluoromethylthiolated amine 81 (Fig. 231). 2.7.2.2 Trifluoromethylthiolation of alkynes and Grignard reagents The reaction of the terminal alkynes with the Billard’s reagent [PhN(Me)SCF3], in the presence of catalytic quantities of LiHMDS, gives the corresponding trifluoromethylthiolated alkynes in high yields. The latter compounds serve as precursors for the Cu(I)-catalyzed 66 Organofluorine Chemistry H N Me N SCF3 SCF3 O O S SCF3 Me Me 77 N 78 79 O H R O 77 , 78 , or 79 SCF3 cat. LiHMDS R N H cat. TMSCl H N R SCF3 R′ RMgCl SCF3 R N R′ SCF3 RSCF3 N H N N SCF3 Me SCF3 -Imipramine (antidepressant) FIGURE 2–30 Billard’s reagents for the trifluoromethylthiolations. alkyneazide cycloaddition reactions (click reactions) for the synthesis of 1,2,3-triazoles (Fig. 230).61 Only a catalytic amount of LiHMDS is required for these reactions, as the conjugate base formed in the catalytic cycle serves as a base for the deprotonation of the alkyne in the subsequent steps. The trifluoromethylthiolation of the Grignard reagents gives the corresponding trifluoromethylthiolated products in moderate yields and the yields are dependent on the choice of the sulfenamide or sulfonamide reagents. The use of the sulfonamide reagent improves yields of these reactions (Fig. 232). 2.7.3 Diethylaminosulfur trifluoridemediated trifluoromethylthiolation of silylenol ethers and β-naphthols β-Naphthols (e.g., 89) and enolsilyl ethers of ketones (e.g., 93) are trifluoromethylthiolated upon reaction with CF3TMS and DAST, using a procedure similar to that of N-trifluoromethylthiolation of primary amines,65 to give their corresponding α-trifluoromethylthiolated β-naphthols (e.g., 90), and α-trifluoromethylthiolated ketones (e.g., 94), respectively Chapter 2 • Electrophilic reactions in the synthesis of organofluorine compounds + CF3SiMe 3 Et N Et 1. Et3N or DIEA (1 equiv) SF3 R 2. RNH2 (DAST) SCF3 81 H N Et 80 Some examples: H N H N 67 H N SCF3 H 3C H N SCF3 Cl H N SCF3 F 90% 80% H N SCF3 SCF3 CH 3 75% 75% 75% +F Me F Me F C Si Me F Mechanistic outline: Et Et Et N S F F R3 N Et F S F NR3 82 (DAST) H Et N F N H F NH2 R S RNH2 Et F CF3 Et N F Et F S F F3 C Et N S F 84 F F Me F Me F C Si Me F F 83 R 3N R3 NH+ F H Et N H F NHR R S F CF3 H N SCF3 81 H + HF Et N 80 FIGURE 2–31 Synthesis of Billard’s reagents [N-(alkyl)trifluoromethanesulfenamides] and a mechanistic outline. (Fig. 233).66 The proposed mechanism for these trifluoromethylthiolations involves the formation of the reactive intermediate 95 in equilibrium with DAST. The reaction of 95 with the trifluoromethyl anion, derived from the fluoride anion activation of CF3TMS, gives diethylamino(trifluoromethyl)sulfur difluoride 96. Electrophilic trifluoromethylthiolation of enolates derived from enolsilyl ethers and β-naphthols with the intermediate 96 then gives the observed products, such as 90 and 92, in a series of steps, analogous to that of N-trifluoromethylthiolation of amines, as shown in Figure 231.65 68 Organofluorine Chemistry H SCF3 LiHMDS (10 mol%) MeO CH3 N Ph SCF3 THF, 0 °C 85 DMF,60 °C, 4 h MeO 86 SCF3 MeO 87 75% 54% LiHMDS (or 4) N N NH NaN 3 (1.5 equiv) Li MeO CH3 N Ph SCF3 Ph CH3 N Li 88 MgCl CH3 N Ph SCF3 THF, 0 °C SCF3 86% CH3 N Ph SCF3 THF, 0 °C MgCl SCF3 10% O O S MgCl Me N SCF3 Me SCF3 THF, 0 °C 63% FIGURE 2–32 Trifluoromethylthiolation of terminal alkynes and Grignard reagents. 2.8 Difluoromethylthiolation Similar to the trifluoromethylthiolated compounds, difluoromethythiolated organic compounds have found applications as pharmaceuticals or agrochemicals. Flomoxef is a cephalomycin-based antibiotic, used in postoperative prophylaxis. It is active against methicillin-resistant and susceptible Staphylococcus aureus and is active against other bacteria, including Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis.67 Pyriprole is used as a veterinary medicine for treating tick infections in dogs (Fig. 234).68 Lu, Shen, and coworkers have developed synthetic methods for the difluoromethylthiolation of amines, thiols, alkynes, and enolates of carbonyl compounds and for the difluoromethylthiolation of aromatic compounds.6 They synthesized the CF2 analog of the Munavalli’s reagent, 98, through the reaction of the N-(chlorothio)phthalimide (97) with the N-heterocyclic carbene(SIPr)stabilized difluoromethyl-silver(I) [SIPrAg(CF2H)]. SCF3 OH DAST/CF3TMS OH DIPEA,DCM –60°C–RT, 18 h 89 90 O OSiMe3 SCF3 DAST/CF3TMS DIPEA,DCM –60°C–RT, 18 h 91 H 3C 92 H 3C CH3 H 3C H 3C H 3C H 3C H 3C H H CH3 DAST/CF3TMS H H 3C F3 CS H H DIPEA,DCM Me 3 SiO H O –60°C, 4 h 93 (silylenol ether of 4-cholesten-3-one) 94 55% (dr: 9:1) Mechanistic outline: Et Et N S F DAST F Et F Et N –Me 3 SiF F F S Et F C SiMe3 F F Et N F S F F3 C F 96 95 OSiMe3 O 91 SCF3 + Multiple steps Et 92 HF + N FIGURE 2–33 Electrophilic trifluoromethylthiolation of enolsilyl ethers and a mechanistic outline. OH O N N N N S N OO N OH O H N OH Me Flomoxef (a cephalomycin antibiotic) H F S F N F F3 C F S HN Cl N N Cl Pyriprole (veterinary medicine for tick infections) FIGURE 2–34 Difluoromethylthiolated compounds used in pharmaceutical and veterinary applications. 70 Organofluorine Chemistry O N SCl 97 O B(OH) 2 SCF2 H i-Pr i-Pr N Cat. CuI and bpy i-Pr Li2 CO3 , diglyme 60°C, 15 h 99 N i-Pr Ag(CF2 H) SIPrAg(CF 2H) O O CO2 R O SCF2H CO2R 100 N SCF2 H K2CO3 , DCM RT, 24 h 98 H N R R' Toluene, 80°C 14–24 h SCF2H R O R N R' 103 H R SCF2H Cat. CuTc and bpy Li2 CO3 , diglyme, 60°C, 15 h SCF2 H TMSCl N H 101 N H 104 RSH RSSCF2H ClCH2CH 2Cl 80–120°C, 16 h ClCH2CH 2Cl 80°C, 16–24 h 102 FIGURE 2–35 Difluoromethylthiolation reactions using the N-(difluoromethyl)phthalimide. bpy, Bipyridine; CuTc, copper(I)-thiophen-2-carboxylate; DCM, dichloromethane; TMSCl, trimethylsilyl chloride. This reagent is shelf-stable, has similar reactivity as for the Munavalli’s reagent, and is a powerful electrophilic difluoromethylthiolation reagent. A wide range of aryl and vinylboronic acids, alkynes, amines, thiols, β-ketoesters, oxindoles, electron-rich aromatics, such as indole, pyrrole, isoxazole, and pyrazole, could be difluoromethylthiolated with this reagent under relatively mild conditions (Fig. 235). Electron-rich aromatics, such as indoles and pyrroles, undergo electrophilic aromatic substitution with this reagent in the presence of catalytic amounts of the mild Lewis acid, trimethylsilyl chloride (TMSCl). Aryl- and vinylboronic acids and terminal alkynes undergo Cu(I)-catalyzed trifluoromethylthiolation with 98 in high yields. Relatively more nucleophilic compounds, such as amines, thiols, β-ketoesters, and oxindoles, react with 98, even in the absence of any organometallic catalyst. References 1. Taylor, S. D.; Kotoris, C. C.; Hum, G. Recent Advances in Electrophilic Fluorination. Tetrahedron 1999, 55, 1243112477. 2. McPake, C. B.; Murray, C. B.; Sandford, G. Continuous Flow Synthesis of Difluoroamine Systems by Direct Fluorination. Aust. J. Chem. 2013, 66, 145150. Chapter 2 • Electrophilic reactions in the synthesis of organofluorine compounds 71 3. Turnbull, M. D.; Carter, N. B.; Dennison, S.; Deacon, J.; Holley, R. Microfluidics-Based Reactors for Safe Fluorinations Using Elemental Fluorine. Chimia 2004, 58, 159162. 4. Umemoto, T.; Zhang, B.; Zhu, T.; Zhou, X.; Zhang, P.; Hu, S.; Li, Y. Powerful, Thermally Stable, One-PotPreparable, and Recyclable Electrophilic Trifluoromethylating Agents: 2,8-Difluoro- and 2,3,7,8Tetrafluoro-S-(trifluoromethyl)dibenzothiophenium Salts. J. Org. Chem. 2017, 82, 77087719. 5. Charpentier, J.; Fruh, N.; Togni, A. Electrophilic Trifluoromethylation by Use of Hypervalent Iodine Reagents. Chem. Rev. (Washington, DC) 2015, 115, 650682. 6. Zhu, D.; Gu, Y.; Lu, L.; Shen, Q. N-Difluoromethylthiophthalimide: A Shelf-Stable, Electrophilic Reagent for Difluoromethylthiolation. J. Am. Chem. Soc. 2015, 137, 1054710553. 7. Hardy, M. A.; Chachignon, H.; Cahard, D. Advances in Asymmetric Di- and Trifluoromethylthiolation and Di- and Trifluoromethoxylation Reactions. Asian J. Org. Chem. 2019, 8, 591609. 8. Barata-Vallejo, S.; Bonesi, S.; Postigo, A. Late Stage Trifluoromethylthiolation Strategies for Organic Compounds. Org. Biomol. Chem. 2016, 14, 71507182. 9. Billard, T. Trifluoromethanesulfenamides: New Reagents for Direct S-CF3 Bond Formation. Chim. Oggi 2016, 34, 1821. 10. Rozatian, N.; Ashworth, I. W.; Sandford, G.; Hodgson, D. R. W. A Quantitative Reactivity Scale for Electrophilic Fluorinating Reagents. Chem. Sci. 2018, 9, 86928702. 11. Toullec, P. Y.; Devillers, I.; Frantz, R.; Togni, A. Relative Electrophilic Fluorinating Power as Assayed by Competitive Catalytic Halogenation Reactions. Helv. Chim. Acta 2004, 87, 27062711. 12. Nyffeler, P. T.; Duron, S. G.; Burkart, M. D.; Vincent, S. P.; Wong, C.-H. Selectfluor: Mechanistic Insight and Applications. Angew. Chem., Int. Ed. 2005, 44, 192212. 13. Lal, G. S.; Pez, G. P.; Syvret, R. G. Electrophilic NF Fluorinating Agents. Chem. Rev. (Washington, DC) 1996, 96, 17371755. 14. Sim, J.; Campbell, M. W.; Molander, G. A. Synthesis of α-Fluoro-α-Amino Acid Derivatives via Photoredox-Catalyzed Carbofluorination. ACS Catal. 2019, 9, 15581563. 15. Singh, R. P.; Shreeve, J. N. M. Recent Highlights in Electrophilic Fluorination with 1-Chloromethyl-4Fluoro-1,4-Diazoniabicyclo[2.2.2]Octane Bis(tetrafluoroborate). Acc. Chem. Res. 2004, 37, 3144. 16. Teare, H.; Robins, E. G.; Kirjavainen, A.; Forsback, S.; Sandford, G.; Solin, O.; Luthra, S. K.; Gouverneur, V. Radiosynthesis and Evaluation of [18F]Selectfluor Bis(triflate). Angew. Chem., Int. Ed. 2010, 49, 68216824. 17. Fuchtner, F.; Steinbach, J. Efficient Synthesis of the 18F-Labeled 3-O-Methyl-6-[18F]Fluoro-L-DOPA. Appl. Radiat. Isot. 2003, 58, 575578. 18. Wood, S. H.; Etridge, S.; Kennedy, A. R.; Percy, J. M.; Nelson, D. J. The Electrophilic Fluorination of Enol Esters Using SelectFluor: A Polar Two-Electron Process. Chem. Eur. J. 2019, 25, 55745585. 19. Kaiser, H. B.; Naclerio, R. M.; Given, J.; Toler, T. N.; Ellsworth, A.; Philpot, E. E. Fluticasone Furoate Nasal Spray: A Single Treatment Option for the Symptoms of Seasonal Allergic Rhinitis. J. Allergy Clin. Immunol. 2007, 119, 14301437. 20. Parri, G.; Nieri, D.; Roggi, M. A.; Vagaggini, B.; Celi, A.; Paggiaro, P. Fluticasone Furoate, Umeclidinium Bromide, and Vilanterol as a Combination Therapy for Chronic Obstructive Pulmonary Disease. Expert Rev. Respir. Med. 2018, 12, 9971005. 21. Malerba, M.; Nardin, M.; Santini, G.; Mores, N.; Radaeli, A.; Montuschi, P. Single-Inhaler Triple Therapy Utilizing the Once-Daily Combination of Fluticasone Furoate, Umeclidinium and Vilanterol in the Management of COPD: The Current Evidence Base and Future Prospects. Ther. Adv. Respir. Dis. 2019, 12 1753466618760779/1753466618760771-1753466618760779/1753466618760779. 72 Organofluorine Chemistry 22. Cherniak, S.; Cyjon, R.; Ozer, I.; Nudelman, I. Process for the Preparation of 17-Desoxy-Corticosteroids WO2012011106A1; Taro Pharmaceutical Industries Ltd.: Israel, 2012. 23. Sessler, C. D.; Rahm, M.; Becker, S.; Goldberg, J. M.; Wang, F.; Lippard, S. J. CF2H, a Hydrogen Bond Donor. J. Am. Chem. Soc. 2017, 139, 93259332. 24. Jin, Y.; Bhattasali, D.; Pellegrini, E.; Forget, S. M.; Baxter, N. J.; Cliff, M. J.; Bowler, M. W.; Jakeman, D. L.; Blackburn, G. M.; Waltho, J. P. α-Fluorophosphonates Reveal How a Phosphomutase Conserves Transition State Conformation Over Hexose Recognition in Its Two-Step Reaction. Proc. Natl. Acad. Sci. U.S.A. 2014, 111, 1238412389. 25. Wnuk, S. F.; Bergolla, L. A.; Garcia, P. I., Jr. Studies Toward the Synthesis of α-Fluorinated Phosphonates via Tin-Mediated Cleavage of α-Fluoro-α-(pyrimidin-2-ylsulfonyl)alkylphosphonates. Intramolecular Cyclization of the α-Phosphonyl Radicals. J. Org. Chem. 2002, 67, 30653071. 26. Yokomatsu, T.; Murano, T.; Akiyama, T.; Koizumi, J.; Shibuya, S.; Tsuji, Y.; Soeda, S.; Shimeno, H. Synthesis of Non-Competitive Inhibitors of Sphingomyelinases with Significant Activity. Bioorg. Med. Chem. Lett. 2003, 13, 229236. 27. Steiner, D. D.; Mase, N.; Barbas, C. F., III Direct Asymmetric α-Fluorination of Aldehydes. Angew. Chem., Int. Ed. 2005, 44, 37063710. 28. Marigo, M.; Fielenbach, D.; Braunton, A.; Kjoersgaard, A.; Jorgensen, K. A. Enantioselective Formation of Stereogenic Carbon-Fluorine Centers by a Simple Catalytic Method. Angew. Chem., Int. Ed. 2005, 44, 37033706. 29. Shibatomi, K.; Kitahara, K.; Okimi, T.; Abe, Y.; Iwasa, S. Enantioselective Fluorination of α-Branched Aldehydes and Subsequent Conversion to α-Hydroxyacetals via Stereospecific C-F Bond Cleavage. Chem. Sci. 2016, 7, 13881392. 30. Huang, H.; Meegalla, S. K.; Lanter, J. C.; Winters, M. P.; Zhao, S.; Littrell, J.; Qi, J.; Rady, B.; Lee, P. S.; Liu, J.; Martin, T.; Lam, W. W.; Xu, F.; Lim, H. K.; Wilde, T.; Silva, J.; Otieno, M.; Pocai, A.; Player, M. R. Discovery of a GPR40 Superagonist: The Impact of Aryl Propionic Acid α-Fluorination. ACS Med. Chem. Lett. 2019, 10, 1621. 31. Ishimaru, T.; Shibata, N.; Horikawa, T.; Yasuda, N.; Nakamura, S.; Toru, T.; Shiro, M. Cinchona Alkaloid Catalyzed Enantioselective Fluorination of Allyl Silanes, Silyl Enol Ethers, and Oxindoles. Angew. Chem., Int. Ed. 2008, 47, 41574161. 32. Souza, L. G.; de O. Domingos, J. L.; de A. Fernandes, T.; Renno, M. N.; Sansano, J. M.; Najera, C.; Costa, P. R. R. Enantioselective Electrophilic Fluorination of α-Aryl-Tetralones Using a Preparation of NFluoroammonium Salts of Cinchonine. J. Fluorine Chem. 2019, 217, 7279. 33. Sun, Y.; Zhou, R.; Xu, H.; Wang, D.; Su, X.; Wang, C.; Ding, Y.; Wang, L.; Chen, Y. Syntheses and Biological Evaluation of BE-43547A2 Analogs Modified at O35 Ester and C15-OH Sites. Tetrahedron 2019, 75, 18081818. 34. Curtis, N. R.; Davies, S. H.; Gray, M.; Leach, S. G.; McKie, R. A.; Vernon, L. E.; Walkington, A. J. Asymmetric Fluorination Approach to the Scalable Synthesis of a SYK Inhibitor. Org. Process Res. Dev. 2015, 19, 865871. 35. Huhmann, S.; Koksch, B. Fine-Tuning the Proteolytic Stability of Peptides with Fluorinated Amino Acids. Eur. J. Org. Chem. 2018, 2018, 36673679. 36. Marsh, E. N. G. Fluorinated Proteins: From Design and Synthesis to Structure and Stability. Acc. Chem. Res. 2014, 47, 28782886. 37. Smits, R.; Cadicamo, C. D.; Burger, K.; Koksch, B. Synthetic Strategies to α-Trifluoromethyl and α-Difluoromethyl Substituted α-Amino Acids. Chem. Soc. Rev. 2008, 37, 17271739. 38. Ulbrich, D.; Daniliuc, C. G.; Haufe, G. Halofluorination of N-Protected α,β-Dehydro-α-Amino Acid Esters a Convenient Synthesis of α-Fluoro-α-Amino Acid Derivatives. J. Fluorine Chem. 2016, 188, 6575. Chapter 2 • Electrophilic reactions in the synthesis of organofluorine compounds 73 39. Gelat, F.; Patra, A.; Pannecoucke, X.; Biju, A. T.; Poisson, T.; Besset, T. N-Heterocyclic Carbene-Catalyzed Synthesis of α-Trifluoromethyl Esters. Org. Lett. 2018, 20, 38973901. 40. Prakash, G. K. S.; Weber, C.; Chacko, S.; Olah, G. A. New Electrophilic Difluoromethylating Reagent. Org. Lett. 2007, 9, 18631866. 41. Prakash, G. K. S.; Ledneczki, I.; Chacko, S.; Olah, G. A. Direct Electrophilic Monofluoromethylation. Org. Lett. 2008, 10, 557560. 42. Zhu, J.; Liu, Y.; Shen, Q. Direct Difluoromethylation of Alcohols with an Electrophilic Difluoromethylated Sulfonium Ylide. Angew. Chem., Int. Ed. 2016, 55, 90509054. 43. Leroux, F. R.; Manteau, B.; Vors, J.-P.; Pazenok, S. Trifluoromethyl Ethers: Synthesis and Properties of an Unusual Substituent. Beilstein J. Org. Chem. 2008, 4 (13), 115. 44. Leo, A.; Hansch, C.; Elkins, D. Partition Coefficients and Their Uses. Chem. Rev. 1971, 71, 525616. 45. Ghiazza, C.; Tlili, A.; Billard, T. Electrophilic Trifluoromethylselenolation of Boronic Acids. Molecules 2017, 22, 833/831833/838. 46. Guo, S.; Cong, F.; Guo, R.; Wang, L.; Tang, P. Asymmetric Silver-Catalysed Intermolecular Bromotrifluoromethoxylation of Alkenes with a New Trifluoromethoxylation Reagent. Nat. Chem. 2017, 9, 546551. 47. Chatalova-Sazepin, C.; Binayeva, M.; Epifanov, M.; Zhang, W.; Foth, P.; Amador, C.; Jagdeo, M.; Boswell, B. R.; Sammis, G. M. Xenon Difluoride Mediated Fluorodecarboxylations for the Syntheses of Di- and Trifluoromethoxyarenes. Org. Lett. 2016, 18, 45704573. 48. Zhou, M.; Ni, C.; Zeng, Y.; Hu, J. Trifluoromethyl Benzoate: A Versatile Trifluoromethoxylation Reagent. J. Am. Chem. Soc. 2018, 140, 68016805. 49. Haas, A.; Moeller, G. Preparation and Reactivity of Tris(trifluoromethylselanyl)carbenium [(CF3Se)3C1] and Trifluoromethylsulfanylacetic Acid Derivatives [(CF3S)3-nCXn(O)OR]. Chem. Ber 1996, 129, 13831388. 50. Munavalli, S.; Rohrbaugh, D. K.; Rossman, D. I.; Berg, F. J.; Wagner, G. W.; Durst, H. D. Trifluoromethylsulfenylation of Masked Carbonyl Compounds. Synth. Commun. 2000, 30, 28472854. 51. Baert, F.; Colomb, J.; Billard, T. Electrophilic Trifluoromethanesulfanylation of Organometallic Species with Trifluoromethanesulfanamides. Angew. Chem., Int. Ed. 2012, 51, 1038210385 S10382/10381S10382/10385. 52. Ma, B.; Shao, X.; Shen, Q. BrØnsted Acid-Catalyzed Electrophilic Trifluoromethylthiolation of Indoles Using Thermally Stable Trifluoromethylthiolating Reagent. J. Fluorine Chem. 2015, 171, 7377. 53. Shao, X.; Wang, X.; Yang, T.; Lu, L.; Shen, Q. An Electrophilic Hypervalent Iodine Reagent for Trifluoromethylthiolation. Angew. Chem., Int. Ed. 2013, 52, 34573460. 54. Alazet, S.; Ismalaj, E.; Glenadel, Q.; Le Bars, D.; Billard, T. Acid-Catalyzed Synthesis of α-Trifluoromethylthiolated Carbonyl Compounds. Eur. J. Org. Chem. 2015, 2015, 46074610. 55. Yang, Y.-D.; Azuma, A.; Tokunaga, E.; Yamasaki, M.; Shiro, M.; Shibata, N. Trifluoromethanesulfonyl Hypervalent Iodonium Ylide for Copper-Catalyzed Trifluoromethylthiolation of Enamines, Indoles, and β-Keto Esters. J. Am. Chem. Soc. 2013, 135, 87828785. 56. Vinogradova, E. V.; Mueller, P.; Buchwald, S. L. Structural Reevaluation of the Electrophilic Hypervalent Iodine Reagent for Trifluoromethylthiolation Supported by the Crystalline Sponge Method for X-Ray Analysis. Angew. Chem., Int. Ed. 2014, 53, 31253128. 57. Gelat, F.; Poisson, T.; Biju, A. T.; Pannecoucke, X.; Besset, T. Trifluoromethylthiolation of α-Chloroaldehydes: Access to Quaternary SCF3-Containing Centers. Eur. J. Org. Chem. 2018, 2018, 36933696. 58. Zhu, S.-Q.; Xu, X.-H.; Qing, F.-L. Oxidative Trifluoromethylthiolation of Terminal Alkynes with AgSCF3. A Convenient Approach to Alkynyl Trifluoromethyl Sulfides. Eur. J. Org. Chem. 2014, 2014, 44534456. 74 Organofluorine Chemistry 59. Chachignon, H.; Kondrashov, E. V.; Cahard, D. Diastereoselective Electrophilic Trifluoromethylthiolation of Chiral Oxazolidinones: Access to Enantiopure α-SCF3 Alcohols. Adv. Synth. Catal. 2018, 360, 965971. 60. Alazet, S.; Billard, T. Electrophilic Aromatic Trifluoromethylthiolation with the Second Generation of Trifluoromethanesulfenamide. Synlett 2015, 26, 7678. 61. Alazet, S.; Zimmer, L.; Billard, T. Base-Catalyzed Electrophilic Trifluoromethylthiolation of Terminal Alkynes. Angew. Chem., Int. Ed. 2013, 52, 1081410817. 62. Glenadel, Q.; Alazet, S.; Baert, F.; Billard, T. Multigram Scale Syntheses of First and Second Generation of Trifluoromethanesulfenamide Reagents. Org. Process Res. Dev. 2016, 20, 960964. 63. Tlili, A.; Alazet, S.; Glenadel, Q.; Billard, T. Copper-Catalyzed Perfluoroalkylthiolation of Alkynes with Perfluoroalkanesulfenamides. Chem. Eur. J. 2016, 22, 1023010234. 64. Alazet, S.; Billard, T.; Ollivier, K. Direct Electrophilic N-Trifluoromethylthiolation of Amines with Trifluoromethanesulfenamide. Beilstein J. Org. Chem. 2013, 9, 23542357. 65. Ferry, A.; Billard, T.; Langlois, B. R.; Bacque, E. Synthesis of Trifluoromethanesulfinamidines and -Sulfanylamides. J. Org. Chem. 2008, 73, 93629365. 66. Saravanan, P.; Anbarasan, P. An Electrophilic Trifluoromethylthiolation of Silylenol Ethers and β-Naphthols with Diethylaminosulfur Trifluoride and (Trifluoromethyl)trimethylsilane. Adv. Synth. Catal. 2018, 360, 28942899. 67. Yang, Q.; Zhang, H.; Cheng, J.; Xu, Z.; Hou, X.; Xu, Y. Flomoxef Showed Excellent In Vitro Activity Against Clinically Important Gram-Positive and Gram-Negative Pathogens Causing Community- and Hospital-Associated Infections. Diagn. Microbiol. Infect. Dis. 2015, 81, 269274. 68. Fourie, J. J.; Horak, I. G.; de la Puente Redondo, V. Efficacy of a Spot-On Formulation of Pyriprole on Dogs Infested with Sarcoptes scabiei. Vet. Rec. 2010, 167, 442445. 3 Free-radical reactions in the synthesis of organofluorine compounds Chapter Outline 3.1 Introduction ................................................................................................................................... 75 3.2 Reagents for the free-radical trifluoromethylation ................................................................... 77 3.3 Decarboxylative fluoroalkylation ................................................................................................ 78 3.3.1 Decarboxylative trifluoromethylation ............................................................................... 78 3.3.2 Decarboxylate difluoromethylation................................................................................... 78 3.4 β-Amino-fluoroalkylation of alkenes .......................................................................................... 80 3.4.1 Cu(I)-catalyzed amino-fluoroalkylation ............................................................................. 80 3.4.2 Fe(II)-catalyzed azido- and amino-trifluoromethylation.................................................. 81 3.4.3 Ru(II)-catalyzed amino-fluoroalkylation ............................................................................ 81 3.5 Fluoroalkylation using sodium triflinate (Langlois reagent) .................................................... 82 3.5.1 Aromatic trifluoromethylation........................................................................................... 84 3.5.2 Hydro-trifluoromethylation of alkenes ............................................................................. 86 3.5.3 Trifluoromethylation of arylboronic acids ........................................................................ 86 3.5.4 Azido-fluoroalkylation of alkenes ..................................................................................... 88 3.5.5 Electrochemical oxy- and amino-trifluoromethylation .................................................... 91 3.5.6 Selective trifluoromethylation of proteins........................................................................ 93 3.6 Photoredox-catalyzed S-fluoroalkylation and arylation ........................................................... 94 3.7 Radical fluoroalkylation of enolates ........................................................................................... 96 References............................................................................................................................................. 98 3.1 Introduction Fluoroalkylation, in particular, trifluoromethylation and difluoromethylation, plays a key role in the design of pharmaceuticals. In some cases, trifluoromethyl moiety significantly enhances metabolic stability and bioavailability of the drug candidates. Sitagliptin, an antidiabetic drug, for example, has an oral bioavailability of 76%, whereas its analog, with CF3 replaced by ethyl moiety (the lead candidate), has negligible oral bioavailability (2%) (Fig. 31).1 The corresponding derivative with a CF2 moiety in place of the CF3 has 39% Organofluorine Chemistry. DOI: https://doi.org/10.1016/B978-0-12-813286-9.00003-1 © 2020 Elsevier Inc. All rights reserved. 75 76 Organofluorine Chemistry F F NH2 O F3 C N N O H N N N F Cl N N Me CF3 N Me F O R NH 2 CNP520 BACE-1 inhibitor; in AD prevention clinical trials at Novartis R = CF3; Sitagliptin (antidiabetic) Oral bioavailability: 76% (R = CF3 ); 39% (R = CF2H); 2% (R = Et) Log D (pH 6.8) = 3.5 pKa = 7.2 F F N CF3 N F3 C Cl O O O N H CF3 HO O H N O H3 C H3 C O CH3 NH N CH3 O Mefloquine (antimalarial drug) Efavirenz (anti-HIV drug) N NH O O O HN S F O CH3 F F Cl N F O H N Cl Voxilaprevir (antihepatitis drug) O O Roflumilast (NSAID for the treatment of COPD) FIGURE 3–1 Selected pharmaceuticals containing CF3 or CF2H substituents. bioavailability, substantially higher than that of the lead compound with the ethyl moiety. Sitagliptin acts as an antidiabetic drug through its inhibition of dipeptidyl peptidase-4 (DPP-4), thereby increasing insulin secretion from the pancreatic beta cells. The DPP-4 inhibitory effect of the sitagliptin and its CF2H analog is also significantly higher than that of the parent lead compound (i.e., with an ethyl group instead of CF3). The trifluoromethyl-substituted aryl rings also may be involved in donor-acceptor ππ stacking interactions with the side-chain aryl moieties at the enzyme active sites, thereby enhancing their binding affinity. The fluoroalkyl moieties adjacent to the amino groups substantially reduce the basicity of the amines, thereby enhancing the lipophilicity and cell permeability of the compounds. Novartis’ CNP520, a drug candidate in clinical trials for the prevention of Alzheimer’s disease, is a β-secretase-I (BACE-1) inhibitor. The effect of the two trifluoromethyl moieties in this drug candidate is to lower the basicity of the compound and thereby to improve the lipophilicity.2,3 Many other pharmaceuticals or drug candidates in clinical trials are trifluoromethyl- or, in some cases, difluoromethyl-containing compounds Chapter 3 • Free-radical reactions in the synthesis of organofluorine compounds 77 (e.g., voxilaprevir, an antihepatitis drug; glecaprevir, an antiviral drug) (Fig. 31) (see Chapter 5: Pharmaceutical applications of organofluorine compounds). Roflumilast, a nonsteroidal antiinflammatory agent, for treating chronic obstructive pulmonary disease, has a difluoromethoxy moiety. Thus in addition to the fluoroalkyl-containing compounds, fluoroalkoxy (RFO)- and also fluoroalkylthio (RFS)-containing compounds, show pharmacological activities. Novel and increasingly more eco-friendly synthetic methods for the fluoroalkylation are continually developed, and it is hoped that the drug discovery would be accelerated through these improved synthetic methods. Fluoroalkyl moieties also impart favorable properties to functional materials, and therefore fluoroalkylation and perfluoroalkylation are increasingly applied in the design of materials, including liquid crystals, biomaterials, high energy materials, and surface-active agents (see Chapter 7: Materials applications of organofluorine compounds). A variety of commercially available electrophilic trifluoromethylating reagents, such as Togni’s reagent and Umemoto’s reagent can be used in the free-radical fluoroalkylation reactions, under photoredox conditions, or using transition metal catalysis. Organometallic catalysis, for example, Ir(III) or Cu(I) catalysis, is used in the decarboxylative trifluoromethylations and difluoromethylations; these reactions proceed through the formation of the fluoroalkyl radicals as the reactive intermediates. Togni’s and Umemoto’s reagents can be used as the sources of trifluoromethyl radicals, under the free-radical conditions (vide infra). Langlois reagent has emerged as the convenient trifluoromethylating reagent for aromatic trifluoromethylations, oxidative- and azido-trifluoromethylation of alkenes, and dehalogenative trifluoromethylation of aryl iodides. These reactions, when carried out under organometallic or photoredox conditions, involve trifluoromethyl radical as the reactive intermediate. Langlois reagent, thus, provides a cost-effective alternative for the trifluoromethylations over the other conventionally used trifluoromethylating agents (vide infra). Langlois reagent also is used for the selective trifluoromethylation of proteins under free-radical conditions. The trifluoromethyl radical, generated from the Langlois reagent, selectively achieves trifluoromethylation of the electron-rich tryptophan residues, as compared to the histidine, tyrosine, and phenylalanine residues (vide infra). 3.2 Reagents for the free-radical trifluoromethylation Some of the widely used reagents for the electrophilic trifluoromethylation, for example, Togni’s and Umemoto’s reagents (Fig. 32), can also generate trifluoromethyl radicals under O O I CF3 Togni's reagent I O I CF3 Togni's reagent II FIGURE 3–2 Structures of Togni’s and Umemoto’s reagents. S CF3 BF 4 Umemoto's reagent 78 Organofluorine Chemistry the photoredox conditions or in the presence of organometallic catalysts. Thus in the freeradical trifluoromethylation reactions, reactive CF3 radicals could be generated through single-electron transfer (SET) redox reactions of CF3I,46 Togni’s reagent,7,8 Umemoto reagent,9 CF3SiMe3 (RuppertPrakash reagent), trifluoroacetic anhydride,10 and sodium triflinate (Langlois reagent).11,12 gem-Difluoromethylation and polyfluoroalkylation can similarly be achieved using the corresponding fluoroalkyl analogs of these reagents.47 3.3 Decarboxylative fluoroalkylation 3.3.1 Decarboxylative trifluoromethylation Under free-radical conditions, through a combination of photoredox and copper/iridium dual catalysis, carboxylic acids undergo decarboxylative trifluoromethylation by the Togni’s reagent, used as a source of the trifluoromethyl free radical, to give their corresponding trifluoromethylated compounds.13 Togni’s reagent I was found to be superior to Togni’s reagent II and Umemoto reagents in the decarboxylative trifluoromethylation of aliphatic carboxylic acids. This synthetic method is of broad scope, tolerating a variety of functional groups, such as olefins, alcohols, carbonyls, alcohols, heterocycles, and strained rings, and therefore provides a means of late-stage functionalization of pharmaceuticals. Pharmaceutically interesting compounds, such as fenbufen, ionazolac, and isoxepac, are decarboxylative trifluoromethylated through this Ir(III)/Cu(II) dual catalysis, in high yields. In this dual catalysis mechanism, the Cu(II)OCOR is oxidized to Cu(III)OCOR by the photoexcited Ir(III) catalyst. Decarboxylation of the Cu(III)-carboxylate, followed by SET to the corresponding intermittent alkyl radical forms the [Cu(III)]R. The latter Cu(III) species, through a series of steps involving Ir(II)-catalyzed reduction to Cu(II) species, followed by reaction with the Togni's reagent, and reductive elimination, forms the RCF3 (Fig. 33). This onestep synthetic method thus gives access to trifluoromethyl analogs of the pharmaceuticals through their late-stage modification. A silver-catalyzed decarboxylative trifluoromethylation, with AgNO3 as the catalyst and K2S2O8 as the oxidant, also involves formation of the transient alkyl radicals, from the carboxylic acids.14 3.3.2 Decarboxylate difluoromethylation Cu(I)-catalyzed gem-difluoromethylation of redox-active carboxylic acid phthalimido esters was accomplished under mild conditions, using the in situ generated Cu(I)(CF2H) reagent [formed through transmetalation of [Zn(II)](CF2H) with Cu(I)Cl].15 The phthalimido esters of the carboxylic acids can also be prepared in situ by the peptide coupling methods. This decarboxylative difluoromethylation reaction tolerates a variety of functional groups, including halogens, aldehydes, esters, nitrogen- and oxygen-containing heterocycles, phenolic hydroxyls, and terminal alkynes (Fig. 34). The electron-withdrawing phthalimido moiety in the Chapter 3 • Free-radical reactions in the synthesis of organofluorine compounds F3 C Ir[dF(CF3 )ppy](4,4' -dCF 3 bpy)PF 6 (1 ); 1 mol% CuCN (20 mol%), bathophen (30 mol%) O R OH O + I CF3 F3C BTMG (0.5 Eq), EtOAc, H2 O blue LEDs, 6 h + PF6 F N N RCF3 79 F F Ir N N F3C F F3C Togni’s reagent I 1 F 3C Selected examples: O CF3 CF3 CF3 Ph N O N F O Cl (From fenbufen) (From ionazolac) (From isoxepac) Mechanistic outline of Ir(III)/Cu(II) dual catalysis: CO2 Ir(III) * Ir(III) [Cu(II)]OCOR [Cu(II)] R [Cu(III)]OCOR SET Ir(II) [Cu(III)]R [Cu(II)]R O [Cu(III)]R OH F O [Cu(II)]OCOR [Cu(II)] I O R = 4-fluoroethyl I CF3 OH I [Cu(III)]R(CF3) CF3 O I F FIGURE 3–3 Decarboxylative trifluoromethylation of carboxylic acids under photoredox and copper/iridium catalysis. bathophen, 4,7-Diphenyl-1,10-phenanthroline; BTMG, 2-tert-butyl-1,1,3,3-tetramethylguanidine. redox-active ester 2 lowers the redox potential of the carboxy moiety. The Cu(I)(CF2)H, through a SET to the redox-active ester 2, gives the transient carboxy radical (R0 CO2•), spontaneous decarboxylation of which forms the corresponding alkyl radical (R0 •). The Cu(II) species 5, through a subsequent single-electron redox reaction with the alkyl radical (R0 •), forms the high-valent Cu(III) intermediate (6), which undergoes reductive elimination to give the difluoromethylated product 3, regenerating the Cu(I) catalyst for further propagation of the catalytic cycle.15 80 Organofluorine Chemistry Cl Cl O Cl O O O OH N bipyridine (20 mol%) Cl O DIC, DMAP (DMPU) 2Zn(CF 2 H)2 CuCl (20 mol%) DMSO, 60 °C, 8 h R R 2 Prepared in situ or isolated CF2H R 3 Selected examples: Br CF2H CF2H N F O F O O CF2 H CF2H H O 85% 88% 80% 94% Mechanistic outline: [Zn]-CF2 H L-Cu(I)-X [Zn]X L-Cu(I)-CF 2H 4 R′CF2 H Redox-active ester 2 3 SET O CO2 + N O L-Cu(III)(CF 2 H)(R′)(X) 6 L-Cu(II)(X)(CF 2 H) . R′ 5 Cl Cl Cl Cl CH 2 R' = R FIGURE 3–4 Cu(I)-catalyzed decarboxylative gem-difluoromethylation of carboxylic acids. DIC, N,N'diisopropylcarbodiimide; DMAP, 4-(N,N-dimethylamino)pyridine; DMPU, N,N'-dimethylpropyleneurea. 3.4 β-Amino-fluoroalkylation of alkenes 3.4.1 Cu(I)-catalyzed amino-fluoroalkylation Cu(I)-catalyzed β-amino-trifluoromethylation of γ-ureido-alkenes (7), using the Togni’s reagent as the source of the trifluoromethyl radical, and a chiral phosphoric acid as a Chapter 3 • Free-radical reactions in the synthesis of organofluorine compounds 81 O O R1 R2 N H N H R' O I CF3 (Togni's reagent) CuCl R1 R2 HN R' N O Chiral phosphoric acid catalyst R R CF3 7 8 R1 O R1 R2 N H R 7 N H R' n-C 4F9 SO2 Cl R2 HN R' N O CuBr (10 mol%) Ag 2CO3 (0.6 equiv) Chiral phosophoric acid R n-C 4 F9 9 FIGURE 3–5 Cu(I)-catalyzed asymmetric amino-trifluoromethylation reactions using Togni’s reagent, and aminoperfluoroalkylation reactions using perfluoroalkylsulfonyl chloride as the source of the fluoroalkyl radicals. catalytic system, gives the corresponding trifluoromethylated aza-heterocycles 8 with high enantiomeric purity (Fig. 35).16 This synthetic method was also extended to aminoperfluoroalkylation of γ-ureido-alkenes to give the perfluoroalkyl-substituted pyrrolidines 9, using perfluoroalkylsulfonyl chlorides, as the source of the perfluoroalkyl radicals, and catalytic amounts of CuBr and a chiral phosphoric acid catalyst.17 In this synthetic approach for the β-(polyfluoroalkyl)amines, Ag2CO3 was added as a mild base to neutralize the HCl byproduct. 3.4.2 Fe(II)-catalyzed azido- and amino-trifluoromethylation Xu et al. have developed Fe(II)-catalyzed azido-trifluoromethylation of alkenes, using Togni’s reagent as a source of trifluoromethyl free-radical intermediate and trimethylsilyl azide (TMSN3) as the azide anion precursor.18 Reduction of the azide moiety to the corresponding amino group could be effected using catalytic hydrogenation in high yields. The mechanism for this azido-trifluoromethylation was shown to involve the Fe(II)-catalyzed generation of the trifluoromethyl radical, which adds to the terminal carbon of the olefins to give the secondary free radical 13. Oxidation of the free radical to the carbocation species by the [Fe(III)], followed by azide anion capture gives the β-(azido)trifluoromethyl compounds, 10 (Fig. 36). 3.4.3 Ru(II)-catalyzed amino-fluoroalkylation Umemoto’s reagent (15), under photoredox conditions, acts as a source of trifluoromethyl radical and thus is useful for the β-azido- or β-amino-trifluoromethylation of alkenes, in the presence of a nucleophilic source, such as trimethylsilyl azide (TMSN3) or an amine. Using Ru(II) as a photocatalyst, and azide anion as the nucleophile, various substituted 82 Organofluorine Chemistry Togni's reagent Fe(OAc) 2 (10 mol%) R R = alky/aryl Ligand 12 (10 mol%) TMSN3 (1.5 equiv) DCM/MeCN, RT 1. H2 , Pd/C R CF3 2. TsOH N3 CF3 NH3 + – OTs N N 12 Ligand O 11 10 87% 86% (R = Ph) O I CF3 Togni's reagent Proposed mechanism: O TMSN3 Ln Fe(II)(OAc) 2 O R Ln Fe(II)(N 3) 2 O I CF3 R CF3 R CF3 13 Ln Fe(III)(N3 )2 (O2CAr) LnFe(III)(N 3 )2 (O 2CAr) Ln Fe(II)(N3)(O2CAr) CF3 R 10 N3 FIGURE 3–6 Fe(II)-catalyzed vicinal azido-trifluoromethylation of alkenes using Togni’s reagent as a source of trifluoromethyl radicals. styrenes, as well as activated and nonactivated (i.e., with electron-donating as well as electron-withdrawing substituents) alkenes, could be transformed into their corresponding β-(azido)trifluoromethyl derivatives (16) in good yields (Fig. 37).19 Similarly, reactions of alkenes with the Umemoto’s reagent (15) and primary amines (or amides such as p-toluenesulfonamide), under these free-radical conditions, give the corresponding β-aminotrifluoromethylated derivatives (17). A likely mechanism for these reactions involves the photo-generated Ru(II) catalyst, which acts as a free-radical initiator, forming trifluoromethyl radical from the Umemoto’s reagent (15). A sequence of reactions involving the trifluoromethyl radical addition to the olefins, followed by Ru(III)-mediated oxidation would give the carbocation intermediate 19, which is trapped by the nucleophilic azide anion or amines to give the corresponding products 16 and 17, respectively. The azide moiety could also be reduced to the corresponding primary amino group, and thus this synthetic strategy is useful for the convenient preparation of a broad range of β-amino-trifluoromethyl compounds. 3.5 Fluoroalkylation using sodium triflinate (Langlois reagent) Langlois reagent (sodium trifluoromethanesulfinate; sodium triflinate; CF3SO2Na) provides a cost-effective means of trifluoromethylation for the synthesis of a wide variety of organic Chapter 3 • Free-radical reactions in the synthesis of organofluorine compounds 83 N3 R' Ru(bpy) 3 (PF6 )2 (5 mol%) R TMSN 3 (3 equiv) blue LEDs DCM, RT 62%–80% yields R = e.g., p-OMe, p-tBu, p-Cl, o-Br R' = e.g., H, Me R R CF3 16 + S CF3 BF4 14 15 NHR' Ru(bpy) 3 (PF6 )2 (5 mol%) R' R R'NH2 (3 equiv) blue LEDs CF3 17 39%–66% yields R' = e.g., aryl, benzyloxycarbonyl (cbz), p-toluenesulfonyl (Ts) Mechanistic outline: Ru(II) R hν R Ru(II)* Ru(III) R (14) CF3 S CF3 R BF4 CF3 18 15 Ru(III) N3 R' R N 3- Ru(II) CF3 R 16 R CF3 19 NHR' R' R RNH2 CF3 17 FIGURE 3–7 β-(Azido)trifluoromethylation and β-amino-trifluoromethylation of alkenes under photoredox conditions. compounds, under free-radical conditions. Free-radical trifluoromethylation of aromatic compounds using this reagent was first demonstrated by Langlois et al. in the early 1990s.20 Baran and coworkers and others have more recently demonstrated the wide applicability of 84 Organofluorine Chemistry R CF3 R O CF3 R R R RCF3 RBF3 K R RB(OH)2 N3 CF3 R R R R R R OR' RCF3 CF3SO2 Na R R I R CF3 CF3 R R CF3 R R R FIGURE 3–8 Various functional group transformations using the Langlois reagent. the Langlois reagent and related fluoroalkyl sulfinates in various functional groups transformations.21 These fluoroalkyl sulfinates (also marketed as diversinates) proved to be of fundamental and practical interest in the modern drug discovery.2227 Langlois reagent has been used extensively for the trifluoromethylation of aromatics,28,29 oxidative-trifluoromethylation of alkenes,3034 hydro-trifluoromethylation of alkenes,30,35 azido-trifluoromethylation of alkenes,36,37 dehalogenative trifluoromethylation of aryl iodides,38 and for the transformation of alkyl- and arylboronic acids39 and organotrifluoroborates40 to the corresponding trifluoromethyl compounds (Fig. 38; vide infra). The area of fluoroalkylation reactions mediated by Langlois reagent continues to expand at a rapid rate.37,4143 3.5.1 Aromatic trifluoromethylation Li et al. have demonstrated photoredox-catalyzed trifluoromethylation of aromatics, using acetone or diacetyl (2,3-butanedione) as the photocatalysts, and Langlois reagent as the source of the trifluoromethyl radical.28 Whereas acetone as a photocatalyst requires irradiation in the UV range, below 330 nm, diacetyl-photo-catalyzed reactions could be performed in the visible range, .400 nm. These photoredox-catalyzed reactions of aromatics, in both cases, tolerate a broad range of substituents on the aromatic ring. Trifluoromethyl derivatives of uridine and deoxyuridine (trifluridine) could be synthesized from the corresponding nucleosides (or deoxynucleosides) in relatively low yields of 38% and 44%, respectively, using acetone as the photosensitizer. Free-radical trifluoromethylation of aromatics using diacetyl (2,3-butanedione) as the photocatalyst, under visible light irradiation, affords the trifluoromethylated products in moderate to high yields. Trifluoromethylated purine derivatives Chapter 3 • Free-radical reactions in the synthesis of organofluorine compounds 85 O Het R CF3 NaSO 2CF3 Arenes or heteroarenes Me Me Het R O >400 nm, air EtOAc:diacetyl (4:1) Diacetyl (photocatalyst) 42%–93% yields Selected examles: OH O Me Me CF3 N H CF3 O 20 21 65% (20 h) 75% (40 h) Me Me N N 23 82% (20 h) O F3 C Me 25 N O OH 27 Uridine-CF3 38% (40 h acetone/UV) O* hν F3 C O S + O Na Me Me O F3 C O O Na 28 H O S O HO OH OH 26 75% (40 h) NH O O Mechanistic outline: Me F3 C HO N H 93% (10 h) O NH N CF3 24 Me 65% (20 h) O CF3 O N N 22 O O CF3 CF3 N N Me Me Me H N N Trifluridine 44% (40 h acetone/UV) R CF3 O + SO 2 O H CF3 CF3 R R -H + CF3 R 30 29 Me + O Na H Me O 31 FIGURE 3–9 Photoredox-catalyzed trifluoromethylation of aromatics. 22 and 23, trifluoromethylated derivative of the aromatics, including phenolic antioxidant (4-trifluoromethyl-2,6-di-tert-butylphenol), 20, and heterocyclic aromatics, such as trifluoromethylated indoles (e.g., 21 and 25), were synthesized under visible light photoredox catalysis using diacetyl as the photocatalyst (Fig. 39). 86 Organofluorine Chemistry A proposed mechanism of this photoredox-catalyzed trifluoromethylation is as follows. Photoexcited diacetyl oxidizes the sodium triflinate to give the trifluoromethylsulfonyl radical, which spontaneously fragments to give the trifluoromethyl (CF3•) radial. The reaction of thus generated trifluoromethyl radical (CF3•) with aromatics gives the corresponding trifluoromethylaryl radical 29, which undergoes redox reaction with the photo-generated diacetyl radical anion 28 to give the arenium cation 30. Spontaneous deprotonation of the latter arenium cation (Wheland intermediate) gives the trifluoromethylated aromatics (Fig. 39). As an alternative approach, using CF3SiMe3 as the source of the trifluoromethyl radical, under oxidative conditions, Surya Prakash and coworkers have developed the direct C(sp2) H trifluoromethylation of enamides, such as naturally occurring isoindolinones, isoquinolinones, and 2-pyrinones.44 3.5.2 Hydro-trifluoromethylation of alkenes Nicewicz and coworkers have achieved hydro-trifluoromethylation of alkenes using the Langlois reagent under photoredox conditions.45 Through this free-radical, anti-Markovnikov reaction, hydro-trifluoromethylation of aliphatic alkenes (terminal and internal) as well as variously substituted styrenes, using the Langlois reagent, in the presence of trifluoroethanol (TFE) and methyl thiosalicylate as hydrogen atom donors, gave moderate yields of the corresponding trifluoromethyl compounds. 9-Mesitylacridinium salt was used as the organophotocatalyst in these reactions (Fig. 310). This hydro-trifluoromethylation is of broad scope, and a range of mono-, di-, and trisubstituted aliphatic and styrenyl alkenes gave the corresponding trifluoromethylated hydrocarbons with high anti-Markovnikov regioselectivity. According to a proposed mechanism, trifluoromethyl radical, generated from the Langlois reagent, undergoes free radical addition to the alkene, forming the relatively more stable secondary alky radical (e.g., an internal free radical from the terminal alkenes). The latter free radical then abstracts the hydrogen atom from the thiol (methyl thiosalicylate) or TFE to give the anti-Markovnikov products of hydro-trifluoromethylation Fig. 310.45 Iridium photoredox catalysis was also used for the hydro-trifluoromethylation of terminal alkenes, using Langlois reagent in methanol solvent.35 Various synthetically useful functional groups, such as ester, aldehyde, ether, sulfone, and aryl boronates, are tolerated and remain unchanged under the reaction conditions. 3.5.3 Trifluoromethylation of arylboronic acids Sanford and coworkers and Beller and coworkers have developed trifluoromethylation of arylboronic acids under photoredox conditions.4,39,46 Copper-mediated trifluoromethylation of arylboronic acids under free-radical conditions, using tert-butylhydroperoxide (tBuOOH) as the oxidant and Langlois reagent as a source of the trifluoromethyl radical, gives the corresponding trifluoromethylaromatics under mild conditions (Fig. 311).39 The trifluoromethylation of arylboronic acids could be performed in the presence of ambient air and moisture without adversely affecting the yields. A related photoredox-catalyzed trifluoromethylation of Chapter 3 • Free-radical reactions in the synthesis of organofluorine compounds CF3 CF3 SO2 Na (1.5 – 3.0 equiv) R R' R = alkyl, aryl R ' = H, alkyl 87 SH R 9-Mes-Acr (5 mol%) methyl thiosalicylate (20 mol%) 450 nm LEDS CHCl3 /TFE (9:1), RT R' OMe H N Me O 9-Mes-Acr Methyl thiosalicylate Selected examples: HO HO CF3 50% Ph CF3 OH Ph OH 51% Mechanistic outline: 9-Mes-Acr 9-Mes-Acr* CF3 9-Mes-Acr RSTFE R R CF3 SO2 Na hν RS . + SO2 . RSH R CF3 CF3 (Hydrogen atom transfer from ArSHl/TFE) FIGURE 3–10 Anti-Markovnikov hydro-trifluoromethylation of alkenes under photoredox conditions. TFE, Trifluoroethanol. arylboronic acids using CF3I as the source of the trifluoromethyl radicals, and [Ru(bpy)3]21 as the photocatalyst, also gives the trifluoromethylarenes in high yields.4 A proposed mechanism46 for the trifluoromethylation of arylboronic acids involves Cu(I)catalyzed formation of the trifluoromethyl radical from the reaction of tert-butylhydroperoxide with the Langlois reagent. The trifluoromethyl radical, upon reaction with Cu(II), forms the Cu(III)CF3 intermediate (32). The later intermediate may undergo transmetalation with arylboronic acid to give the CF3Cu(III)Ar (33), which then undergoes spontaneous reductive elimination to give the trifluoromethylarenes, regenerating the Cu(I) species for the continuation of the catalytic cycle. Alternatively, transmetalation of the Cu(II) species with arylboronic acids gives ArCu(II) (34), which upon SET to the trifluoromethyl radical may form the CF3Cu(III)Ar (33) (Fig. 311). 88 Organofluorine Chemistry B(OH)2 NaSO2 CF3 (3 equiv) CF3 CuCl (1 equiv), TBHP (5 equiv) R R DCM or MeOH Selected examples: CF3 CF3 CF3 CF3 MeO Ph N H MeO 85% 94% O 74% CF3 O 80% 67% Mechanistic outline: F3C t-BuO O S O t-BuO + SO2 Ln Cu(II) + OH F3 C t-BuOOH Ln Cu(I) O S O ArB(OH) 2 CF3 Ln Cu(III)CF3 32 B(OH) 2X ArB(OH) 2 ArCu(II)L n 34 B(OH) 2X ArCF3 CF3 Ln CF3 Cu(III)Ar 33 FIGURE 3–11 Trifluoromethylation of arylboronic acids using Langlois reagent. The Cu(I)-catalyzed reaction of aryl- or vinyltrifluoroborate salts with Langlois reagent, in the presence of tert-butyl hydroperoxide, also affords the corresponding trifluoromethylsubstituted compounds in high yields. In these reactions, CuCl was found to be the optimal catalyst, as compared to other copper salts (Fig. 312).47 The aryl- and vinyltrifluoroborate salts are, in turn, synthesized from the corresponding boronic acids and KHF2. 3.5.4 Azido-fluoroalkylation of alkenes β-(Azido)trifluoromethyl compounds are versatile intermediate for the synthesis of the corresponding β-(trifluoromethyl)amines and also as substrates in the Cu(I)-catalyzed Chapter 3 • Free-radical reactions in the synthesis of organofluorine compounds R KHF2 /MeOH/H2 O B(OH) 2 R BF3 K NaSO 2CF3 /TBHP/CuCl CF3 R' DCM/H2 O/MeOH RT 6–15 h R' R' R 89 74%–94% FIGURE 3–12 Cu(I)-catalyzed trifluoromethylation of vinyl (or aryl) trifluoroborates. O Me N O O F N OH Me H 2N Me O Br CF3 F3 C NH 2 34 35 AAK1 kinase inhibitor Antimicrobial agent FIGURE 3–13 Structures of some biologically important β-(trifluoromethyl)amines. azidealkyne cycloaddition reactions (click chemistry) for further functionalization. The trifluoromethyl group attenuates the basicity of the β-trifluoromethyl amines and increases the lipophilicity of the compounds. Through appropriate choice of the fluoroalkyl groups, the lipophilicity and solubility characteristics can be fine-tuned for optimal biological activity. The β-(amino)trifluoromethyl moiety is a component of some biologically and pharmaceutically interesting compounds, such as compound 34, an adaptor-associated kinase 1 (AAK1) inhibitor, and compound 35, an antimicrobial agent (Fig. 313).18,48,49 Langlois reagent is a cost-effective reagent for the transformation of alkenes to the corresponding β-(azido)trifluoromethyl compounds. Furthermore, various sodium fluoroalkyl sulfinates could be used as the source of the fluoroalkyl free radicals (monofluoroalkyl, difluoroalkyl, trifluoromethyl, and polyfluoroalkyl radicals) in the free-radical fluoroalkylation reactions. Zhang and coworkers have developed synthetic methodology for the azidofluoroalkylation of alkenes, using the Langlois reagent and its analogs, in the presence of trimethylsilyl azide, Cu(I) catalyst, and tert-butyl peroxybenzoate (TBPB) as the free-radical initiator.50 Their observations showed that other peroxide radical initiators were not as successful as TBPB in these reactions. The proposed mechanism involves the Cu(I)-catalyzed formation of the fluoroalkyl freeradicals (•CF3 and •CHF2) that add to the olefins to give the internal radicals, 42. Oxidative addition of the Cu(II)N3 (formed in the first step) to the radical 42, followed by reductive elimination of the Cu(III) complex 43, then gives the β-(azido)fluoroalkyl compounds (37), regenerating the Cu(I) catalyst in the process (Fig. 314).50 The β-(azido)trifluoromethyl compounds formed in these reactions, for example, compounds 38 and 39, could be further transformed into the corresponding triazoles 40 and 41, respectively, using the Sharpless azide-alkyne click chemistry. Interestingly, use of sodium alkylsulfinates (nonfluorinated compounds) in these reactions results in the β-(azido)sulfonylation to give 44, and not the expected β-(azido) 90 Organofluorine Chemistry O R FSO 2Na (1.5 equiv)/TMS N 3 R' CuCl/TBPB (1.5 equiv)/MeCN/H 2 O 16 h, 30 °C –60 °C R N3 R' R RF O 37 O TBPB 36 R, R ' = aryl, alkyl Some examples: Ph Ph N3 N3 EtO CF3 EtO CF2 H H 3C MeO MeO N N N N N N CF3 CF2H Ph 38 39 5 40 41 68% 70% 50% 40% Mechanistic outline: O Cu(I) O O Me 3SiN 3 Na + O NaSO 2R F O RF O TBPB OSiMe 3 + Cu(II)N 3 R' R N3 R' R RF 37 –Cu(I) R' N3 (III)Cu R R' Cu(II)N 3 RF 43 36 R RF 42 FIGURE 3–14 β-(Azido)fluoroalkylation of alkenes, and a mechanistic outline. alkylation, as the reaction of the alkylsulfinates proceeds through the formation of the corresponding alkylsulfonyl radicals (RSO2•), which do not extrude sulfur dioxide to form the alkyl radicals. In other words the nonfluorinated alkylsulfonyl radicals, unlike the fluoroalkylsulfonyl radicals, do not form the corresponding alkyl radicals, in accordance with the relatively lower stability of the alkyl radicals as compared to the fluoroalkyl radicals (Fig. 315). A Mn(III)-catalyzed version of the β-(azido)trifluoromethylation of terminal alkenes has been demonstrated using the Langlois reagent as the source of the trifluoromethyl radical.37 The β-(azido)trifluoromethyl compounds (46) could be further transformed into the β-aminotrifluoromethyl compounds (47), or used as the starting materials for the Cu(I)-catalyzed azidealkyne cycloaddition reactions (CuAAC; click reactions), to give the corresponding 1,2,3-triazoles (48) (Fig. 316).37 Chapter 3 • Free-radical reactions in the synthesis of organofluorine compounds N3 R"SO2Na (1.5 equiv) R' R TMSN3 CuCl/TBPB (1.5 equiv) MeCN/H 2O 16 h, 30 ºC–60 ºC R -e – R"SO2 Na R" 91 R' O S R" O 44 R" = alkyl O S O R" O S O R . FIGURE 3–15 Formation of the β-(azido)sulfones from the reactions of alkenes with sodium alkylsulfinates. CF3 SO2 Na (2 equiv) TMSN3 (3 equiv) Mn(OAc) 2 (20 mol%),TBHP (3 equiv) MeO CF3 MeO MeCN, air, 45 °C 45 N3 46 Ph3 P THF/H2 O CuSO4 Sodium ascorbate H2 O/tBuOH, 70 °C CF3 NH2 MeO CF3 N MeO 47 N N 48 Ph FIGURE 3–16 β-(Azido)trifluoromethylation of terminal alkenes and the transformation of the azido-moiety into the amines or triazoles. 3.5.5 Electrochemical oxy- and amino-trifluoromethylation Electrochemical oxy-trifluoromethylation and amino-trifluoromethylation of alkenes were achieved using Langlois reagent (sodium trifluoromethanesulfinate; CF3SO2Na), using a carbon anode and a Pt cathode. A variety of β-alkoxy and β-acyloxy trifluoromethyl compounds could be synthesized from the reaction of styrenes with alcohols, carboxylic acids, and amines through this electrochemical synthesis in an undivided cell under constant current conditions (Fig. 317).51 A free-radical mechanism was proposed for these electrochemical trifluoromethylation reactions based on the electron spin resonance (ESR) spectroscopic studies and mechanistic 92 Organofluorine Chemistry Oxy-trifluoromethylations: CF3 RO Carbon anode and Pt cathode, 15 mA + CF3 SO2 Na n-Bu 4BF4 , ROH/Y(OTf) 3, RT, 3 h R = e.g., Me,85%; Et, 60%; Selected examples: Cl CF3 O O O O CF3 CF3 CF3 CF3 60% 53% H CH3 O O 53% 59% Amino-trifluoromethylations: CF3 R 2 NH + CF3 SO2 Na R 2N Carbon anode and Pt cathode, 15 mA n-Bu 4BF4 Y(OTf)3, CH3CN/CH2Cl2, RT, 3 h R R Selected examples: N N CF3 Cl N N CF3 N N Br CF3 R 40% 43% 39% Mechanistic outline: CF3 -e – CF3 SO2 – . CF3 + SO2 CF3 (anodic oxidation) -e – (anodic oxidation) ROH or R 2NH + CF3 -H + X CF3 X = OR or NR2 FIGURE 3–17 Electrochemical oxy-trifluoromethylation and amino-trifluoromethylation of styrenes. studies, using a radical trapping agent DMPO (5,5-dimethyl-1-pyrroline N-oxide). No ESR signal could be detected from the reaction mixtures under the standard reaction conditions. However, the reaction mixture, in the absence of the alkenes (e.g., styrene), showed a strong Chapter 3 • Free-radical reactions in the synthesis of organofluorine compounds 93 ESR signal attributable to the DMPO adduct of the trifluoromethyl radical.51 Thus the trifluoromethyl radicals, formed through a single-electron electrochemical oxidation of the Langlois reagent, react with the styrene and aryl-substituted styrenes to form their corresponding more stable β-trifluoromethyl radicals. The latter β-trifluoromethyl radicals upon further electrochemical oxidation to the corresponding carbocations, followed by nucleophilic capture, give the β-alkoxy- or β-amino-trifluoromethyl compounds. The use of yttrium triflate [Y(OTf)3] as a Lewis acid catalyst gave relatively higher yields (B80%) than without the Lewis acid (B65%), implying prior coordination of the Lewis acid with the nucleophile that is captured by the carbocation intermediate. The Lewis acidnucleophile adduct may also complex to the alkene, thereby activating it for the electrophilic addition of the trifluoromethyl radical. 3.5.6 Selective trifluoromethylation of proteins The radical trifluoromethylation of proteins, using the Langlois reagent as the source of trifluoromethyl radical, affords the trifluoromethyl-modified proteins, under physiological conditions.52 The high selectivity of the trifluoromethyl radical is shown by the selective trifluoromethylation of the relatively electron-rich tryptophan residues as compared to histidine, tyrosine, and phenylalanine residues. A 19F NMR competition assay for the equimolar mixture of various amino acids, at ,40% conversion, showed that the predominant product arises from the regioselective C2-aryl trifluoromethylation of tryptophan, with minor products arising from the trifluoromethylation of cysteine, phenylalanine, and tyrosine (Fig. 318). FIGURE 3–18 Limited-conversion competition 19F NMR assay of natural amino acids (0.03 mmol), revealing chemoand regioselectivity toward Trp with minor products (Cys and lower levels of Phe, His, Tyr isomers). Adapted from Imiolek, M.; Karunanithy, G.; Ng, W. -L.; Baldwin, A. J.; Gouverneur, V.; Davis, B. G. Selective Radical Trifluoromethylation of Native Residues in Proteins. J. Am. Chem. Soc. 2018, 140, 15681571. 94 Organofluorine Chemistry NH CF3 SO2 Na (200 equiv) TBHP (12.5 equiv) 100 mM NH 4OAc, pH 6–8 NH F 3C FIGURE 3–19 Selective radical trifluoromethylation of tryptophan residues in proteins, using Langlois reagent. The radical trifluoromethylation of proteins, using this protocol, affords greater than 50% conversions to the C2-aryl tryptophan residues at a moderate pH of about 6 (Fig. 319).52 At pH 6.0, histidine residues are protonated and thereby become less reactive with the electrophilic trifluoromethyl radical, thus increasing selectivity toward tryptophan trifluoromethylation by 30-fold. Under optimal conditions, this chemoselective radical trifluoromethylation of tryptophan residues proceeds in 10 min, although it does not show absolute selectivity over other aromatic amino acid residues. This method allows direct access to 19F NMR experiments and in vitro protein modifications. 3.6 Photoredox-catalyzed S-fluoroalkylation and arylation S-Trifluoromethylation and S-perfluoroalkylation of thiol moieties can be effected through visible light photoredox catalysis, using a Ru(II) photocatalyst. N-Boc-cysteine methyl ester (49), under these conditions, in batch- and continuous flow reactors, gave the corresponding S-fluoroalkyl derivatives (50) in moderate yields and at relatively short reaction times (2 h in batch and 4 min in flow reactors). The proposed mechanism for this fluoroalkylation involves reductive formation of the fluoroalkyl radicals by the Ru(I) catalyst, which is generated in the presence of tetramethylethylenediamine under photoredox catalysis. The cysteine thiolate 51, reversibly formed under the basic conditions, undergoes oxidative addition to the fluoroalkyl radical (RF•), forming the sulfur radical anion 52. The radical anion 52, upon SET to the fluoroalkyl iodide (RFI) then gives the product 50 and the fluoroalkyl radical (RF•), which, in turn, propagates the catalytic cycle through its reaction with the thiolate anion 51 (Fig. 320).53 Selective functionalization of the cysteine thiol moiety in a pentapeptide derivative (53) could be achieved under metal-free photoredox conditions (and also under physiological conditions, in a phosphate buffer medium), using Eosin Y as the photocatalyst, to give the corresponding 4-fluoroaryl- or 4-(trifluoromethoxy)aryl derivative 54 (Fig. 321). The diazonium cation required in these reactions is generated, in situ, in a microflow reactor from the corresponding anilines using tert-butyl nitrite.54 The reaction is tolerant to unprotected serine and lysine residues and involves the photoredox-catalyzed formation of intermediate aryl free radicals from the diazonium ions. Chapter 3 • Free-radical reactions in the synthesis of organofluorine compounds O O RFI MeO MeO SH OtBu HN Ru(II), TMEDA, MeCN SR F OtBu HN Blue LED irradiation O O 50 49 RF = e.g., –CF 3, –CF2 CF2 CF3, –CF2 CO2Et 60%–84% yields O Mechanistic outline: MeO S OtBu HN 51 RFI Ru(I) O TMEDA Ru(II)* RF O MeO Ru(II) RFI MeO S OtBu HN O O hν RF MeO SR F OtBu HN O Chain propagation O O 52 51 SR F OtBu HN 50 FIGURE 3–20 Free-radical S-fluoroalkylation under photoredox conditions. O HN NH 2 X O HS NH O H N HO O H N N H O O N H OH (X = F, OCF3) N N NH 2 Eosyn Y, phosphate buffer, RT White CFL irradiation, 30 min O 53 N-acyl-cys-gly-ser-ser-lys-NH2 O HN NH 2 O S X NH H N HO O N H O H N O O N H OH 54 (X = F, OCF3) FIGURE 3–21 Selective functionalization of cysteine thiol moiety in a pentapeptide derivative. NH 2 O 95 96 Organofluorine Chemistry 3.7 Radical fluoroalkylation of enolates Radical trifluoromethylation of lithium- or titanium-enolates, or diethylzinc (Et2Zn) complexed silyl enol ethers can be accomplished using iodotrifluoromethane (CF3I) and the free-radical initiator triethylborane (Et3B), in the presence of oxygen. These reactions, however, have limited substrate scope and give low yields in some cases, due to the competing defluorination of the α-CF3 ketones by the enolate anions or other bases (Fig. 322).5558 Free-radical trifluoromethylation of chiral N-acyl-oxazolidinones, however, gives the corresponding α-trifluoromethylated carbonyl compounds in moderate to good yields and with high diastereoselectivities. The presence of the bulky substituents on the oxazolidinone moiety in these reactions disfavors the accompanying defluorination reactions.59 Chiral N-acyl oxazolidinones could be fluoroalkylated using trifluoromethyl iodide or perfluoroalkyl iodides and a binary mixture of transition metal reagents, ZrCl4 and [Ph3P]3Ru(II) Cl2, with high diastereoselectivities. The α-fluoroalkylated N-acyl-oxazolidinones can be hydrolyzed (using LiOH and aqueous H2O2) or reduced (using LiAlH4 in THF) to the corresponding carboxylic acids (9) and primary alcohols (10), respectively, in high yields and with high enantiomeric purity (enantiomeric ratios .97:3).60 The proposed mechanism for these trifluoromethylation and perfluoroalkylation reactions involves SET redox reactions, mediated by Ru(II) (Fig. 323).60 Thus the enolate ion formed from the N-acyl-oxazolidinone is complexed to the ZrCl4 to give the Zr(IV) enoalate 61. Ru (II)-catalyzed SET reduction of trifluoromethyl iodide gives the trifluoromethyl radical, which undergoes free-radical addition to the enolate complex 61 to give the radical anion 62. Subsequent Ru(III)-catalyzed oxidation of the radical anion 62 then gives the α-trifluoromethylated N-acyl-oxazolidinone. R1 OSiMe3 R2 H O 1. Et 2Zn/THF 2. CF3I/Et3 B/O2 R2 R1 CF3 R1 R2 R1 H O OM R2 CF3 M = e.g., Li FIGURE 3–22 Radical trifluoromethylation of enolates. OLi CF3I/Et3 B/O2 R2 R1 F F Chapter 3 • Free-radical reactions in the synthesis of organofluorine compounds O O O N R FI ZrCl 4 (1.5 equiv); Et3N Ph [Ph 3P] 3RuCl2 (7 mol%) DCM, 45 °C, 16 h O N O CF3 O O RF Ph N O Ph 74%; dr 9:1 O O O N O N F F F C 4 F9 C 3 F7 57 N O 56 O O O O 55 Selected examples: O 97 F Ph F Ph Ph 58 59 60 74%; dr 24:1 71%; dr 24:1 73%; dr 98:2 F F O Mechanistic outline: O O O + ZrCl4 N O O Ph Et3 N ZrCl 4 LiOH/H 2 O2 63 O CF3 N CF3 55 HO Ph 56 LiAlH 4 /THF HO CF3 64 Cl4 Zr O O O L nRu(II) N CF3 Ph 61 CF3 I L nRu(III) Cl4 Zr O O O N CF3 Ph 62 FIGURE 3–23 Trifluoromethylation and perfluroalkylation of the chiral enolates, mediated by Ru(II)-catalyzed redox reactions. 98 Organofluorine Chemistry References 1. Kim, D.; Kowalchick, J. E.; Edmondson, S. D.; Mastracchio, A.; Xu, J.; Eiermann, G. J.; Leiting, B.; Wu, J. K.; Pryor, K. D.; Patel, R. A.; He, H.; Lyons, K. A.; Thornberry, N. A.; Weber, A. E. TriazolopiperazineAmides as Dipeptidyl Peptidase IV Inhibitors: Close Analogs of JANUVIA (Sitagliptin Phosphate). Bioorg. Med. Chem. Lett. 2007, 17, 33733377. 2. Neumann, U.; Ufer, M.; Jacobson, L. H.; Rouzade-Dominguez, M.-L.; Huledal, G.; Kolly, C.; Lueoend, R. M.; Machauer, R.; Veenstra, S. J.; Hurth, K.; Rueeger, H.; Tintelnot-Blomley, M.; Staufenbiel, M.; Shimshek, D. R.; Perrot, L.; Frieauff, W.; Dubost, V.; Schiller, H.; Vogg, B.; Beltz, K.; Avrameas, A.; Kretz, S.; Pezous, N.; Rondeau, J.-M.; Beckmann, N.; Hartmann, A.; Vormfelde, S.; David, O. J.; Galli, B.; Ramos, R.; Graf, A.; Lopez Lopez, C. The BACE-1 Inhibitor CNP520 for Prevention Trials in Alzheimer’s Disease. EMBO Mol. Med. 2018, 10, e9316. 3. Dobrowolska Zakaria, J. A.; Vassar, R. J. A Promising, Novel, and Unique BACE1 Inhibitor Emerges in the Quest to Prevent Alzheimer’s Disease. EMBO Mol. Med. 2018, 10, e9717. 4. Ye, Y.; Sanford, M. S. Merging Visible-Light Photocatalysis and Transition-Metal Catalysis in the CopperCatalyzed Trifluoromethylation of Boronic Acids with CF3I. J. Am. Chem. Soc. 2012, 134, 90349037. 5. Iqbal, N.; Jung, J.; Park, S.; Cho, E. J. Controlled Trifluoromethylation Reactions of Alkynes Through Visible-Light Photoredox Catalysis. Angew. Chem., Int. Ed. 2014, 53, 539542. 6. Iqbal, N.; Choi, S.; Kim, E.; Cho, E. J. Trifluoromethylation of Alkenes by Visible Light Photoredox Catalysis. J. Org. Chem. 2012, 77, 1138311387. 7. Wang, X.; Studer, A. Iodine(III) Reagents in Radical Chemistry. Acc. Chem. Res. 2017, 50, 17121724. 8. Wang, F.; Wang, D.; Wan, X.; Wu, L.; Chen, P.; Liu, G. Enantioselective Copper-Catalyzed Intermolecular Cyanotrifluoromethylation of Alkenes via Radical Process. J. Am. Chem. Soc. 2016, 138, 1554715550. 9. Mizuta, S.; Verhoog, S.; Engle, K. M.; Khotavivattana, T.; O’Duill, M.; Wheelhouse, K.; Rassias, G.; Medebielle, M.; Gouverneur, V. Catalytic Hydrotrifluoromethylation of Unactivated Alkenes. J. Am. Chem. Soc. 2013, 135, 25052508. 10. Beatty, J. W.; Douglas, J. J.; Cole, K. P.; Stephenson, C. R. J. A Scalable and Operationally Simple Radical Trifluoromethylation. Nat. Commun. 2015, 6 7919 pp. 11. Yu, X.-L.; Chen, J.-R.; Chen, D.-Z.; Xiao, W.-J. Visible-Light-Induced Photocatalytic Azotrifluoromethylation of Alkenes with Aryldiazonium Salts and Sodium Triflinate. Chem. Commun. (Cambridge, UK) 2016, 52, 82758278. 12. Fang, J.; Wang, Z.-K.; Wu, S.-W.; Shen, W.-G.; Ao, G.-Z.; Liu, F. Photoredox-Catalysed Chloro-, Bromoand Trifluoromethylthio-Trifluoromethylation of Unactivated Alkenes with Sodium Triflinate. Chem. Commun. (Cambridge, UK) 2017, 53, 76387641. 13. Kautzky, J. A.; Wang, T.; Evans, R. W.; MacMillan, D. W. C. Decarboxylative Trifluoromethylation of Aliphatic Carboxylic Acids. J. Am. Chem. Soc. 2018, 140, 65226526. 14. Tan, X.; Liu, Z.; Shen, H.; Zhang, P.; Zhang, Z.; Li, C. Silver-Catalyzed Decarboxylative Trifluoromethylation of Aliphatic Carboxylic Acids. J. Am. Chem. Soc. 2017, 139, 1243012433. 15. Zeng, X.; Yan, W.; Zacate, S. B.; Chao, T.-H.; Sun, X.; Cao, Z.; Bradford, K. G. E.; Paeth, M.; Tyndall, S. B.; Yang, K.; Kuo, T.-C.; Cheng, M.-J.; Liu, W. Copper-Catalyzed Decarboxylative Difluoromethylation. J. Am. Chem. Soc. 2019, 141, 1139811403. 16. Lin, J.-S.; Dong, X.-Y.; Li, T.-T.; Jiang, N.-C.; Tan, B.; Liu, X.-Y. A Dual-Catalytic Strategy to Direct Asymmetric Radical Aminotrifluoromethylation of Alkenes. J. Am. Chem. Soc. 2016, 138, 93579360. 17. Lin, J.-S.; Wang, F.-L.; Dong, X.-Y.; He, W.-W.; Yuan, Y.; Chen, S.; Liu, X.-Y. Catalytic Asymmetric Radical Aminoperfluoroalkylation and Aminodifluoromethylation of Alkenes to Versatile EnantioenrichedFluoroalkyl Amines. Nat. Commun. 2017, 8 14841 pp. Chapter 3 • Free-radical reactions in the synthesis of organofluorine compounds 99 18. Zhu, C.-L.; Wang, C.; Qin, Q.-X.; Yruegas, S.; Martin, C. D.; Xu, H. Iron(II)-Catalyzed Azidotrifluoromethylation of Olefins and N-Heterocycles for Expedient Vicinal Trifluoromethyl Amine Synthesis. ACS Catal. 2018, 8, 50325037. 19. Dagousset, G.; Carboni, A.; Magnier, E.; Masson, G. Photoredox-Induced Three-Component Azido- and Aminotrifluoromethylation of Alkenes. Org. Lett. 2014, 16, 43404343. 20. Langlois, B. R.; Laurent, E.; Roidot, N. Trifluoromethylation of Aromatic Compounds with Sodium Trifluoromethanesulfinate Under Oxidative Conditions. Tetrahedron Lett. 1991, 32, 75257528. 21. Smith, J. M.; Dixon, J. A.; deGruyter, J. N.; Baran, P. S. Alkyl Sulfinates: Radical Precursors Enabling Drug Discovery. J. Med. Chem. 2019, 62, 22562264. 22. Smith, J. M.; Harwood, S. J.; Baran, P. S. Radical Retrosynthesis. Acc. Chem. Res. 2018, 51, 18071817. 23. Li, J.; Zhang, X.; Xiang, H.; Tong, L.; Feng, F.; Xie, H.; Ding, J.; Yang, C. C-H Trifluoromethylation of 2-Substituted/Unsubstituted Aminonaphthoquinones at Room Temperature with Bench-Stable (CF3SO2)2Zn: Synthesis and Antiproliferative Evaluation. J. Org. Chem. 2017, 82, 67956800. 24. Kuttruff, C. A.; Haile, M.; Kraml, J.; Tautermann, C. S. Late-Stage Functionalization of Drug-Like Molecules Using Diversinates. ChemMedChem 2018, 13, 983987. 25. Gnaim, S.; Scomparin, A.; Li, X.; Baran, P. S.; Rader, C.; Satchi-Fainaro, R.; Shabat, D. Tagging the Untaggable: A Difluoroalkyl-Sulfinate Ketone-Based Reagent for Direct C-H Functionalization of Bioactive Heteroarenes. Bioconjugate Chem. 2016, 27, 19651971. 26. Fujiwara, Y.; Dixon, J. A.; O’Hara, F.; Funder, E. D.; Dixon, D. D.; Rodriguez, R. A.; Baxter, R. D.; Herle, B.; Such, N.; Collins, M. R.; Ishihara, Y.; Baran, P. S. Practical and Innate Carbon-Hydrogen Functionalization of Heterocycles. Nature (London, UK) 2012, 492, 9599. 27. Blakemore, D. C.; Castro, L.; Churches, I.; Rees, D. C.; Thomas, A. W.; Wilson, D. M.; Wood, A. Organic Synthesis Provides Opportunities to Transform Drug Discovery. Nat. Chem. 2018, 10, 383394. 28. Li, L.; Mu, X.; Liu, W.; Wang, Y.; Mi, Z.; Li, C.-J. Simple and Clean Photoinduced Aromatic Trifluoromethylation Reaction. J. Am. Chem. Soc. 2016, 138, 58095812. 29. Abdiaj, I.; Bottecchia, C.; Alcazar, J.; Noel, T. Visible-Light-Induced Trifluoromethylation of Highly Functionalized Arenes and Heteroarenes in Continuous Flow. Synthesis 2017, 49, 49784985. 30. Cui, B.; Sun, H.; Li, L.; Duan, L.; Li, Y.-M.; Xu, Y. Mn(OAc)3-Mediated Hydrotrifluoromethylation of Unactivated Alkenes Using CF3SO2Na as the Trifluoromethyl Source. J. Org. Chem. 2018, 83, 60156024. 31. Lu, Q.; Liu, C.; Huang, Z.; Ma, Y.; Zhang, J.; Lei, A. Relay Cooperation of K2S2O8 and O2 in Oxytrifluoromethylation of Alkenes Using CF3SO2Na. Chem. Commun. (Cambridge, UK) 2014, 50, 1410114104. 32. Shen, W.-G.; Wu, Q.-Y.; Gong, X.-Y.; Ao, G.-Z.; Liu, F. A Facile Method for Hydroxytrifluoromethylation of Alkenes with Langlois Reagent and DMSO. Green Chem. 2019, 21, 29832987. 33. Shi, L.; Yang, X.; Wang, Y.; Yang, H.; Fu, H. Metal-Free Trifluoromethylation and Arylation of Alkenes. Domino Synthesis of Oxindole Derivatives. Adv. Synth. Catal. 2014, 356, 10211028. 34. Wei, W.; Wen, J.; Yang, D.; Liu, X.; Guo, M.; Dong, R.; Wang, H. Metal-Free Direct Trifluoromethylation of Activated Alkenes with Langlois’ Reagent Leading to CF3-Containing Oxindoles. J. Org. Chem. 2014, 79, 42254230. 35. Zhu, L.; Wang, L.-S.; Li, B.; Fu, B.; Zhang, C.-P.; Li, W. Operationally Simple Hydrotrifluoromethylation of Alkenes with Sodium Triflinate Enabled by Ir Photoredox Catalysis. Chem. Commun. (Cambridge, UK) 2016, 52, 63716374. 36. Panday, P.; Garg, P.; Singh, A. Manganese-Dioxide-Catalyzed Trifluoromethylation and Azidation of Styrenyl Olefins via Radical Intermediates. Asian J. Org. Chem. 2018, 7, 111115. 100 Organofluorine Chemistry 37. Zhang, Y.; Han, X.; Zhao, J.; Qian, Z.; Li, T.; Tang, Y.; Zhang, H.-Y. Synthesis of β-Trifluoromethylated Alkyl Azides via a Manganese-Catalyzed Trifluoromethylazidation of Alkenes with CF3SO2Na and TMSN3. Adv. Synth. Catal. 2018, 360, 26592667. 38. Yang, Y.; Xu, L.; Yu, S.; Liu, X.; Zhang, Y.; Vicic, D. A. Triphenylphosphine-Mediated Deoxygenative Reduction of CF3SO2Na and Its Application for Trifluoromethylthiolation of Aryl Iodides. Chem. Eur. J. 2016, 22, 858863. 39. Ye, Y.; Kunzi, S. A.; Sanford, M. S. Practical Method for the Cu-Mediated Trifluoromethylation of Arylboronic Acids with CF3 Radicals Derived from NaSO2CF3 and tert-Butyl Hydroperoxide (TBHP). Org. Lett. 2012, 14, 49794981. 40. Presset, M.; Oehlrich, D.; Rombouts, F.; Molander, G. A. Copper-Mediated Radical Trifluoromethylation of Unsaturated Potassium Organotrifluoroborates. J. Org. Chem. 2013, 78, 1283712843. 41. Rong, J.; Ni, C.; Hu, J. Metal-Catalyzed Direct Difluoromethylation Reactions. Asian J. Org. Chem. 2017, 6, 139152. 42. Lefebvre, Q. Toward Sustainable Trifluoromethylation Reactions: Sodium Triflinate under the Spotlight. Synlett 2017, 28, 1923. 43. Borah, A. J.; Yan, G. Decarboxylative Functionalization of Cinnamic Acids. Org. Biomol. Chem. 2015, 13, 80948115. 44. Krishnamurti, V.; Munoz, S. B.; Ispizua-Rodriguez, X.; Vickerman, J.; Mathew, T.; Surya Prakash, G. K. C (sp2)-H Trifluoromethylation of Enamides Using TMSCF3: Access to Trifluoromethylated Isoindolinones, Isoquinolinones, 2-Pyridinones and Other Heterocycles. Chem. Commun. (Cambridge, UK) 2018, 54, 1057410577. 45. Wilger, D. J.; Gesmundo, N. J.; Nicewicz, D. A. Catalytic Hydrotrifluoromethylation of Styrenes and Unactivated Aliphatic Alkenes via an Organic Photoredox System. Chem. Sci. 2013, 4, 31603165. 46. Li, Y.; Wu, L.; Neumann, H.; Beller, M. Copper-Catalyzed Trifluoromethylation of Aryl- and Vinylboronic Acids with Generation of CF3-radicals. Chem. Commun. (Cambridge, UK) 2013, 49, 26282630. 47. Dubbaka, S. R.; Salla, M.; Bolisetti, R.; Nizalapur, S. Copper-Mediated Trifluoromethylation of Aryl-, Heteroaryl-, and Vinyltrifluoroborates with Langlois’ Reagent. RSC Adv. 2014, 4, 64966499. 48. Li, Q.; Wang, W.; Berst, K. B.; Claiborne, A.; Hasvold, L.; Raye, K.; Tufano, M.; Nilius, A.; Shen, L. L.; Flamm, R.; Alder, J.; Marsh, K.; Crowell, D.; Chu, D. T. W.; Plattner, J. J. Synthesis and Structure-Activity Relationships of 2-Pyridones: II. 8-(Fluoro-Substituted Pyrrolidinyl)-2-Pyridones as Antibacterial Agents. Bioorg. Med. Chem. Lett. 1998, 8, 19531958. 49. Vrudhula, V.M.; Pan, S.; Rajamani, R.; Nara, S.J.; Karatholuvhu, M.S.; Maishal, T.K.; Ditta, J.L.; Dzierba, C.D.; Bronson, J.J.; Macor, J.E. Preparation of Aryl Lactam Kinase Inhibitors Useful in Inhibiting AAK1 WO2014022167A1. Assignee: Syngene International Limited, India; Bristol-Myers Squibb Company, USA, 2014. 50. Xiong, Y.; Sun, Y.; Zhang, G. Copper-Catalyzed Synthesis of β-Azido Sulfonates or Fluorinated Alkanes: Divergent Reactivity of Sodium Sulfinates. Org. Lett. 2018, 20, 62506254. 51. Zhang, L.; Zhang, G.; Wang, P.; Li, Y.; Lei, A. Electrochemical Oxidation with Lewis-Acid Catalysis Leads to Trifluoromethylative Difunctionalization of Alkenes Using CF3SO2Na. Org. Lett. 2018, 20, 73967399. 52. Imiolek, M.; Karunanithy, G.; Ng, W.-L.; Baldwin, A. J.; Gouverneur, V.; Davis, B. G. Selective Radical Trifluoromethylation of Native Residues in Proteins. J. Am. Chem. Soc. 2018, 140, 15681571. 53. Bottecchia, C.; Wei, X.-J.; Kuijpers, K. P. L.; Hessel, V.; Noel, T. Visible Light-Induced Trifluoromethylation and Perfluoroalkylation of Cysteine Residues in Batch and Continuous Flow. J. Org. Chem. 2016, 81, 73017307. 54. Bottecchia, C.; Rubens, M.; Gunnoo, S. B.; Hessel, V.; Madder, A.; Noel, T. Visible-Light-Mediated Selective Arylation of Cysteine in Batch and Flow. Angew. Chem., Int. Ed. 2017, 56, 1270212707. Chapter 3 • Free-radical reactions in the synthesis of organofluorine compounds 101 55. Itoh, Y.; Mikami, K. Facile Radical Trifluoromethylation of Lithium Enolates. Org. Lett. 2005, 7, 48834885. 56. Kawauchi, S.; Hayashi, Y.; Tomita, Y.; Hashimoto, R.; Honda, K.; Itoh, Y.; Mikami, K. Theoretical Study on Radical Trifluoromethylation of Silyl Enol Ethers Accelerated via Complexation with Dialkylzinc. Heterocycles 2015, 90, 907917. 57. Mikami, K.; Tomita, Y.; Ichikawa, Y.; Amikura, K.; Itoh, Y. Radical Trifluoromethylation of Ketone Silyl Enol Ethers by Activation with Dialkylzinc. Org. Lett. 2006, 8, 46714673. 58. Tomita, Y.; Ichikawa, Y.; Itoh, Y.; Kawada, K.; Mikami, K. Zincate-Type Enolate for Radical α-Trifluoromethylation. Tetrahedron Lett. 2007, 48, 89228925. 59. Iseki, K.; Nagai, T.; Kobayashi, Y. Diastereoselective Trifluoromethylation of Chiral Imide Enolates with Iodotrifluoromethane Mediated by Triethylborane. Tetrahedron: Asymmetry 1994, 5, 961974. 60. Herrmann, A. T.; Smith, L. L.; Zakarian, A. A Simple Method for Asymmetric Trifluoromethylation of NAcyl Oxazolidinones via Ru-Catalyzed Radical Addition to Zirconium Enolates. J. Am. Chem. Soc. 2012, 134, 69766979. 4 Organotransition metal catalysis in the synthesis of organofluorine compounds Chapter Outline 4.1 Introduction ............................................................................................................................... 104 4.2 Pd-catalyzed fluorination of aryl halides and triflates.......................................................... 105 4.3 Transition metalcatalyzed CH fluorination ....................................................................... 106 4.3.1 Aryl fluorination .............................................................................................................. 106 4.3.2 Benzylic fluorination ....................................................................................................... 108 4.3.3 Fluoroalkylation of hydrazones ..................................................................................... 111 4.4 Au(I)-catalyzed hydrofluorination of alkenes and alkynes................................................... 114 4.5 Ni-catalyzed fluoroalkylation of aromatics ............................................................................ 116 4.5.1 Fluoroalkylation of arylsilanes ....................................................................................... 116 4.5.2 Aryl difluoromethylation ................................................................................................ 118 4.6 Ag(II)-catalyzed oxidative ring-opening fluorination of cyclic amines ................................ 121 4.7 Ag(I)-catalyzed decarboxylative fluorination......................................................................... 123 4.8 Cu(I)-mediated dediazoniative difluoromethylation ............................................................. 124 4.9 Fluoroalkylation of arylboronic acids and esters ................................................................... 125 4.9.1 Copper-mediated trifluoromethylation ........................................................................ 125 4.9.2 Cu(I)-catalyzed trifluoromethylation of arylboronate esters ...................................... 126 4.9.3 Pd(0)-catalyzed difluoroalkylation of arylboronic acids .............................................. 126 4.10 Cu(I)-catalyzed fluoroalkylation of aryl halides ..................................................................... 126 4.11 Ni-catalyzed trifluoromethylthiolation ................................................................................... 127 4.12 Pd(II)-catalyzed (amino)trifluoromethoxylation..................................................................... 129 References........................................................................................................................................... 131 Organofluorine Chemistry. DOI: https://doi.org/10.1016/B978-0-12-813286-9.00004-3 © 2020 Elsevier Inc. All rights reserved. 103 104 Organofluorine Chemistry 4.1 Introduction Nearly one-third of the pharmaceuticals are fluorinated compounds, and a vast majority of these compounds have either fluoroaryl moieties or fluoroalkyl moieties. A single fluorine on the aromatic ring, at the metabolic degradation sites, often improves metabolic stability of the drug candidates. The widely prescribed pharmaceuticalsatorvastatin and rosuvastatin (cholesterol-lowering drugs), safinamide (for treating Parkinson’s disease), ciprofloxacin (an antibacterial drug), flurbiprofen (a nonsteroidal antiinflammatory agent), and dacomitinib (an anticancer drug for treating non-small-scale lung cancers)have a monofluorinated aryl ring (Fig. 41). Many pharmaceuticals, including the widely prescribed antimalarial drug ciprofloxacin and antidepressant drug fluoxetine, are trifluoromethylated compounds. Difluoromethylated pharmaceuticals include roflumilast (for treating chronic obstructive pulmonary disease), pantoprazole (for treating gastrointestinal diseases), tafluprost (for treating glaucoma), and maraviroc (anti-HIV drug) (see Chapter 5: Pharmaceutical applications of organofluorine compounds). FIGURE 4–1 Structures of selected pharmaceuticals containing a fluoroaryl moiety. Chapter 4 • Organotransition metal catalysis in the synthesis 105 Fluorination of aryl rings can be achieved through the BalzSchiemann reaction [Cu (I)-catalyzed dediazoniative fluorination of aryldiazonium salts],1 nucleophilic aromatic substitution (SNAr) of aryl halides (a nontransition metalcatalyzed reaction, limited to deactivated aromatics),2 Pd-catalyzed fluorination of aryl halides and triflates,3 or Pdcatalyzed CH activation using a directing group, such as pyridine moiety, in proximity to the fluorination site.4 Transition metalmediated or transition metalcatalyzed fluorination and fluoroalkylation reactions are especially useful in the late-stage fluorination of pharmaceuticals and in the synthesis of 18F-labeled positron emission tomography (PET) tracers (see Chapter 6: Synthesis and applications of 18F-labeled compounds).5 Fluoroalkyl groups afford favorable pharmacokinetic properties to the drug candidates, because of their effectiveness in improving lipophilicity, membrane permeability, and oxidative stability. The efficient synthetic methods for fluorinations and fluoroalkylations, achievable by organometallic catalysis, expand the synthetic strategies for the fluorinated drugs and also provide access to the late-stage synthesis of pharmaceuticals and 18Flabeled compounds for PET. 4.2 Pd-catalyzed fluorination of aryl halides and triflates Buchwald and coworkers, in 2009, developed the Pd(0)-catalyzed ipso-fluorination of aryl bromides or triflates, in the presence of a sterically crowded ligand, t-Bu-Brettphos (1).6 Although oxidative addition of aryl halides (triflates) to Pd(0) is relatively fast, the reductive elimination to form the aryl fluorides is too slow and is not practicable for the synthetic applications, using the conventional triarylphosphine ligands. However, Buchwald and coworkers have demonstrated that the sterically crowded phosphine ligands accelerate this reductive elimination and thus achieved the synthetically useful Pd(0)-catalyzed arylfluorination reactions. However, relatively high temperatures (80 C130 C) are still required for these aryl fluorinations. This Pd(0)-catalyzed trifluoromethylation reaction was improved using even more sterically crowded ligand adamantly-Brettphos (2).7 A variety of pharmaceutically interesting phenolic compounds, such as estrone and vitamin E (α-tocopherol), were deoxyfluorinated through Pd(0) catalysis in a two-step process, involving the conversion of the phenols to the triflates, followed by Pd(0)-catalyzed ipsoarylfluorination (Fig. 41). Whether these deoxyfluorinated versions of the estrone or vitamin E have comparable pharmacological effects as that of the parent compounds is yet to be established. This aryl-fluorination reaction involves oxidative addition of the aryl triflate (or bromide) to Pd(0) to give the Ar(X)Pd(II) intermediate (3), which upon transmetalation with CsF forms the Ar(F)Pd(II) intermediate (4). Reductive elimination of the latter Pd(II) intermediate, facilitated by the sterically crowded ligands, such as t-Bu-BrettPhos or adamantly-BrettPhos, gives the corresponding fluoroaromatic compounds, regenerating the Pd(0) catalyst that reenters the catalytic cycle (Fig. 42). 106 Organofluorine Chemistry FIGURE 4–2 Pd-catalyzed fluorination of aryl halides (or triflates). 4.3 Transition metalcatalyzed CH fluorination 4.3.1 Aryl fluorination Aryl fluorination through CH activation serves as an atom economical synthetic method and provides a convenient synthetic strategy for the late-stage fluorination of pharmaceuticals. Pd (II)-catalyzed CH fluorination of aromatics, using the Pd(II) complex 5, proceeds under relatively mild conditions to give the fluorinated aromatics. Aromatic substrates, consisting of electron-releasing groups (such as alkoxy groups), as well as mildly electron-withdrawing substituents (such as halogens), give the regio-isomeric mixture of fluoroaryl compounds in moderate to good yields (Fig. 43).8 For example, through this Pd catalysis, bromobenzene gives isomeric mixture of o- and p-fluorobromobenzene. Similarly, procymidone (a pesticide) gives Chapter 4 • Organotransition metal catalysis in the synthesis FIGURE 4–3 Pd-catalyzed electrophilic aromatic CH fluorination. 107 108 Organofluorine Chemistry fluorination at the ortho- and para-positions with respect to the amino moiety. This synthetic method can be used in the late-stage fluorination of pharmaceuticals or agrochemicals, such as fluorinated versions of the procymidone and butyl ciprofibrate, a lipid-lowering drug. In these aryl fluorinations, unlike most other organometallic reactions involving CH bond activation, arylorganometallic intermediates are not formed as the intermediates. Instead, a reactive FPd(IV) intermediate, 6, is formed as the transient species through the Selectfluor-mediated oxidation of the Pd(II) complex. A single electron transfer (SET) from the high-valent Pd(IV) complex 6 to the arenes forms a fluoride-bridged aryl radical intermediate 7. This aryl radical intermediate, 7, through a fluoride-coupled intramolecular electron transfer, that is, electron transfer from the aryl radical to the Pd(III), forms the fluoroarenium cation 8, regenerating the Pd(II) catalyst. In effect, this two-step sequence is equivalent to the transfer of “F1” from the high-valent Pd(IV) intermediate to the arenes to form the fluoroarenium cation (8). A subsequent deprotonation of the fluoroarenium cation by a base (e.g., F2) then gives the fluoroaromatic compound. This mechanistic outline is substantiated by density functional theory calculations.8 4.3.2 Benzylic fluorination 4.3.2.1 Mn(III)-catalyzed benzylic fluorination Benzylic hydrogens in pharmaceuticals are prone to metabolic oxidation, and therefore fluorination of benzylic hydrogens provides a means of enhancing the metabolic stability as well as potency and bioavailability of the drug candidates. Benzylic fluorination can be achieved using electrophilic fluorinating agents, such as Selectfluor and N-fluorobenzenesulfonimide, using organometallic catalysis.912 The organometallic catalysis also allows latestage benzylic fluorination of pharmaceutically interesting compounds. Groves and coworkers developed regioselective benzylic fluorination using Mn(III)(salen) Cl (1) as the catalyst and iodosobenzene as the oxidant in the presence of Et3N3HF. Using this method, pharmaceutically interesting compounds, such as ibuprofen, celestolide, δ-tocopherol, and homophenylalanine were selectively fluorinated at the benzylic site in moderate yields (Fig. 44).11 The proposed mechanism involves oxidation of the Mn(III) complex (9) to the Mn(V) complex (10) by iodosobenzene, followed by abstraction of the benzylic hydrogen to form the benzylic radical. Fluoride ion exchange of Mn(IV)-OH (11) to give Mn(IV)-F (12), followed by the recombination of fluorine to the benzylic radical then gives the corresponding benzyl fluorides, regenerating the Mn(III) catalyst (9). This methodology was adapted for the synthesis of the pharmaceutically interesting 18F-labeled compounds, such as ibuprofen [a nonselective cyclooxygenase (COX) inhibitor], rasagiline (monoamine oxidase-B inhibitor), dopamine (neurotransmitter), celecoxib (a COX-2 inhibitor), and enalaprilat (angiotensin-converting enzyme inhibitor), using the no-carrier-added [18F]fluoride (see Chapter 6: Synthesis and applications of 18F-labeled compounds).13,14 4.3.2.2 Pd(II)-catalyzed benzylic fluorination Pd(II)-catalyzed enantioselective benzylic CH fluorination 2-alkylbenzaldehydes (13) was achieved, using N-fluoro(2,4,6-trimethyl)pyridinium tetrafluoroborate (14) as the source of Chapter 4 • Organotransition metal catalysis in the synthesis N 109 N Mn t-Bu O Cl t-Bu H t-Bu O t-Bu F ( 9) 20 mol% R2 R2 PhIO, Et 3 N.3HF/AgF MeCN, 50 °C, 6–9 h R1 R1 Selected examples: O F F F F CO2 Me NPhth MeO 2 C Ph 48% 58% F-ibuprfen ester 55% F-celestolide 67% F-homophenylalanine Mechanistic outline: PhIO N N Mn III O O Cl t-Bu t-Bu t-Bu PhI t-Bu 9 O Mn V R-F Cl 10 R R-H F Mn IV R OH Cl 12 Mn IV Cl F– 11 FIGURE 4–4 Mn(III)-catalyzed benzylic fluorination. electrophilic fluorine (F1) as well as the oxidizing agent, in the presence of the catalytic amounts of a chiral α-amino acid diethylamide (15). The reaction gives moderate to low yields of the fluorinated products (e.g., 1820) along with a minor by-product 17 (Fig. 45).15 110 Organofluorine Chemistry H2N O H BF 4– + R1 15 O 20 mol% O Pd(OAc 2) (10 mol%) H 14 H + OCOC 6F5 R1 Bu4 NPF6 (50 mol%) R1 R2 C 6F5 CO2H (5.0 equiv) 13 O F N F R2 N R2 16 Benzene (0.5 M) 17 70 °C, 24 h Minor byproduct Examples: O F3 C H F 61% (91% ee) F N R2 R2 36% (86% ee) 19 18 20 + Mechanistic outline: t-Bu Pd(OAc) 2 –AcOH H R1 14 t-Bu NEt2 NF O Pd IV OAc R2 "F+" 22 NEt2 N t-Bu O Inner sphere fluorination H 21 R2 F3 C 58% (90% ee) R2 H H H F R1 O O O2 N C 6 F5CO2 O N OCOC 6 F5 SN 2 –Pd(II) NEt2 – H R2 O O H t-Bu NEt 2 H OCOC 6F 5 F R1 N R2 H O 16 H (Major product) R1 13 R2 R2 23 F O R1 R2 17 (Minor byproduct) FIGURE 4–5 Pd-catalyzed enantioselective benzylic fluorination via CH oxidative addition. 24 t-Bu NEt2 H 2N O + 15 Chapter 4 • Organotransition metal catalysis in the synthesis 111 A mechanistic rationalization of the enantioselective benzylic fluorination is as follows.15 The N-fluoropyridinium salt (14) oxidizes Pd(II) to Pd(IV), which undergoes benzylic CH insertion from the chiral imine 21, followed by elimination of acetic acid to give 22. The Pd (IV) complex 22 undergoes reductive elimination, through inner sphere fluorine transfer, to give the benzylic fluoride 23. Reversible equilibration of 23 with the starting 2alkylbenzaldehyde 13 forms the benzylic fluorination product 16. A minor byproduct, 17, is formed from the competitive SN2 reaction of the Pd(IV) intermediate 22 with the weakly nucleophilic (perfluoro)benzoate anion. Because of the relatively low nucleophilicity of the perfluorobenzoate, the latter SN2 reaction is disfavored over the reductive elimination to give 23 (Fig. 45).15 The use of a sterically crowded (amino)amide transient directing group (15) is critical for achieving high enantioselectivity in these reactions. That is, the CH insertion of 21 to Pd(IV), as well as CF bond formation through reductive elimination, occurs from the side opposite to the bulky tert-butyl substituent in the chiral auxiliary. 4.3.3 Fluoroalkylation of hydrazones 4.3.3.1 Difluoroalkylation of hydrazones The sp2-CH difluoroalkylation, trifluoromethylation, and perfluoroalkylation of hydrazones can be achieved using organometallic catalysis, in tandem, with photoredox catalysis. These fluoroalkylation reactions, involving fluoroalkyl radical addition to the imino carbon, followed by SET from the resulting nitryl radical to the high-valent transition metal [e.g., Ir(IV)], and then deprotonation of the nitrenium cation, constitute indirect CH activation (vide infra; Fig. 46). Difluoroalkylation of aldehyde hydrazones, such as morpholino hydrazones of aromatic and aliphatic aldehydes, could be achieved under the photoredox conditions in the presence of an Ir(III) photo-catalyst [fac-Ir(ppy)3] to give the corresponding difluoroalkyl hydrazones.16 In these reactions the imino CH bond is substituted by the difluoroalkyl moiety, in a multistep process. Ethyl bromodifluoroacetate reacts with aliphatic and aromatic aldehyde hydrazones under these photochemical conditions to afford the corresponding difluoroalkylation products in high yields, and these reactions can also be carried out in a one-pot procedure from the reaction mixture, consisting of the aldehydes, N-aminomorpholine, CF2BrCO2Et, and the Ir(III) catalyst (Fig. 46). This visible-light photocatalyzed reaction provides an indirect route to CH activation leading to the difluoroalkylation. The mechanistic rationale for this photoredox reaction is as follows.16 A SET from the photoexcited Ir(III) to the ethyl bromodifluoroacetate generates the transient gem-difluoroalkyl radical species 28, which undergoes free-radical addition to the hydrazones, forming the hydrazinyl radical intermediates 29. The later radical species is oxidized by Ir(IV) formed in the first step (through a SET mechanism), to the corresponding nitrenium cation 30, regenerating the Ir(III) photocatalyst. Finally, deprotonation of the nitrenium cation 30 by a base (e.g., Br2) gives the corresponding fluoroalkyl-substituted hydrazones 26. 112 Organofluorine Chemistry FIGURE 4–6 [Ir(III)] photoredoxcatalyzed difluoroalkylation of hydrazones using ethyl bromodifluoroacetate. ppy 5 2-phenylpyridine. Chapter 4 • Organotransition metal catalysis in the synthesis 113 FIGURE 4–7 Au(I)-catalyzed fluoroalkylations of hydrazones. dppm 5 1,1-bis(diphenylphosphino)methane Au(I)-catalyzed photoredox reactions can also be used for the sp2-CH difluoroalkylation and perfluoroalkylation of hydrazones, using the difluoroalkyl- and perfluoroalkyl bromides (e.g., 34), or diethyl bromo(difluoromethyl)phosphonate (37).17 Reduction of these gem-difluoroalkyl-substituted hydrazones gives the β-hydrazino carboxylic acid esters (36) and gem-difluoromethylated β-hydrazino phosphonic acid esters (39). These gem-difluoromethylated carboxylic acid and phosphonic acid derivatives have potential biological and medicinal applications, such as in the design of peptide isosteres and enzyme inhibitors (Fig. 47). 4.3.3.2 Trifluoromethylation of hydrazones Aldehyde N, N-(dialkyl)hydrazones react with Togni’s reagent, in the presence of catalytic amounts of CuCl to give the corresponding trifluoromethyl-substituted hydrazones (Fig. 48). This trifluoromethylation reaction was suggested to involve a SET from Cu(I) to the Togni’s reagent to generate the trifluoromethyl radical, which reacts with the imino carbon of the hydrazone, forming the hydrazinyl radical, as in the case of the gem-difluoromethylations, described earlier. Oxidation of this radical by Cu(II), followed by deprotonation from the neighboring carbon would then give the α-trifluoromethyl hydrazones.18 The fluoroalkyl hydrazones, if transformed into their corresponding amines, would give biologically and pharmaceutically interesting α-(fluoroalkyl)amines. The reduced basicity of the amines through incorporation of the fluoroalkyl moieties would enhance their lipophilicity and metabolic stability. 114 Organofluorine Chemistry FIGURE 4–8 Cu(I)-catalyzed α-trifluoromethylation of hydrazones using Togni’s reagent. FIGURE 4–9 Fluorinated peptide bioisosteres as transition state analog inhibitors of proteases. 4.4 Au(I)-catalyzed hydrofluorination of alkenes and alkynes Fluroalkenes are biologically interesting compounds. For example, fluoroalkene peptide bioisosteres, such as Cbz-Glyψ[(Z)-CF 5 CH]Leu (41) are inhibitors of endopeptidase thermolysin, a Zn-dependent metalloproteinase, produced by the Gram-positive bacteria (Fig. 49).19 The synthesis of the peptide isosteres 41 usually involves a multistep process. An attractive strategy for these compounds would be the hydrofluorination of the corresponding alkynes. Sadighi and coworkers demonstrated the (NHC)Au(I)-catalyzed trans-hydrofluorination of alkynes in the presence of triethylamine tris(hydrofluoride) [Et3N (HF)3] (Fig. 410).20 The π-complex of the alkynes with Au(I) species (44) was isolated and characterized by single-crystal X-ray crystallography. Nucleophilic addition of fluoride anion to this cationic Au(I)alkyne complex gives the trans-fluoroalkenyl-Au(I) species 45, which undergoes stereoselective protolysis, in the presence of Et3N (HF)3 to give the corresponding fluoroalkenes. The formation of the trans-fluoroalkenyl-Au(I) intermediate 45 was confirmed by 1H NMR spectroscopy. Due to the relatively weaker acidity of the Et3N (HF)3, strong acid catalysts, such as amine-triflic acid and KHSO4, are used as additives for enhancing the rates of these reactions. In some cases, these reactions are not completely regioselective. However, the major product contains fluorine vicinal to the more strongly electron-releasing aryl rings Chapter 4 • Organotransition metal catalysis in the synthesis Et3 N.(HF)3 /KHSO4 R1 R2 PhNMe 2 .HOTf (10 mol%) Au(I) catalyst (2.5 mol%) 42 115 H SIPr Au O R1 R2 Au(I) catalyst F 43 DCE, RT, 18–30 h i-Pr i-Pr 74%–86% R 1 = for example, Ph, 4-OMePh, n-C 5H 11 N R 2 = for example, Ph, n-C 5 H 11 N i-Pr i-Pr SIPr (NHC) Proposed mechanistic outline: L R1 Au X R2 X = e.g., F L AuL H F AuL Au X R1 F + R2 44 F F AuL H R1 R1 R2 F 45 R2 F 46 FIGURE 4–10 Au(I)-catalyzed hydrofluorination of alkynes and a proposed mechanism. (Fig. 410). Hydrofluorination of alkynes with amineHF reagents, in the absence of the Au (I) catalysis, on the other hand, results in the predominant formation of the dihydrofluorination products, that is, the gem-difluoro compounds, as the major products, because the intermediate fluoroalkenes are rapidly hydrofluorinated to give the corresponding gem-difluoro compounds.21 Hammond and coworkers, using a phthalimido-Au(I) complex 47 as the organometallic catalyst and HF/DMPU [1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone] reagent, achieved regioselective hydrofluorination of alkynes. The reaction scope and regioselectivity of the hydrofluorination of the alkynes is dramatically improved when 65%:35% wt/wt HF/ DMPU (i.e., 12:1 mol ratio of HF:DMPU) is used in these reactions. A variety of aryl- and alkyl-substituted terminal and internal alkynes give the corresponding hydrofluorination products in moderate to high yields (Fig. 411).22 In these Au(I)-catalyzed hydrofluorination reactions, the phthalimide ligand in the Au(I) catalyst 47 serves as a good leaving group in the presence of the amineHF complex, forming the Au(I)1 as the active catalyst. The Au(I)1 forms a π-complex with the alkyne (42), to give 44. The alkyneAu1 complex 14 then undergoes regio- and stereoselective trans-hydrofluorination to give fluoroalkenes, 46. The π-complexes 44 were isolated and characterized through single-crystal X-ray crystallography.20 The fluoroalkenes 43 are unreactive under the abovementioned reaction conditions. However, in the presence of Lewis acids, such as Ga(OTf)3, or Bronsted acids, such as KHSO4, the fluoroalkenes react with DMPU/HF and Au(I) catalyst to give the corresponding gem-difluoro compounds in high yields (Fig. 412).22 116 Organofluorine Chemistry n FIGURE 4–11 Au(I)-catalyzed hydrofluorination of alkynes using DMPU/HF amine complex. 4.5 Ni-catalyzed fluoroalkylation of aromatics 4.5.1 Fluoroalkylation of arylsilanes Ni-catalyzed fluoroalkylation of arylsilanes or (heteroaryl)silanes, using monofluoroalkyl bromides (RCHFBr) or gem-difluoroalkyl bromides (RCF2Br), affords the corresponding Chapter 4 • Organotransition metal catalysis in the synthesis 117 FIGURE 4–12 Au(I)-catalyzed dihydrofluorination of alkynes in the presence of a strong acid. monofluoroalkyl- or gem-(difluoroalkyl)arenes in moderate to high yields. After careful optimization of the reaction conditions, it was found that the 4,40 -di-tert-butyl-2,20 -bipyridyl ligand (dtbbpy) is critical for achieving high yields.23 This reaction has a wide substrate scope, including aryl and heteroaryl substrates, and is tolerant to various functional groups, such as ketone, ester, halogen, and ether moieties. Both electron-withdrawing as well as electron-donating groups on the aryl ring favored the fluoroalkylation reactions (Fig. 413). This reaction allows late-stage fluoroalkylations and thus is useful for the synthesis of fluorine-containing pharmaceuticals and their derivatives. Thus ezetimibe, a cholesterol-lowering drug that lowers intestinal cholesterol absorption, could be fluoroalkylated through its triethoxysilane derivative. The following proposed mechanism rationalizes the Ni(I)-catalyzed fluoroalkylation of aromatics.23 The (Dtbbpy)Ni(I)Cl complex (51), formed from the Ni(II)(dme)Cl2, is the active catalyst in these reactions. The fluoride anioninitiated formation of the aryl carbanions from the arylsilanes [ArSi(OEt)3], followed by transmetalation with (dtbbpy)Ni(I)Cl (51) gives (dtbbpy)Ni(I)-Ar intermediate (52). [Ni(I)]Ar intermediate (52) then undergoes oxidative addition to the fluoroalkyl substrates to give the [Ni(III)] intermediate 54. The subsequent reductive elimination of 54 gives the (fluoroalkyl)arenes, regenerating the Ni(I) catalyst. Radical-trapping experiments suggested the involvement of a transient [Ni(II)] species, associated with the fluoroalkyl free radical (53), for these fluoroalkylations (Fig. 414).23 118 Organofluorine Chemistry ′ ′ FIGURE 4–13 Ni-catalyzed fluoroalkylation of arylsilanes. dtbbpy 5 4,4’-di-tert-butyl-2,2’-bipyridyl. 4.5.2 Aryl difluoromethylation Ni(0)-catalyzed cross-coupling of various substituted (hetero)aryl chlorides using chlorodifluoromethane (CHF2Cl), an inexpensive industrial chemical, gives the corresponding difluoromethylated products in moderate to high yields. A variety of functional groups on the aromatics, such as amines, esters, imines, and ketones, are tolerated in these Ni(0)-catalyzed difluoromethylations.24 Through this innovative synthetic methodology, Zhang and coworkers have synthesized a large variety of difluoromethylated versions of the pharmaceuticals, starting from their corresponding aryl chlorides. Chlorine-containing pharmaceuticals, such as fenofibrate (cardiovascular drug), chlorodiphenhydramine (antihistamine Chapter 4 • Organotransition metal catalysis in the synthesis 119 FIGURE 4–14 Mechanistic rationale for the Ni-catalyzed fluoroalkylation of aromatics. dtbbpy 5 4,4’-di-tert-butyl2,2’-bipyridyl. and anticholinergic), buclizine (antihistamine), tolvaptan (hyponatremia agent), clofibrate (cardiovascular disease drug), clomipramine (antidepressant), loratadine (antihistaminergic agent), empagliflozin (treatment of type II diabetes) were transformed into the corresponding difluoromethylated compounds (Fig. 415). These new fluorinated versions of the pharmaceuticals are useful in the structure-activity studies toward developing more effective pharmaceuticals, as well as in the synthesis of the 18F-labeled compounds for PET applications. Preliminary mechanistic studies suggested that the reaction proceeds through the oxidative addition of aryl chlorides to Ni(0), which is formed through reduction of Ni(II) by Zn metal. The resulting Ar-Ni(II)-Cl species through a SET with the difluoromethyl FIGURE 4–15 Ni(0)-catalyzed difluoromethylation of chloroarenes, and synthesis of difluoromethylated versions of pharmaceuticals. Chapter 4 • Organotransition metal catalysis in the synthesis 121 [Ni(0)] Zn ArCl [Ni(I)] [Ar-Ni(II)-Cl] ArCF2 H Zn [Ar-Ni(III)-CF2 H(Cl)] [Ar-Ni(I)] [Ar-Ni(II)-Cl CHF2H ClCF2H FIGURE 4–16 Proposed mechanism for the Ni(0)-catalyzed difluoromethylation of aryl chlorides. radical intermediate (•CF2H), presumably generated through a single-electron reduction of ClCF2H by the Ni(I) catalyst, forms the Ar-Ni(III)-CF2H species. The reductive elimination of the latter Ar-Ni(III)-CF2H intermediate then gives the difluoromethylarenes (Ar-CF2H) (Fig. 416). 4.6 Ag(II)-catalyzed oxidative ring-opening fluorination of cyclic amines Sarpong and coworkers used the so-called deconstructive approach in the fluorinative ringopening of the N-benzoylazacycloalkanes (or other N-acyl-protected cyclic amines), such as piperidines, azetidines, and pyrroles, using the reagent combination of Selectfluor and silver tetrafluroborate (AgBF4) to give the corresponding linear, terminal alkyl fluorides (Fig. 417).25 While in most cases, the formyl group is attached at the amide nitrogen in the product, in some cases, such as N-benoylazetidine and N-benzoyl-2-alkylazacyclohexanes, the N-formyl group is lacking in the product. Although this reaction is applicable to fourmembered and other larger rings, unexpectedly, the five-membered ring N-acyl amines do not give the ring-opened fluorination products. This deconstructive ring-opening fluorination reaction was further developed toward practical applications in the synthesis of fluorinated peptide derivatives.25,26 Thus using pipecolic acid amides as the N-terminal protecting group of the peptides (or amino acids), the corresponding ring-opened fluoroalkyl-substituted unnatural peptides (e.g., 62) are formed in good yields. The ring-opening fluorination synthetic strategy was also used for the 122 Organofluorine Chemistry F Selectfluor (4 equiv) AgBF4 (4 equiv) acetone:H 2O (1:9); 40 °C 1 h N Ph N O N Ph N F O O Cl 2BF4 – Selectfluor 56 81% 55 F Above conditions NH H3 C N H3 C Ph Ph O O 57 58 81% Above conditions N F NH Ph Ph O O 59 60 40 % F H N N O Ph O O HN OCH 3 RT, 15 h O Ph 63 Acetone:h2O (1:1) RT, 15 h F F F N H3 C O OCH 3 62 50% Selectfluor (4 equiv) AgBF4 (0.25 equiv) N O O Ph 61 H3 C H N Above conditions Ph 64 OH O H3 C N Ph O O 65 FIGURE 4–17 Ring-opening fluorination of cyclic amines to give the linear fluoroalkyl (gem-difluoroalkyl) amides. preparation of the terminal gem-difluoroalkyl amides (e.g., 65) from the corresponding Nacyl enamines, such as 63. Fluorohydrin 64 was proposed as the reaction intermediate in this reaction (Fig. 417). One of the two probable mechanisms for the deconstructive fluorination of the N-acyl cyclic amines, suggested by Sarpong and coworkers, involves the Ag(II)-catalyzed SET oxidation of the N-acyl cyclic amine (55), forming the corresponding N-acylaminium radical cation (66).25 The Ag(II), required for this transformation, is formed through Selectfluormediated oxidation of the Ag(I). Selectfluor radical cation abstracts the α-CH hydrogen atom of the radical cation 66 to give the imine 67, hydration of which forms the hemiaminal 68. Complexation of Ag(II) ion with 68 gives the intermediate 69, which undergoes Chapter 4 • Organotransition metal catalysis in the synthesis 123 FIGURE 4–18 Probable mechanism for the deconstructive ring-opening fluorination of N-acyl cyclic amines. homolytic CC bond cleavage to form the terminal alkyl radical 70. The latter alkyl radical 70 then abstracts the fluorine atom from the Selectfluor to give the terminal alkyl fluoride, regenerating the Selectfluor radical cation for further propagation of the catalytic cycle (Fig. 418). 4.7 Ag(I)-catalyzed decarboxylative fluorination Decarboxylative fluorination of carboxylic acids using Selectfluor and AgNO3 gives the corresponding alkyl fluorides. The decarboxylative fluorination of the (carboxymethyl)thio ester 71 gives fluticasone propionate in high yields, under mild reaction conditions (Fig. 419).27 The proposed mechanism involves Selectfluor-mediated oxidation of the Ag(I) to F-Ag(III), which undergoes a SET redox reaction with the carboxylic acid 71 to form F-Ag(II) and the alkyl radical 72. F-Ag(II) then transfers the fluorine atom to 72 to give the fluticasone propionate, regenerating the Ag(I) catalyst. 124 Organofluorine Chemistry FIGURE 4–19 Ag(I)-catalyzed decarboxylative fluorination in the synthesis of fluticasone propionate. 4.8 Cu(I)-mediated dediazoniative difluoromethylation Goossen and coworkers have developed Sandmeyer-type of dediazoniationdifluoromethylation reaction of aromatic amines, using in situ generated Cu-CHF2 reagent. The Cu-CHF2 reagent was synthesized in situ from the reaction of copper thiocyanate and CHF2TMS in dimethylformamide (DMF) in the presence of CsF. This reaction was demonstrated to involve a free-radical pathway through mechanistic studies, using the difluoromethylation of 2-(allyloxy)diazonium tetrafluoroborate as the substrate. The formation of the cyclized product in this reaction indicates that the reaction goes through a free-radical mechanism (Fig. 420).28 Chapter 4 • Organotransition metal catalysis in the synthesis 125 FIGURE 4–20 Sandmeyer-type of dediazoniative difluoromethylation of arylamines. FIGURE 4–21 Cu-catalyzed trifluoromethylation of arylboronic acids. 4.9 Fluoroalkylation of arylboronic acids and esters 4.9.1 Copper-mediated trifluoromethylation Cu(II)-catalyzed trifluoromethylation of arylboronic acids using TMSCF3 proceed under mild conditions to afford the trifluoromethyl aromatics in moderate to good yields.29 The reaction is useful for the trifluoromethylation of a variety of aromatic and heteroaromatic compounds, such as quinoline, thiazole, and indole derivatives (Fig. 421). In the absence of oxygen, when exposed only to air, the trifluoromethylated products are formed in much lower yields. 126 Organofluorine Chemistry FIGURE 4–22 Cu(I)-catalyzed trifluoromethylation of arylboronate esters. 4.9.2 Cu(I)-catalyzed trifluoromethylation of arylboronate esters Hartwig and coworkers have developed the Cu(I)-catalyzed trifluoromethylation of arylboronate esters to give the corresponding trifluoromethylarenes, using the (phen)CuCF3 (phen 5 1,10-phenanthroline) reagent. The Cu(I)CF3 reagent could be synthesized from the complexation of tBuOCu(I) with 1,10-phenanthroline, followed by reaction with CF3TMS, in high yields.30,31 Perfluoroalkylcopper(I) reagents, under similar reaction conditions, give the corresponding perfluoroalkylarenes (Fig. 422). 4.9.3 Pd(0)-catalyzed difluoroalkylation of arylboronic acids Pdmediated difluoroalkylation using difluoroalkyl halides and arylboronic acids affords the corresponding difluoroalkyl derivatives in high yields (Fig. 423).32,33 The Pd(0)-catalyzed gem-difluoroallylation provides access to the late-stage synthesis of biologically active compounds, such as steroidal compound 78.33 The mechanism of these reactions is similar to that of the Pd(0)-catalyzed Suzuki reactions and involves oxidative addition of the difluoroalkyl halides to the Pd(0), ligand exchange from the arylboronic acid, and finally reductive elimination to give the difluoroalkyl aromatics. 4.10 Cu(I)-catalyzed fluoroalkylation of aryl halides Cu(I)-catalyzed trifluoromethylation and pentafluoroethylation of aryl iodides can be achieved under relatively mild conditions, whereas the corresponding reactions of aryl bromides or chlorides require more drastic conditions.34 Hartwig and coworkers have Chapter 4 • Organotransition metal catalysis in the synthesis 127 FIGURE 4–23 Transition metalcatalyzed difluoroalkylation of arylboronic acid. achieved trifluoromethylation and pentafluoroethylation of aryl bromides, including heteroaryl bromides, using phen(RF)Cu(I) complexes (phen 5 1,10-phenanthroline) (Fig. 424A).35 Grushin and coworkers have generated CuCF3 from fluoroform and used in the trifluoromethylation of aryl iodides and bromides, in the presence of Et3N3HF as a stabilizing agent.36 This ligand-less CuCF3 could be used in the trifluoromethylation of a wide range of iodoarenes, bromoarenes, such as pyridine, pyrimidine, pyrazine, and thiazole derivatives, and bromoarenes bearing electron-withdrawing groups (Fig. 424B). Hu and coworkers have generated pentafluoroethyl-Cu(I) reagent [(CF3CF2)Cu(I)], in situ, by the reaction of CuCl with CF3TMS in the presence of KF and pyridine in DMF solvent, at relatively high temperature. The formation of the CuCF2CF3 may involve the intermediate formation of the difluoromethylene carbene (:CF2) from the initially formed CF3Cu(I), and its insertion into the CuCF3 bond to give the CuCF2CF3.37 This in situ generated reagent reacts with various aryl iodides to give their corresponding pentafluoroethyl derivatives in good yields (Fig. 424C). 4.11 Ni-catalyzed trifluoromethylthiolation Trifluoromethylthiolated compounds have found applications as pharmaceuticals, agrochemicals, and as veterinary medicines. Synthetic methods for the electrophilic trifluoromethylthiolations have been extensively investigated, and there are many commercially available electrophilic trifluoromethylthiolating reagents (see Chapter 2: Electrophilic reactions in the synthesis of organofluorine compounds). 128 Organofluorine Chemistry FIGURE 4–24 Cu(I)-catalyzed trifluoromethylation and pentafluoroethylation of aryl halides: (A) trifluoromethylatin of ArI and ArBr, mediated by (phen)CuCF3 (Hartwig and coworkers); (B) trifloromethyhlation of ArI and ArBr, using ligand-less CF3Cu (Grushin and coworkers); and (C) pentafluoroethylation of ArI using the in situ generated CuCF2CF3 (Hu and coworkers). phen 5 1,10-phenanthroline Schoenebeck and coworkers have developed Ni-catalyzed trifluoromethylthiolation of aryl and vinyl triflates, using (Me4N)SCF3 as the trifluoromethylthiolating agent (Fig. 425). This organometallic approach for the trifluoromethylthiolation is applicable for the synthesis of biologically interesting compounds, such as flavanones and estrone derivatives and serves as an alternative approach to the electrophilic trifluoromethylthiolations (see Chapter 2: Electrophilic reactions in the synthesis of organofluorine compounds). These Ni(0)-catalyzed trifluoromethylthiolations presumably proceeds through oxidative addition of the aryl triflates to Ni(0), transmetalation, and reductive elimination of the ArSCF3. Chapter 4 • Organotransition metal catalysis in the synthesis 129 FIGURE 4–25 Ni-catalyzed trifluorothiomethylation of aryl triflates. 4.12 Pd(II)-catalyzed (amino)trifluoromethoxylation Introduction of the trifluoromethoxy group into organic compounds is challenging as the nucleophilic CF3O2 anion is prone to dissociate into fluoride anion and fluorophosgene (COF2). Transition metaltrifluoromethoxide complexes also have a tendency to undergo β-fluoride elimination to give COF2. AgOCF3, on the other hand, is stable to dissociation of fluoride anion and is useful in the trifluoromethoxylation of alkenes. The oxidative (amino)cyclization of N-tosyl-2,2-dialkyl-4-pentenamines (e.g., 79, 81, 83, and 85), in the presence of AgOCF3, catalytic amounts of Pd(II) catalyst, and Selectfluor as the oxidant, gives the corresponding trifluoromethoxy piperidines in moderate-to-high yields. The unsubstituted compound 79 (when R and R0 5 H) failed to cyclize in these reactions, implying that the gem-dialkyl groups exert angle-suppression effect (ThropeIngold effect) on the linear chain, favoring the cyclization (Fig. 426).38 A possible mechanism was suggested to involve a reversible (amino)palladation, followed by oxidation of the resulting secondary alkylPd(II) intermediate (87) by Selectfluor to give the high-valent (alkyl)(OCF3)Pd(IV) complex (88), which spontaneously undergoes reductive elimination to give the 3-(trifluoromethoxy)piperidines (Fig. 426). Apparently, the reductive elimination of the high-valent Pd(IV)(alkyl)(OCF3) (88) is relatively faster than the 130 Organofluorine Chemistry FIGURE 4–26 Pd(II)-catalyzed (amino)trifluoromethoxylation of N-tosyl-4-pentenamines, in the presence of AgOCF3 and Selectfluor; Ts 5 4-methylbenzenesulfonyl. β-elimination of the fluoride in these reactions, so that the major products are the trifluoromethoxylated compounds. Liu and coworkers confirmed the intermediacy of the Pd(IV) (OCF3) intermediates in these reactions by the single-crystal X-ray analysis of the intermediate and by NMR studies.38 Chapter 4 • Organotransition metal catalysis in the synthesis 131 References 1. Salehi Marzijarani, N.; Snead, D. R.; McMullen, J. P.; Levesque, F.; Weisel, M.; Varsolona, R. J.; Lam, Y.-h; Liu, Z.; Naber, J. R. One-Step Synthesis of 2-Fluoroadenine Using Hydrogen Fluoride Pyridine in a Continuous Flow Operation. Org. Process Res. Dev. 2019, 23, 15221528. 2. Neumann, C. N.; Hooker, J. M.; Ritter, T. Concerted nucleophilic aromatic substitution with 19F- and 18F. Nature (London, U.K.) 2016, 534, 369373. 3. Sather, A. C.; Buchwald, S. L. The Evolution of Pd0/PdII-Catalyzed Aromatic Fluorination. Acc. Chem. Res. 2016, 49, 21462157. 4. Lou, S.-J.; Chen, Q.; Wang, Y.-F.; Xu, D.-Q.; Du, X.-H.; He, J.-Q.; Mao, Y.-J.; Xu, Z.-Y. Selective CH Bond Fluorination of Phenols with a Removable Directing Group: Late-Stage Fluorination of 2-Phenoxyl Nicotinate Derivatives. ACS Catal. 2015, 5, 28462849. 5. Preshlock, S.; Tredwell, M.; Gouverneur, V. 18F-Labeling of Arenes and Heteroarenes for Applications in Positron Emission Tomography. Chem. Rev. (Washington, DC, U.S.) 2016, 116, 719766. 6. Watson, D. A.; Su, M.; Teverovskiy, G.; Zhang, Y.; Garcia-Fortanet, J.; Kinzel, T.; Buchwald, S. L. Formation of ArF From LPdAr(F): Catalytic Conversion of Aryl Triflates to Aryl Fluorides. Science (Washington, DC, U.S.) 2009, 325, 16611664. 7. Lee, H. G.; Milner, P. J.; Buchwald, S. L. An Improved Catalyst System for the Pd-Catalyzed Fluorination of (Hetero)aryl Triflates. Org. Lett. 2013, 15, 56025605. 8. Yamamoto, K.; Li, J.; Garber, J. A. O.; Rolfes, J. D.; Boursalian, G. B.; Borghs, J. C.; Genicot, C.; Jacq, J.; van Gastel, M.; Neese, F.; Ritter, T. Palladium-Catalysed Electrophilic Aromatic CH Fluorination. Nature (London, U.K.) 2018, 554, 511514. 9. Nodwell, M. B.; Bagai, A.; Halperin, S. D.; Martin, R. E.; Knust, H.; Britton, R. Direct Photocatalytic Fluorination of Benzylic CH Bonds with N-Fluorobenzenesulfonimide. Chem. Commun. (Cambridge, U.K.) 2015, 51, 1178311786. 10. Koperniku, A.; Liu, H.; Hurley, P. B. Mono- and Difluorination of Benzylic Carbon Atoms. Eur. J. Org. Chem. 2016, 2016, 871886. 11. Liu, W.; Groves, J. T. Manganese Catalyzed CH Halogenation. Acc. Chem. Res. 2015, 48, 17271735. 12. Bloom, S.; McCann, M.; Lectka, T. Photocatalyzed Benzylic Fluorination: Shedding "Light" on the Involvement of Electron Transfer. Org. Lett. 2014, 16, 63386341. 13. Huang, X.; Liu, W.; Ren, H.; Neelamegam, R.; Hooker, J. M.; Groves, J. T.; Late Stage.; Benzylic, C.-H Fluorination with [18F]Fluoride for PET Imaging. J. Am. Chem. Soc. 2014, 136, 68426845. 14. Liu, W.; Huang, X.; Placzek, M. S.; Krska, S. W.; McQuade, P.; Hooker, J. M.; Groves, J. T. Site-Selective 18 F Fluorination of Unactivated CH Bonds Mediated by a Manganese Porphyrin. Chem. Sci. 2018, 9, 11681172. 15. Park, H.; Verma, P.; Hong, K.; Yu, J.-Q. Controlling Pd(IV) Reductive Elimination Pathways Enables Pd (II)-Catalysed Enantioselective C(sp3)-H Fluorination. Nat. Chem. 2018, 10, 755762. 16. Xu, P.; Wang, G.; Zhu, Y.; Li, W.; Cheng, Y.; Li, S.; Zhu, C. Visible-Light Photoredox-Catalyzed CH Difluoroalkylation of Hydrazones Through an Aminyl Radical/Polar Mechanism. Angew. Chem., Int. Ed. 2016, 55, 29392943. 17. Xie, J.; Zhang, T.; Chen, F.; Mehrkens, N.; Rominger, F.; Rudolph, M.; Hashmi, A. S. K. Gold-Catalyzed Highly Selective Photoredox C(sp2)-H Difluoroalkylation and Perfluoroalkylation of Hydrazones. Angew. Chem., Int. Ed. 2016, 55, 29342938. 18. Pair, E.; Monteiro, N.; Bouyssi, D.; Baudoin, O. Copper-Catalyzed Trifluoromethylation of N,NDialkylhydrazones. Angew. Chem., Int. Ed. 2013, 52, 53465349. 19. Bartlett, P. A.; Otake, A. Fluoroalkenes as Peptide Isosteres: Ground State Analog Inhibitors of Thermolysin. J. Org. Chem. 1995, 60, 31073111. 132 Organofluorine Chemistry 20. Akana, J. A.; Bhattacharyya, K. X.; Mueller, P.; Sadighi, J. P. Reversible CF Bond Formation and the AuCatalyzed Hydrofluorination of Alkynes. J. Am. Chem. Soc. 2007, 129, 77367737. 21. Olah, G. A.; Li, X. Y.; Wang, Q.; Prakash, G. K. S. Synthetic Methods and Reactions. 169. Poly-4Vinylpyridinium Poly(Hydrogen Fluoride): A Solid Hydrogen Fluoride Equivalent Reagent. Synthesis 1993, 693699. 22. Okoromoba, O. E.; Han, J.; Hammond, G. B.; Xu, B. Designer HF-Based Fluorination Reagent: Highly Regioselective Synthesis of Fluoroalkenes and gem-Difluoromethylene Compounds From Alkynes. J. Am. Chem. Soc. 2014, 136, 1438114384. 23. Wu, Y.; Zhang, H.-R.; Cao, Y.-X.; Lan, Q.; Wang, X.-S. Nickel-Catalyzed Monofluoroalkylation of Arylsilanes via Hiyama Cross-Coupling. Org. Lett. 2016, 18, 55645567. 24. Xu, C.; Guo, W.-H.; He, X.; Guo, Y.-L.; Zhang, X.-Y.; Zhang, X. Difluoromethylation of (Hetero)aryl Chlorides with Chlorodifluoromethane Catalyzed by Nickel. Nat. Commun. 2018, 9, 110. 25. Roque, J. B.; Kuroda, Y.; Goettemann, L. T.; Sarpong, R. Deconstructive Fluorination of Cyclic Amines by Carbon-Carbon Cleavage. Science (Washington, DC, U.S.) 2018, 361, 171174. 26. Roque, J. B.; Kuroda, Y.; Gottemann, L. T.; Sarpong, R. Deconstructive Diversification of Cyclic Amines. Nature (London, U.K.) 2018, 564, 244248. 27. Zhou, J.; Jin, C.; Su, W. Improved Synthesis of Fluticasone Propionate. Org. Process Res. Dev. 2014, 18, 928933. 28. Matheis, C.; Jouvin, K.; Goossen, L. J. Sandmeyer Difluoromethylation of (Hetero-)arenediazonium Salts. Org. Lett. 2014, 16, 59845987. 29. Senecal, T. D.; Parsons, A. T.; Buchwald, S. L. Room Temperature Aryl Trifluoromethylation via CopperMediated Oxidative Cross-Coupling. J. Org. Chem. 2011, 76, 11741176. 30. Morimoto, H.; Tsubogo, T.; Litvinas, N. D.; Hartwig, J. F. A Broadly Applicable Copper Reagent for Trifluoromethylations and Perfluoroalkylations of Aryl Iodides and Bromides. Angew. Chem., Int. Ed. Engl. 2011, 50, 37933798. 31. Zhang, C. Advances in Trifluoromethylation or Trifluoromethylthiolation with Copper CF3 or SCF3 Complexes. J. Chem. Sci. (Berlin, Ger.) 2017, 129, 17951805. 32. Feng, Z.; Xiao, Y.-L.; Zhang, X. Transition-Metal (Cu, Pd, Ni)-Catalyzed Difluoroalkylation via CrossCoupling with Difluoroalkyl Halides. Acc. Chem. Res. 2018, 51, 22642278. 33. Min, Q.-Q.; Yin, Z.; Feng, Z.; Guo, W.-H.; Zhang, X. Highly Selective gem-Difluoroallylation of Organoborons with Bromodifluoromethylated Alkenes Catalyzed by Palladium. J. Am. Chem. Soc. 2014, 136, 12301233. 34. Lantano, B.; Torviso, M. R.; Bonesi, S. M.; Barata-Vallejo, S.; Postigo, A. Advances in Metal-Assisted NonElectrophilic Fluoroalkylation Reactions of Organic Compounds. Coord. Chem. Rev. 2015, 285, 76108. 35. Mormino, M. G.; Fier, P. S.; Hartwig, J. F. Copper-Mediated Perfluoroalkylation of Heteroaryl Bromides with (phen)CuRF. Org. Lett. 2014, 16, 17441747. 36. Lishchynskyi, A.; Novikov, M. A.; Martin, E.; Escudero-Adan, E. C.; Novak, P.; Grushin, V. V. Trifluoromethylation of Aryl and Heteroaryl Halides with Fluoroform-Derived CuCF3: Scope, Limitations, and Mechanistic Features. J. Org. Chem. 2013, 78, 1112611146. 37. Xie, Q.; Li, L.; Zhu, Z.; Zhang, R.; Ni, C.; Hu, J. From C1 to C2: TMSCF3 as a Precursor for Pentafluoroethylation. Angew. Chem., Int. Ed. 2018, 57, 1321113215. 38. Chen, C.; Chen, P.; Liu, G. Palladium-Catalyzed Intramolecular Aminotrifluoromethoxylation of Alkenes. J. Am. Chem. Soc. 2015, 137, 1564815651. 5 Pharmaceutical applications of organofluorine compounds Chapter Outline 5.1 Introduction ............................................................................................................................... 134 5.1.1 Bloodbrain permeability .............................................................................................. 136 5.1.2 Metabolic stability and bioavailability .......................................................................... 137 5.1.3 ππ Stacking interactions............................................................................................... 140 5.2 Antibacterial pharmaceuticals.................................................................................................. 141 5.2.1 Fluoroquinolones............................................................................................................. 142 5.2.2 Tetracyclines..................................................................................................................... 145 5.3 Antidiabetic pharmaceuticals................................................................................................... 146 5.3.1 Sitagliptin ......................................................................................................................... 146 5.3.2 Carmegliptin .................................................................................................................... 150 5.3.3 Canagliflozin .................................................................................................................... 151 5.4 Anti-Alzheimer pharmaceuticals.............................................................................................. 152 5.4.1 BACE-1 inhibitors ............................................................................................................. 153 5.4.2 γ-Secretase inhibitors and modulators .......................................................................... 159 5.5 Anti-HIV pharmaceuticals ......................................................................................................... 163 5.5.1 Bictegravir ........................................................................................................................ 163 5.5.2 Doravirine......................................................................................................................... 165 5.6 Antimalarial pharmaceuticals................................................................................................... 165 5.6.1 Tafenoquine..................................................................................................................... 165 5.6.2 Mefloquine....................................................................................................................... 166 5.7 Anticancer pharmaceuticals ..................................................................................................... 167 5.7.1 Dacomitinib.................................................................................................................... 167 5.7.2 Lorlatinib ........................................................................................................................ 169 5.7.3 Cobimetinib.................................................................................................................... 171 5.7.4 Abemaciclib.................................................................................................................... 172 5.7.5 PARP inhibitors: rucaparib (Rubraca) and olaparib (Lynparza) ................................. 172 5.7.6 Taxoid anticancer agents .............................................................................................. 173 5.7.7 Fulvestrant...................................................................................................................... 177 5.7.8 Enasidenib ...................................................................................................................... 178 5.7.9 Nonsteroidal antiandrogens (apalutamide, bicalutamide, and flutamide) ............. 181 Organofluorine Chemistry. DOI: https://doi.org/10.1016/B978-0-12-813286-9.00005-5 © 2020 Elsevier Inc. All rights reserved. 133 134 Organofluorine Chemistry 5.7.10 BRAF and mitogen-activated protein kinase kinase enzyme inhibitors in cancer treatment ....................................................................................................................... 183 5.8 Antiviral pharmaceuticals......................................................................................................... 185 5.8.1 Tecovirimat....................................................................................................................... 185 5.8.2 Sofosbuvir......................................................................................................................... 187 5.8.3 Ledipasvir ......................................................................................................................... 189 5.8.4 Glecaprevir and pibrentasvir .......................................................................................... 190 5.8.5 Voxilaprevir ...................................................................................................................... 194 5.8.6 Letermovir (Prevymis)...................................................................................................... 194 5.9 Fluorinated pharmaceuticals for cardiovascular diseases ..................................................... 195 5.9.1 Statin drugs ...................................................................................................................... 195 5.9.2 Ezetimibe.......................................................................................................................... 196 5.9.3 Nebivolol .......................................................................................................................... 196 5.9.4 Antiplatelet drugs ........................................................................................................... 197 5.10 Antiinflammatory pharmaceuticals ......................................................................................... 199 5.10.1 Nonsteroidal antiinflammatory agents ....................................................................... 199 5.10.2 Celecoxib ........................................................................................................................ 200 5.10.3 Corticosteroids ............................................................................................................... 200 5.11 Antidepressants......................................................................................................................... 202 References........................................................................................................................................... 204 5.1 Introduction Incorporation of fluorine or fluorinated moieties as bioisosteres in the lead compounds has emerged as the major focus of the drug design efforts. In 2018 nearly one-third of the FDAapproved drugs, that is, 18 out of the 59 drugs approved, were fluorine- or fluoroalkyl(aryl) moietycontaining compounds. Due to the relatively small van der Waals radius of fluorine (1.47 Å for fluorine; 1.20 Å for hydrogen; 1.23 Å for sp2-hybridized oxygen; and 1.43 Å for the sp3-hybridized oxygen) and its high electronegativity (EN 5 3.98, highest of all the elements), gem-difluoromethyl (CF2) moiety is bioisosteric with respect to the oxygen in ethers and alcohols, and trifluoromethyl (CF3) substituent is bioisosteric with respect to chloro, bromo, and cyano moieties. It is a common practice in drug design to replace the oxidatively unstable CH bonds by CF bonds because of the relatively stronger CF bond strength and biochemical stability. The drug candidates appropriately modified by the fluorine and fluoroalkyl(aryl) moieties, including CF2 and CF3 moieties, often show improved pharmacokinetic and pharmacodynamic properties. Fluorine is more lipophilic than the hydrogen, hydroxyl, and carbonyl moieties, is not as polarizable as other halogen atoms, and exhibits limited halogen-bonding effects. Due to these favorable steric and stereoelectronic effects, fluorine and fluoroalkyl groups (e.g., CF2) could be used as bioisosteres of hydrogen, carbonyl, and hydroxyl groups. Furthermore, the fluoroalkyl substituents provide improved Chapter 5 • Pharmaceutical applications of organofluorine compounds 135 lipophilicity and modulate acidity and basicity of the pharmacophores, thereby enhancing the bioavailability of the fluorinated drug candidates.1,2 Fluorine atom is a biomimetic (bioisosteric) of hydrogen atom and thus replacement of CF3 moiety in place of CH3 moiety would not significantly alter pharmacokinetic properties, such as P-glycoprotein (P-gp) recognition, membrane permeability, and lipophilicity. On the other hand, CF3, CF2, OCF3, or OCF2H groups enhance oxidative stability, when placed at or adjacent to the CH bonds that are prone to enzymatic oxidation. For example, fluorination or fluoroalkoxylation of the aromatic ring in the taxoid compounds would result in enhanced metabolic stability and potency (vide infra).3 Many of the blockbuster drugs, including the cholesterol-lowering drug atorvastatin (Lipitor), are fluorinated compounds (Fig. 51). Atorvastatin was the third most prescribed drug as of 2016.4 The structurally related statins, rosuvastatin, fluvastatin, and cerivastatin, all have p-fluorophenyl moiety attached to the heterocyclic aromatic ring, and these statin drugs have high affinity to the 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, with half-maximal inhibitory constant (IC50), ranging from 5 to 28 nM.5 An X-ray crystal structure of the atorvastatin bound to HMG-CoA reductase shows orthogonal multipolar interactions6,7 of the fluorine with the Arg590 guanidino carbon (3.1 Å) and polar (or hydrogen bonding) interactions with the Arg590 guanidino moiety and Ser661 hydroxyl group. This demonstrates the OH OH F O O N O N N S O N OH NH OH OH OH O F Rosuvastatin IC 50 = 5 nM Atorvastatin IC 50 = 8 nM F F OH OH N OH Fluvastatin IC 50 = 28 nM OH O O OH O OH N Cerivastatin IC 50 = 10 nM FIGURE 5–1 Structure of atorvastatin and structurally related statins, the widely used cholesterol-lowering drugs. The IC50 values shown are for the inhibition of the HMG-CoA reductase; HMG-CoA, 3-Hydroxy-3-methylglutaryl coenzyme A; IC50, half-maximal inhibitory constant. 136 Organofluorine Chemistry FIGURE 5–2 Expanded view of the X-ray structure of atorvastatin bound to HMG-CoA reductase, showing the multipolar and hydrogen bonding interactions of the aryl fluorine with Arg590 and Ser660 residues; multipolar and hydrogen bond distances (Å) are shown on the dotted lines; the structure was created using UCSF Chimera software; PDB 1HWK; HMG-CoA, 3-Hydroxy-3-methylglutaryl coenzyme A. stabilizing effect of the aryl fluorine, involving multipolar and hydrogen-bonding interactions. The stabilization energies for the multipolar interactions of the fluorine were estimated to be in the order of 0.30.6 kcal/mol (Fig. 52).8 5.1.1 Bloodbrain permeability When fluorine or fluoroalkyl groups are placed proximal to the amino groups, the basicity of the amines is significantly attenuated, and therefore the lipophilicity and membrane permeability of these compounds are enhanced. For example, the quinuclidine 1 is a potent α7 nicotinic acetylcholine receptor (nAChR; involved in the long-term memory) agonist, but due to its relatively high basicity, compound 1 has poor bloodbrain barrier (BBB) permeability, probably because of the increased transporter-mediated efflux. The fluorine-substituted analog 2 has a dramatic decrease in the basicity (ΔpKa 5 2.5), thereby substantially improved bloodbrain penetration, as determined by a Caco-2 (human epithelial colorectal adenocarcinoma cell line) permeability assay. Thus, the Caco-2 P-gp efflux ratios for the compounds 1 and 2 are 6.9 and 0.6, respectively (Fig. 53).9 The P-gp-mediated efflux ratios of greater than 3 are generally indicative of the compounds being P-gp substrates and, therefore, are not retained in the brain tissues in sufficient concentrations for the drug to be active.10 Based on this empirical criterion, in order to be effective central nervous system (CNS) drugs, the efflux ratios should be less than 3. Although compound 2 is a relatively less potent α7 nAChR agonist than compound 1, these results nevertheless indicate the importance of modulating the basicity of the amino groups for improved bloodbrain permeability.9 Chapter 5 • Pharmaceutical applications of organofluorine compounds N N O N N 1 pKa = 10.1 137 O F 2 pKa = 7.6 Caco-2 efflux ratios: 6.9 0.6 FIGURE 5–3 Structures of quinolonequinuclidine moiety containing α7 nAChR agonists, and the effect of basicity on the bloodbrain permeability, as measured by the Caco-2 efflux ratio (the lower the pKa, the lower the efflux ratio, and thereby the higher the bloodbrain permeability); nAChR, Nicotinic acetylcholine receptor. As indicated above, in order for the drug candidate to show optimal BBB penetration, the compound should have high passive permeability across the BBB and decreased efflux transporter liability (i.e., the lower the efflux ratio, the better the BBB penetration). Substitution of fluorine at the strategic positions increases lipophilicity and thereby increases passive permeability of the drug candidate, and also decreases basicity of the amino groups, as shown earlier in the case of an α7 nAChR agonist. Silverman and coworkers designed analogs of a lead compound 3 (selective inhibitor of human neuronal nitric oxide synthase) with enhanced lipophilicity through incorporation of an additional aryl CF bond in the linker fragment. Furthermore, the basicity of the terminal tertiary amine moiety decreases with increasing ring strain (i.e., attenuated flexibility of the amino group). The least strained amine with the openchain tertiary-amino moiety (compound 3) has the highest basicity among the series of its analogs, N-methyl-azacyclopentane (4) and N-methyl-azacyclobutane (5; the most strained in the series). As a result of these structural optimizations, the Caco-2 efflux ratios for compounds 3, 4, and 5 are in the decreasing order of 5.9, 2.1, and 0.8, respectively (Fig. 54).11 The selectivity of the compounds 4 and 5 toward human nitric oxide synthase are retained or slightly enhanced (Ki 5 21 and 23 nM for compounds 4 and 5, respectively) as compared to the parent lead compound 3 (Ki 5 30 nM). The passive permeabilities of the compounds 4 and 5, as measured by PAMPA-BBB (parallel artificial membrane permeability for the BBB) assay, are also enhanced as compared to the lead compound 3: 14.8 3 1026, 17.0 3 1026, 16.3 3 1026 cm/s, respectively, for compounds 3, 4, and 5.11 In general, CNS positive drugs have effective permeability values higher than 4.0 3 1026 cm/s.11 5.1.2 Metabolic stability and bioavailability The fluorine substitution, in general, results in improved pharmacokinetic and pharmacodynamic properties for the drug candidates. Often, the incorporation of a single fluorine on the aryl rings provides enhanced metabolic stability and enhanced potency to the pharmaceuticals, 138 Organofluorine Chemistry H2 N N N H2 N N N F F F 4 3 Efflux ratio (Caco-2): 2.1 Efflux ratio (Caco-2): 5.9 H2 N N N F F 5 Efflux ratio (Caco-2): 0.8 FIGURE 5–4 Optimization of hnNOS inhibitor lead compound 3 for effective bloodbrain barrier penetration; hnNOS, Human neuronal nitric oxide synthase. as compared to those of the structurally similar nonfluorinated compounds. Similarly, replacement of the alkyl groups by the fluoroalkyl moieties could result in significantly improved metabolic stability and thereby, bioavailability. As an illustrative example, sitagliptin (Januvia), a widely prescribed drug to treat diabetes, has substantially improved bioavailability as compared to the analogous pharmacophores with a methyl or ethyl substituent on the triazole ring (6). Sitagliptin exerts its antidiabetic effect through inhibition of the dipeptidyl peptidase-IV (DPP-IV) enzyme. Sitagliptin exerts its antihyperglycemic effect (i.e., lowering of the blood-glucose levels) by inhibiting the DPP-IV enzyme. The latter enzyme degrades the incretins GLP-1 (glucagon-like peptide-1) and gastric inhibitory peptide, the gastrointestinal hormones, whose function is to stimulate insulin secretion and reduce the amount of glucose produced by the liver, as needed. Through inhibition of the DPP-IV enzyme, sitagliptin helps elevate the incretin levels and thereby increase the insulin secretion from the pancreatic β-cells and decrease blood-glucose levels.12 The substantially high oral bioavailability of sitagliptin (by about 76%) as compared to its difluoromethyl (CF2H) and ethyl analogues is because of the metabolically inert trifluoromethyl (CF3) moiety in the triazole ring. The corresponding difluoromethyl and ethyl analogs are prone to relatively fast biodegradation. Thus, whereas the bioavailability of the ethylsubstituted analog 7 is only 2% of the total administration, the bioavailability of the Chapter 5 • Pharmaceutical applications of organofluorine compounds 139 F F F NH2 O N N N CF3 Sitagliptin Oral bioavailability: NH2 O N F IC50 for DPP-4 inhibition: F F N F 76% NH2 O N N N N F N CH2 CH3 7 29 nM N N CF2H 6 13 nM F 37 nM 39% 2% FIGURE 5–5 Pharmacokinetics of the antidiabetic drug sitagliptin and its structural analogs. difluoromethyl analog 6 is relatively enhanced (39%) (Fig. 55).11 The strong CF bond of the CF3 moiety resists biodegradation as compared to the relatively weaker CH bonds in accordance with these observed trends in bioavailability (vide infra). Sitagliptin has relatively improved DPP-IV inhibitory effect (IC50 5 13 nM) as compared to the related difluoromethyl and ethyl analogs (IC50 5 29 and 37 nM for 6 and 7, respectively; Fig. 55).13 Sitagliptin illustrates a case in which the trifluoromethyl group increases bioavailability due to its enhanced metabolic stability and at the same time is more potent, with a relatively lower IC50 value for the DPP-IV enzyme inhibition, as compared to the difluoromethyl and ethyl analogs. The gem-difluoromethylene moiety in 1,3-dioxoles protects them from CYP450mediated oxidative degradation. Cystic fibrosis drugs, lumacaftor and tezacaftor, serve as the examples of the drugs, which contain the gem-difluoro-1,3-dioxole moiety to enhance their metabolic stability. Lumacaftor (VX-809; Vertex Pharmaceuticals) is used to treat cystic fibrosis patients, as a combination drug with ivacaftor (also called Orkambi).14 In cystic fibrosis the cystic fibrosis transmembrane conductance regulator (CFTR) protein, a cyclic adenosine monophosphatedependent anion-gate channel, is misfolded due to the genetic defect and is unable to transport fluids across the epithelium.15 Genetic mutation resulting in the deletion of phenylalanine at the 509 residue (F509del) is the key factor in the misfolding of the CFTR protein.16 Lumacaftor acts as a chaperone for the correct protein folding of the CFTR and its trafficking to the cell surface, thus restoring the ion-gate channels and thereby alleviating the symptoms of cystic fibrosis. The 3,3-difluoro-1,3-dioxole moiety in this compound helps attenuate the metabolic instability to the cytochrome P450 enzymes. In contrast, the nonfluorinated 1,3-dioxoles as well as the catechol derivatives are prone to oxidative degradation by the CYP450 enzymes. The CYP450 enzymes oxidize the nonfluorinated 1,3-dioxole moiety to the carbene intermediate that complexes to the CYP450 iron centers and thereby inhibit these enzymes, whereas in the gem-difluoro analog, such carbene formation is disfavored as it cannot be 140 Organofluorine Chemistry F F F O F O O O OH H N H N N O OH N O F O HO HO Lumacaftor O O H H Tezacaftor O CYP450 R CYP450 O Fe(III) O O R O O CYP450 R Carbene-ligated CYP450 (deactivated enzyme) F F R (Carbene formation is prevented and does not deactivate the enzyme) FIGURE 5–6 Structures of the cystic fibrosis pharmaceuticals, lumacaftor and tezacaftor; the difluoromethylenebenzodioxole moiety is metabolically more stable to the cytochrome P450 oxidase. readily oxidized.2 Vertex Pharmaceuticals’ next-generation CFTR potentiator, tezacaftor (FDA-approved in 2018), is a structural analog of lumacaftor with improved bioavailability (Fig. 56).17 Fluorination of aryl rings seem to afford optimal pharmacokinetic properties for the FDAapproved pharmaceuticals baloxavir marboxil (Xofluza; ROCHE AGE; for the treatment of influenza A and B),18 fostamatinib (Tavalisse; Regel Pharmaceuticals, Inc., for chronic immune thrombocytopenia), and safinamide [Xadago; a monoamine oxidase-B (MAO-B) inhibitor used in the treatment of Parkinson’s disease] (Fig. 57).19 5.1.3 ππ Stacking interactions N-3,5-Bis(trifluoromethyl)benzylcarboxamides (e.g., in TAK-637), due to hindered rotation across the amide CN bond, exist as atropisomers. Due to the strong electron-withdrawing effect of the two CF3 groups, the bis(trifluoromethyl)aryl ring is electron deficient and Chapter 5 • Pharmaceutical applications of organofluorine compounds F S O F P O N O N N Me Me O O O Me Baloxavir Marboxil O (Xofluza) (for the treatment of influenza A and B) O H N N N O O O H N O Me F 141 H N F N OH OH N O O Me Me O Fostamatinib (Tavalisse) (for the treatment of chronic immune thrombocytopenia) O NH2 Me Safinamide (Xadago) (for the treatment of Parkinson's disease) FIGURE 5–7 Structures of selected FDA-approved drugs consisting of fluorinated aromatics. thereby is involved in the donor-acceptor π2π-stacking interactions with the adjacent electron-donating aryl rings.20 This conformational bias is responsible for the excellent neurokinin-1 (NK1) antagonistic activity of TAK-637. Presumably, similar π2π-stacking interaction is responsible for the NK1-receptor antagonistic characteristics of fosnetupitant (netupitant; Akynzeo), an FDA-approved combination drug for the treatment of acute nausea and vomiting induced by the cancer chemotherapy (Fig. 58).21,22 5.2 Antibacterial pharmaceuticals Organoarsenicals and sulfa drugs were among the first antibacterial drugs developed in early 1900. These highly toxic drugs were later replaced by a number of complex, naturally occurring antibiotics, including penicillin, streptomycin, and erythromycin. As a consequence of rapid evolution of drug-resistant pathogenic bacteria, many of these antibacterials are increasingly ineffective in many cases. Semisynthetic modifications of these antibacterials play a dominant role in the current antibacterial drug discovery. Erythromycin is used for Gram-positive bacterial infections. It is one of the most prescribed antibiotics and is in the United Nations’ list of essential medicines. However, erythromycin has low oral bioavailability and relatively short in vivo half-life, and is unstable under acidic conditions. The chemical instability is attributed to the reaction of the C6 and C12 hydroxy groups with the C9-ketone moiety, forming spiroketal derivatives.23,24 142 Organofluorine Chemistry OOH P O O O N Me N F N+ N N N F F O O F N Me F3 C F F CF3 TAK-637 Fosnetupitant FIGURE 5–8 Structures of NK1 antagonists, TAK-637, and fosnetupitant (an FDA-approved drug), illustrating the possible π2π stacking interactions; NK1, Neurokinin-1. 9 O O H HO F HO OH 12 OH OH 6 O O O O O O O O O HO O OH O O N HO O N O HO HO Flurithromycin Erythromycin-A FIGURE 5–9 Structures of the broad-spectrum antibiotics, erythromycin and flurithromycin, a C8-fluorinated version of the erythromycin-A. Flurithromycin is a second-generation erythromycin, differing from erythromycin by the presence of a single fluorine on the C8 carbon (Fig. 59). The fluorine enhances metabolic stability, and bioavailability of the drug, as compared to the erythromycin. The enhanced metabolic stability may presumably be due to the decreased basicity of the C6-hydroxy group as well as due to the destabilization of the intermediate C9-carbocation intermediate, thereby disfavoring the spiroketal formation. 5.2.1 Fluoroquinolones In general, quinolone and fluoroquinolone antibiotics are among the most widely used drugs worldwide and because of their extensive use over decades, quinolone-resistant bacterial Chapter 5 • Pharmaceutical applications of organofluorine compounds 143 strains have steadily increased. FDA-approved fluoroquinolone antibiotics include levofloxacin (Levaquin), ciprofloxacin (Cipro), moxifloxacin (Avelox), ofloxacin, sparfloxacin, and gemifloxacin (Factive) (Fig. 510). Although these fluoroquinolones are effective antibacterial agents and are widely used worldwide for the treatment of bacterial infections, the potential risks posed by the use of the drugs, such as permanent disabling side effects involving tendons, muscles, joints, and the CNS, outweigh their therapeutic benefits in some cases, according to the FDA announcements in 2016 and 2018.2527 In some serious bacterial infections, such as plague and bacterial pneumonia, the benefits of fluoroquinolone antibiotics, however, outweigh their risks, according to these FDA guidelines. According to the FDA’s warning about the adverse effects of fluoroquinolones in certain patients (nine aortic aneurysm events per 100,000 people per year in the general population to 300 aortic aneurysm events per 100,000 people per year in individuals at highest risk), “the fluoroquinolone O O O F O F OH OH N N N O Me O N N HN HN Me Ciprofloxacin O N Me Me N HO O O N O Levofloxacin ((S)-isomer of ofloxacin) Ofloxacin (racemic) Me F N NH N HO Me F O O Me O N NH2 Sparfloxacin FIGURE 5–10 Structures of selected FDA-approved fluoroquinolones. Me N N F F O N Gemifloxacin O N N HO N OH H 2N Norfloxacin Me O F HO N F O O Moxifloxacin H NH H 144 Organofluorine Chemistry antibiotics can increase the occurrence of rare but serious events of ruptures or tears in the main artery of the body, called aorta. These tears, called aortic dissections, or ruptures of an aortic aneurysm can lead to dangerous bleeding or even death.”25 The mechanisms of the toxicity of fluoroquinolones, and, in particular, the effect of the fluorine moiety on the toxicity, are not clearly delineated. Thus, it is now even more important to understand the mechanistic basis of the therapeutic as well as the adverse effects of these fluoroquinolone drugs, so that improved drug-resistant and safe antibiotics could be designed. Levofloxacin is an enantiomerically pure version [levorotatory, (S)-configuration] of oflaxacin. Levofloxacin, moxifloxacin, and sparfloxacin show potent activity against some Gram-positive and Gram-negative bacteria. These quinolones as well as ciprofloxacin (also active against Gram-positive bacteria) have been widely used in respiratory tract infections, chronic bronchitis, and community-acquired pneumonia, among other bacterial infections. The quinolone antibiotics also have favorable pharmacokinetics. Especially due to the superior pharmacokinetics of levofloxacin, as compared to the other members of this class, a single pill-a-day suffices for the treatment of bacterial infections.28 However, as described earlier, in some cases, these fluoroquinolone antibiotics exhibit severe adverse effects, and the recent FDA warnings recommend the use of alternate antibiotics when other treatment options are available. Delafloxacin (Baxdela; Rib-X Pharmaceuticals) is an FDA-approved fluoroquinolone antibiotic that is used to treat multidrug-resistant (MDR) Gram-negative and Gram-positive bacterial infections (Fig. 511). The preclinical and clinical trial data suggest delafloxacin does not cause the drug-related adverse effects, that is, those adverse effects typically associated with other fluoroquinolone antibiotics. It was concluded that the use of delafloxacin for treating acute, bacterial skin infections and skin structure infections in over 1400 patients for up to 14 days is as effective as that of vancomycin with or without aztreonam.29,30 However, delafloxacin is structurally similar to the other fluoroquinolones and therefore is susceptible to the emergence of possible future drug-resistant newer bacterial-resistant strains. It is also active against methicillin-resistant Staphylococcus aureus and several other fluoroquinoloneresistant bacterial strains. O O F OH N HO N Cl F N H2 N F Delafloxacin (Baxdela) FIGURE 5–11 Structure of delafloxacin (Baxdela), an FDA-approved antibacterial drug against multidrug-resistant bacterial infections. Chapter 5 • Pharmaceutical applications of organofluorine compounds 145 H O N N O F O O H O H O H OH 2 Mg2+ H 2O O NH H O H OH Ser 84 Glu88 FIGURE 5–12 Schematic illustration of moxifloxacin at the active site of the bacterial topoisomerase IV. 5.2.1.1 Mechanism of action of fluoroquinolones The fluoroquinolones are bactericidal drugs, that is, they kill bacteria. The quinolone antibiotics inhibit DNA replication in the bacterial cells, by inhibiting the bacterial DNA gyrase (topoisomerase II) and topoisomerase IV (a type IIA topoisomerase), which are unique to the bacterial cells but not the eukaryotic cells. The latter bacterial type II toposiomerases are heterotetrameric enzymes consisting of two A subunits and two B subunits. Human type II topoisomerases, in contrast to the bacterial type IIA topoisomerases, are homodimeric and thus are not the targets of quinolone antibiotics.28 These topoisomerases II and IV are critical to the bacterial DNA replication. X-ray crystal structures of moxifloxacin bound to the topoisomerase IV show that noncatalytic Mg21 is chelated to the quinoline carbonyl and the carboxylate moieties and is bound to four water molecules in an octahedral environment. The Mg21 bound water molecules, in turn, are involved in hydrogen bonding with the protein Ser84 and Glu88 residues (Fig. 512). The Mg21-promoted ππ-stacking interactions of quinolone with the base pairs, at the DNA cleavage sites, results in the inhibition of the type IIA topoisomerase. Accordingly, mutations at the Ser84 and Glu88 sites in topoisomerase IV give drug resistance to the quinolone drugs.31 5.2.2 Tetracyclines Systematic structural variation of the antibiotics, through the incorporation of fluorine or fluoroalkyl substituents, provides an opportunity to design drug-resistant antibiotics, such as tetracyclines, fluoroquinolones, penicillins, cephalosporins, and carbapenems. Tetracycline antibiotics have been used for decades to treat bacterial infections resulting from both Gram-positive and Gram-negative bacteria. Over the years, bacteria acquired resistance to these tetracycline antibiotics. The drug resistance for tetracyclines is mostly due to the active drug efflux, and ribosomal protection afforded by the drug-resistant bacteria, the mechanisms that are most commonly found in Gram-positive bacteria, such as S. aureus and Streptococcus spp. Some of the widely used synthetic analogs of the naturally occurring tetracyclines for treating bacterial infections include chlorotetracycline, doxycycline, and fluorocyclines (Fig. 513). Eravacycline (Xerava, Tetraphase Pharmaceuticals), a tetracyclic antibiotic, consisting of a fluorine in the D-ring, was approved by FDA in 2018 for complicated intra-abdominal 146 Organofluorine Chemistry HO D OH H C B OH Cl HO OH N H H OH N H O O A OH O H H 7 D N H 9 OH O NH2 O OH OH O OH OH OH O OH O Doxycycline N O O N OH Chlorotetracycline Tetracycline F OH N H NH2 NH2 OH OH H O C B A OH OH OH O NH2 O Eravacycline (FDA approved in 2018) FIGURE 5–13 Structures of some of the widely used tetracycline antibiotics; eravacycline is the next-generation fluorotetracycline antibiotic that is active against multidrug-resistant bacterial strains. infections in adult patients.32,33 It shows potent antibacterial activity against MDR Grampositive and Gram-negative bacterial strains. However, some bacteria, such as Enterococcus spp., show resistance to eravacycline. Tetraphase Pharmaceuticals’ synthesis of the eravacycline and other fluorocyclines with a variety of the amino substituents at the C9-carbon is outlined in Fig. 514. The D-ring precursor 9 was synthesized from the 2-mexthoxy-5-fluorobenzoic acid (8), through regioselective deprotonation using s-BuLi, followed by methylation using methyl iodide, esterification of the carboxy moiety, demethylation of the ether moiety using BBr3, followed by protection of the phenolic hydroxy group as tert-butyloxycarbonyl derivative (t-Boc), using di(tert-butyl) dicarbonate [(Boc)2O]. The tandem MichaelDieckmann cyclization of the fluoroarene 9 with the enone 10, followed by deprotection and installation of the C9-amino substituents gave eravacycline. Using this general procedure, various C9-acetamido derivatives were synthesized and tested for their efficacy as antibacterial agents.33 Among many such derivatives, eravacycline exhibited the optimal and potent antibacterial activity against MDR bacteria and was finally approved by FDA for clinical use in 2018. 5.3 Antidiabetic pharmaceuticals 5.3.1 Sitagliptin As described in Section 5.1, inhibitors of DPP-IV are among the effective therapeutics for type 2 diabetes. The type 2 diabetes is characterized by hyperglycemia that leads to various diabetes-induced complications such as diabetic retinopathy, diabetic neuropathy, and Chapter 5 • Pharmaceutical applications of organofluorine compounds H 1. F F 1. s-BuLi CO2H OMe 2. 3. 4. 5. CO2Ph Ot-Boc O B A N O OH O OBn 2. Deprotections 3. Derivatizations 9 8 F H H N O O N N 10 Michael–Dieckmann annulation CH3 MeI Esterification BBr 3 (Boc) 2 O 147 D N 9 H OH C B A OH OH OH O NH2 O Eravacycline FIGURE 5–14 Outline of the synthesis of eravacycline; Bn, Benzyl; (Boc)2O, di(tert-butyl)dicarbonate; t-Boc, tertbutyloxycarbonyl. F F F NH 2 O N N N N CF3 Sitagliptin (Januvia) DPP-IV inhibtor; antidiabetic drug FIGURE 5–15 Structure of sitagliptin, a DPP-IV inhibitor, and a widely used antidiabetic drug. cardiovascular complications. GLP-1 induces pancreatic secretion of insulin, and inhibits glucagon secretion, thus reversing hyperglycemic state in the body. However, GLP-1 peptide is rapidly cleaved by the DPP-IV enzyme in the diabetic cases. Therefore drugs targeting inhibition of the DPP-IV enzyme provide an attractive means for the treatment of diabetes 2 cases. Sitagliptin (Januvia), a DPP-IV inhibitor, is among the widely used FDA-approved antidiabetic drugs (Fig. 515). During the optimization of the drug candidate, it was found that the 2,4,5-trifluorophenyl substituent in sitagliptin showed relatively enhanced IC50 value (18 nM) for the inhibition of the DPP-IV, as compared to the 2,5-difluorophenyl and 148 Organofluorine Chemistry FIGURE 5–16 Expanded view of the X-ray structure of sitagliptin bound to DPP-IV; Phe357 and Tyr662 aromatic rings have orthogonal π2π-stacking interactions with the electron-deficient triazole and trifluorophenyl moieties, respectively (highlighted by arrows). Hydrogen bond distances (in Å) to the primary amino group are shown in dotted lines. The structure was created using UCSF Chimera software, PDB 1X70. 3,4-difluorophenyl analogs (IC50 values of 27 and 128 nM, respectively).34,35 Sitagliptin showed relatively improved oral bioavailability as compared to the other fluoroaryl derivatives, which led to its successful development of the antidiabetic drug. An X-ray crystal structure of sitagliptin, embedded in DPP-IV shows that it is located in the S1 hydrophobic pocket and that the electron-withdrawing trifluoromethyl triazole- and 2,4,5-trifluorophenyl moieties are involved in π2π stacking interactions with the Phe357 and Tyr662 aromatic rings, respectively (Fig. 516). The primary amino group has hydrogen bonding interactions with the side-chain carboxylic acids of Glu205, Glu206, and the hydroxy moiety of Tyr662 residues.35,36 5.3.1.1 Synthesis of sitagliptin Merck group’s green synthesis of sitagliptin is shown in Fig. 517.37 In this greener synthetic method, as compared to the conventional multistep process,38 one-pot conversion of 2,4,5-trifluorophenylacetic acid (11) to the enamine amide 17 was achieved in high yields. Thus, the Chapter 5 • Pharmaceutical applications of organofluorine compounds 149 CO2 + acetone F F O OH + O F O O O t-BuCOCl i-Pr 2NEt F F F Cat. DMAP/MeCN CF3CO2 H O H+ 13 O 12 11 OH O Cl H2 N O N N N CF3 14 F F F O C F 15 H O F O NH4 OAc MeCN/MeOH O F N N N + 16 N HN N CF3 N N CF3 F F F NH2 O F Rh(COD) 2OTf (5 mol%) N N N 17 F NH2 O F N N N N CF3 Fe P(t-Bu) 2 P(t-Bu) 2 Sitagliptin N CF3 99% Conversion; 95% ee (t-Bu JOSPHOS); 10 mol% MeOH, H 2 (90 psi, 50 °C) FIGURE 5–17 Synthesis of sitagliptin; DMAP 5 4-(N,N-dimethylamino)pyridine. pivaloyl anhydride of trifluorophenylacetic acid 11, formed from the in situ reaction of 11 with pivaloyl chloride (t-BuCOCl), undergoes nucleophilic acyl substitution reaction [SN2(Ac)] with the enolate of the Meldrum’s acid (2,2-dimethyl-1,3-dioxane-4,6-dione; 12) to give the enol 13. The in situ reaction of 13 with trifluoroacetic acid, in the presence of the triazole salt 14, gives the β-keto amide 16. Through kinetic studies, it was observed that the acid-catalyzed decomposition of the compound 13 gives the ketene 15, which rapidly undergoes nucleophilic addition reaction with the triazole 14 to give the β-keto amide 16. Without any further workup, addition of NH4OAc and methanol to the reaction mixture gives the enamine amide 17. Thus, this one-pot, multistep reaction sequence gives compound 17 directly without the necessity of the intermediate workup procedures or compound purifications, affording greener synthetic route to this compound. Rh(I)-catalyzed asymmetric hydrogenation of the enamine amide 17 then affords the sitagliptin in high conversions and enantioselectivity.37 150 Organofluorine Chemistry OMe OMe H H 2N N N F O Carmegliptin FIGURE 5–18 Structure of carmegliptin. FIGURE 5–19 X-ray crystal structure of carmegliptin bound to the DPP-IV; hydrogen bonds are shown as dotted lines; DPP-IV, Dipeptidyl peptidase-IV. Adapted from Mattei, P.; Boehringer, M.; Di Giorgio, P.; Fischer, H.; Hennig, M.; Huwyler, J.; Kocer, B.; Kuhn, B.; Loeffler, B.M.; MacDonald, A.; et al. Discovery of Carmegliptin: A Potent And Long-Acting Dipeptidyl Peptidase IV Inhibitor for the Treatment of Type 2 Diabetes. Bioorg. Med. Chem. Lett. 2010, 20, 11091113. 5.3.2 Carmegliptin Carmegliptin (Fig. 518) was developed as an effective DPP-IV inhibitor for treating type 2 diabetes.39,40 The fluoromethyl moiety in the carmegliptin was found to be the optimal substituent that interacts with the hydrophobic S1 pocket of the enzyme. Carmegliptin inhibits DPP-IV with an IC50 of 6.8 nM, as compared to its nonfluorinated methyl analog with an IC50 of 13 nM.39 Furthermore, carmegliptin exhibits a unique pharmacokinetic profile with optimal renal and hepatic excretion. Phase 1 and 2 clinical studies of this compound proved it to be a safe and orally administrable compound. A single crystal X-ray structure of the carmegliptin, bound to the DPP-IV enzyme, shows hydrogen-bonding interactions of the amide carbonyl oxygen with Tyr662, Asn710, and Arg125, and that the primary amino group has hydrogen-bonding interactions with Glu206 and Glu205 residues.39 The fluoromethyl moiety occupies the hydrophobic S1 pocket of the enzyme (Fig. 519). The amide carbonyl effectively mimics the P2-amide carbonyl of the GLP-1. Chapter 5 • Pharmaceutical applications of organofluorine compounds 151 FIGURE 5–20 Synthesis of carmegliptin, a DPP-IV inhibitor; DPP-IV, Dipeptidyl peptidase-IV. OH Me HO O HO HO S OH F HO OH O Cl O Me OH Canagliflozin Dapagliflozin FIGURE 5–21 Structures of the SGLT-2 inhibitors, canagliflozin and dapagliflozin, the FDA-approved drugs for the treatment of type 2 diabetes; SGLT-2, Sodiumglucose cotransporter 2. 5.3.2.1 Synthesis of carmegliptin The synthesis of carmegliptin is shown in Fig. 520.39 Regioselective reduction of the enantiomerically pure (S)-paraconic acid (18), followed by deoxyfluorination using the Deoxofluor [bis(2-methoxyethyl)aminosulfur trifluoride], gave the fluoromethyl lactone 19 in 67% yield for the two steps. The fluoromethyl lactone 19 was transformed into compound 20 by reaction with thionyl chloride in the presence of ZnCl2 and was then reacted with enantiomerically pure 21 to give the carmegliptin, isolated as the hydrochloride salt. 5.3.3 Canagliflozin Canagliflozin (Invokana; Janssen Pharmaceuticals; Fig. 521) was approved by FDA in 2013 for the treatment of type 2 diabetes and to reduce the risks associated with cardiovascular events. Type 2 diabetes increases the risks of cardiovascular dysfunction, leading to 152 Organofluorine Chemistry exacerbation of heart failure. Canagliflozin is a sodiumglucose cotransporter 2 (SGLT-2) inhibitor. It reduces the glycated hemoglobin (HbA1c) levels in type 2 diabetes and improves the left-ventricular diastolic function within 3 months of treatment.41 The (fluoroaryl)thiophene moiety contributes to enhanced metabolic stability for canagliflozin. Canagliflozin shows significant antihyperglycemic effects as compared to several analogous C-glucosides in diabetic mice models.42 The antihyperglycemic effect of this drug is attributable to the inhibition of SGLT-2, resulting in the decreased reabsorption of the filtered glucose.43 Some of these flozin families of SGLT2 inhibitors (e.g., dapagliflozin) also inhibit the SGLT-2 in the pancreatic α-cells, triggering the glucagon secretion and hepatic gluconeogenesis and thereby decreasing plasma glucose induced by fasting.44 5.4 Anti-Alzheimer pharmaceuticals Alzheimer’s disease (AD) has the highest global burden, with an estimated annual global cost of US$ 818 billion. It is estimated that by 2030, the number of AD patients would rise to over 70 million worldwide. The global cost for treating and caring for AD patients exceeds that of other major diseases, such as cancer and diabetes.45 Whereas the number of deaths due to cardiovascular diseases and HIV infections has dramatically reduced since 2000 due to the state-of-the-art therapies, AD cases remain to increase as there are no pharmaceuticals that can cure AD to date (Fig. 522). Over 90% of the AD cases are of nongenetic (i.e., sporadic) origin and the mechanisms of the onset of sporadic AD are not well defined. According to amyloid hypothesis, the formation and accumulation of the aggregates of amyloid-β (Aβ) protein in the brains of the AD patients are considered to be the key contributing factor for the onset of the disease. As the neuronal activity is increased, the synapses release increasing concentrations of the soluble 100 2015 2030 1500 1000 500 Percentage change in deaths in the United States since 2000 Estimated global cost (billion US$) 2000 Someone in the United States develops Alzheimer’s disease every 66 s 75 50 25 0 –25 –50 –75 0 Alzheimer’s disease Cancer Diabetes Alzheimer’s disease Breast Cardiovascular cancer disease HIV FIGURE 5–22 Comparison of AD statistics with other major diseases; AD, Alzheimer’s disease. Adapted from McDade, E.; Bateman, R.J. Stop Alzheimer’s Before It Starts. Nature (London) 2017, 547, 153155. Chapter 5 • Pharmaceutical applications of organofluorine compounds 153 Aβ peptide. If the rate of the breakdown of the soluble Aβ peptides is lower than that of formation, the soluble Aβ peptides are accumulated and are eventually transformed into the Aβ aggregates, also called amyloid plaques. The Aβ plaques have further downstream effects, including synaptic damage, tau-protein hyperphosphorylation, and mitochondrial damage.46 Excessive formation of amyloid plaques leads to overactivation of the TREM2, the gene that expresses the microglial receptor protein TREM2 (triggering receptor expressed on myeloid cells-2) and thereby the microglial removal of the damaged synapses and surrounding tissues. Extensive loss of the synapses along the axon results in the loss of neuronal function. Hence, removing the amyloid plaques during the late stage of AD has no significant beneficial effect on memory restoration or on delaying the progression of AD.46 The Aβ plaque deposition occurs about 15 years earlier than the onset of the AD symptoms. Based on this hypothesis, major pharmaceutical companies developed the BACE-1 inhibitors [a β-site amyloid protein (β-amyloid) cleaving enzyme], γ-secretase inhibitors (GSIs), and γ-secretase modulators (GSMs) toward the attenuation of the Aβ formation. However, clinical trials of several of these pharmaceuticals targeting Aβ peptides were not successful in reducing the AD symptoms, despite their effectiveness in attenuating the Aβ plaque formation. It is uncertain whether Aβ is the primary causative factor or other downstream events are involved in the neurodegeneration. According to the tau hypothesis as a causative factor for AD, Aβ-induced downstream events lead to the tau hyperphosphorylation and formation of the paired helical filaments that aggregate to form the intracellular neurofibrillary tangles, with consequent neuronal disintegration.47 According to the cell-cycle hypothesis as a causative factor for AD, the Aβ oligomers may induce aberrant cell-cycle reentry of neuronal cells, resulting in neuronal cell death.48 A number of tau kinases are involved in the aberrant cell-cycle reentry of the neuronal cells. These tau kinases, when overactivated, likely due to the downstream events arising from the Aβ peptides, lead to the aberrant cell-cycle entry. Due to the lack of a definitive mechanistic understanding of the causative factors for the sporadic AD, there have been no therapeutics for this disease to date. The only FDAapproved drugs, rivastigmine, galantamine, donepezil (cholinesterase inhibitors), and memantine (NMDA receptor antagonist), are used for the palliative treatment with little effect on the memory loss and disease progression (Fig. 523). 5.4.1 BACE-1 inhibitors Most of the current drug development by major pharmaceutical companies is based on the BACE-1 (a β-amyloid cleaving enzyme; β-secretase) inhibitors. BACE-1 is an aspartyl protease that is involved in the formation of the brain-soluble Aβ peptide oligomers. Sequential cleavage of the amyloid precursor protein (APP; a transmembrane protein), first by the BACE-1 and then by γ-secretase, results in the formation of these soluble Aβ peptides, which in turn, form neurotoxic oligomeric fibrils and aggregates, called Aβ plaques, in the brains of affected individuals. The γ-secretase cleaves the APP at various sites between the 36 and 43 residues to give Aβ peptides of varying chain lengths. Of these Aβ peptides, the major 154 Organofluorine Chemistry O MeO O Et N Me Me N Me Me O MeO N Rivastigmine Donepezil Me N NH2 Me HO O Galantamine Me O Me Memantine FIGURE 5–23 Structures of cholinesterase inhibitors—rivastigmine, donepezil, galantamine, and NMDA receptor antagonist memantine—currently used as palliative treatments of AD; AD, Alzheimer’s disease. isoforms formed are Aβ140 and Aβ142. Aβ142 is the most toxic peptide of all these Aβ peptides, and decreasing ratios of Aβ142 to the other Aβ peptides were shown to have a beneficial effect in attenuating the Aβ plaque formation in vivo. It was, therefore, suggested that drug discovery strategies that target reduction of Aβ140 actually worsen the disease, and that the selective increase in Aβ140 levels, over that of Aβ142, may, in fact, reduce the risk of AD pathogenesis.49 On the other hand, the peptide fragments arising from the α-secretase cleavage, followed by β-secretase cleavage, are nonneurotoxic (Fig. 524). In normal and healthy individuals the clearance of the initially formed soluble Aβ peptides is at the same rate as their formation. On the other hand, as described earlier, when there is an imbalance in the Aβ clearance and formation mechanisms, the brain-soluble Aβ peptides aggregate to form the Aβ plaques in the brain tissues, resulting in the neuronal loss and thereby cognitive decline. However, none of the drug candidates targeted at BACE-1 inhibitors were proven successful in the clinical trials.50 Merck’s phase 3 studies of verubecestat were halted because of the ineffectiveness of this drug candidate in reducing or reversing cognitive decline and due to the drug-related adverse effects (vide infra). The clinical trials of this drug in the early stages (prodromal) of AD were also halted because of the lack of positive outcomes. Eli Lilly and AstraZeneca’s phase 3 clinical trials on a BACE-1 inhibitor, lanabecestat (a nonfluorinated compound), were also halted due to the expected lack of positive outcomes of this drug candidate. Eli Lilly halted its phase 3 clinical trials on the BACE-1 inhibitor atabecestat due to the liver toxicity that this drug candidate exerts (Fig. 525). All of these BACE-1 inhibitors are effective in reducing or clearing Aβ plaques in brain and in Chapter 5 • Pharmaceutical applications of organofluorine compounds 155 FIGURE 5–24 Schematic illustration of the formation of the Aβ oligomers and Aβ aggregates through sequential cleavage of APP by β- and γ-secretases; Aβ, Amyloid-β; APP, amyloid precursor protein. cerebrospinal fluid (CSF). Presumably, there is an irreversible synapse loss and neurodegeneration at the mid-to-late stages of the AD so that further reducing the formation of the Aβ plaques has no observable effect in reducing the AD symptoms. In order to be effective AD therapeutics the BACE-1 inhibitors should be highly selective and ideally should not inhibit the structurally closely related BACE-2, whose inhibition leads to adverse effects. The adverse effects caused by the BACE-1 inhibitor pharmaceuticals (that were in clinical trials) are ascribed to their competing BACE-2 inhibition. 156 Organofluorine Chemistry NH F N HN H N Me F O Me N S O O N H 2N OCH3 Lanabcestat O N N N H Me S N H3 C Verubecestat (MK-8931) F NH2 N Me C N Atabecestat FIGURE 5–25 Structures of verubecestat, lanabecestat, and atabecestat, the BACE inhibitors that were in the phase 3 clinical trials. F3C O Cl H N N O N N Me F Me CF3 O In vivo hydrolysis NH2 CNP520 BACE-1 inhibitor; in AD prevention clinical trials at Novartis H 2N N N Me F Me CF3 NH2 22 LogD (pH 6.8) = 3.5 pKa = 7.2 FIGURE 5–26 Structures of CNP520, a drug in clinical trials for the prevention of AD in susceptible individuals, and its metabolite 22, formed via hydrolysis of the amide moiety; AD, Alzheimer’s disease. 5.4.1.1 CNP520 as an Alzheimer’s diseasepreventive drug Novartis is currently undertaking the early clinical trials for CNP520 (Novartis Pharmaceuticals; Fig. 526), a highly selective BACE-1 inhibitor for normal and healthy individuals who are susceptible to the development of AD, but have not yet developed AD symptoms despite having Aβ depositions in the brains, as a preventive approach for treating AD.50,51 CNP520 has favorable pharmacokinetic and pharmacodynamic properties with a log D (at pH 6.8) of 3.5 and a pKa of 7.2. Apparently, the trifluoromethyl moiety reduces the basicity of the amino moiety, such that the lipophilicity at pH 6.8 is substantially increased. In vivo hydrolysis of CNP520 forms o-aminopyridine metabolite 22, which, unlike aniline derivatives, has no known safety hazard. Chapter 5 • Pharmaceutical applications of organofluorine compounds 157 In vitro studies on animal models as well as in clinical studies of presymptomatic patients indicate that CNP520 is an exquisitely safe therapeutic agent for the prevention of AD at the early stages of the disease, that is, prior to the onset of the AD symptoms. CNP520 has high selectivity for BACE-1 over BACE-2 (about threefold more selective to BACE-1) and other structurally closely related aspartate proteases, such as human cathepsin D and human cathepsin E. Most of the BACE-1 inhibitor pharmaceuticals have equipotent selectivity against BACE-2, and thereby, leads to hair depigmentation. In animal models, CNP520 reduced Aβ40 production by 90% and there was no depigmentation of the hair. Unlike the amyloid-based immunotherapeutic drugs, which are associated with cerebral microhemorrhages (CMH), CNP520 did not increase CMH frequency or severity relative to vehicle-treated animals (i.e., control animal models treated with the solvent used to dissolve the drug, e.g., saline or dimethyl sulfoxide, without using the actual drug). APP transgenic mice exhibited reduced Aβ load and reduced neuroinflammation with the use of the CNP520. Clinical trials of CNP520 in humans showed dose-dependent reduced levels of Aβ and sAPPβ (soluble APP-β, a product of APP cleavage by β-secretase) in the CSF.51 An X-ray structure of the CNP520, bound to the active site of the BACE-1, shows hydrogen-bonding interaction of the active site Asp32 and Asp228 side-chain carboxy moieties with the amidine moieties.51 The CF3 moiety adjacent to the amidine moiety exhibits hydrogen-bonding interaction with the Asp228 side-chain carboxylic acid (2.9 Å) (Fig. 527). 5.4.1.2 Verubecestat, a BACE-1 inhibitor Verubecestat (MK-8931; Merck & Co.), a 3-imino-1,2,4-thiadiazinane derivative (Fig. 528), is a β-amyloid cleaving enzyme (BACE-1; an aspartyl protease) inhibitor. Verubecestat had been in clinical trials at Merck & Co., since 2012 for the treatment of mild-to-moderate and early-stage (prodromal) AD. It has relatively high selectivity for BACE-1, although it is nonselective over the closely related protein BACE-2. Verubecestat has high selectivity for BACE-1, as compared to the other key aspartyl proteases, such as cathepsin D, and it lowers CSF and brain Aβ levels in rats and nonhuman primates and CSF Aβ levels in humans.52 However, the phase 3 clinical trials on mild-to-moderate AD as well as prodromal AD were halted as verubecestat did not have any positive outcome on patients with mild-to-moderate AD (i.e., it was ineffective in reducing the cognitive decline), while having drug-related adverse effects, such as falls and injuries, sleep disturbance, and hair-color change.53 Prolonged treatment with BACE-1 inhibitors, including verubecestat, results in dendritic spine loss, even though they help to reduce the Aβ levels in the brains of AD patients. There are two types of dendritic spines, transient dendritic spines with a lifetime of about 4 days and stable spines, persisting for more than 8 days. The short-lived dendritic spines are involved in learning, while the stable dendritic spines are associated with long-term memory. Herms and coworkers showed a significant reduction of Aβ140 as well as the dendritic spines in the brains of mouse AD models, when treated with verubecestat. However, the loss of spine density was recovered after verubecestat withdrawal.54 Metabolic degradation products of verubecestat, N2-desmethyl compound 23 and aniline metabolite 24, have about 158 Organofluorine Chemistry FIGURE 5–27 Expanded view of the X-ray structure of CNP520 in complex with BACE-1 at the active site; the dotted lines indicate hydrogen-bonding interactions of the Asp32 (with guanidine nitrogens) and Asp228 side-chain carboxy group with the CF3 fluorine; the structure was created using UCSF Chimera software; PDB 6EQM. 100-fold less BACE-1 inhibitory activity than the verubecestat (Fig. 528). Verubecestat and many of the other BACE-1 inhibitors having a basic amino moiety in close proximity to a hydrophobic group inhibit cardiac delayed-rectifier K1 (IKR) channel, encoded by human ether-a-go-go-related gene. The drug-induced inhibition of the IKR channel sometimes can lead to arrhythmia and sudden death.52 Chapter 5 • Pharmaceutical applications of organofluorine compounds F N H N O NH 1 Me HN 2 N S O O Me F 159 NH F N HN H N Metabolic transformation Me F O NH S O O 23 Verubecestat (MK-8931) NH HN H 2N + Me F Me N S O O 24 FIGURE 5–28 Structures of verubecestat, a BACE-1 inhibitor, and its major metabolic degradation products. An X-ray structure of verubecestat bound to BACE-1 (Fig. 529) reveals extensive hydrogen-bonding interactions of the amidineimino moiety with Asp289 and Asp93 sidechain carboxylic acid moieties. The aryl fluorine has hydrophobic interactions with the backbone CH bonds of the Phe169 and Tyr132 residues.52 5.4.2 γ-Secretase inhibitors and modulators Inhibition or modulation of γ-secretase helps reduce the Aβ burden. However, GSIs have been abandoned because of their mechanism-based inhibitory effect on various other enzymes, resulting in severe drug-related adverse effects.55 Two of the key GSIs, semagacestat (Eli Lilly; a nonfluorinated compound) and avagacestat (BristolMyersSquibb; Fig. 530), were tested in late-stage clinical trials but were abandoned due to the adverse effects associated with these drug candidates. In particular, the notch-signaling inhibition by GSIs impairs notch processing and leads to adverse effects, such as skin cancer, that were observed in the phase 3 clinical trials of semagacestat.56 Two phase 2 trials of avagacestat were terminated by BristolMyersSquibb in 2012, because the patients treated with this drug had comparable disease progression and brain atrophy as compared to those treated with a placebo. Furthermore, treatment with avagecestat resulted in adverse effects, including squamous- and basal-cell skin cancers (nonmelanoma skin cancers).57 The drug discovery effort in the area of GSIs is now shifted to GSMs due to the competing notch-signaling inhibition of the GSIs and the consequent drug-related severe adverse effects, including skin cancers. Many of these GSM drug candidates showed poor drug-like properties, although they are effective GSMs and thus potential AD therapeutics. The ongoing research efforts in this area are targeted toward increasing the GSMs’ potency and efficacy with improved drug-like properties, such as relatively low c Log P (,5), high CNS 160 Organofluorine Chemistry FIGURE 5–29 Expanded view of the X-ray structure of verubecestat bound to BACE-1 at the active site; there are extensive hydrogen-bonding interactions of the imino-nitrogen with the Asp289 and Asp93 residues, and aryl fluorine has hydrophobic interactions with the backbone CH bonds of Phe169 and Tyr132; the structure was created using UCSF Chimera software, PDB 5HU1. multiparameter optimization scores, and high sp3 character.55 The lipophilicity of the molecule should be adjusted such that it should have sufficient aqueous solubility and membrane permeability for enhanced bioavailability. Drug candidates that have c Log P higher than 5 have poor aqueous solubility and therefore have poor oral bioavailability. Relatively higher sp3-hybridized carbon content (preferably with a fractional sp3 character, fsp3, greater than 0.38 during the phase 1 clinical trials) gives the drug candidates natural productlike characteristics and thereby enhances their drug-like properties.58 GSMs regulate the γ-secretase activity so that the formation of the Aβ142 peptides is attenuated. The GSMs do not show inhibitory effect on γ-secretase, and therefore the notch Chapter 5 • Pharmaceutical applications of organofluorine compounds 161 F OH O H N O Me CF3 O N N H N N O O Me N S NH2 O O Cl Semagestat Avagacestat FIGURE 5–30 Structures of semagestat and avagacestat, the GSIs, whose late-stage clinical trials were abandoned; GSIs, γ-Secretase inhibitors. Me N N N OMe Me NH Me O N N H N Me N N N N N NH H Me Me F F BMS-932481 BPN-15606 FIGURE 5–31 Structure of BMS-932481 and BPN-15606, the GSMs; GSMs, γ-Secretase modulators. inhibition, typically associated with GSIs, is avoided. Thus, there are fewer adverse effects with the GSMs as compared to those with GSIs. GSMs have distinct binding sites on the APP than GSIs, and the enzyme is modulated such that the Aβ142/Aβ140 ratio is decreased, and other relatively nontoxic, shorter Aβ peptides are formed at the expense of the neurotoxic Aβ142 peptide.59 By diverting the mechanism of formation of the neurotoxic Aβ142 to the less toxic, shorter Aβ peptides, GSMs may prove to be therapeutics for AD. BristolMyersSquibb’s GSM, BMS-932481 (Fig. 531), modulated the Aβ peptides in the plasma and CSFs in preclinical studies. Upon treatment with BMS-932481, the relative levels of Aβ140 and Aβ142 were attenuated, while the levels of the relatively nontoxic Aβ137 and Aβ138 were increased in the CSF of healthy volunteers. Therefore BMS932481 is a GSM rather a GSI. Further development of this drug was halted due to the insufficient safety margin.60 BPN-15606, a GSM modulator (Fig. 531), significantly lowers the Aβ142 levels in the CNSs of rats and mice and reduces Aβ neuritic plaque in AD transgenic mouse model, attenuates Aβ142, and attenuates phosphorylation of the tau protein at the threonine 181 (pThr181 Tau). This drug candidate was granted the investigational new drug status for future human clinical trials.61 Other GSMs that progressed to clinical trials but were not 162 Organofluorine Chemistry Cl O Cl HO OH F F O Itanapraced (CHF-5074) Tarenflurbil (R-flurbiprofen) FIGURE 5–32 Structures of itanapraced and tarenflurbil, the GSMs that were not successful in clinical trials; GSMs, γ-Secretase modulators. Me Me OH O O OH Cl OH F3 C O O F CF3 Ibuprofen Flurbiprofen EVP-0015962 FIGURE 5–33 Structures of NSAID-based GSMs. GSMs, γ-Secretase modulators; NSAID, nonsteroidal antiinflammatory drug. successful include Chiesi’s itanapraced (CHF 5074; EC50 5 40 μM) and a first-generation nonsteroidal antiinflammatory drug (NSAID), tarenflurbil (R-flurbiprofen; EC50 5 300 μM) (Fig. 532).62 5.4.2.1 Nonsteroidal antiinflammatory drugs as γ-secretase modulators NSAIDs, such as sulindac sulfide, ibuprofen, and flurbiprofen (a fluorobiphenyl analog of ibuprofen), lower the Aβ142 levels while elevating Aβ-38 levels at relatively high concentrations. Thus, these NSAIDs, when used as GSMs, have potential benefit for managing the AD symptoms, although the first-generation NSAID-derived GSMs have relatively low potencies and undesirable neuropharmacokinetics. As allosteric modulators, these GSMs induce conformational changes in the γ-secretase, thus modifying their effect on Aβ142 formation.63 Clinical studies of COX-1/COX-2-nonselective (e.g., naproxen) and COX-2-selective NSAIDs (e.g., rofecoxib and celecoxib) in AD patients revealed their ineffectiveness as AD therapeutics. These NSAIDs were even suggested to be detrimental because of their inhibitory effect on brain microglia, which help in the clearance of the Aβ peptides.64 Relatively more potent flurbiprofen analogs, such as EVP-0015962 (EnVivo Pharmaceuticals; Fig. 533), have proven to be more effective GSMs, that is, they divert the Aβ142-forming mechanism to the formation of shorter Aβ peptides. EVP-0015962 lowered Chapter 5 • Pharmaceutical applications of organofluorine compounds 163 Aβ1-42 levels in human H4 neuroglioma cells with an EC50 for Aβ142 clearance of 67 nM and increased the relatively nonneurotoxic Aβ-38 deposition by 1.7-fold.65 Although this drug candidate has positive outcomes in the transgenic Tg2576 mice animal models, clinical trials have not been reported so far. The goal of designing the improved versions of NSAID-derived GSMs is to enhance their GSM activity and to attenuate the NSAIDassociated cyclooxygenase (COX-1 and COX-2) inhibitory effect so that the gastrointestinal toxicity is diminished. 5.5 Anti-HIV pharmaceuticals HIV, a retrovirus that replicates through insertion of its genome into the host-cell DNA, is responsible for the onset of AIDS (acquired immune deficiency syndrome) disease. Replication of HIV virus is highly error-prone, resulting in the formation of multiple strains of drug-resistant virus. In order to overcome the rapid drug resistance of HIV virus, combination drugs that act on multiple viral targets, consisting of the reverse transcriptase inhibitors, integrase inhibitors, and protease inhibitors, also called as highly active antiretroviral therapy (HAART), are being developed. In 2018 FDA approved the Gilead’s combination drug Biktarvy for the treatment of HIV infections. Biktarvy includes two HIV nucleoside analog reverse transcriptase inhibitors, emtricitabine (20 ,30 -dideoxy-5-fluoro-30 -thiacytidine) and tenofovir alafenamide fumarate,66,67 and an integrase strand transfer inhibitor (a class of integrase inhibitors), bictegravir68 (Fig. 534). Emtricitabine was previously marked as a triple-combination drug with tenofovir and efavirenz by BristolMyersSquibb (approved by FDA in 2006). Efavirenz is a nonnucleoside reverse transcriptase inhibitor (NNRTI), whereas emtricitabine and tenofovir are the nucleoside analog reverse transcriptase inhibitors; hence, the combination of these drugs in a single dosage would minimize the viral drug resistance. Emtricitabine, tenofovir, and efavirenz, along with their fixed-dose combinations, are listed as part of the essential medicines by the World Health Organization.69 5.5.1 Bictegravir Bictegravir is an integrase strand transfer inhibitor. Integrases are viral enzymes that are involved in the incorporation of the viral genome into the host DNA strand and therefore by deactivating these integrase enzymes, HIV replication is attenuated. The hydrophobic (2,4,6trifluoromethyl)benzyl moiety in bictegravir contributes to its efficient binding to the plasma proteins, thereby minimizing undesirable interactions with other drugs and enhancing its solubility and half-life time.70 Modeling studies showed that the trifluorobenzyl moiety exhibits ππ hydrophobic stacking interactions with the cytosine on the 30 -end of the viral DNA.71 These calculations also showed high flexibility of the oxazepane ring, allowing enhanced conformational mobility to the molecule and thereby tight binding to various integrase strand transferaseresistant mutants, such as G118R and S119R mutants. Three other FDA-approved integrase strand transfer inhibitors, raltegravir, elvitegravir, and dolutegravir (Fig. 535), 164 Organofluorine Chemistry H 2N N O O N N F O N H N OH S F O H O O Emtricitabine F F OH Bictegravir O O O O P O N NH O HO N F3 C OH N Cl O N O NH2 N H O Efavirenz Tenofovir alafenamide fumarate FIGURE 5–34 Structures of emtricitabine, bictegravir, tenofovir alafenamide, and efavirenz; biktarvy (Gilead Sciences) is a triple-combination anti-HIV drug consisting of emtricitabine, bictegravir, and tenofovir fumarate, while atripia (BristolMyersSquibb) is a triple-combination drug consisting of efavirenz, emtricitabine, and tenofovir. O OH F O O F N N N H N N N H N O N F O O O OH H N O Raltegravir (FDA-approved integrase strand transfer inhibitor Dolutegravir (FDA-approved integrase strand transfer inhibitor) Cl F O Na+ O O OH O N OH Elvitegravir (FDA-approved integrase strand transfer inhibitor) F F O O H N N N O O Cabotegravir (integrase strand transfer inhibitor; in clinical trials) FIGURE 5–35 Structures of some of the clinically active integrase strand transfer inhibitors. Chapter 5 • Pharmaceutical applications of organofluorine compounds 165 potently inhibit replication of the wild-type HIV-1. However, resistant mutants can rapidly arise when using these drugs.71 Dolutegravir is among the widely used drugs for the combination antiretroviral therapy. Carbotegravir (Fig. 535), another structurally related integrase inhibitor, is in advanced clinical trials for the treatment of HIV-1 infections.72,73 Of particular interest, all these integrase inhibitors have electron-acceptor fluoroaryl moieties that are vital to the ππ hydrophobic stacking interactions with the viral DNA. 5.5.2 Doravirine Doravirine (MK-1439; Pifeltro; Merck), an NNRTI, was approved by FDA in 2018 for the treatment of HIV infections. It is a single-dose combination drug consisting of lamivudine and tenofovir disoproxil fumarate. This three-drug combination provides an alternative treatment strategy to the other widely used HIV drugs. Doravirine shows superior pharmacokinetic and safety profile over efavirenz in cases involving neuropsychiatric and cutaneous adverse effects and has relatively low potential for drugdrug interactions. 7476 An X-ray crystal structure of doravirine bound to the wild-type reverse transcriptase shows edge-to-face ππ stacking of the aryloxy moiety with the Trp 229, and a face-to-face ππ stacking interaction with the Tyr188. The trifluoromethyl moiety has hydrophobic van der Waals interactions with the Tyr188 and Val189 backbone (Fig. 536).75,76 The binding mode of doravirine to the active site is similar to that of efavirenz, except that the Tyr181 is rotated 90 degrees in efavirenz. 5.6 Antimalarial pharmaceuticals Malaria remains one of the deadliest infectious diseases worldwide, especially in developing and underdeveloped countries, despite the early success of the quinine-based drugs in eradicating the disease. Artemisinin and its derivatives, including the watersoluble artesunate, showed promise in countering the drug resistance and are being used as combination drugs with other quinine-based antimalarial agents.77 Due to the emergence of the drug-resistant plasmodium strains against the widely used antimalarial drug chloroquine, structurally related quinoline-based drugs, such as primaquine and fluoroquinolones (the widely used antibacterial agents), have emerging interest as antimalarial drugs (Fig. 537).78,79 5.6.1 Tafenoquine Tafenoquine (Krintafel; GlaxoSmithKline; Fig. 537), a primaquine analog, consisting of the 5-(m-trifluoromethylphenoxy)quinoline as the pharmacophore, was approved by FDA in 2018 for the treatment of malaria and for the prevention of malaria (prophylaxis) for travelers and people living in the malaria-endemic regions. Tafenoquine shows improved safety and therapeutic profile as compared to the antimalarial drug primaquine and can be used against 166 Organofluorine Chemistry FIGURE 5–36 Structure of doravirine (MK-1439; Merck & Co.), a nonnucleoside reverse transcriptase inhibitor, and expanded view of the X-ray structure of doravirine (ball and stick), bound to the wild-type HIV reverse transcriptase; ππ stacking of Tyr188 (face-to-face) and Trp 229 (edge-to-face) with the aryloxy moiety of doravirine are shown as hashed lines; the structure was created using UCSF Chimera software, PDB 4NGC. the currently drug-resistant plasmodium strains.80,81 The drug is prescribed in the racemic form. Tafenoquine and related 5-aryloxyquinoline analogs of the antimalarial drug primaquine have improved metabolic stability as compared to the primaquine. In general, 5-(4-trifluoromethylphenoxy)-4-methylprimaquines are several-fold more potent inhibitors of monoamine oxidase-A and MAO-B.82 This increased metabolic stability apparently translates into the effectiveness of tafenoquine against the drug-resistant strains of plasmodium species. 5.6.2 Mefloquine The mechanism of antimalarial action of the mefloquine and other quinine pharmaceuticals has evaded the scientific community.83 Nordlund and coworkers have demonstrated the purine nucleoside phosphorylase enzyme as the target of quinine and mefloquine drugs, using thermal shift assay coupled with the mass spectrometry (MS-CETSA), and through structural studies using the recombinant protein.84 Quinine and mefloquine bind to the active site of the enzyme with nanomolar affinity, attenuating the parasite protein synthesis. Chapter 5 • Pharmaceutical applications of organofluorine compounds 167 FIGURE 5–37 Structures of chloroquine, primaquine, artemisinin, artesunate, artemether, mefloquine, and tafenoquine, the antimalarial agents. The cocrystal X-ray structure indicates that the trifluoromethyl moieties of mefloquine have hydrophobic interactions with the active-site amino acids, stabilizing the ligandprotein interactions (Fig. 538).84 5.7 Anticancer pharmaceuticals 5.7.1 Dacomitinib In 2018, FDA approved dacomitinib (VIZIMPRO; Pfizer Pharmaceutical Company) for the first-line treatment of metastatic nonsmall-cell lung carcinoma (NSCLC), arising from the mutations in the epidermal growth factor receptor (EGFR), involving exon 19 deletion or L858R mutation (i.e., Lys858 substituted by Arg) in the exon 21, as detected by an FDAapproved test. The tumor progressionfree survival for dacomitinib was 14.7 months, as compared to 9.2 months when gefitinib anticancer drug was used.85 Another NSCLC drug, lorlatinib (Lorbrena; Lorviqua; Pfizer Pharmaceuticals, Inc.) was also FDA approved in 2018 for the treatment of anaplastic lymphoma kinase (ALK) positive metastatic NSCLC (Fig. 539). Dacomitinib is an orally bioavailable second-generation, pan-HER tyrosine kinase (EGFRs HER1, HER2, and HER4) inhibitor and is also an investigational drug for the treatment of glioblastoma.86 An X-ray structure of T790M mutant EGFR bound to dacomitinib shows the 168 Organofluorine Chemistry FIGURE 5–38 Structure of mefloquine (ball and stick) bound to PfPNP, showing hydrophobic interactions of the trifluoromethyl moieties; The hydrophobic interactions (F-C distances ranging from 2.8 to 3.3 Å) of the CF3 groups are shown by the dotted lines; the structure was created using UCSF Chimera software, PDB 5ZNI; PfPNP, Purine nucleoside phosphorylase. F H N N O O CH3 Cl HN N N Dacomitinib FIGURE 5–39 Structure of dacomitinib, an FDA-approved drug for the treatment of NSCLC; NSCLC, Nonsmall-cell lung carcinoma. formation of a covalent bond for the cysteine-790, arising from the Michael reaction to the α,β-unsaturated amide moiety (i.e., acrylamide moiety) of dacomitinib (Figs. 540 and 541).87 Similar cysteine adducts of the EGFR inhibitors, such as afatinib, an FDA-approved drug for the treatment of NSCLC, were revealed through X-ray structure analysis and also confirmed by MS.88 The role of the fluorine moieties in these compounds is apparently to enhance the pharmacokinetic properties (i.e., increased lipophilicity and metabolic stability) Chapter 5 • Pharmaceutical applications of organofluorine compounds 169 O O EGFR-Cys O N O N EGFR L858R/T790M S N N H O N HN O N H Cl N N HN Cl F Afatinib F Cysteine adduct of afatinib F F EGFR- Cys797-SH H N N O Cl HN N O N O CH 3 H N N EGFRCys797S O CH 3 Cl HN N N Michael adduct of dacomitinib with Cys797-SH of the EGFR kinase Dacomitinib F O HN N O O Cl N EGFR L858R/T790M No inhibitory effect N Gefitinib FIGURE 5–40 Formation of the Michael adducts of the cysteine residues with EGFR inhibitors afatinib and dacomitinib; EGFR, Epidermal growth factor receptor. of the drug molecules. Analogs of these compounds lacking the acrylamide moiety, such as gefitinib (Iressa, AstraZenica, and Teva), are ineffective in inhibiting these doubly mutated EGFR enzymes (i.e., with L858R/T790M mutations), although gefitinib is effective in inhibiting L858R mutants of EGFR and was approved by FDA (in 2003) for treating nonsmall-cell lung cancers. 5.7.2 Lorlatinib Standard initial treatments for NSCLC include first-line treatment with crizotinib (consisting of a fluoroaryl moiety) or ceritinib (a nonfluorine compound). However, secondary mutations of the ALK domain occur because of the acquired resistance to these drugs. More potent 170 Organofluorine Chemistry FIGURE 5–41 Expanded structure of the active site region of T790M mutant of EGFR kinase, bound to dacomitinib (ball and stick) through covalent bond formation with Cys797; the terminal pyrrolidine moiety is not revealed in this X-ray crystal structure; the structure was created using UCSF Chimera software, PDB 4I24. second-generation treatments have clinical benefit, although most patients develop resistance to the second-generation tyrosine kinase inhibitors. Lorlatinib is a tyrosine kinase inhibitor directed at the ALK-positive and c-ros oncogene 1 (ROS1) kinases (Fig. 542). Phase 1 and phase 2 clinical trials of lorlatinib showed that it shows durable response with a median duration of response of 12.4 months for ALK-positive advanced NSCLC patients, for most of whom CNS metastasis also progressed after the second-line of treatment with other drugs.89 The NSCLC metastasis is also transmitted to the brain, and permeation of the BBB is critical for any pharmaceutical compound to treat the brain tumors. Positron emission tomography studies, using the 11C- and 18F-labeled lorlatinib, demonstrated high brain permeability for lorlatinib (see Chapter 6: Synthesis and applications of 18F-labeled compounds).90 Chapter 5 • Pharmaceutical applications of organofluorine compounds CH 3 N N C N NH F Cl Cl Cl O Me H 2N N O S O N N N H NH N HN F N Larotectinib (Vitrakvi; for NTRK gene fusionpositive solid tumors) N N H N Me N H N O O Lorlatinib (second-generation drug for NSCLC) N C N N H N H 2N CH3 O N O F H 3C Ceritinib (first-line treatment for NSCLC) N N OH N O F Crizotinib (first-line treatment for NSCLC) N N N 171 F N N O O F N Talazoparib (Talzenna; for BRCA-mutated HER2-negative breast cancer) Cl H N O F F F Ivosidenib (Tibsovo; for acute myeloid leukemia and cholangiocarcinoma) FIGURE 5–42 Structures of larotrectinib, talazoparib, and ivosidenib—FDA-approved anticancer drugs. Three other fluorine-containing heterocyclic anticancer drugs were approved by FDA in 2018: larotrectinib (Vitrakvi) for the treatment of NTRK gene fusion-positive solid tumors, talazoparib (Talzenna) for BRCA (breast cancer)-mutated HER2-negative breast cancer, ivosidenib (Tibsovo), and ivosidenib (Tibsovo) for the treatment of acute myeloid leukemia (AML) and cholangiocarcinoma (bile duct cancer) (Fig. 542). 5.7.3 Cobimetinib Cobimetinib (Cotellic; Exelixis and Genentech/Roche) was approved by FDA (in 2015) for the treatment of various cancers, including melanoma and breast cancer.91 Cobimetinib inhibits mitogen-activated protein kinase (MAPK), which is overactivated in human tumors. It is used as a combination drug with vemurafenib (Genentech and DaiichiSankyo) for the treatment of unresectable or metastatic BRAF V600 mutation positive melanoma (progression-free survival for 9.9 months for the combination drug versus 6.2 months for placebo plus vemurafenib recipients). Cobimetinib in humans is metabolized by cytochrome P450 enzymes and excreted as the glucuronide conjugates, but the fluoroaryl rings are not 172 Organofluorine Chemistry N F H N F F N NH I O N OH H O Cl F F Cobimetinib O HN S O Vemurafenib FIGURE 5–43 Structures of cobimetinib and vemurafenib, anticancer drugs used in combination to treat melanoma. oxidized during this P450-mediated oxidation, reflecting the enhanced oxidative stability afforded by the aryl fluorines.91 Vemurafenib (Zelboraf; Roche) was approved by FDA in 2011 for the treatment of V600E-mutated late-stage melanoma (Fig. 543).92,93 5.7.4 Abemaciclib Abemaciclib (Verzinio; Eli Lilly) was approved by FDA in 2017 for the treatment of breast cancers. It is a selective CDK4 and CDK6 inhibitor and thereby deactivates the retinoblastoma protein, a key enzyme involved in cell-cycle progression, leading to the apoptosis of tumor cells. It is the first CDK inhibitor drug for treating breast cancers.9496 The lipophilicity enhancement afforded by fluorines in abemaciclib, with a c Log P of 5.5, allows the molecule to bind more effectively in the ATP cleft, as compared to the other nonfluorinated CDK4/ CDK6 drugs, palbociclib (c Log P 2.7) and ribociclib (c Log P 2.3).96 X-ray crystal structure of abemaciclib, bound to CDK6 enzyme, shows hydrogen-bonding interactions of the fluorine with ε-amino group of the Lys43 residue (3.5 Å) and exhibits hydrophobic interactions with Phe98 (Fig. 544).96 5.7.5 PARP inhibitors: rucaparib (Rubraca) and olaparib (Lynparza) Rucaparib (Rubraca; Clovis Oncology) is an inhibitor of poly[adenosine diphosphate (ADP)ribose] polymerases 1 and 2 (PARP-1 PARP-2) and was approved by FDA in 2016 for treating advanced ovarian cancers involving BRCA1 (breast cancer type 1) and BRCA2 (breast cancer type 2) mutations.97,98 Rucaparib also suppresses the lactate dehydrogenasemediated transformation of pyruvate to lactate in A2780 cells, resulting in the suppression of the ovarian cancer cell growth (Fig. 545).99 X-ray crystallographic structure of rucaparib complexed to human PARP-1 shows hydrophobic interactions of the fluorine with Glu988, Ala898, and Phe897 residues (Fig. 546).100 PARP enzymes are involved in DNA repair and their inhibition prevents DNA repairs, leading to cell death in cancers. Olaparib (Lynparza; AstraZeneca and Merck & Co.), a nonselective PARP-1 inhibitor, was approved by FDA in 2014 for the treatment of advanced ovarian and breast cancers with BRCA mutations.101,102 Chapter 5 • Pharmaceutical applications of organofluorine compounds 173 FIGURE 5–44 Expanded X-ray crystal structure of abemaciclib (ball and stick), bound to CDK6; the hydrogen bonding (with Lys43) and the hydrophobic interactions (with Phe98) of the fluorines and the NH interactions with the His100 moiety, ranging from 3.4 to 3.5 Å, are shown by the dotted lines; the structure was created using UCSF Chimera software, PDB 5L2S. O O H N O HN CH3 F NH N N N H N F O Rucaparib (Rubraca; Clovis Oncology) Olaparib (Lynparza; AstraZeneca and Merck & Co.) FIGURE 5–45 Structures of rucaparib and olaparib, the FDA-approved drugs for treating advanced ovarian cancers, associated with BRCA mutations. 5.7.6 Taxoid anticancer agents Paclitaxel (Taxol) and its synthetic analog docetaxel are among the widely used anticancer drugs, often used as combination therapeutics along with other anticancer agents. These compounds and various other derivatives currently in development have limited scope in the treatment of cancers due to their lack of tumor specificity or acquired multidrug 174 Organofluorine Chemistry FIGURE 5–46 Expanded X-ray crystal structure of rucaparib (ball and stick) bound to human PARP-1; the hydrophobic and polar interactions of the fluorine (2.8 to 3.5 Å) are shown as dotted lines; the structure was created using UCSF Chimera software, PDB 4RV6. resistance. Ojima and coworkers have synthesized various fluorinated analogs of paclitaxel as potential cancer therapeutics through the reaction of the semisynthetic taxol derivatives with a β-lactam derivative, as shown in Fig. 547.3,103110 The fluorinated taxoids have substantially improved cell viability in MCF-7/PTX human breast cancer cells. Whereas paclitaxel has a cell viability IC50 of about 2 μM, the m-trifluoromethoxy derivatives SB-T-121205, SB-T121405, and SB-T-121605 have IC50 values in the range of 1935 nM. These compounds and other fluorinated versions, including m-difluoromethoxy derivatives, have comparable cytotoxicity for various cancer cell lines, and in some cases, two orders of potency magnitude greater for drug-resistant cancer cell lines, as compared to paclitaxel. Especially with LCC6MDR breast cancer cell lines, the fluorinated versions (e.g., SB-T-121405, SB-T-121605, SB-T121705, and SB-T-121206) have substantially potent cancer-cell toxicity IC50 values of about 12.5 nM as compared to an IC50 value of 619 nM for paclitaxel.3 Furthermore, some of these fluorinated versions, such as SB-T-121205, were able to suppress the growth, migration, and invasion of MCF-7/PTX human breast cancer cell line, and thus prevented metastasis and suppressed epithelialmesenchymal transition.110 5.7.6.1 Tumor-targeted drug delivery of the fluorinated taxoids Ojima and coworkers designed a mechanism-based self-immolative disulfide linker, conjugated with biotin (a vitamin), as the tumor-targeting moiety for targeted delivery of the Chapter 5 • Pharmaceutical applications of organofluorine compounds 175 O O O NH O O OH NH O O O O OH O O O O OH O O O OH OH O O OH O O O OH Docetaxel Paclitaxel (Taxol) O TIPSO O O O R O O N O O OH O O O NH Ot-Bu O HO O O OH R OH O O O O OH O O O OH 1. LiHMDS, THF, –40 °C O 2. HF/Py, MeCN/Py 0 °C - RT X X SB-121205; SB-121305; SB-121405; SB-121605; SB-121705; R R R R R = Me, X = OCF 3 = Et, X = OCF 3 = c-Pr, X = OCF 3 = NMe 2 , X = OCF 3 = OMe, X = OCF 3 SB-121206; SB-121306; SB-121406; SB-121606; SB-121706; R R R R R = Me, X = OCHF 2 = Et, X = OCHF 2 = c-Pr, X = OCHF 2 = NMe 2 , X = OCHF2 = OMe, X = OCHF 2 FIGURE 5–47 Structures of paclitaxel (taxol) and docetaxel; and synthesis of fluorinated versions of paclitaxel as potent anticancer agents. taxoids.111 Vitamin receptors are overexpressed at the cell surfaces in cancer cells, and thus it would be expected that the biotin-conjugated drugs would selectively bind to the tumor cells over the normal cells and then internalized through the receptor-mediated endocytosis. The intracellularly abundant glutathione (GSH) mediates the taxoid drug release from the biotin conjugate through a cascade of reactions, involving disulfide bond cleavage, followed 176 Organofluorine Chemistry FIGURE 5–48 Time-resolved 19F NMR spectra for the disulfide linker cleavage and thiolactonization process of probe 1 (2.5 mM) in 30% DMSO in D2O, beginning at 1 h after the addition of 6 equiv of GSH at 25 C with 15 min intervals (128 scans/spectrum); DMSO, Dimethyl sulfoxide; GSH, glutathione. Adapted from Seitz, J.D.; Vineberg, J.G.; Wei, L.; Khan, J.F.; Lichtenthal, B.; Lin, C.-F.; Ojima, I. Design, Synthesis and Application of Fluorine-Labeled Taxoids as 19F NMR Probes for the Metabolic Stability Assessment of Tumor-Targeted Drug Delivery Systems. J. Fluorine Chem. 2015, 171, 148161. by thiolactonization to give the fluorobenzothiolactone as a byproduct. The cytotoxicity of the cancer drugs is thereby significantly attenuated. Time-resolved 19F NMR spectroscopy allows monitoring the controlled release of the taxoid drug SB-T-12145 from its biotin-conjugated compound, BLT-F2 (Fig. 548).111 Immediately after addition of GSH, the disulfide bond of the biotin-conjugated compound BLT-F2 is reduced to give the taxoid 3-A (in Fig. 548). The latter intermediate taxoid then incrementally forms the fluorobenzothiolactone (structure 9 in Fig. 548), as shown by the time-dependent increase in the intensity of the signal at δ19F 2 116.3, corresponding to the thiolactone 9 (in Fig. 548), and a corresponding decrease in intensity of the signal at δ19F 2 119.2, ascribed to the taxoid 3-A (in Fig. 548). The absorption at δ19F112.5 corresponds to the aryl fluorine of the fluoro-taxoid SB-T-12145, and, it overlaps with that of the biotin conjugate BLT-F2 (Fig. 548). Chapter 5 • Pharmaceutical applications of organofluorine compounds 177 FIGURE 5–49 Structure of the fluorouracil-phosphoramidite module, used as the building block for the automated and modular synthesis of aptamerdrug conjugates; DMTr 5 4,40 -dimethoxytrityl. 5.7.6.2 Drug delivery through aptamerdrug conjugates DNA aptamerdrug conjugates provide a means of delivering drug molecules with high specificity into the cancer cells. Tan and coworkers have synthesized aptamerdrug conjugates consisting of the anticancer drug, 5-fluorouracil, and a photocleavable 2-nitrobenzyl group as the linker.112 The fluorouracil-phosphoramidite module (Fig. 549) was synthesized using solidphase synthesis, and using this module, automated and modular synthesis of aptamerdrug conjugates was achieved at the 50 -end of the aptamer. These aptamerdrug conjugates showed high specificity to the targeted cancer cells. Various other drug candidates could also be incorporated in these aptamerdrug conjugates. The aptamerdrug conjugates are relatively more selective drug-delivery agents as compared to the widely used antibodydrug conjugates. These aptamerdrug conjugates are also stable compounds, as dry powders, and are relatively less toxic. Using this synthetic approach, the drug molecules could be incorporated in a chained mode at the 50 -end or incorporated at the predesigned sites on a DNA synthesizer.112 The aptamer-taxoid-based approach is also useful for the tumor-specific drug delivery of taxoids, and for improving aqueous solubility of the hydrophobic docetaxel.111,113,114 5.7.7 Fulvestrant Fulvestrant (Faslodex; AstraZeneca) is an FDA-approved (approved in 2002) anticancer drug. It is an estradiol derivative having a terminal pentafluoroethyl moiety on the side chain, which imparts lipophilic properties to the drug (Fig. 550). Fulvestrant is used to treat hormone receptorpositive metastatic breast cancer (hormone receptors are either estrogen or progesterone receptors). Fulvestrant is a steroidal estrogen antagonist and offers advantage over the antiestrogen tamoxifen, as the latter compound can act as an estrogen agonist in some cases. Thus, by downregulating the estrogen receptors (ERs), fulvestrant is active in tamoxifen-resistant breast cancers.115 Fulvestrant also helps degrade the ERs through its hydrophobic effect on the surface of the receptors (vide infra). Fulvestrant is designed such that the endogenous ER ligand, 17β-estradiol, which acts as an estrogen antagonist, is tethered to the hydrophobic side chain with a terminal fluoroalkyl 178 Organofluorine Chemistry Me OH Me OH H H H H H O S H HO HO Estradiol (endogenous ER ligand) F F F F F Fulvestrant FIGURE 5–50 Structures of estradiol and fulvestrant. moiety, the latter, mediating the ER degradation through its hydrophobic effect. Fulvestrant, upon binding with the ERs, induces structural changes in the protein and enhances hydrophobicity, mimicking the partially unfolded protein and thereby recruiting endogenous chaperones that would unfold the protein. The unfolded protein is then shuttled for proteasome degradation.116 Thus, fulvestrant, in addition to being an estrogen antagonist, also helps degrade ERs through its hydrophobic effect on the surface of the receptors. As a selective ER degrader (SERD), fulvestrant exhibits high specificity to the target protein (ER) and thereby provides advantages over the widely used anticancer drug tamoxifen, with decreased risk of endometrial cancer. Newer generation of SERDs address the poor oral bioavailability and poor systemic exposure of the fulvestrant.117 5.7.7.1 Synthesis of fulvestrant A kilogram-scale synthesis of fulvestrant is carried out, starting from the pentafluoropentanol (25). The key step for the synthesis of fulvestrant involves the stereoselective 1,6-addition of an organocuprate derived from 29 to the 17β-acetoxyestra-4,6-dien-3-one (30), followed by Cu(II)-mediated aromatization of the A-ring, and alkaline hydrolysis of the ester moiety, to give compound 32. (Fig. 551).118 The H2O2-mediated oxidation of the thio ether 32, followed by repeated recrystallizations (which also remove the unwanted 7β-isomer formed as a minor byproduct) gave the fulvestrant, as a crystalline compound, in high purity. 5.7.8 Enasidenib Enasidenib (Agios Pharmaceuticals; AG-221) was approved by FDA in 2017 to treat relapsed or refractory AML. AML is characterized by attenuated cellular differentiation, through a disruption in the citric acid cycle, thereby resulting in the formation of immature cells. AML therapies are therefore targeted to promoting cellular differentiation. Enasidenib exerts its therapeutic effect through inhibition of the mutated version of isocitrate dehydrogenase-2 (IDH2) enzyme. The mutated form of the IDH2, found in certain cancer cells, is involved in the reduction of the α-ketoglutarate, a product of the normal citric acid cycle, to the (R)-2hydroxyglutarate (2-HG); the wild-type IDH2 enzyme, unlike the mutated version of IDH2, does not reduce the α-ketoglutarate to 2-hydroxyglutaric acid. The IDH2 mutations are also found in premalignant disorders, such as myelodysplastic syndrome. Through inhibition of Chapter 5 • Pharmaceutical applications of organofluorine compounds HO 1. MsCl, Et3N, MeCN CF2CF3 HO MsO SH (27) CF2CF3 26 25 HO S Ph3P/Br2/MeCN CF2CF3 28 Br S CF2CF3 1. Mg/THF, 4–10 °C 2. CuCl, –34 °C 3. Me 29 OAc H –34 °C H H O (30) Me OAc H H 1. CuBr2/LiBr/Ac2O 2. NaOH H S O CF2CF3 31 Me OH H H 1. H2O2 H HO S CF2CF3 O S CF2CF3 32 Me OH 17 H H H HO 3 7 Fulvestrant FIGURE 5–51 Industrial-scale synthesis of fulvestrant. 179 2. Purification 180 Organofluorine Chemistry FIGURE 5–52 X-ray structure of enasidenib bound to IDH2 (PDB 5I96), showing the tetrel bonding between the CF3 carbon and Asp312 (distance in Å). Adapted with permission from Garcia-Llinas, X.; Bauza, A.; Seth, S.K.; Frontera, A. Importance of R-CF3 O Tetrel Bonding Interactions in Biological Systems. J. Phys. Chem. A 2017, 121, 53715376. ©2017, American Chemical Society. the IDH2 mutants (IC50 1020 nm), enasidenib suppresses the formation of 2-HG and induces cellular differentiation in human AML cells in xenograft mouse models.119 Noncovalent bonding interaction between a CF3 carbon and asp-312 oxygen, also called σ-hole tetrel bonding, was observed in the X-ray crystal structure of the enasidenib bound to the IDH2, and this tetrel bonding was substantiated by ab initio calculations.120 In other words the CF3 carbon is able to act as an electrophilic center, forming noncovalent bonding interactions with the Lewis basic aspartate 312 oxygen (Fig. 552). Synthesis of enasidenib was achieved in five steps, starting from 6-trifluoromethyl-2pyridinecarboxylic acid (33) (Fig. 553).121 Thus, 6-trifluoromethylpyridine-2-carboxylic acid (33), upon esterification, followed by reaction of the ester 34 with biuret (imidodicarbonic diamide) gives the 6-(6-trifluoromethyl-2-yl)-1,3,5-triazine-2,4-dione (35). Reaction of compound 35 with PCl5 in phosphoryl chloride (POCl3) gives the 2,4-dichloro-6-(6-trifluoromethylpyridin-2-yl)-1,3,5-triazine (36). Sequential aromatic nucleophilic substitution reactions (SNAr) Chapter 5 • Pharmaceutical applications of organofluorine compounds 181 FIGURE 5–53 Synthesis of enasidenib. of the triazine 36 with 2-(trifluoromethyl)-4-pyridinamine (37) and 1-amino-2-methyl-2-propanol gives enasidenib. 5.7.9 Nonsteroidal antiandrogens (apalutamide, bicalutamide, and flutamide) Apalutamide (Erleada; Johnson and Johnson) was approved by FDA in 2018 for the treatment of nonmetastatic castration-resistant prostate cancer (CRPC) (NM-CRPC).122 Related antiandrogens flutamide (Eulexin; Schering Plough) and bicalutamide (Casodex, AstraZeneca) were approved by FDA in 1989 and 1995, respectively, for the treatment of metastatic prostate cancer. Apalutamide, bicalutamide, and flutamide are nonsteroidal antiandrogens (i.e., androgen antagonists), and the structures of all these three compounds have a trifluoromethyl group adjacent to an electron-withdrawing moiety (CN or NO2) in the aromatic ring (Fig. 554). 182 Organofluorine Chemistry O F H3 C N N N H N O O C N S Flutamide (Eulexin) Apalutamide (Erleada) C O O O S HO N H N CF3 O- CF3 N H CF3 O N+ O H3 C N H F S N C N N CF3 O F Bicalutamide (Casodex) Enzalutamide (Xtandi) FIGURE 5–54 Structure of apalutamide (Erleada) and flutamide (Eulexin) for the treatment of nonmetastatic castration-resistant prostate cancer. Bicalutamide is among the widely used nonsteroidal antiandrogens for the treatment of metastatic CRPC.122 Although the prognosis of CRPC patients has significantly improved after the introduction of these and other related drugs—including docetaxel, immunotherapy agents, and radiopharmaceuticals—targeting multiple aspects of the disease, identifying the best sequence of these pharmaceuticals in the combination therapy still remains a challenge. An X-ray structure of the bicalutamide bound to the W741L mutant androgen receptor shows a bent conformation for the bicalutamide, with an intramolecular hydrogen bonding of the sulfonyl oxygen with the chiral hydroxy group, which also has hydrogen-bonding interaction with Asn705 side-chain amide nitrogen (2.5 Å).123 The trifluoromethyl group has hydrophobic interactions with Phe746, Leu873, Val746, and Met745 residues, and the cyanonitrogen is in the hydrogen-bonding distance with respect to the Arg752 side-chain nitrogen (3.0 Å) and Gln711 side-chain oxygen (3.4 Å). Thus, the binding of the bicalutamide to the androgen receptor is dominated mostly by hydrophobic interactions (Fig. 555). 5.7.9.1 Enzalutamide Enzalutamide (4-[3-[4-cyano-3-(trifluoromethyl)phenyl]-5,5-dimethyl-4-oxo-2-thioxoimidazolidin1-yl]-2-fluoro-N-methylbenzamide; Xtandi; Astellas Pharma Europe B.V.) is structurally closely related to apalutamide. These compounds have in common the thioxo-imidazolidinone ring and N-methyl-o-fluorobenzamide moiety. Whereas apalutamide consists of trifluoromethyl and cyano moieties in the pyridyl ring, enzalutamide has these moieties on the phenyl ring. Enzalutamide is approved by the European Medicines Agency (EMA) for the treatment of NM-CRPC.122 The synthesis of enzaltumide was elegantly achieved in three steps starting from 4-cyano-3trifluoromethylaniline (39). Reaction of compound 39 with thiophosgene gives the corresponding Chapter 5 • Pharmaceutical applications of organofluorine compounds 183 FIGURE 5–55 Expanded view of the X-ray structure of R-bicalutamide (ball and stick) bound to the W741L mutant androgen receptor; hydrogen-bonding interactions of the cyano nitrogen and the hydrophobic interactions of the CF3 moiety are shown with dotted lines; binding of the bicalutamide to the active site is dominated by hydrophobic interactions; the structure was created using UCSF Chimera software, PDB 1Z95. isothiocyanates 40. The isocyanate 40 upon condensation with the β-amino ester 41 gives enzalutamide, in high yield. Compound 41, in turn, was synthesized from 2-fluoro-4-bromobenzoic acid, involving conversion of the carboxylic acid moiety into the N-methylamide, using a mixture of thionyl chloride and methylamine, followed by Cu(I)-catalyzed aryl-amination (Fig. 556).124 5.7.10 BRAF and mitogen-activated protein kinase kinase enzyme inhibitors in cancer treatment In 2018 FDA approved the combination therapy using binimetinib (Mektovi; ARRY-162; Array BioPharma) and encorafenib (Braftovi; Novartis; Array BioPharma) for the treatment of unresectable or metastatic melanoma, involving BRAF V600E or V600K mutations (Fig. 557).125 Encorafenib is a BRAF inhibitor and binimetinib is a MAPK kinase enzyme (MEK) inhibitor (phosphorylating the MAPK); both drugs are developed by Array BioPharma. 184 Organofluorine Chemistry F O OH Br 1. SOCl2/DMF, CH3NH 2 O 2. NH2 O F CN CN S CF3 Me CF3 Cl Cl Heptane, 10 °C to 46 °C NH 2 N 15 h 92% 39 40 CuI O N H O N H O Me 41 DMSO, RT to 84 °C C 82% S F O S H 3C N H N C N N CF3 O Enzalutamide FIGURE 5–56 Synthesis of enzalutamide. O S Br HN O F F N N F NH H N N N O Cl H N N OH N O N H O O Binimetinib (Mektovi) Encorafenib FIGURE 5–57 Structures of binimetinib and encorafenib, the combination drugs for the treatment of melanoma. Chapter 5 • Pharmaceutical applications of organofluorine compounds 185 Array BioPharma and Genentech have developed a clinical candidate GDC-0994 (42) as an effective inhibitor of extracellular signal-regulated kinase kinases (ERK1/2).126 Activation of the RAS/ RAF/MEK/ERK (MAPK) signal transduction pathways is common to a large subset of human cancers, including BRAF-mutant-resistant metastatic melanoma. Although small molecule inhibitors of BRAF and MEK, such as binimetinib and encorafenib, are currently effective in the treatment of BRAF-mutant metastatic cancers, these drugs may have a limited duration of efficacy in some patients due to the pathway-reactivating mutations. Selective inhibitors of ERK1/2 kinases, such as GDC-0994, in concert with the RAF and MEK inhibition, thus may have improved efficacy against various cancers. An analog of the GDC-0994 with a trifluoromethyl moiety in the pyrazole ring (43) proved to be a relatively less effective ERK1/2 inhibitor (Fig. 558). The fluorochlorophenyl ring has hydrophobic interactions with the Tyr36 side chain in the glycine-rich loop at the active site, and the 5-aminopyrazole has hydrogen bondingstabilizing interactions with the Lys114 residue.126 5.8 Antiviral pharmaceuticals 5.8.1 Tecovirimat Tecovirimat (ST-246; Tpoxx; SIGA Technologies) is an orally active antiviral pharmaceutical, active against orthopoxviruses, including smallpox and monkeypox (Fig. 559). It was F3C N N N Me N H N N N Cl O N Me N H Cl O N F F OH OH GDC-0994 42 43 ERK1 IC 50 = 74 nM ERK2 IC 50 = 52 nM ERK1 IC 50 = 6.1 nM ERK2 IC 50 = 3.1 nM FIGURE 5–58 Structures of the clinical candidate GDC-0994 and its analog (as anticancer drugs). H H N O O O N H CF 3 Tecovirimat (ST-246; Tpoxx) (antiviral against smallpox and monkeypox) FIGURE 5–59 Structure of tecovirimat, an antiviral drug. 186 Organofluorine Chemistry approved by FDA in 2018 as a prophylaxis agent and for the treatment of smallpox, caused by variola virus in adults and pediatric patients weighing greater than 13 kg. This drug was developed by SIGA Technologies in collaboration with the US Department of Health and Human Services, Biomedical Advances Research and Development Authority, and is stockpiled in the US strategic national stockpile as a preventive measure.127,128 Tecovirimat inhibits the activity of orthopoxvirus VP37 envelopewrapping protein, thereby preventing the formation of the egress-competent virions and suppressing the spread of virions in the host cells. Tecovirimat showed insignificant adverse events in a clinical study on 449 adult volunteers at a dose of 600 mg twice daily for 14 days.129 X-ray crystallographic details of this compound bound to viral proteins are not available, and the role of the trifluoromethyl moiety in its drug action is therefore not known with certainty. 5.8.1.1 Synthesis of tecovirimat SIGA Technologies’ synthesis of tecovirimat is outlined in Fig. 560.130 Reaction of maleic anhydride with N-(tert-butoxycarbonyl)hydrazine, followed by deprotection, gives N-aminomaleimide 45 in good yields. Acyl nucleophilic substitution reaction of the N-aminomaleimide (45) with 4-(trifluoromethyl)benzoyl chloride gives compound 46, which undergoes FIGURE 5–60 Synthesis of tecovirimat. Chapter 5 • Pharmaceutical applications of organofluorine compounds 187 DielsAlder reaction with 1,3,5-cycloheptatriene to afford the tecovirimat. In the final step the cycloheptatriene is in reversible equilibrium with bicyclo[4.1.0]heptadiene under the reaction conditions, and the DielsAlder reaction of the latter bicyclo[4.1.0]heptadiene with the maleimide derivative 46 gives the tecovirimat. 5.8.2 Sofosbuvir Sofosbuvir (Sovaldi; Gilead Sciences) is an FDA-approved orally bioavailable drug for the treatment of hepatitis C, used as combination therapy with other small molecule inhibitors, such as ribavirin and velpatasvir (Fig. 561). Sofosbuvir was a blockbuster drug in 2015 with sales exceeding 5 billion US dollars. Sofosbuvir is transformed into its active drug form, a uridine triphosphate analog, in vivo. It is a potent nucleotide analog inhibitor of the hepatitis C virus (HCV) nonstructural protein 5B (NS5B), an RNA-dependent RNA polymerase, with an EC50 of 0.15 μM. HCV cannot replicate in the absence of the latter NS5B polymerase enzyme, and thus, most drugs developed for treating hepatitis C are either nucleoside analog polymerase inhibitors that bind at the active site of the enzyme (e.g., sofosbuvir) or nonnucleoside polymerase inhibitors that bind at the allosteric site. The deficiency in the proofreading during the RNA-polymerase-initiated RNA replication results in a high spontaneous mutation rate, necessitating combination therapy. This antiviral drug induces an S282T mutation in the NS5B, lowering the binding affinity of the sofosbuvir-derived active cytidine-analogous triphosphate, and thereby attenuating its replicon activity. Cytidine versions of sofosbuvir (mericitabine, 47 and 48 in Fig. 561) inhibit the HCV NS5B protein by a similar mechanism, and some of these analogs exhibit similar half-maximal effective concentration, EC50, as that of sofosbuvir.131 These cytidine analogs of sofosbuvir may provide promising alternative candidates for HCV treatment, including the treatment of HCV resulting from the drug-resistant mutation NS5B S282T, formed through the resistance acquired from the sofosbuvir. The combination therapy using sofosbuvir plus daclatasvir plus ribavirin for 12 or 24 weeks showed excellent outcome with a 91%89% HCV patient survival rates in a wider patient trial.132 The active form of sofosbuvir, 20 -deoxy-20 -α-fluoro-20 -β-methyl-uridine-50 -triphosphate (51) is formed through a series of metabolic processes in the liver. The first-pass metabolism of sofosbuvir in the liver generates terminal carboxylic acid 49, which upon further metabolism gives the 20 -deoxy-20 -α-fluoro-20 -β-methyl-uridine-50 -phosphate 50. The latter compound, 50, is inactive in inhibiting the RNA-dependent NS5B RNA polymerase enzyme, until it is further transformed into the triphosphate, mediated by uridinecytidine monophosphate kinase and nucleoside diphosphate kinase (Fig. 562).133 The phosphoramidate prodrug is designed to enhance its metabolic lifetime and potency, and as described above, the active drug form 51 is released through a series of enzymatic reactions in the liver, a site where the drug action is involved. The triphosphate 51 binds to the catalytic site of NS5B protein, thereby resulting in the RNApolymerase chain termination. This chain termination is presumably due to the basicity-lowering effect of the 30 -OH group by the proximal fluorine, so that its reactivity for further chain propagation is drastically reduced. 188 Organofluorine Chemistry NH2 O N NH N O N P O H O O O N O O O O Me OH O O Me O O F F Mericitabine (cytidine analog of sofosbuvir) Sofosbuvir EC50 (NS5B enzyme): 0.15 μM 1.11 μM O NH2 N N O N P O H O O O N O O Me N O N P O H O O O OH R NH F O O Me O F 48; R = e.g., Propyl (cytidine analog of sofosbuvir) 47 (cytidine analog of sofosbuvir) 0.24 μM EC50 (NS5B enzyme): 0.66 μM Me O Me O NH2 N N O H N N HO OH Me O NH O O O NH Me Me N HO Me O O N N NH N O Ribavarin Velpatasvir FIGURE 5–61 Structures of sofosbuvir, mericitabine, and cytidine analogs of sofosbuvir, 47 and 48, with EC50 values for the HCV NS5B polymerase inhibition; structures of ribavirin and velpatasvir are also shown; EC50, Half-maximal effective concentration; HCV, hepatitis C virus. Chapter 5 • Pharmaceutical applications of organofluorine compounds O O NH NH O O N O O N P O H O O Me N P O H O O O Me OH (hydrolysis of phosphoramidate ester) F N O HO Liver metabolism O OH 189 F 49 Sofosbuvir (prodrug) O O NH NH O HO P O HO N O Enzyme-catalyzed phosphorylations O Me OH N O O O HO P O P O P O OH OH OH O Me OH F O F 51 50 Active form generated through in vivo metabolism FIGURE 5–62 Liver metabolism in the generation of the active drug form 49 from sofosbuvir. F F N N N N H O N H O HN NH O H 3C N O O O CH3 Ledipasvir FIGURE 5–63 Structure of ledipasvir, used as a combination therapy with sofosbuvir. 5.8.3 Ledipasvir A combination drug of sofosbuvir and ledipasvir (Fig. 563) is marketed as Harvoni (Gilead), which is approved by FDA for adult as well as pediatric patients. Ledipasvir is active against the HCV protein mutations, S282T, induced by sofosbuvir, and similarly, sofosbuvir is active against the mutations induced by the ledipasvir, as a result of which HCV genotype 1infected patients are cured in 1224 weeks, with a success rate of 94%99%.133 190 Organofluorine Chemistry 5.8.3.1 Synthesis of ledipasvir Gilead Sciences’ synthesis of ledipasvir is summarized in Fig. 564.134 A key aspect of this synthetic strategy involves the gem-difluorination of the fluorene 1 in one step using N-fluorobenzenesulfonimide (NFSI; 53) as the electrophilic fluorinating agent. Thus, gem-difluorination of the fluorine 52, using NFSI in the presence of potassium hexamethyldisilazide (KHMDS), gives the gem-difluoro compound 54. Selective metalation at the aryl-I bond in 54, using isopropylmagnesium bromide, followed by acylation with Weinreb amide 55 gives the compound 56. Alkylation of Boc-proline 57 by compound 56 gives an intermediate ketoester, which upon reaction with ammonium acetate gives the imidazole 58. SuzukiMiyaura reaction of the compound 58 with the boronic ester 59 gives compound 60. Deprotection of the t-Boc moiety in compound 60, followed by peptide coupling with the N-(methoxycarbonyl)valine (61) then gives the ledipasvir. 5.8.4 Glecaprevir and pibrentasvir Glecaprevir (ABT-493; AbbVie, Inc.) in combination with pibrentasvir (ABT-530; AbbVie, Inc.) has been fast-track approved by FDA in 2017 for treating the major HCV genotypes 16 under the trade name Mavyret (Fig. 565). Safety and efficacy studies of Mavyret in the phase 2 and phase 3 clinical trials showed that overwhelming majority of HCV cases (92% 100%) were completely cured after 1216 weeks of treatment.135,136 In patients with chronic hepatitis C viral disease, the inflammation of the liver associated with this disease may result in decreased liver function and eventual liver failure. The combination drug Mavyret is one of the leading breakthrough therapies for treating hepatitis infections due to HCV genotype 1, a most common hepatitis infection in the United States; according to the FDA statistics, approximately 75% of the US hepatitis infections are due to the HCV genotype 1. A realworld effectiveness study of this drug combination was assessed in Italy with 726 HCV patients and the results showed, in general, excellent effectiveness and safety when administered for the duration of an 8-, 12-, or 16-week period.137 With a sustained virologic response of 99.2% (with a relatively small population having posttreatment relapse), this relatively large-scale clinical trial proved to be overall effective.137 Glecaprevir is a nonstructural protein 3/4A protease inhibitor, whereas pibrentasvir is a nonstructural protein 5A (NS5A) inhibitor. The NS5A helps in the viral RNA replication and viral self-assembly, and thus NS5A inhibitors are widely sought out in the antiviral drug design, especially the combination drugs that target multiple viral proteases. The currently available NS5B inhibitors, such as sofosbuvir, exhibit high potency only when used in combination with other complementary pharmaceuticals (such as ribavirin and interferon), as the viral proteins develop immunity to these drugs, through relatively rapid mutations, when the drug is given as a single component. The NS5A is proline-rich, and a large number of the small-molecule HCV drugs targeting this protein consist of the proline- or structurally related moiety, such as pyrrolidine. Glecaprevir has one pyrrolidine moiety, whereas pibrentasvir has three such pyrrolidine-based bioisostere moieties. In pibrentasvir, a symmetric dimeric version, the 6-fluorobenzimidazole serves as a linker connecting two pyrrolidine moieties. Chapter 5 • Pharmaceutical applications of organofluorine compounds I O O S N F S O O (53) Br F F 1. i-PrMgCl I Br 2. KHMDS, THF O Cl 54 52 N ( 55 ) F F 1. t-Boc H O N OH O ( 57 ) Br K 2 CO 3 , KI acetone Cl 56 2. NH O B O 191 OMe Me F F t-Boc H N N N H Br 58 NH 4OAc, toluene F F H N N t-Boc (59) t-Boc H N N N H H H N N N t-Boc 60 Pd(OAc) 2, PPh 3, NaHCO3, DME F F 1. HCl/dioxane/CH2 Cl2 2. HO O HATU, i-Pr 2 NEt DMF t-Boc H N N N H H H N N N O NHCO 2Me Ledipasvir NHCO 2Me (61) FIGURE 5–64 Synthesis of ledipasvir. KHMDS 5 potassium hexamethyldisilazide; HATU 5 [O-(7-azabenzotriazol-1yl)-N,N,N0 ,N0 -tetramethyluronium hexafluorophosphate]. The difluoroaryl moiety in pibrentasvir has an important modulating effect on the potency and pharmacokinetics of these drugs and this drug is substantially more potent than its nonfluorinated analog. The fluorine atoms exert their enhanced potency by attenuating the 192 Organofluorine Chemistry F N N F F O N O O H H O O H3 C S N H N H H3 C NH O O N N NH H 3C O F F O H O N H N O F F N O NH N N O O HN H 3C O CHF2 O CH 3 CH3 O CH 3 Pibrentasvir Glecaprevir EC 50 for genotype 1a: 3 pM N R R' H3 C O F F O NH O N N NH H 3C N NH N N O O HN H 3C O O CH 3 CH3 O CH 3 R = H, R' = F (62); EC50 for genotype 1a: 18 pM R = R' = F (63); EC 50 for genotype 1a: 5 pM FIGURE 5–65 Structures of glecaprevir and pibrentasvir, the anti-HCV drug constituents of the combination drug Mavyret; also shown are the analogs of pibrentasvir, 62 and 63; HCV, Hepatitis C virus. basicity of the adjacent piperidine moiety. Pibrentasvir exhibits relatively enhanced EC50 value of 3 pM for the genotype 1a.138 The basicity-attenuating effect of fluorines was clearly evident in the structure-activity studies of the corresponding piperidine analogs 62 and 63. The IC50 value for the inhibition of HCV stable replicons (genotype 1a) for 62 is 18 pM, whereas it is 5 pM for the difluoro analog 63 (Fig. 565).138 Pibrentasvir is prepared in a multistep synthesis, starting from 3,4,5-trifluoronitrobenzene (64) (Fig. 566).138,139 The nitro group facilitates the aromatic nucleophilic substitution Chapter 5 • Pharmaceutical applications of organofluorine compounds Pibrentasvir FIGURE 5–66 Synthesis of pibrentasvir; HATU, O-(7-Azabenzotriazol-1-yl)-N,N,N0 ,N0 -tetramethyluronium hexafluorophosphate. 193 194 Organofluorine Chemistry (SNAr) of the para-fluorine in compound 64 by the piperidine-4-one acetal to give compound 65. Conversion of 65 to the corresponding vinyl triflate, followed by SuzukiMiyaura cross-coupling affords compound 68. Consecutive SN2 reactions of 68 with the 1,4-dimesylate 69 gives the pyrrolidine product 70. Pd(0)-catalyzed BuchwaldHartwig amination of compound 70 using N-t-Boc-prolinamide, followed by catalytic hydrogenation, gives compound 71 that undergoes acid-catalyzed dehydrative cyclization to give compound 72. Deprotection of the Boc group in 72, followed by HATU [O-(7-azabenzotriazol-1-yl)-N,N,N0 , N0 -tetramethyluronium hexafluorophosphate]-mediated peptide coupling with N-methoxycarbonyl-threonine methyl ether affords the pibrentasvir. 5.8.5 Voxilaprevir Voxilaprevir (Gilead Sciences) was approved by FDA in 2017 for the treatment of hepatitis C in combination with sofosbuvir and velpatasvir (Vosevi). It is a nonstructural protease 3/4A (NS3/4A protease) inhibitor (Fig. 567). A cocrystal structure of voxilaprevir with GT3 surrogate (GT1 D168Q) NS3/4A protease shows hydrophobic interaction of the macrocyclic gemdifluoromethylene moiety with the Arg155 alkyl moiety.80 Due to this hydrophobic interaction and improved metabolic stability, voxilaprevir exhibits improved potency as compared to the compound lacking the macrocyclic gem-difluoromethylene moiety. 5.8.6 Letermovir (Prevymis) Letermovir (Prevymis; Merck & Co.) is an orally available nonnucleoside inhibitor of the pUL56 subunit of the viral terminase complex of cytomegalovirus (CMV) and was approved by FDA in 2017 for the treatment of CMV-specific viral infections in allogeneic hematopoietic stem-cell-transplant patients.140 Merck’s enantioselective synthesis of letermovir is accomplished through the bistriflamide 74 (a weak Brønsted acid)catalyzed intramolecular aza-conjugate addition reaction of the F F N O O O H3C H3C N O CH 3 NH N CH 3 O NH O O O HN S F O CH 3 F Voxilaprevir FIGURE 5–67 Structure of voxilaprevir, an NS3/4A protease inhibitor and antihepatitis drug. Chapter 5 • Pharmaceutical applications of organofluorine compounds 195 OTf NHTf NHTf O MeO MeO OTf HN N F O HO N F N N OMe CF3 N ( 74 ) 5 mol% CF3 MeO N N OMe then NaOH 73 Letermovir (Prevymis) 95% (96.7:3.3 er) FIGURE 5–68 Enantioselective Michael addition in the synthesis of letermovir. compound 73, followed by hydrolysis of the ester moiety. Enantioselectivities as high as 96:7:3.3 were obtained using this chiral Brønsted-acid catalysis.141 This synthetic method is amenable for the synthesis of a large variety of 3,4-dihydroquinazoline moietycontaining compounds (Fig. 568). 5.9 Fluorinated pharmaceuticals for cardiovascular diseases 5.9.1 Statin drugs The statin class of drugs lower cholesterol levels by inhibiting the HMG-CoA reductase, the rate-limiting enzyme in the early stages of the biosynthesis of cholesterol. The fluorinecontaining statin drugs are the blockbuster drugs (based on the sales) and are among the most prescribed medicines, used for the treatment of hypercholesterolemia, for preventing cardiovascular disease. Fluorinated statin drugs include atorvastatin (Lipitor; Pfizer Pharmaceuticals), rosuvastatin (Crestor; AstraZenica), and fluvastatin (Lescol; Novartis Pharmaceutical Corporation) (Fig. 569). The statin drugs, atorvastatin and rosuvastatin, are the 3rd and 37th most prescribed drugs, respectively, as of 2016.4 Other widely prescribed nonfluorinated statins include lovastatin (Mevacor), pravastatin (Pravachol), simvastatin (Zocor), and pitavastatin (Livalo). Despite the amazing success of the statin class of drugs in preventing cardiovascular diseases, a meta-analysis of the drug usage now reveals drug-related adverse effects, such as the new onset of diabetes. By inhibiting the biosynthesis of cholesterol in the early stages, the statin drugs decrease the levels of coenzyme Q10, farnesyl pyrophosphate, geranylgeranyl pyrophosphate, and dolichol—the downstream products arising from the HMG CoA reductasecatalyzed reactions. The depletion of these compounds results in the inactivation of the intracellular insulin signal transduction pathways, which, in turn, contributes to the loss of 196 Organofluorine Chemistry Me Me OH OH O F Me O N OH NH F O N N S Me O N Me OH Me OH OH O Rosuvastatin Atorvastatin F OH N Me Me OH OH O Fluvastatin FIGURE 5–69 Structures of fluorinated statin drugs, atorvastatin, rosuvastatin, and fluvastatin. glucose homeostasis and the drug-induced onset of type 2 diabetes. Elucidating the precise mechanisms for the underlying development of type 2 diabetes would help in the design of a new class of statins with minimal side effects.142,143 5.9.2 Ezetimibe Ezetimibe (Zetia; Merck & Co.) is a nonstatin drug, used for the treatment of hypercholesterolemia and hyperlipidemia, usually in combination with other statin drugs, such as atorvastatin and simvastatin.144 It was the 144th most prescribed drug, as of 2016.4 Unlike statin inhibitors, which inhibit the cholesterol biosynthesis in the early stages, ezetimibe is an inhibitor of intestinal cholesterol absorption, and thus lowers the total plasma cholesterol levels. The enhanced metabolic stability of ezetimibe, as compared to a nonfluorinated analog SCH 48461 (Fig. 570), is ascribed to the fluorine effect.145 The para-arylfluorine blocks the enzymic oxidation site and apparently also acts as a bioisostere of the hydrogen and methoxy moieties.145 5.9.3 Nebivolol Nebivolol (Bystolic; Allergan Pharmaceuticals) was approved by FDA in 2016 for the treatment of hypertension (Fig. 571). It is a β-blocker (β-antagonist) and has a similar blood pressurelowering effect as that of the other β-blockers, with relatively lower adverse effects. Chapter 5 • Pharmaceutical applications of organofluorine compounds OH 197 O O N N F OCH 3 F H3 CO HO SCH 48461 Ezetimibe ED 50 = 2.2 mg/kg ED 50 = 0.04 mg/kg FIGURE 5–70 Structure of ezetimibe, used for the treatment of hypercholesterolemia and hyperlipidemia, and comparison of its effectiveness with a nonfluorinated analog SCH 48461 in lowering the liver cholesterol ester levels in cholesterol-fed hamsters. F F O OH N H O OH Nebivolol (β−blocker; to treat high blood pressure) FIGURE 5–71 Structure of nebivolol, a β-antagonist, prescribed for the treatment of hypertension. It enhances bioavailability for nitric oxide, through its activation of endothelial nitric oxide synthase (eNOS) and inducible nitric oxide synthase (iNOS), and thereby exhibits vasodilatory effect. Furthermore, nebivolol functions as an antioxidant and decreases markers of oxidative stress, thereby modulating the endothelial dysfunction and lowering the hypertension.146 5.9.4 Antiplatelet drugs 5.9.4.1 Cangrelor Cangrelor (Kengreal; Kengrexal; The Medicines Company) is an antiplatelet drug, used through intravenous injection. Cangrelor (Fig. 572) is a P2Y12 (an ADP receptor on platelet cell membrane) inhibitor, and was approved by FDA in 2015 for reducing periprocedural thrombotic events during surgery. It is a fast-acting drug as it is not a prodrug. Vorapaxar (Zontivity; SCH 530348; Merck & Co.) is an FDA-approved (in 2014) antiplatelet drug. Vorapaxar (Fig. 572) is a thrombin receptor (protease-activated receptor PAR-1) antagonist, and is prescribed for patients with myocardial infarction (MI) and peripheral 198 Organofluorine Chemistry CF3 Me S Me N H H O O N N H OH O O H Me Cl Cl OH P P P OH O OH O OH O O Cangrelor N Vorapaxar F F HN N N S H O N HO N S H N O F N N N HO O OH HO Ticagrelor FIGURE 5–72 Structures of cangrelor, vorapaxar, and ticagrelor, the FDA-approved antiplatelet drugs. arterial disease. It has demonstrated benefit in the prevention of the recurrent thrombic effects, including MI and stroke.147 Among all the known PAR-1 inhibitors, vorapaxar is the only drug that is approved by FDA for the prevention of recurrent ischemic events in patients with peripheral artery disease and MI.148 Ticagrelor (Brillinta; AstraZeneca; Fig. 572) is a reversible, direct-acting platelet-aggregation inhibitor. It exerts its anti-platelet-aggregation effect through allosteric inhibition of the ADP receptor P2Y12 and is a more effective drug as compared to clopidogrel (Plavix), a nonfluorinated antiplatelet drug.149 5.9.4.2 Riociguat Riociguat (Adempas; Bayer Pharmaceuticals; Fig. 573) is an orally available, soluble guanylate cyclase inhibitor and is a first-in-class drug, approved by FDA in 2013, for the treatment of chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension.150 Chronic thromboembolic pulmonary hypertension arises from a complication of pulmonary embolism, leading to right heart failure and death. Riociguat is recommended as a medical therapy in the complicated, inoperable cases for treating this disease.151 Chapter 5 • Pharmaceutical applications of organofluorine compounds 199 F N N N N H 2N N N Me NH2 O Me O Riociguat FIGURE 5–73 Structure of riociguat, a drug for chronic pulmonary hypertension. 5.10 Antiinflammatory pharmaceuticals 5.10.1 Nonsteroidal antiinflammatory agents Steroidal as well as NSAIDs are usually nonselective COX-1 and COX-2 inhibitors. Both of these cyclooxygenases are involved in the biosynthesis of prostaglandins, the compounds responsible for the inflammation at the sites of inflammation. Whereas COX-2 is inducible, and expressed at the sites of inflammation, COX-1 is expressed in most tissues, including gastrointestinal mucosa. The COX-1 inhibition, therefore, results in gastrointestinal side effects, as observed in the prolonged use of NSAIDs, such as aspirin. The COX-1 inhibitors, on the other hand, inhibit platelet aggregation and thus reduce the risk of cardiovascular complications. Although selective COX-2 inhibitors eliminate the gastrointestinal side effects associated with COX-1 inhibition, COX-2 inhibition results in the inhibition of prostacyclin synthesis, thereby promoting thrombosis, resulting in adverse cardiovascular events, such as heart attack and stroke.152 NSAIDs, such as aspirin (O-acetylsalicyclic acid), ibuprofen, naproxen, and flurbiprofen (a fluorinated NSAID), are nonselective COX inhibitors and are associated with gastrointestinal side effects. Celecoxib (a fluorinated NSAID), rofecoxib (Vioxx; Merck & Co.), and valdecoxib (Bextra; Pfizer) are selective COX-2 inhibitors, thereby minimizing the gastrointestinal adverse effects associated with the COX-1 inhibitors. Rofecoxib (used to treat osteoarthritis), a nonfluorinated selective COX-2 inhibitor, was withdrawn from the market due to the serious drug-related adverse effects, including heart attack and stroke. Pfizer has withdrawn valdecoxib from the market due to the drug-induced heart attack and stroke risks. Celecoxib inhibits autoimmune encephalomyelitis in COX-2-deficient mice, thus demonstrating that it can act also exhibit COX-2-independent pathways.153 Roflumilast, an NSAID, is approved by FDA and EMA for the treatment of inflammatory diseases, such as chronic obstructive pulmonary disease (COPD) and chronic bronchitis (Fig. 574).154 200 Organofluorine Chemistry O NH 2 S O N F3 C F F Me OH N Cl O F N O H N Cl O O Me Celecoxib Flurbiprofen Roflumilast FIGURE 5–74 Structures of fluorinated NSAIDs—celecoxib, flurbiprofen, and roflumilast; NSAIDs, Nonsteroidal antiinflammatory drugs. 5.10.2 Celecoxib Celecoxib (Celebrex; Pfizer) is an NSAID, prescribed for the treatment of osteoarthritis and rheumatoid arthritis. It is widely prescribed and is now available in the generic form after the original patent expiration. It is the 120th best selling drug as of 2016.4 Celecoxib is a selective cyclooxygenase-2 (COX-2) inhibitor and thereby interferes with the COX-2mediated biosynthesis of arachidonic acid, the precursor compound for the biosynthesis of prostaglandins, which are responsible for inflammation at the sites of inflammation. As described earlier, COX-1 is expressed in almost every tissue, whereas COX-2 is expressed at the sites of inflammation. Thus, COX-2-selective inhibitors attenuate the inflammation at the specific inflammation sites. The nonfluorinated COX-2-selective inhibitors rofecoxib (Vioxx; Merck & Co.) and valdecoxib (Bextra; Pfizer), on the other hand, proved to be too hazardous to use and are withdrawn, so that celecoxib is the only marketed COX-2 selective inhibitor for treating rheumatoid arthritis and osteoarthritis. An X-ray crystal structure of celecoxib at the active site of the S121P mutant (PDB 5JW1) reveals predominant hydrophobic interactions of the CF3 moiety with the side chain CH bonds (Fig. 575).155 5.10.3 Corticosteroids Fluorinated steroidal antiinflammatory agents are widely prescribed for the treatment of inflammatory skin diseases and as inhalation drugs against asthma. Some of these fluorinated steroidal antiinflammatory agents are antagonists for estrogen or the progesterone receptors and thereby are used as anticancer agents. For example, fulvestrant, an ER antagonist, is used as a second-line therapy for advanced breast cancers. Dexamethasone, a fluorinated steroidal antiinflammatory agent is used as an antiemetic as well as an anticancer agent for the treatment of multiple myeloma.156,157 As topical antiinflammatory agents, these drugs are often used interchangeably for various conditions, such as skin rashes, skin allergies, and dermatitis. Some of the corticosteroids, such as fluticasone, are used as nasal sprays for the treatment of asthma and COPD and allergic rhinitis.158,159 Chapter 5 • Pharmaceutical applications of organofluorine compounds 201 O NH 2 S O F3C N N Celecoxib Me FIGURE 5–75 Expanded view of the X-ray structure of celecoxib (ball and stick), bound to the S121P COX-2 mutant; hydrophobic interactions of the CF3 moiety with the side chain CH bonds (3.43.5 Å) are shown as dotted lines; the structure was created using UCSF Chimera software, PDB 5JW1. Many of the corticosteroidal drugs are fluorine-containing compounds, and are used in treating various inflammatory conditions. The therapeutic effects of various fluorine-containing corticosteroidal drugs are as follows: flunisolide (AeroBid) is a corticosteroid, used for the treatment of allergic rhinitis through its effect on the activation of glucocorticoid receptors. Amcinonide (Cyclocort) is a topical glucocorticoid for the treatment of atopic dermatitis and allergic contact dermatitis. Fluorometholone is a glucocorticoid used for the ophthalmic treatment of eye inflammation and various skin disorders. Desoximetasone (Topisolone) is a topical corticosteroid used for the treatment of skin allergies, such as rashes and itching. Triamcinolone (Kenalog) is a synthetic glucocorticoid, used for the treatment of various allergies, arthritis, asthma, and COPD. Fluticasone propionate (Flonase), a glucocorticoid, is used as a topical antiinflammatory agent and for the treatment of asthma and COPD. Fluticasone furoate, as a combination drug with vilanterol (a β-agonist), is used in chronic bronchitis and emphysema. Fluticasone furoate as well as fluticasone propionate are also effective against allergic rhinitis.158,159 As of 2016, fluticasone propionate is the 16th most prescribed drug (Fig. 576).4 The synthesis of these corticosteroids, in general, involves Selectfluor-mediated fluorination of the dienol acetate (e.g., 75) to install the fluorine at C6 and ring-opening hydrofluorination of the C9C11 epoxide for installing the fluorine at C9 as the key steps (Fig. 577) (see Chapter 2: Electrophilic reactions in the synthesis of organofluorine compounds).160 The terminal fluoromethyl group in fluticasone is introduced by Selectfluor/Ag(I)-mediated 202 Organofluorine Chemistry Me O O Me HO Me 9 F Me HO Me O O H O H F H F Flunisolide H O Me O 5 Fluorometholone Amcinonide OH O O HO HO Me Me F Me HO O HO Me OH F H O O OH H F O O HO O O H O HO O HO S F Me H O O F Desoximetasone Triamcinolone Fluticasone propionate O HO Me F O O O Me O S F Me HO H Me H HO OH Me F H O O F Fluticasone furoate Dexamethasone FIGURE 5–76 Structures of widely used steroidal antiinflammatory pharmaceuticals. decarboxylative fluorination (see Chapter 4: Organotransition metal catalysis in the synthesis of organofluorine compounds).161 5.11 Antidepressants A vast majority of the antidepressant pharmaceuticals are selective serotonin reuptake inhibitors (SSRIs). The widely prescribed SSRI-based antidepressants, including fluvoxamine, paroxetine, citalopram, escitalopram (chirally pure, S-isomer of citalopram), and fluoxetine are fluorinated compounds (Fig. 578). The SSRIs delay the serotonin reuptake so that the O O O Me O HO 11 OH H Me O Me Me 1. Selectfluor H AcO O OH H 9 F 2. HF/H2O OH H 6 F 75 76 O F HO Me OH O Me S O O Me H F O Me HO Me Selectfluor, AgNO3 Acetone/H2O, 45 °C H –CO2 O F S O O H Me H O F F Fluticasone propionate 92.7% 77 FIGURE 5–77 Synthesis of corticosteroids. CF3 F O HN H3 C O O O N N O O F NH 2 Fluvoxamine N Citalopram (racemic) Paroxetine F N (S) O F3 C O N H N Escitalopram ((S)-Citalopram) Fluoxetine FIGURE 5–78 Structure of SSRI antidepressant drugs—fluvoxamine, paroxetine, citalopram, escitalopram, and fluoxetine; SSRI, Selective serotonin reuptake inhibitor. 204 Organofluorine Chemistry inhibitory neutotransmitter serotonin persists longer at synaptic junctions, thereby enhancing the efficacy of the neurotransmission, with concomitant decrease in anxiety disorders in patients. SSRIs are among the most prescribed drugs, with citalopram being the 21st most prescribed drug and fluoxetine, the 29th, as of 2016.4 Fluvoxamine (Luvox), now a generically available drug, is used for the treatment of anxiety disorders.162 It is an SSRI inhibitor and has a similar efficacy as that of paroxetine and citalopram. Fluoxetine (Prozac; Eli Lilly), also a generically available antidepressant drug, is listed among the United Nations’ essential drugs. In 2016 it was the 29th most prescribed drug.4 References 1. Gillis, E. P.; Eastman, K. J.; Hill, M. D.; Donnelly, D. J.; Meanwell, N. A. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015, 58, 83158359. 2. Meanwell, N. A. Fluorine and Fluorinated Motifs in the Design and Application of Bioisosteres for Drug Design. J. Med. Chem. 2018, 61, 58225880. 3. Haranahalli, K.; Honda, T.; Ojima, I. Recent Progress in the Strategic Incorporation of Fluorine Into Medicinally Active Compounds. J. Fluorine Chem. 2019, 217, 2940. 4. The Top 300 of 2019, Provided by the ClinCalc DrugStats; Web content as of: 10/25/2019; ,https://clincalc.com/DrugStats/Top300Drugs.aspx.. 5. Istvan, E. S.; Deisenhofer, J. Structural Mechanism for Statin Inhibition of HMG-CoA Reductase. Science (Washington, DC) 2001, 292, 11601164. 6. Paulini, R.; Mueller, K.; Diederich, F. Orthogonal Multipolar Interactions in Structural Chemistry and Biology. Angew. Chem., Int. Ed. 2005, 44, 17881805. 7. Persch, E.; Dumele, O.; Diederich, F. Molecular Recognition in Chemical and Biological Systems. Angew. Chem., Int. Ed. 2015, 54, 32903327. 8. Xing, L.; Keefer, C.; Brown, M. F. Fluorine Multipolar Interaction: Toward Elucidating Its Energetics in Binding Recognition. J. Fluorine Chem. 2017, 198, 4753. 9. McDonald, I. M.; Mate, R. A.; Zusi, F. C.; Huang, H.; Post-Munson, D. J.; Ferrante, M. A.; Gallagher, L.; Bertekap, R. L.; Knox, R. J.; Robertson, B. J.; Harden, D. G.; Morgan, D. G.; Lodge, N. J.; Dworetzky, S. I.; Olson, R. E.; Macor, J. E. Discovery of a Novel Series of Quinolone α7 Nicotinic Acetylcholine Receptor Agonists. Bioorg. Med. Chem. Lett. 2013, 23, 16841688. 10. Rankovic, Z. CNS Drug Design: Balancing Physicochemical Properties for Optimal Brain Exposure. J. Med. Chem. 2015, 58, 25842608. 11. Do, H. T.; Li, H.; Chreifi, G.; Poulos, T. L.; Silverman, R. B. Optimization of Blood-Brain Barrier Permeability with Potent and Selective Human Neuronal Nitric Oxide Synthase Inhibitors Having a 2Aminopyridine Scaffold. J. Med. Chem. 2019, 62, 26902707. 12. Drucker, D. J.; Nauck, M. A. The Incretin System: Glucagon-Like Peptide-1 Receptor Agonists and Dipeptidyl Peptidase-4 Inhibitors in Type 2 Diabetes. Lancet 2006, 368, 16961705. 13. Kim, D.; Kowalchick, J. E.; Edmondson, S. D.; Mastracchio, A.; Xu, J.; Eiermann, G. J.; Leiting, B.; Wu, J. K.; Pryor, K. D.; Patel, R. A.; He, H.; Lyons, K. A.; Thornberry, N. A.; Weber, A. E. TriazolopiperazineAmides as Dipeptidyl Peptidase IV Inhibitors: Close Analogs of JANUVIA (Sitagliptin Phosphate). Bioorg. Med. Chem. Lett. 2007, 17, 33733377. 14. Kuk, K.; Taylor-Cousar, J. L. Lumacaftor and Ivacaftor in the Management of Patients with Cystic Fibrosis: Current Evidence and Future Prospects. Ther. Adv. Respir. Dis. 2015, 9, 313326. Chapter 5 • Pharmaceutical applications of organofluorine compounds 205 15. Fiore, M.; Cossu, C.; Capurro, V.; Picco, C.; Ludovico, A.; Mielczarek, M.; Carreira-Barral, I.; Caci, E.; Baroni, D.; Quesada, R.; Moran, O. Small Molecule-Facilitated Anion Transporters in Cells for a Novel Therapeutic Approach to Cystic Fibrosis. Br. J. Pharmacol. 2019, 176, 17641779. 16. Wang, X. R.; Li, C. Decoding F508del Misfolding in Cystic Fibrosis. Biomolecules 2014, 4, 498509. 17. Schneider, E. K. Cytochrome P450 3A4 Induction: Lumacaftor Versus Ivacaftor Potentially Resulting in Significantly Reduced Plasma Concentration of Ivacaftor. Drug Metab. Lett. 2018, 12, 7174. 18. Heo, Y.-A. Baloxavir: First Global Approval. Drugs 2018, 78, 693697. 19. Bussel, J.; Arnold, D. M.; Grossbard, E.; Mayer, J.; Trelinski, J.; Homenda, W.; Hellmann, A.; Windyga, J.; Sivcheva, L.; Khalafallah, A. A.; Zaja, F.; Cooper, N.; Markovtsov, V.; Zayed, H.; Duliege, A.-M. Fostamatinib for the Treatment of Adult Persistent and Chronic Immune Thrombocytopenia: Results of Two Phase 3, Randomized, Placebo-Controlled Trials. Am. J. Hematol. 2018, 93, 921930. 20. Ishichi, Y.; Ikeura, Y.; Natsugari, H. Amide-Based Atropisomers in Tachykinin NK1-Receptor Antagonists: Synthesis and Antagonistic Activity of Axially Chiral N-Benzylcarboxamide Derivatives of 2,3,4,5Tetrahydro-6H-pyrido[2,3-b][1,5]oxazocin-6-one. Tetrahedron 2004, 60, 44814490. 21. Aapro, M.; Zhang, L.; Yennu, S.; LeBlanc, T. W.; Schwartzberg, L. Preventing Chemotherapy-Induced Nausea and Vomiting with Netupitant/Palonosetron, the First Fixed Combination Antiemetic: Current and Future Perspective. Future Oncol. 2019, 15, 10671084. 22. Celio, L.; Fabbroni, C. Pro-Netupitant/Palonosetron (IV) for the Treatment of Radio-and-ChemotherapyInduced Nausea and Vomiting. Expert Opin. Pharmacother. 2018, 19, 12671277. 23. Wright, P. M.; Seiple, I. B.; Myers, A. G. The Evolving Role of Chemical Synthesis in Antibacterial Drug Discovery. Angew. Chem., Int. Ed. 2014, 53, 88408869. 24. Zhu, S.-Q.; Xu, X.-H.; Qing, F.-L. Oxidative Trifluoromethylthiolation of Terminal Alkynes with AgSCF3. A Convenient Approach to Alkynyl Trifluoromethyl Sulfides. Eur. J. Org. Chem. 2014, 2014, 44534456. 25. FDA Drug Safety Communication; FDA Warns About Increased Risk of Ruptures or Tears in the Aorta Blood Vessel with Fluoroquinolone Antibiotics in Certain Patients; content current as of: 12/21/2018; ,https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-increased-risk-ruptures-ortears-aorta-blood-vessel-fluoroquinolone-antibiotics.. 26. FDA News Release; FDA Updates Warnings for Fluoroquinolone Antibiotics; content current as of: 8/15/ 2016; ,https://www.fda.gov/news-events/press-announcements/fda-updates-warnings-fluoroquinoloneantibiotics.. 27. FDA Drug Safety Communication: FDA Advises Restricting Fluoroquinolone Antibiotic Use for Certain Uncomplicated Infections; Warns About Disabling Side Effects That Can Occur Together; content current as of: 09/25/2018; ,https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-advises-restricting-fluoroquinolone-antibiotic-use-certain.. 28. Aldred, K. J.; Kerns, R. J.; Osheroff, N. Mechanism of Quinolone Action and Resistance. Biochemistry 2014, 53, 15651574. 29. Voulgaris, G. L.; Voulgari, M. L.; Falagas, M. E. Developments on Antibiotics for Multidrug Resistant Bacterial Gram-Negative Infections. Expert Rev. Anti-Infect. Ther. 2019, 17, 387401. 30. Bassetti, M.; Righi, E.; Pecori, D.; Tillotson, G. Delafloxacin: An Improved Fluoroquinolone Developed Through Advanced Molecular Engineering. Future Microbiol. 2018, 13, 10811094. 31. Wohlkonig, A.; Chan, P. F.; Fosberry, A. P.; Homes, P.; Huang, J.; Kranz, M.; Leydon, V. R.; Miles, T. J.; Pearson, N. D.; Perera, R. L.; Shillings, A. J.; Gwynn, M. N.; Bax, B. D. Structural Basis of Quinolone Inhibition of Type IIA Topoisomerases and Target-Mediated Resistance. Nat. Struct. Mol. Biol. 2010, 17, 11521153. 32. Scott, L. J. Eravacycline: A Review in Complicated Intra-Abdominal Infections. Drugs 2019, 79, 315324. 33. Xiao, X.-Y.; Hunt, D. K.; Zhou, J.; Clark, R. B.; Dunwoody, N.; Fyfe, C.; Grossman, T. H.; O’Brien, W. J.; Plamondon, L.; Ronn, M.; Sun, C.; Zhang, W.-Y.; Sutcliffe, J. A. Fluorocyclines. 1. 7-Fluoro-9- 206 Organofluorine Chemistry Pyrrolidinoacetamido-6-Demethyl-6-Deoxytetracycline: A Potent, Broad Spectrum Antibacterial Agent. J. Med. Chem. 2012, 55, 597605. 34. Hagmann, W. K. The Many Roles for Fluorine in Medicinal Chemistry. J. Med. Chem. 2008, 51, 43594369. 35. Kim, D.; Wang, L.; Beconi, M.; Eiermann, G. J.; Fisher, M. H.; He, H.; Hickey, G. J.; Kowalchick, J. E.; Leiting, B.; Lyons, K.; Marsilio, F.; McCann, M. E.; Patel, R. A.; Petrov, A.; Scapin, G.; Patel, S. B.; Roy, R. S.; Wu, J. K.; Wyvratt, M. J.; Zhang, B. B.; Zhu, L.; Thornberry, N. A.; Weber, A. E. (2R)-4-Oxo-4-[3(Trifluoromethyl)-5,6-Dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]-1-(2,4,5-Trifluorophenyl)butan-2amine: A Potent, Orally Active Dipeptidyl Peptidase IV Inhibitor for the Treatment of Type 2 Diabetes. J. Med. Chem. 2005, 48, 141151. 36. Edmondson, S. D.; Mastracchio, A.; Mathvink, R. J.; He, J.; Harper, B.; Park, Y.-J.; Beconi, M.; Di Salvo, J.; Eiermann, G. J.; He, H.; Leiting, B.; Leone, J. F.; Levorse, D. A.; Lyons, K.; Patel, R. A.; Patel, S. B.; Petrov, A.; Scapin, G.; Shang, J.; Roy, R. S.; Smith, A.; Wu, J. K.; Xu, S.; Zhu, B.; Thornberry, N. A.; Weber, A. E. (2S,3S)-3-Amino-4-(3,3-difluoropyrrolidin-1-yl)-N,N-dimethyl-4-Oxo-2-(4-[1,2,4]triazolo[1,5-a]-Pyridin-6ylphenyl)butanamide: A Selective A-Amino Amide Dipeptidyl Peptidase IV Inhibitor for the Treatment of Type 2 Diabetes. J. Med. Chem. 2006, 49, 36143627. 37. Hansen, K. B.; Hsiao, Y.; Xu, F.; Rivera, N.; Clausen, A.; Kubryk, M.; Krska, S.; Rosner, T.; Simmons, B.; Balsells, J.; Ikemoto, N.; Sun, Y.; Spindler, F.; Malan, C.; Grabowski, E. J. J.; Armstrong, J. D. Highly Efficient Asymmetric Synthesis of Sitagliptin. J. Am. Chem. Soc. 2009, 131, 87988804. 38. Biftu, T.; Scapin, G.; Singh, S.; Feng, D.; Becker, J. W.; Eiermann, G.; He, H.; Lyons, K.; Patel, S.; Petrov, A.; Sinha-Roy, R.; Zhang, B.; Wu, J.; Zhang, X.; Doss, G. A.; Thornberry, N. A.; Weber, A. E. Rational Design of a Novel, Potent, and Orally Bioavailable Cyclohexylamine DPP-4 Inhibitor by Application of Molecular Modeling and X-Ray Crystallography of Sitagliptin. Bioorg. Med. Chem. Lett. 2007, 17, 33843387. 39. Mattei, P.; Boehringer, M.; Di Giorgio, P.; Fischer, H.; Hennig, M.; Huwyler, J.; Kocer, B.; Kuhn, B.; Loeffler, B. M.; MacDonald, A.; Narquizian, R.; Rauber, E.; Sebokova, E.; Sprecher, U. Discovery of Carmegliptin: A Potent and Long-Acting Dipeptidyl Peptidase IV Inhibitor for the Treatment of Type 2 Diabetes. Bioorg. Med. Chem. Lett. 2010, 20, 11091113. 40. Kuhn, B.; Guba, W.; Hert, J.; Banner, D.; Bissantz, C.; Ceccarelli, S.; Haap, W.; Korner, M.; Kuglstatter, A.; Lerner, C.; Mattei, P.; Neidhart, W.; Pinard, E.; Rudolph, M. G.; Schulz-Gasch, T.; Woltering, T.; Stahl, M. A Real-World Perspective on Molecular Design. J. Med. Chem. 2016, 59, 40874102. 41. Matsutani, D.; Sakamoto, M.; Kayama, Y.; Takeda, N.; Horiuchi, R.; Utsunomiya, K. Effect of Canagliflozin on Left Ventricular Diastolic Function in Patients with Type 2 Diabetes. Cardiovasc. Diabetol. 2018, 17, 73. 42. Nomura, S.; Sakamaki, S.; Hongu, M.; Kawanishi, E.; Koga, Y.; Sakamoto, T.; Yamamoto, Y.; Ueta, K.; Kimata, H.; Nakayama, K.; Tsuda-Tsukimoto, M. Discovery of Canagliflozin, A Novel C-Glucoside with Thiophene Ring, as Sodium-Dependent Glucose Co-Transporter 2 Inhibitor for the Treatment of Type 2 Diabetes Mellitus. J. Med. Chem. 2010, 53, 63556360. 43. Donnan, K.; Segar, L. SGLT2 Inhibitors and Metformin: Dual Antihyperglycemic Therapy and the Risk of Metabolic Acidosis in Type 2 Diabetes. Eur. J. Pharmacol. 2019, 846, 2329. 44. Bonner, C.; Kerr-Conte, J.; Gmyr, V.; Queniat, G.; Moerman, E.; Thevenet, J.; Beaucamps, C.; Delalleau, N.; Popescu, I.; Malaisse, W. J.; Sener, A.; Deprez, B.; Abderrahmani, A.; Staels, B.; Pattou, F. Inhibition of the Glucose Transporter SGLT2 with Dapagliflozin in Pancreatic Alpha Cells Triggers Glucagon Secretion. Nat. Med. (NY) 2015, 21, 512517. 45. McDade, E.; Bateman, R. J. Stop Alzheimer’s Before It Starts. Nature (London) 2017, 547, 153155. 46. Edwards, F. A. A Unifying Hypothesis for Alzheimer’s Disease: From Plaques to Neurodegeneration. Trends Neurosci. 2019, 42, 310322. 47. Lindsley, C. W.; Hooker, J. M. Beyond the Amyloid Hypothesis of Alzheimer’s Disease: Tau Pathology Takes Center Stage. ACS Chem. Neurosci 2018, 9, 2519. Chapter 5 • Pharmaceutical applications of organofluorine compounds 207 48. Kozlov, S.; Afonin, A.; Evsyukov, I.; Bondarenko, A. Alzheimer’s Disease: As It Was in the Beginning. Rev. Neurosci. (Berlin) 2017, 28, 825843. 49. Kim, J.; Onstead, L.; Randle, S.; Price, R.; Smithson, L.; Zwizinski, C.; Dickson, D. W.; Golde, T.; McGowan, E. Aβ40 Inhibits Amyloid Deposition In Vivo. J. Neurosci. 2007, 27, 627633. 50. Dobrowolska Zakaria, J. A.; Vassar, R. J. A Promising, Novel, and Unique BACE1 Inhibitor Emerges in the Quest to Prevent Alzheimer’s Disease. EMBO Mol. Med. 2018, 10, e9717. 51. Neumann, U.; Ufer, M.; Jacobson, L. H.; Rouzade-Dominguez, M.-L.; Huledal, G.; Kolly, C.; Lueoend, R. M.; Machauer, R.; Veenstra, S. J.; Hurth, K.; Rueeger, H.; Tintelnot-Blomley, M.; Staufenbiel, M.; Shimshek, D. R.; Perrot, L.; Frieauff, W.; Dubost, V.; Schiller, H.; Vogg, B.; Beltz, K.; Avrameas, A.; Kretz, S.; Pezous, N.; Rondeau, J.-M.; Beckmann, N.; Hartmann, A.; Vormfelde, S.; David, O. J.; Galli, B.; Ramos, R.; Graf, A.; Lopez Lopez, C. The BACE-1 Inhibitor CNP520 for Prevention Trials in Alzheimer’s Disease. EMBO Mol. Med. 2018, 10, e9316. 52. Scott, J. D.; Li, S. W.; Brunskill, A. P. J.; Chen, X.; Cox, K.; Cumming, J. N.; Forman, M.; Gilbert, E. J.; Hodgson, R. A.; Hyde, L. A.; Jiang, Q.; Iserloh, U.; Kazakevich, I.; Kuvelkar, R.; Mei, H.; Meredith, J.; Misiaszek, J.; Orth, P.; Rossiter, L. M.; Slater, M.; Stone, J.; Strickland, C. O.; Voigt, J. H.; Wang, G.; Wang, H.; Wu, Y.; Greenlee, W. J.; Parker, E. M.; Kennedy, M. E.; Stamford, A. W. Discovery of the 3-Imino1,2,4-Thiadiazinane 1,1-Dioxide Derivative Verubecestat (MK-8931)-A β-Site Amyloid Precursor Protein Cleaving Enzyme 1 Inhibitor for the Treatment of Alzheimer’s Disease. J. Med. Chem. 2016, 59, 1043510450. 53. Egan, M. F.; Kost, J.; Tariot, P. N.; Aisen, P. S.; Cummings, J. L.; Vellas, B.; Sur, C.; Mukai, Y.; Voss, T.; Furtek, C.; Mahoney, E.; Mozley, L. H.; Vandenberghe, R.; Mo, Y.; Michelson, D. Randomized Trial of Verubecestat for Mild-to-Moderate Alzheimer’s Disease. N. Engl. J. Med. 2018, 378, 16911703. 54. Blume, T.; Filser, S.; Jaworska, A.; Moschke, K.; Lichtenthaler, S. F.; Herms, J.; Blain, J.-F.; Koenig, G.; Moschke, K.; Lichtenthaler, S. F.; Lichtenthaler, S. F.; Herms, J.; Herms, J. BACE1 Inhibitor MK-8931 Alters Formation but Not Stability of Dendritic Spines. Front Aging Neurosci 2018, 10, 229. 55. Bursavich, M. G.; Harrison, B. A.; Blain, J.-F. Gamma Secretase Modulators: New Alzheimer’s Drugs on the Horizon? J. Med. Chem. 2016, 59, 73897409. 56. Doody, R. S.; Raman, R.; Farlow, M.; Iwatsubo, T.; Vellas, B.; Joffe, S.; Kieburtz, K.; He, F.; Sun, X.; Thomas, R. G.; Aisen, P. S.; Siemers, E.; Sethuraman, G.; Mohs, R. A Phase 3 Trial of Semagacestat for Treatment of Alzheimer’s Disease. N. Engl. J. Med. 2013, 369, 341350. 57. Coric, V.; Pilcher, G.; Colby, S.; Kerselaers, W.; Dockens, R.; Soares, H.; Kaplita, S.; Luo, F.; Cedarbaum, J. M.; Albright, C.; Berman, R. M.; Salloway, S.; van, D. C. H.; Dubois, B.; Andreasen, N.; Brody, M.; Curtis, C.; Soininen, H.; Thein, S.; Shiovitz, T.; Ferris, S.; Pachai, C.; Bracoud, L.; Mintun, M.; Grill, J. D.; Marek, K.; Seibyl, J.; Feldman, H. H. Targeting Prodromal Alzheimer Disease with Avagacestat: A Randomized Clinical Trial. JAMA Neurol 2015, 72, 13241333. 58. Lovering, F.; Bikker, J.; Humblet, C. Escape From Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009, 52, 67526756. 59. Xia, W. γ-Secretase and Its Modulators: Twenty Years and Beyond. Neurosci. Lett. 2019, 701, 162169. 60. Soares, H. D.; Gasior, M.; Toyn, J. H.; Wang, J.-S.; Hong, Q.; Berisha, F.; Furlong, M. T.; Raybon, J.; Lentz, K. A.; Sweeney, F.; Zheng, N.; Akinsanya, B.; Berman, R. M.; Thompson, L. A.; Olson, R. E.; Morrison, J.; Drexler, D. M.; Macor, J. E.; Albright, C. F.; Ahlijanian, M. K.; AbuTarif, M. The γ-Secretase Modulator, bms-932481, Modulates Aβ Peptides in the Plasma and Cerebrospinal Fluid of Healthy Volunteers. J. Pharmacol. Exp. Ther. 2016, 358, 138150. 61. Wagner, S. L.; Rynearson, K. D.; Duddy, S. K.; Zhang, C.; Nguyen, P. D.; Becker, A.; Vo, U.; Masliah, D.; Monte, L.; Klee, J. B.; Echmalian, C. M.; Xia, W.; Quinti, L.; Johnson, G.; Lin, J. H.; Kim, D. Y.; Mobley, W. C.; Rissman, R. A.; Tanzi, R. E. Pharmacological and Toxicological Properties of the Potent Oral Γ-Secretase Modulator BPN-15606. J. Pharmacol. Exp. Ther. 2017, 362, 3144. 208 Organofluorine Chemistry 62. Peng, H.; Talreja, T.; Xin, Z.; Cuervo, J. H.; Kumaravel, G.; Humora, M. J.; Xu, L.; Rohde, E.; Gan, L.; Jung, M.-y; Shackett, M. N.; Chollate, S.; Dunah, A. W.; Snodgrass-Belt, P. A.; Arnold, H. M.; Taveras, A. G.; Rhodes, K. J.; Scannevin, R. H. Discovery of BIIB042, A Potent, Selective, and Orally Bioavailable γ-Secretase Modulator. ACS Med. Chem. Lett 2011, 2, 786791. 63. Crump, C. J.; Johnson, D. S.; Li, Y.-M. Development and Mechanism of γ-Secretase Modulators for Alzheimer’s Disease. Biochemistry 2013, 52, 31973216. 64. Imbimbo, B. P. An Update on the Efficacy of Non-Steroidal Anti-Inflammatory Drugs in Alzheimer’s Disease. Expert Opin. Invest. Drugs 2009, 18, 11471168. 65. Rogers, K.; Felsenstein, K. M.; Hrdlicka, L.; Tu, Z.; Albayya, F.; Lee, W.; Hopp, S.; Miller, M.-J.; Spaulding, D.; Yang, Z.; Hodgdon, H.; Nolan, S.; Wen, M.; Costa, D.; Blain, J.-F.; Freeman, E.; De Strooper, B.; Vulsteke, V.; Scrocchi, L.; Zetterberg, H.; Portelius, E.; Hutter-Paier, B.; Havas, D.; Ahlijanian, M.; Flood, D.; Leventhal, L.; Shapiro, G.; Patzke, H.; Chesworth, R.; Koenig, G. Modulation of γ-Secretase by EVP0015962 Reduces Amyloid Deposition and Behavioral Deficits in Tg2576 Mice. Mol. Neurodegener. 2012, 7, 61. 66. Shafran, S.; Di Perri, G.; Esser, S.; Lelievre, J. D.; Parczewski, M. Planning HIV Therapy to Prevent Future Comorbidities: Patient Years for Tenofovir Alafenamide. HIV Med. 2019, 20, 116. 67. Bednasz, C. J.; Venuto, C. S.; Ma, Q.; Daa, E. S.; Sax, P. E.; Fischl, M. A.; Collier, A. C.; Smith, K. Y.; Tierney, C.; Acosta, E. P.; Mager, D. E.; Morse, G. D. Race/Ethnicity and Protease Inhibitor Use Influence Plasma Tenofovir Exposure in Adults Living with HIV-1 in AIDS Clinical Trials Group Study A5202. Antimicrob. Agents Chemother. 2019, 63, e0163801618. 68. Mandal, S.; Prathipati, P. K.; Belshan, M.; Destache, C. J. A Potential Long-Acting Bictegravir Loaded Nano-Drug Delivery System for HIV-1 Infection: A Proof-of-Concept Study. Antiviral Res. 2019, 167, 8388. 69. WHO Model List of Essential Medicines, 18th list; content current as of 10/25/2019; ,https://www.who. int/medicines/publications/essentialmedicines/18th_EML.pdf.. 70. Tsiang, M.; Jones, G. S.; Goldsmith, J.; Mulato, A.; Hansen, D.; Kan, E.; Tsai, L.; Bam, R. A.; Stepan, G.; Stray, K. M.; Niedziela-Majka, A.; Yant, S. R.; Yu, H.; Kukolj, G.; Cihlar, T.; Lazerwith, S. E.; White, K. L.; Jin, H. Antiviral Activity of Bictegravir (GS-9883), A Novel Potent HIV-1 Integrase Strand Transfer Inhibitor with an Improved Resistance Profile. Antimicrob. Agents Chemother. 2016, 60, 70867097. 71. Smith, S. J.; Zhao, X. Z.; Burke, T. R., Jr; Hughes, S. H. Efficacies of Cabotegravir and Bictegravir Against Drug-Resistant HIV-1 Integrase Mutants. Retrovirology 2018, 15, 37. 72. Jacobson, J. M.; Flexner, C. W. Universal Antiretroviral Regimens: Thinking Beyond One-Pill-Once-a-Day. Curr. Opin. HIV AIDS 2017, 12, 343350. 73. Elliot, E.; Chirwa, M.; Boffito, M. How Recent Findings on the Pharmacokinetics and Pharmacodynamics of Integrase Inhibitors Can Inform Clinical Use. Curr. Opin. Infect. Dis. 2017, 30, 5873. 74. Colombier, M.-A.; Molina, J.-M. Doravirine: A Review. Curr. Opin. HIV AIDS 2018, 13, 308314. 75. Cote, B.; Burch, J. D.; Asante-Appiah, E.; Bayly, C.; Bedard, L.; Blouin, M.; Campeau, L.-C.; Cauchon, E.; Chan, M.; Chefson, A.; Coulombe, N.; Cromlish, W.; Debnath, S.; Deschenes, D.; Dupont-Gaudet, K.; Falgueyret, J.-P.; Forget, R.; Gagne, S.; Gauvreau, D.; Girardin, M.; Guiral, S.; Langlois, E.; Li, C. S.; Nguyen, N.; Papp, R.; Plamondon, S.; Roy, A.; Roy, S.; Seliniotakis, R.; St-Onge, M.; Ouellet, S.; Tawa, P.; Truchon, J.-F.; Vacca, J.; Wrona, M.; Yan, Y.; Ducharme, Y. Discovery of MK-1439, An Orally Bioavailable Non-Nucleoside Reverse Transcriptase Inhibitor Potent Against a Wide Range of Resistant Mutant HIV Viruses. Bioorg. Med. Chem. Lett. 2014, 24, 917922. 76. Burch, J. D.; Sherry, B. D.; Gauthier, D. R., Jr.; Campeau, L.-C. Discovery and Development of Doravirine: An Investigational Next Generation Non-Nucleside Reverse Transcriptase Inhibitor (NNRTI) for the Treatment of HIV. ACS Symp. Ser. 2016, 1239, 175205. 77. Sa, J. M.; Kaslow, S. R.; Krause, M. A.; Melendez-Muniz, V. A.; Salzman, R. E.; Kite, W. A.; Zhang, M.; Moraes Barros, R. R.; Mu, J.; Han, P. K.; Mershon, J. P.; Figan, C. E.; Caleon, R. L.; Rahman, R. S.; Gibson, Chapter 5 • Pharmaceutical applications of organofluorine compounds 209 T. J.; Amaratunga, C.; Nishiguchi, E. P.; Breglio, K. F.; Engels, T. M.; Velmurugan, S.; Ricklefs, S.; Straimer, J. Artemisinin Resistance Phenotypes and K13 Inheritance in a Plasmodium falciparum cross and Aotus Model. Proc. Natl. Acad. Sci. U.S.A. 2018, 115, 1251312518. 78. Fan, Y.-L.; Cheng, X.-W.; Wu, J.-B.; Liu, M.; Zhang, F.-Z.; Xu, Z.; Feng, L.-S. Antiplasmodial and Antimalarial Activities of Quinolone Derivatives: An Overview. Eur. J. Med. Chem. 2018, 146, 114. 79. Beteck, R. M.; Smit, F. J.; Haynes, R. K.; N’Da, D. D. Recent Progress in the Development of Anti-Malarial Quinolones. Malar. J. 2014, 13, 339 310 pp. 80. Taylor, J. G.; Zipfel, S.; Ramey, K.; Vivian, R.; Schrier, A.; Karki, K. K.; Katana, A.; Kato, D.; Kobayashi, T.; Martinez, R.; Sangi, M.; Siegel, D.; Tran, C. V.; Yang, Z.-Y.; Zablocki, J.; Yang, C. Y.; Wang, Y.; Wang, K.; Chan, K.; Barauskas, O.; Cheng, G.; Jin, D.; Schultz, B. E.; Appleby, T.; Villasenor, A. G.; Link, J. O. Discovery of the Pan-Genotypic Hepatitis C Virus NS3/4A Protease Inhibitor Voxilaprevir (GS-9857): A Component of Vosevi. Bioorg. Med. Chem. Lett. 2019, 29, 24282436. 81. Ratner, M. FDA Approves First Single-Dose Antimalarial. Nat. Biotechnol. 2018, 36, 785. 82. Chaurasiya, N. D.; Ganesan, S.; Nanayakkara, N. P. D.; Dias, L. R. S.; Walker, L. A.; Tekwani, B. L. Inhibition of Human Monoamine Oxidase A and B by 5-Phenoxy 8-Aminoquinoline Analogs. Bioorg. Med. Chem. Lett. 2012, 22, 17011704. 83. Dow, G.; Smith, B. The Blood Schizonticidal Activity of Tafenoquine Makes an Essential Contribution to Its Prophylactic Efficacy in Nonimmune Subjects at the Intended Dose (200 mg). Malar. J. 2017, 16, 209. 84. Dziekan, J. M.; Yu, H.; Chen, D.; Dai, L.; Wirjanata, G.; Larsson, A.; Prabhu, N.; Sobota, R. M.; Bozdech, Z.; Nordlund, P. Identifying Purine Nucleoside Phosphorylase as the Target of Quinine Using Cellular Thermal Shift Assay. Sci. Transl. Med. 2019, 11, eaau3174. 85. FDA Approves Dacomitinib for Metastatic Non-small Cell Lung Cancer; content current as of: 12/14/ 2018; ,https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-dacomitinib-metastaticnon-small-cell-lung-cancer-0.. 86. Sepulveda, J. M.; Sanchez-Gomez, P.; Vaz Salgado, M. A.; Gargini, R.; Balana, C. Dacomitinib: An Investigational Drug for the Treatment of Glioblastoma. Expert Opin. Invest. Drugs 2018, 27, 823829. 87. Gajiwala, K. S.; Feng, J.; Ferre, R.; Ryan, K.; Brodsky, O.; Weinrich, S.; Kath, J. C.; Stewart, A. Insights Into the Aberrant Activity of Mutant EGFR Kinase Domain and Drug Recognition. Structure (Oxford) 2013, 21, 209219. 88. Solca, F.; Dahl, G.; Zoephel, A.; Bader, G.; Sanderson, M.; Klein, C.; Kraemer, O.; Himmelsbach, F.; Haaksma, E.; Adolf, G. R. Target Binding Properties and Cellular Activity of Afatinib (BIBW 2992), An Irreversible ErbB Family Blocker. J. Pharmacol. Exp. Ther. 2012, 343, 342350. 89. Solomon, B. J.; Besse, B.; Bauer, T. M.; Felip, E.; Soo, R. A.; Camidge, D. R.; Chiari, R.; Bearz, A.; Lin, C.C.; Gadgeel, S. M.; Riely, G. J.; Tan, E. H.; Seto, T.; James, L. P.; Clancy, J. S.; Abbattista, A.; Martini, J.-F.; Chen, J.; Peltz, G.; Thurm, H.; Ignatius Ou, S.-H.; Shaw, A. T. Lorlatinib in Patients with ALK-Positive Non-Small-Cell Lung Cancer: Results From a Global Phase 2 Study. Lancet Oncol., 19. ; 201816541667. 90. Collier, T. L.; Maresca, K. P.; Normandin, M. D.; Richardson, P.; McCarthy, T. J.; Liang, S. H.; Waterhouse, R. N.; Vasdev, N. Brain Penetration of the ROS1/ALK Inhibitor Lorlatinib Confirmed by PET. Mol. Imaging 2017, 16, 13. 91. Garnock-Jones, K. P. Cobimetinib: First Global Approval. Drugs 2015, 75, 18231830. 92. Karoulia, Z.; Gavathiotis, E.; Poulikakos, P. I. New Perspectives for Targeting RAF Kinase in Human Cancer. Nat. Rev. Cancer 2017, 17, 676691. 93. Ascierto, P. A.; Kirkwood, J. M.; Grob, J.-J.; Simeone, E.; Grimaldi, A. M.; Maio, M.; Palmieri, G.; Testori, A.; Marincola, F. M.; Mozzillo, N. The Role of BRAF V600 Mutation in Melanoma. J. Transl. Med. 2012, 10, 85. 94. de Lartigue, J. Abemaciclib Becomes First CDK Inhibitor to Clinch Single-Agent Approval for Breast Cancer. J. Community Supportive Oncol. 2018, 16, e2e4. 210 Organofluorine Chemistry 95. Corona, S. P.; Generali, D. Abemaciclib: A CDK4/6 Inhibitor for the Treatment of HR 1 /HER2 2 Advanced Breast Cancer. Drug Des., Dev. Ther 2018, 12, 321330. 96. Chen, P.; Lee, N. V.; Hu, W.; Xu, M.; Ferre, R. A.; Lam, H.; Bergqvist, S.; Solowiej, J.; Diehl, W.; He, Y.-A.; Yu, X.; Nagata, A.; Van Arsdale, T.; Murray, B. W. Spectrum and Degree of CDK Drug Interactions Predicts Clinical Performance. Mol. Cancer Ther. 2016, 15, 22732281. 97. Berchuck, A.; Secord, A. A.; Moss, H. A.; Havrilesky, L. J. Maintenance Poly(ADP-Ribose) Polymerase Inhibitor Therapy for Ovarian Cancer: Precision Oncology or One Size Fits All? J. Clin. Oncol. 2017, 35, 39994003. 98. Dal Molin, G. Z.; Westin, S. N.; Coleman, R. L. Rucaparib in Ovarian Cancer: Extending the Use of PARP Inhibitors in the Recurrent Disease. Future Oncol. 2018, 14, 31013110. 99. Nonomiya, Y.; Noguchi, K.; Katayama, K.; Sugimoto, Y. Novel Pharmacological Effects of Poly(ADPRibose) Polymerase Inhibitor Rucaparib on the Lactate Dehydrogenase Pathway. Biochem. Biophys. Res. Commun. 2019, 510, 501507. 100. Thorsell, A.-G.; Ekblad, T.; Karlberg, T.; Loew, M.; Pinto, A. F.; Tresaugues, L.; Moche, M.; Cohen, M. S.; Schuler, H. Structural Basis for Potency and Promiscuity in Poly(ADP-Ribose) Polymerase (PARP) and Tankyrase Inhibitors. J. Med. Chem. 2017, 60, 12621271. 101. Wu, J.; Xiao, S.; Yuan, M.; Li, Q.; Xiao, G.; Wu, W.; Ouyang, Y.; Huang, L.; Yao, C. PARP Inhibitor ReSensitizes Adriamycin Resistant Leukemia Cells Through DNA Damage and Apoptosis. Mol. Med. Rep. 2019, 19, 7584. 102. Jain, P. G.; Patel, B. D. Medicinal Chemistry Approaches of Poly ADP-Ribose Polymerase 1 (PARP1) Inhibitors as Anticancer Agents—A Recent Update. Eur. J. Med. Chem. 2019, 165, 198215. 103. Yamazaki, T.; Taguchi, T.; Ojima, I. Unique Properties of Fluorine and Their Relevance to Medicinal Chemistry and Chemical Biology; John Wiley & Sons Ltd, 2009. In: Fluorine in Medicinal Chemistry and Chemical Biology, I. Ojima, Ed., pp. 346. 104. Pepe, A.; Kuznetsova, L.; Sun, L.; Ojima, I. Fluoro-Taxoid Anticancer Agents; John Wiley & Sons Ltd, 2009. In: Fluorine in Medicinal Chemistry and Chemical Biology, I. Ojima, Ed., pp. 117139. 105. Ojima, I.; Lichtenthal, B.; Lee, S.; Wang, C.; Wang, X. Taxane Anticancer Agents: A Patent Perspective. Expert Opin. Ther. Pat. 2016, 26, 120. 106. Ojima, I.; Das, M. Recent Advances in the Chemistry and Biology of New Generation Taxoids. J. Nat. Prod. 2009, 72, 554565. 107. Ojima, I.; Chen, J.; Sun, L.; Borella, C. P.; Wang, T.; Miller, M. L.; Lin, S.; Geng, X.; Kuznetsova, L.; Qu, C.; Gallager, D.; Zhao, X.; Zanardi, I.; Xia, S.; Horwitz, S. B.; Mallen-St Clair, J.; Guerriero, J. L.; Bar-Sagi, D.; Veith, J. M.; Pera, P.; Bernacki, R. J. Design, Synthesis, and Biological Evaluation of New-Generation Taxoids. J. Med. Chem. 2008, 51, 32033221. 108. Kuznetsova, L. V.; Pepe, A.; Ungureanu, I. M.; Pera, P.; Bernacki, R. J.; Ojima, I. Syntheses and Structure-Activity Relationships of Novel 30 -Difluoromethyl and 30 -Trifluoromethyl-Taxoids. J. Fluorine Chem. 2008, 129, 817828. 109. Kuznetsova, L.; Sun, L.; Chen, J.; Zhao, X.; Seitz, J.; Das, M.; Li, Y.; Veith, J. M.; Pera, P.; Bernacki, R. J.; Xia, S.; Horwitz, S. B.; Ojima, I. Synthesis and Biological Evaluation of Novel 30 -Difluorovinyl Taxoids. J. Fluorine Chem. 2012, 143, 177188. 110. Zheng, X.; Wang, C.; Xing, Y.; Chen, S.; Meng, T.; You, H.; Ojima, I.; Dong, Y. SB-T-121205, A NextGeneration Taxane, Enhances Apoptosis and Inhibits Migration/Invasion in MCF-7/PTX Cells. Int. J. Oncol. 2017, 50, 893902. 111. Seitz, J. D.; Vineberg, J. G.; Wei, L.; Khan, J. F.; Lichtenthal, B.; Lin, C.-F.; Ojima, I. Design, Synthesis and Application of Fluorine-Labeled Taxoids as 19F NMR Probes for the Metabolic Stability Assessment of Tumor-Targeted Drug Delivery Systems. J. Fluorine Chem. 2015, 171, 148161. 112. Wang, R.; Zhu, G.; Mei, L.; Xie, Y.; Ma, H.; Ye, M.; Qing, F.-L.; Tan, W. Automated Modular Synthesis of Aptamer-Drug Conjugates for Targeted Drug Delivery. J. Am. Chem. Soc. 2014, 136, 27312734. Chapter 5 • Pharmaceutical applications of organofluorine compounds 211 113. Kim, M.; Kim, D.-M.; Kim, K.-S.; Jung, W.; Kim, D.-E. Applications of Cancer Cell-Specific Aptamers in Targeted Delivery of Anticancer Therapeutic Agents. Molecules 2018, 23 830/831-830/820. 114. Chen, N; Zhu, Y; Feng, Z; Wang, Q; Qin, S; Quan, K; Huang, J; Liu, J; Yang, X; Wang, K. Selection of Aptamers for Hydrophobic Drug Docetaxel to Improve Its Solubility. ACS Appl. Bio Mater. 2018, 1, 168174. 115. Howell, A.; Osborne, C. K.; Morris, C.; Wakeling, A. E. ICI 182,780 (Faslodex) Development of a Novel, “Pure” Antiestrogen. Cancer (NY) 2000, 89, 817825. 116. Lai, A. C.; Crews, C. M. Induced Protein Degradation: An Emerging Drug Discovery Paradigm. Nat. Rev. Drug Discovery 2017, 16, 101114. 117. Hu, J.; Hu, B.; Wang, M.; Xu, F.; Miao, B.; Yang, C.-Y.; Wang, M.; Liu, Z.; Hayes, D. F.; Chinnaswamy, K.; Delproposto, J.; Stuckey, J.; Wang, S. Discovery of ERD-308 as a Highly Potent Proteolysis Targeting Chimera (PROTAC) Degrader of Estrogen Receptor (ER). J. Med. Chem. 2019, 62, 14201442. 118. Brazier, E. J.; Hogan, P. J.; Leung, C. W.; O’Kearney-McMullan, A.; Norton, A. K.; Powell, L.; Robinson, G. E.; Williams, E. G. Fulvestrant: From the Laboratory to Commercial-Scale Manufacture. Org. Process Res. Dev. 2010, 14, 544552. 119. Yen, K.; Travins, J.; Wang, F.; David, M. D.; Artin, E.; Straley, K.; Padyana, A.; Gross, S.; De La Barre, B.; Tobin, E.; Chen, Y.; Nagaraja, R.; Choe, S.; Jin, L.; Konteatis, Z.; Cianchetta, G.; Saunders, J. O.; Salituro, F. G.; Quivoron, C.; Opolon, P.; Bawa, O.; Saada, V.; Paci, A.; Broutin, S.; Bernard, O. A.; de Botton, S.; Marteyn, B. S.; Pilichowska, M.; Xu, Y. X.; Fang, C.; Jiang, F.; Wei, W.; Jin, S.; Silverman, L.; Liu, W.; Yang, H.; Dang, L.; Dorsch, M.; Penard-Lacronique, V.; Biller, S. A.; Su, S.-S. M. AG-221, A First-in-Class Therapy Targeting Acute Myeloid Leukemia Harboring Oncogenic IDH2 Mutations. Cancer Discovery 2017, 7, 478493. 120. Garcia-Llinas, X.; Bauza, A.; Seth, S. K.; Frontera, A. Importance of RCF3 O Tetrel Bonding Interactions in Biological Systems. J. Phys. Chem. A 2017, 121, 53715376. 121. Zhang, S. Method for Preparing 6-(Aryl or Heteroaryl)-1,3,5-Triazine-2,4-Diol and 6-(Aryl or Heteroaryl)-1,3,5-Triazine-2,4-Diamine. WO2017024134A1; AgiosPharmaceuticals, Inc., 2017. 122. Pagliuca, M.; Buonerba, C.; Fizazi, K.; Di Lorenzo, G. The Evolving Systemic Treatment Landscape for Patients with Advanced Prostate Cancer. Drugs 2019, 79, 381400. 123. Bohl, C. E.; Gao, W.; Miller, D. D.; Bell, C. E.; Dalton, J. T. Structural Basis for Antagonism and Resistance of Bicalutamide in Prostate Cancer. Proc. Natl. Acad. Sci. U.S.A. 2005, 102, 62016206. 124. Jain, R.P.; Angelaud, R.; Thompson, A.; Lamberson, C.; Greenfield, S. Processes for the Synthesis of Diarylthiohydantoin and Diarylhydantoin Compounds. WO2011106570A1; Medivation Prostate Therapeutics, Inc., 2011. 125. Shirley, M. Encorafenib and Binimetinib: First Global Approvals. Drugs 2018, 78, 12771284. 126. Blake, J. F.; Burkard, M.; Chan, J.; Chen, H.; Chou, K.-J.; Diaz, D.; Dudley, D. A.; Gaudino, J. J.; Gould, S. E.; Grina, J.; Hunsaker, T.; Liu, L.; Martinson, M.; Moreno, D.; Mueller, L.; Orr, C.; Pacheco, P.; Qin, A.; Rasor, K.; Ren, L.; Robarge, K.; Shahidi-Latham, S.; Stults, J.; Sullivan, F.; Wang, W.; Yin, J.; Zhou, A.; Belvin, M.; Merchant, M.; Moffat, J.; Schwarz, J. B. Discovery of (S)-1-(1-(4-Chloro-3-Fluorophenyl)-2Hydroxyethyl)-4-(2-((1-Methyl-1H-Pyrazol-5-yl)amino)pyrimidin-4-yl)pyridin-2(1H)-One (GDC-0994), An Extracellular Signal-Regulated Kinase 1/2 (ERK1/2) Inhibitor in Early Clinical Development. J. Med. Chem. 2016, 59, 56505660. 127. Hoy, S. M. Tecovirimat: First Global Approval. Drugs 2018, 78, 13771382. 128. Damon, I. K.; Damaso, C. R.; McFadden, G. Are We There Yet? The Smallpox Research Agenda Using Variola Virus. PLoS Pathog. 2014, 10, e1004108 1004103 pp. 129. Grosenbach, D. W.; Honeychurch, K.; Rose, E. A.; Chinsangaram, J.; Frimm, A.; Maiti, B.; Lovejoy, C.; Meara, I.; Long, P.; Hruby, D. E. Oral Tecovirimat for the Treatment of Smallpox. N. Engl. J. Med. 2018, 379, 4453. 212 Organofluorine Chemistry 130. Dai, D. Process for the Preparation of Tecovirimat. WO2014028545A1; Siga Technologies, Inc., 2014. 131. Zhen, L.; Dai, L.; Wen, X.; Yao, L.; Jin, X.; Yang, X.-W.; Zhao, W.; Yu, S.-Q.; Yuan, H.; Wang, G.; Sun, H. Discovery of Novel Nucleotide Prodrugs with Improved Potency Against HCV Variants Carrying NS5B S282T Mutation. J. Med. Chem. 2017, 60, 60776088. 132. Marciano, S.; Haddad, L.; Reggiardo, M. V.; Peralta, M.; Vistarini, C.; Marino, M.; Descalzi, V. I.; D’Amico, C.; Figueroa Escuti, S.; Gaite, L. A.; Perez Ravier, R.; Longo, C.; Borzi, S. M.; Galdame, O. A.; Bessone, F.; Fainboim, H. A.; Frias, S.; Cartier, M.; Gadano, A. C. Effectiveness and Safety of original and Generic Sofosbuvir for the Treatment of Chronic Hepatitis C: A Real World Study. J. Med. Virol. 2018, 90, 951958. 133. Keating, G. M. Ledipasvir/Sofosbuvir: A Review of Its Use in Chronic Hepatitis C. Drugs 2015, 75, 675685. 134. Link, J. O.; Taylor, J. G.; Xu, L.; Mitchell, M.; Guo, H.; Liu, H.; Kato, D.; Kirschberg, T.; Sun, J.; Squires, N.; Parrish, J.; Kellar, T.; Yang, Z.-Y.; Yang, C.; Matles, M.; Wang, Y.; Wang, K.; Cheng, G.; Tian, Y.; Mogalian, E.; Mondou, E.; Cornpropst, M.; Perry, J.; Desai, M. C. Discovery of Ledipasvir (GS-5885): A Potent, Once-Daily Oral NS5A Inhibitor for the Treatment of Hepatitis C Virus Infection. J. Med. Chem. 2014, 57, 20332046. 135. FDA News Release; FDA Approves Mavyret for Hepatitis C; content current as of: 03/28/2018; ,https:// www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm570038.htm.. 136. Krishnan, P.; Pilot-Matias, T.; Schnell, G.; Tripathi, R.; Ng, T. I.; Reisch, T.; Beyer, J.; Dekhtyar, T.; Irvin, M.; Xie, W.; Larsen, L.; Mensa, F. J.; Collins, C. Pooled Resistance Analysis in Patients with Hepatitis C Virus Genotype 1 to 6 Infection Treated with Glecaprevir-Pibrentasvir in Phase 2 and 3 Clinical Trials. Antimicrob. Agents Chemother. 2018, 62, e01249-18. 137. D’Ambrosio, R.; Pasulo, L.; Puoti, M.; Vinci, M.; Schiavini, M.; Lazzaroni, S.; Soria, A.; Gatti, F.; Menzaghi, B.; Aghemo, A.; Capelli, F.; Rumi, M. G.; Morini, L.; Giorgini, A.; Pigozzi, M. G.; Rossini, A.; Maggiolo, F.; Pan, A.; Memoli, M.; Spinelli, O.; Del Poggio, P.; Saladino, V.; Spinetti, A.; De Bona, A.; Capretti, A.; Uberti-Foppa, C.; Bonfanti, P.; Terreni, N.; Menozzi, F.; Colombo, A. E.; Giglio, O.; Centenaro, R.; Borghi, M.; Baiguera, C.; Picciotto, V.; Landonio, S.; Gori, A.; Magnani, C.; Noventa, F.; Paolucci, S.; Lampertico, P.; Fagiuoli, S. Real-World Effectiveness and Safety of Glecaprevir/Pibrentasvir in 723 Patients with Chronic Hepatitis C. J. Hepatol. 2019, 70, 379387. 138. DeGoey, D. A.; Kati, W. M.; Hutchins, C. W.; Donner, P. L.; Krueger, A. C.; Randolph, J. T.; Motter, C. E.; Nelson, L. T.; Patel, S. V.; Matulenko, M. A.; Keddy, R. G.; Jinkerson, T. K.; Gao, Y.; Liu, D.; Pratt, J. K.; Rockway, T. W.; Maring, C. J.; Hutchinson, D. K.; Flentge, C. A.; Wagner, R.; Tufano, M. D.; Betebenner, D. A.; Sarris, K.; Woller, K. R.; Wagaw, S. H.; Califano, J. C.; Li, W.; Caspi, D. D.; Bellizzi, M. E.; Carroll, W. A. WO2012051361A2012051361 Heterocyclic Peptide Compounds as Antiviral Agents and Their Preparation and Use in the Treatment of Hepatitis C Virus Infection; Abbott Laboratories, 2012. 139. Wagner, R.; Randolph, J. T.; Patel, S. V.; Nelson, L.; Matulenko, M. A.; Keddy, R.; Pratt, J. K.; Liu, D.; Krueger, A. C.; Donner, P. L.; Hutchinson, D. K.; Flentge, C.; Betebenner, D.; Rockway, T.; Maring, C. J.; Ng, T. I.; Krishnan, P.; Pilot-Matias, T.; Collins, C.; Panchal, N.; Reisch, T.; Dekhtyar, T.; Mondal, R.; Stolarik, D. F.; Gao, Y.; Gao, W.; Beno, D. A.; Kati, W. M. Highlights of the Structure-Activity Relationships of Benzimidazole Linked Pyrrolidines Leading to the Discovery of the Hepatitis C Virus NS5A Inhibitor Pibrentasvir (ABT-530). J. Med. Chem. 2018, 61, 40524066. 140. Letermovir, U.S. National Library of Medicine, National Center for Biotechnology Information; PubChem; content current as of 10/25/2019; ,https://pubchem.ncbi.nlm.nih.gov/compound/ Letermovir.. 141. Chung, C. K.; Liu, Z.; Lexa, K. W.; Andreani, T.; Xu, Y.; Ji, Y.; Di Rocco, D. A.; Humphrey, G. R.; Ruck, R. T. Asymmetric Hydrogen Bonding Catalysis for the Synthesis of Dihydroquinazoline-Containing Antiviral, Letermovir. J. Am. Chem. Soc. 2017, 139, 1063710640. 142. Brault, M.; Ray, J.; Gomez, Y.-H.; Mantzoros, C. S.; Daskalopoulou, S. S. Statin Treatment and NewOnset Diabetes: A Review of Proposed Mechanisms. Metab., Clin. Exp. 2014, 63, 735745. Chapter 5 • Pharmaceutical applications of organofluorine compounds 213 143. Kim, D.-W.; Kim, D.-H.; Park, J.-H.; Choi, M.; Kim, S.; Kim, H.; Seul, D.-E.; Park, S.-G.; Jung, J.-H.; Han, K.; Park, Y.-G. Association Between Statin Treatment and New-Onset Diabetes Mellitus: A Population Based Case-Control Study. Diabetol Metab Syndr 2019, 11, 30. 144. Ezetimibe, Drugs.Com; content current as of 10/25/2019; ,https://www.drugs.com/monograph/ezetimibe.html.. 145. Rosenblum, S. B.; Huynh, T.; Afonso, A.; Davis, H. R., Jr; Yumibe, N.; Clader, J. W.; Burnett, D. A. Discovery of 1-(4-fluorophenyl)-(3R)-[3-(4-fluorophenyl)-(3S)-hydroxypropyl]-(4S)-(4-Hydroxyphenyl)2-Azetidinone (SCH 58235): A Designed, Potent, Orally Active Inhibitor of Cholesterol Absorption. J. Med. Chem. 1998, 41, 973980. 146. Wojciechowski, D.; Papademetriou, V. β-Blockers in the Management of Hypertension: Focus on Nebivolol. Expert Rev. Cardiovasc. Ther. 2008, 6, 471479. 147. Chackalamannil, S. Case History: Vorapaxar (Zontivity): A First-in-Class Protease-Activated Receptor-1 (PAR-1) Antagonist Antiplatelet Agent. Med. Chem. Rev. 2016, 51, 397418. 148. Moon, J. Y.; Franchi, F.; Rollini, F.; Angiolillo, D. J. Role for Thrombin Receptor Antagonism with Vorapaxar in Secondary Prevention of Atherothrombotic Events: From Bench to Bedside. J. Cardiovasc. Pharmacol. Ther. 2018, 23, 2337. 149. Wallentin, L.; Becker, R. C.; Budaj, A.; Cannon, C. P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; Mahaffey, K. W.; Scirica, B. M.; Skene, A.; Steg, P. G.; Storey, R. F.; Harrington, R. A. Ticagrelor Versus Clopidogrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2009, 361, 10451057. 150. Garnock-Jones, K. P. Riociguat: A Review of Its Use in Patients with Chronic Thromboembolic Pulmonary Hypertension or Pulmonary Arterial Hypertension. Drugs 2014, 74, 20652078. 151. Kim, N. H.; Delcroix, M.; Jais, X.; Madani, M. M.; Matsubara, H.; Mayer, E.; Ogo, T.; Tapson, V. F.; Ghofrani, H.-A.; Jenkins, D. P. Chronic Thromboembolic Pulmonary Hypertension. Eur. Respir. J. 2019, 53, 1801915. 152. Weintraub, W. S. Safety of Non-Steroidal Anti-Inflammatory Drugs. Eur. Heart J. 2017, 38, 32933295. 153. Di Penta, A.; Chiba, A.; Alloza, I.; Wyssenbach, A.; Yamamura, T.; Villoslada, P.; Miyake, S.; Vandenbroeck, K. A Trifluoromethyl Analogue of Celecoxib Exerts Beneficial Effects in Neuroinflammation. PLoS One 2013, 8, e83119. 154. Alexa, I.; Alexa-Stratulat, T.; Antoniu, S.; Antohe, I.; Arghir, O.; Grigorescu, C. Roflumilast in Patients with Advanced Chronic Obstructive Pulmonary Disease: Towards a Better-Targeted Use. Expert Opin. Pharmacother. 2019, 20, 9193. 155. Dong, L.; Yuan, C.; Orlando, B. J.; Malkowski, M. G.; Smith, W. L. Fatty Acid Binding to the Allosteric Subunit of Cyclooxygenase-2 Relieves a Tonic Inhibition of the Catalytic Subunit. J. Biol. Chem. 2016 (291), 2564125655. 156. Jasem, Y. A.; Thiemann, T.; Gano, L.; Oliveira, M. C. Fluorinated Steroids and Their Derivatives. J. Fluorine Chem. 2016, 185, 4885. 157. Harousseau, J.-L.; Attal, M.; Leleu, X.; Troncy, J.; Pegourie, B.; Stoppa, A.-M.; Hulin, C.; Benboubker, L.; Fuzibet, J.-G.; Renaud, M.; Moreau, P.; Avet-Loiseau, H. Bortezomib Plus Dexamethasone as Induction Treatment Prior to Autologous Stem Cell Transplantation in Patients with Newly Diagnosed Multiple Myeloma: Results of an IFM Phase II Study. Haematologica 2006, 91, 14981505. 158. May, J. R.; Dolen, W. K. Evaluation of Intranasal Corticosteroid Sensory Attributes and Patient Preference for Fluticasone Furoate for the Treatment of Allergic Rhinitis. Clin. Ther. 2019, 41, 15891596. 159. Flunisolide, DRUGBANK, content current as of 10/25/2019; ,https://www.drugbank.ca/drugs/ DB00180.. 214 Organofluorine Chemistry 160. Cherniak, S.; Cyjon, R.; Ozer, I.; Nudelman, I. Process for the Preparation of 17-Desoxy-Corticosteroids. WO2012011106A1, Israel: Taro Pharmaceutical Industries Ltd., 2012. 161. Zhou, J.; Jin, C.; Su, W. Improved Synthesis of Fluticasone Propionate. Org. Process Res. Dev. 2014, 18, 928933. 162. Figgitt, D. P.; McClellan, K. J. Fluvoxamine: An Updated Review of Its Use in the Management of Adults with Anxiety Disorders. Drugs 2000, 60, 925954. 6 Synthesis and applications of 18 F-labeled compounds Chapter Outline 6.1 Introduction ............................................................................................................................... 216 6.2 Synthetic methods for radiofluorination................................................................................ 220 6.2.1 Synthesis of 18F-labeled reagents................................................................................... 221 6.3 Sharpless click reactions for positron emission tomography tracers................................... 225 6.3.1 Protein and oligonucleotide triazole positron emission tomography tracers ........... 227 6.3.2 18 F-octreotate positron emission tomography tracers for tumor imaging ................ 228 6.3.3 Strain-promoted click chemistry..................................................................................... 229 6.4 Staudinger ligation reactions for positron emission tomography tracers .......................... 232 6.5 Radiofluorination via aromatic nucleophilic substitution ..................................................... 235 6.5.1 [18F]fluoro-(1)-biotin........................................................................................................ 236 6.5.2 L-3,4-Dihydroxy-6-[18F]fluorophenylalanine (6-[18F]L-DOPA) ....................................... 238 6.5.3 γ-Aminobutyric acid transporter positron emission tomography tracers .................. 238 6.5.4 Radiofluorination of phenolic compounds ................................................................... 239 6.6 Transition metalmediated radiofluorination....................................................................... 243 6.6.1 Mn(III)-catalyzed radiofluorinations .............................................................................. 243 6.6.2 Pd-catalyzed radiofluorinations ..................................................................................... 246 6.6.3 Au(III) catalysis for the synthesis of [18F]trifluoromethyl compounds ........................ 246 6.6.4 Ni(II)-catalyzed radiofluorinations ................................................................................. 247 6.6.5 Cu(I)-catalyzed radiofluorinations.................................................................................. 250 6.7 Radiofluorination via diaryliodonium salts ............................................................................ 250 6.7.1 Cu(I)-catalyzed radiofluorination of diaryliodonium salts........................................... 252 6.7.2 Radiofluorination via iodonium ylides .......................................................................... 253 6.8 Enzymatic fluorination reactions for [18F]-labeled positron emission tomography tracers ......................................................................................................................................... 256 6.8.1 50 -Fluoro-50 -deoxyadenosine and 5-fluororibose.......................................................... 256 6.8.2 Fluorinase-catalyzed synthesis of [18F]50 -deoxy-50 -fluoroadenosine-biotin conjugate ......................................................................................................................... 257 6.8.3 50 -Fluoro-50 -deoxyadenosine-RGD conjugate in cancer detection .............................. 257 6.9 Positron emission tomography tracers in Alzheimer’s disease ............................................ 258 6.9.1 [18F]Flortaucipir (a neurofibrillary tangle biomarker) .................................................. 259 Organofluorine Chemistry. DOI: https://doi.org/10.1016/B978-0-12-813286-9.00006-7 © 2020 Elsevier Inc. All rights reserved. 215 216 Organofluorine Chemistry 6.9.2 2-(4-Aminoaryl)quinoline-based 18F-labeled positron emission tomography tracers (THK series)....................................................................................................................... 261 6.9.3 Tropomyosin receptor kinase targeted 18F-positron emission tomography.............. 263 6.10 18 F-positron emission tomography tracers in cancer diagnosis ........................................... 264 6.10.1 [18F]-(R)-lorlatinib........................................................................................................... 264 6.10.2 Cyclic RGDYK (arginine-glycine-aspartic acid-tyrosine-lysine) dimer-derived positron emission tomography tracers ....................................................................................... 265 References........................................................................................................................................... 271 6.1 Introduction Positron emission tomography (PET) imaging using 18F-PET tracers has emerged as a powerful diagnostic tool for cancers,1 Alzheimer disease (AD) and other neurological disorders,2 and atherosclerotic lesions.3 The preferred use of the 18F-PET tracers over the other conventionally used C-11-based PET tracers is largely due to the relatively long half-life of 18F and the favorable pharmacokinetic properties of the organofluorine compounds. Several 18F-PET tracers are now available either through commercial sources or via in-house synthesis at the site of PET-administration using automated synthetic strategies. 18F isotope has a half-life of 110 min, which is significantly higher than the half-lives of the other pharmaceutically significant PET tracers: 11C (10 min), 13N (10 min), 15O (2 min), 14O (1.2 min), and decays almost exclusively by positron emission (97% β1 emission). Furthermore, 18F isotope has relatively the lowest positron energy of 0.635 MeV (as compared to 0.96, 1.19, and 1.723 MeV, respectively, for 11C, 13N, and 15O) and the shortest tissue penetration range (B2 mm), and thus the radiation toxicity to the patients receiving 18F-PET is minimal to negligible. 68 Ga (t1/2 5 68 min), 82Rb (t1/2 5 1.27 min), and 177Lu (t1/2 5 28.4 min) are among the other radioisotopes that are used as PET tracers. As described earlier, 18F-labeled PET imaging agents offer obvious advantage of their relatively longer half-life times and also the feasibility of transportation of the compounds from the generating facilities to the PET-administering sites. Some of the illustrative FDA-approved PET tracers are shown in Fig. 61. The small molecule PET tracer fluciclovine (Axumin) is FDA approved for imaging of prostate-specific antigen (PSA) levels in prostate cancer. 177Lu DOTA-TATE (Lutathera) and 68Ga DOTA-TATE are FDA-approved PET imaging agents for the somatostatin receptor (SSTR)-positive gastroenteropancreatic neuroendocrine tumors, and second-generation PET tracers of these theranostics (i.e., compounds acting as PET-diagnostic as well as therapeutic agents) with improved pharmacokinetic effects are continuously being developed.4,5 Pittsburgh compound B [(2-(4-[11C]methylamino)phenyl)6-hydroxybenzothiazole (PiB)], a thioflavin T-derived compound,6,7 is the first FDA-approved PET tracer for PET imaging of the amyloid plaques and is widely used. Other more recently FDA-approved 18F-labeled PET tracers for imaging amyloid plaques include florbetaben (Neuraceq),810 flutemetamol (Vizamyl),1012 and florbetapir (Amyvid).13,14 These radiotracers are now in routine use along with 2-[18F]fluoro-2-deoxy-D-glucose ([18F]FDG) and PiB to monitor the extent of Chapter 6 • Synthesis and applications of 18F-labeled compounds 217 amyloid plaque formation in cases of AD as well as other dementias that involve amyloid pathology, such as Parkinson’s disease. [18F]FDG is among the earliest developed 18F-PET tracers and is the first FDA-approved 18 F-labeled compound for PET imaging in the clinical settings. The [18F]FDG is being OH HO O OH HO HO 11 CH3 NH S N 18 F Pittsburgh compound B (PiB) (for PET imagiing of Aβ levels in the brain) 18 [ F]-2-Deoxy-2-fluoro-D-glucose (for monitoring glucose metabolism in cancers and in the brain) 18 F 18 F CH3 HO O Florbetaben (Neuraceq) (for PET imaging of Aβ levels in the brain) H N Flutemetamol (Vizamyl) (for PET imaging of Aβlevels in the brain) CH3 O 18 18 F O CH 3 NH S N O O H N F O O N H Florbetapir ( 18F-AV-45; Amyvid) (for PET imaging of Aβ levels in the brain) OH NH2 Fluciclovine (Axumin) (for PET imaging of prostate-specific antigen (PSA) levels in prostate cancer) OH NH O O O N O (III) M N NH O O O NH2 HN N N H S N O O O O H N N H N H S HN O OH H 3C OH OH O O M = 177Lu: 177 M = 68Ga: 68 Ga DOTA-TATE (for PET imaging of somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumors Lu DOTA-TATE (Lutathera) (for PET imaging of somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumors) FIGURE 6–1 Illustrative examples of the FDA-approved 18F-, 177Lu-, 68Ga-, and 11C-labeled PET tracers. PET, Positron emission tomography. 218 Organofluorine Chemistry widely used for monitoring glucose metabolism, and thereby in the diagnosis of cancers and neurological disorders. Since glucose metabolism is dramatically elevated in cancerous cells, 18F-labeled glucose allows monitoring the tumor progression and tumor metastasis. On the other hand, unusually low levels of its uptake in the brain indicate hypometabolism in the brain neuronal cells and therefore serve as a diagnostic marker in monitoring the extent and progression of AD.15 Cancerous cells have abnormally high glucose metabolism and thus [8F] FDG, in combination with computed tomography (CT) scans, would reveal the anatomical structure along with glucose regional metabolism during a single combined scan. Thus, the [8F]FDG PETCT scans would serve as diagnostic criterion for detecting cancers as well as for monitoring various other pathophysiological processes.16,17 Due to its ready availability through automated one-step synthesis, and due to its superior pharmacokinetic properties, [18F]FDG is widely used as a PET tracer, and also as a building block for other glucosederived PET imaging agents, in the detection of and in the monitoring of the disease progression in cancer, neurological disorders, including AD and cardiovascular diseases. Often, as described above, the PET is combined with CT (PET/CT) to gain metabolic as well as anatomical information at the sites of lesion. Magnetic resonance imaging (MRI) in combination with the PET gives similar and complementary information of the disease progression as that of the PET/CT, as shown in a comparative analysis of these techniques in the diagnosis of the lung cancer (Fig. 62). Moreover, MRI technique does not involve the patients being exposed to the ionizing radiation, unlike that for the CT, and in certain cases, PET/MRI gives additional information. For example, the PET/MRI in lung cancers shows both the main lesion and the lung pleural retraction (Fig. 62F), whereas PET/CT shows only the main lesion (Fig. 62C). However, due to the current technical difficulties in the recording of MRI, often both PET/CT and PET/MRI are recorded for a patient to gain complementary information on the sites of lesion.18 With rapid advances in the MRI, it is hoped that the PET/MRI would completely replace PET/CT. The FDG PET/CT has been conventionally used to monitor the clinical progress of the nonsmall-cell lung cancers (NSCLC). Ganem and coworkers have demonstrated through 18 F-PET/CT monitoring that standardized uptake value (SUVmax) and the tumor volume of the 18F-FDG were substantially lowered upon radiation treatment of the patients with stage IIA left lung adenocarcinoma (a subtype of nonsmall-cell lung carcinoma).19 The SUVmax of 18 F-FDG decreased from 9.6 to 4.2 after radiation therapy, coincident with the decrease in the tumor size. This positive correlation of the SUVmax of the 18F tracer versus the tumor size was also demonstrated in other lung cancers, including adenocarcinoma, squamous cell carcinoma, and small-cell carcinoma.20,21 There is an emerging interest in developing clinically useful, disease-specific PET imaging agents. For example, 6-[18F]fluoro-A-85380 binds to the nicotinic acetylcholine receptors (nAChRs) with very high affinity and was in preclinical trials as a PET tracer for imaging nACHRs.22,23 The bloodbrain barrier penetration is an important criterion for the drug candidate to be used as a PET tracer in the clinical settings. A comparative study using the [11C]befloxatone (a PET tracer for the monoamine oxidase A in the brain), in in vitro as well as in vivo studies, showed that the 6-[18F]fluoro-A-85380 PET tracer effectively penetrates the bloodbrain barrier. Chapter 6 • Synthesis and applications of 18F-labeled compounds 219 FIGURE 6–2 Images for a 58-year-old female with lung cancer confirmed by pathology. 18F-FDG uptake was observed in PET images (A and D); both CT (B) and T2-weighted MRI (T2MRI) (E) showed the main lesion (shown by the upper arrow). However, only T2MRI (E) showed the pleural retraction accompanied by lung cancer (as shown by the lower arrow). PET/CT (C) showed only the main lesion, while PET/MR (F) showed both the main lesion and the pleural retraction. CT, Computed tomography; [18F]FDG, 2-[18F]fluoro-2-deoxy-D-glucose; MRI, magnetic resonance imaging; PET, positron emission tomography. Adapted from Hu, Z.; Yang, W.; Liu, H.; Wang, K.; Bao, C.; Song, T.; Wang, J.; Tian, J. From PET/CT to PET/MRI: Advances in Instrumentation and Clinical Applications. Mol. Pharm. 2014, 11, 37983809, Copyright 2014, American Chemical Society. This comparative PET imaging technique provides an accurate estimate of the rate constant for the passage of the free ligand from the plasma into the brain (k1), and the rate constant for the passage of the free ligand from the brain to the plasma (k2) (Fig. 63).24 Florbetapir (Amyvid Pharmaceutical) is an FDA-approved PET tracer for the detection of the amyloid beta (Aβ) plaques in the brains and is among the widely used PET tracers in the diagnosis of the AD. A florbetapir PET image of one control (i.e., non-AD case) and two AD cases, in conjunction with Aβ antibody 4G8 immunohistochemistry, shows clear distinction between these patients.14 The AD cases are evidenced by relatively higher uptake of the PET tracer, indicating relatively larger areas of Aβ plaques, as also supported by the immunohistochemistry. [18F]FDG imaging is complementary to that of the other PET tracers that target Aβ plaques or neurofibrillary tangles (NFTs), including florbetapir, as it indicates the extent of glucose metabolism, which is inversely related to the levels of the Aβ plaques or NFTs. Among the other selective Aβ-binding PET imaging agents that are in preclinical trials, the [18F]FIBT, an 18F-labeled imidazo[2,1-b]benzothiazole PET tracer, has comparable 220 Organofluorine Chemistry 18 F N HN O [18F]-A85380 FIGURE 6–3 Coregistered PETMRI images representing the k1 obtained in human after intravenous injection of [11C]befloxatone (left) and [18F]fluoro-A-85380 (right). The PET images representing the K1 are obtained as follows. PET image obtained at 1 min postinjection (mean value between 30 and 90 s) is considered as independent of the receptor binding. This image (in Bq/mL) is corrected from the vascular fraction (Fv in Bq/mL, considered as 4% of the total blood concentration at 1 min) and divided by the arterial plasma input function [AUC0-1 min of the plasma concentration, in (Bq min)/mL]. The resulting parametric image, expressed in min21, represents an index of the K1 parameter of the radiotracer. MRI, Magnetic resonance imaging; PET, positron emission tomography. Adapted from Mabondzo, A.; Bottlaender, M.; Guyot, A.-C.; Tsaouin, K.; Deverre, J.R.; Balimane, P.V. Validation of In Vitro Cell-Based Human Blood-Brain Barrier Model Using Clinical Positron Emission Tomography Radioligands to Predict In Vivo Human Brain Penetration. Mol. Pharm. 2010, 7, 18051815, Copyright 2010, American Chemical Society. binding to the Aβ plaques as that for the PiB. [18F]FIBT, used in combination with the MRI (PET/MRI), distinguishes an AD case from a control human subject (Fig. 64).25 6.2 Synthetic methods for radiofluorination A variety of new synthetic methods are continually developed for the preparation of 18 F-labeled compounds,26,27 although most of the recent advances in the organofluorine synthetic routes have not yet been optimized or adapted for the synthesis of PET tracers. Synthetic methods involving late-stage incorporation of the 18F label have obvious advantages because of the relatively short half-life of the 18F isotope.28 For example, nucleophilic radiofluorination of α-diazocarbonyl compounds using the no-carrier-added [18F]fluoride anion to give the corresponding [18F]fluoro carbonyl compounds is one such late-stage fluorination strategy.29 Other late-stage radiofluorination reactions (vide infra) for the synthesis of PET tracers include Cu(I)-mediated CH fluorination of electron-rich arenes (or heteroarenes) using hypervalent iodonium reagents,30 PhenoFluor-mediated radiofluorination of phenolic compounds,31,32 Mn(III)-catalyzed radiofluorination of aliphatic CH bonds,33 Chapter 6 • Synthesis and applications of 18F-labeled compounds 18 F S N H 221 N O N Me [18F]FIBT FIGURE 6–4 First human brain PET/MR images with [18F]FIBT. PET images of a patient with moderate AD (top) and a subject defined as control (psychometric testing within normal limits, normal FDG PET, and normal CSF τ, τ , and Aβ142 levels) (bottom) coregistered to their corresponding T1-weighted MPRAGE MR images as taken with the Siemens Biograph in a fully dynamic 90 min PET/MR study. 7090 min postinjection frames are shown as axial (left), sagittal (middle), and coronal (right) views. PET data was transformed to SUVRs using the cerebellum as reference region (scale on left side). Images from the patient with tracer distribution typical for AD (top) show strong contrast to images from the subject (bottom) who was cleared of any signs of neurodegeneration with a tracer distribution typical for healthy controls. Vertical color bar indicates lookup-table “Cold” (taken from Pmod) between ratio values of 1.0 (i.e., equality) and 2.0. AD, Alzheimer’s disease; CSF, cerebrospinal fluid; PET, positron emission tomography; SUVRs, standardized uptake value ratios. Adapted from Hooshyar Yousefi, B.; Manook, A.; Grimmer, T.; Arzberger, T.; von Reutern, B.; Henriksen, G.; Drzezga, A.; Foerster, S.; Schwaiger, M.; Wester, H.-J. Characterization and First Human Investigation of FIBT, a Novel Fluorinated Aβ Plaque Neuroimaging PET Radioligand. ACS Chem. Neurosci. 2015, 6, 428437, Copyright 2015, American Chemical Society. Pd-catalyzed radiofluorination of arylboronic acids to give the aryl [18F]fluorides,34 and complexation of [Al]-18F or [B]-18F moieties into the macrocyclic ligands, such as 1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA) (vide infra) (Fig. 65).9,35 6.2.1 Synthesis of 18F-labeled reagents A recent review summarizes the synthesis of the electrophilic, free radical, and nucleophilic reagents that are extensively used in the synthesis of the PET tracers.28 A vast majority of the 18F-labeled radiotracers have been synthesized through nucleophilic substitution reactions using the [18F]fluoride anion. The [18F]fluoride ion is synthesized by the nuclear 222 Organofluorine Chemistry 1. Radiofluorination of α-diazo compounds: α-18 F-esters and amides mol%) R′ FIGURE 6–5 Selected synthetic methods for the late-stage radiofluorination (vide infra). reaction 18O(p,n)18F, by bombarding [18O]water with high-energy protons in the cyclotrons, and this reagent is called “no-carried-added” fluoride anion since it is not contaminated with externally added 19F-fluoride ion. The aqueous-solvated fluoride thus produced is unreactive in the SN2 reactions and thus needs to be activated in a multistep process by eluting the solution through ion-exchange columns with MeCN/H2O solution containing countercations, such as K1 and R4N1. Typically, the macrocyclic Kryptofix2.2.2 cryptand Chapter 6 • Synthesis and applications of 18F-labeled compounds 223 (a K1-complexing phase-transfer agent) is used for this purpose, and the resulting Kryptofix-K2.2.2-18F is subsequently transferred into a polar aprotic solvents [such as acetonitrile and dimethylformamide (DMF)] or in some cases polar protic solvents (sterically hindered alcohols, such as tert-butyl alcohol)36 for radiofluorination of the compounds through SN2 or SNAr reactions. For example, an 18F-labeled captopril, an angiotensinconverting enzyme (ACE) inhibitor, could be synthesized in a relatively late-stage SN2 reaction using K18F/Kryptofix2.2.2 in acetonitrile. Subsequent treatment with aqueous NaOH and neutralization would result in the hydrolysis of the ester and thioester moieties to give the 18F-PET tracer (Fig. 66).37 O O O N S H3 C H MeO 2C 18 OSO 2CF3 1. K F/kryptofix-2.2.2/CH3 CN 2. NaOH (2 M) 3. HCl (conc.) HS N H3 C H HO2C 18F [18 F]fluorocaptopril (18 F-ACE inhibitor) FIGURE 6–6 Synthesis of an18F-labeled captopril (ACE inhibitor). ACE, Angiotensin-converting enzyme. For reactions with water-sensitive compounds, presynthesized acid [18F]fluorides (e.g., acetyl [ ]fluoride, prepared from the reaction of acetic anhydride with [18F]fluoride anion) can be used.2 Doyle and coworkers have synthesized a thermally stable, selective deoxyfluorination reagent, PyFluor (2-pyridylsulfonyl fluoride), which is effective in the deoxyfluorination of alcohols on a preparative scale, and developed a corresponding 18F-labeled version, [18F]PyFluor, for the radiofluorination of acohols.38 Thus, elution of the acetonitrile solution of 2-pyridylsulfonylchloride through an [18F]KF/Kryptofix-K2.2.2-containing anion-exchange cartridge gives the anhydrous [18F]PyFluor. Tetra-O-benzyl-D-glucose upon reaction with [18F]PyFluor gave 18F-labeled D-glucose derivative ([18F]FDG-benzyl ether) in 15% radiochemical conversion. This deoxyfluorination reaction involves the initial rapid formation of the sulfonate ester of the alcohols, followed by relatively slow SN2 reaction with the fluoride anion. The activation of alcohols toward reaction with PyFluor was achieved through the relatively sterically crowded, nonnucleophilic bases, such as 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) and 7-methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene (MTBD). Minor byproducts derived from DBU or MTBD are usually formed along with the desired PET tracers, and these impurities can be easily separated in the final purification step (Fig. 67). Thus, [18F]PyFluor serves as an alternative reagent to the commonly used [18F]fluoride ion, although it has not yet been automated for the synthesis of PET tracers of radiopharmaceuticals in the clinical settings, perhaps due to the involvement of additional steps for its preparation. Various electrophilic and free-radical fluorinating agents can be prepared from the reaction of [18F]F2 gas with amines, alcohols, and carboxylic acids. Thus, the high electrophilic reactivity of the elemental fluorine is tamed to provide effective radiofluorinating agents. 18 224 Organofluorine Chemistry O O S Cl N [18F]KF/Kryptofix-K2.2.2 MeCN, 80 °C, 5 min 2-Pyridylsulfonyl chloride O O S N [18F]PyFluor O O S 18 F N OBn OBn BnO BnO O BnO BnO 18F OH CH3 MeCN, 80 °C, 20 min N N OBn O OBn 18 Tetra-O-benzyl- O O S O [18F]F N F N D-glucose MTBD OBn BnO BnO O 18 F OBn 2-[ 18F]fluoro-2-deoxyglucose derivative ([18 F]FDG tetrabenzyl ether) RCC: 15% FIGURE 6–7 Synthesis of [18F]PyFluor and its reaction with tetra-O-benzyl-D-glucose to give the corresponding 18 F-labeled product. The [18F]F2 is generated from the nuclear reactions 20Ne(d,α)18F and 18O(p,n)18F using the elemental, gaseous neon, and O2, each diluted with [19F]F2 gas as the coadditive for the extraction of the [18F]F2. The [18F]F2 produced in this manner is contaminated with 19 F-labeled F2, and therefore this reagent is called carrier-added [18F]fluorine gas. In general, radiofluorinations using the no-carrier-added [18F]fluoride reagents give relatively higher specific activities over those using carrier-added fluorinating reagents and are thus the preferred reagents for radiofluorination. Elemental [18F]F2 is transformed into various electrophilic and free-radical fluorinating agents, such as 18F-labeled acetyl hypofluorite, trifluoromethyl hypofluorite, N-fluoropyridinium salts, Selectfluor, N-fluorobenzenesulfonimide (NFSI), and N-fluoropyridin-2-one, that are useful for the radiofluorination reactions undercontrolled reaction conditions (Fig. 68). Thus, for example, the elemental 18 F-fluorine is allowed to react with N-chloromethyl-1,4-diazabicyclo(2.2.2)octyl triflate and (PhSO2)2NH to give [18F]Selectfluor and [18F]NFSI, respectively. These reagents can be used in the electrophilic fluorination of enolsilyl ethers to give the corresponding α-fluorocarbonyl compounds and fluorination of allylsilanes to give the corresponding allyl fluorides.39 18F-labeled Selectfluor bis(triflate) achieves fluorodestannylation of arylstannanes to give the corresponding 18F-labeled aromatic fluorides.40 The reaction conditions for the radiofluorination using 18F-selectfluor are much milder, and the radiofluorination is more selective than for the radiofluorinations using elemental [18F]F2. Chapter 6 • Synthesis and applications of 18F-labeled compounds F3 C O 18 O F 225 O 18 F Trifluoromethyl hypofluorite Acetyl hypofluorite [18F]XeF 2 F3 C OH O Xe OH O OSO 2CF3 18 N F OO O S N O Pyridine/NaOSO2 CF3 Na 18 [ F]F2 [18F]N-fluoropyridine S OO O S N 18 F S Cl OSIMe3 [18F]NFSI N N N HClO4 TfO LiOTf Cl O 18 N N F N [18F]FClO3 [18F]N-fluoropyridin-2-one [1 8F]Perchloryl fluoride 18 2TfO F [18 F]Selectfluor FIGURE 6–8 Conversion of the elemental [18F]F2 into various electrophilic and free-radical fluorinating agents. Electrophilic radiotrifluoromethylation can be achieved using 18F-labeled Umemoto reagents. Thus, a [18F]-labeled Umemoto reagent 3 can be used for the chemoselective radiotrifluoromethylation of thiol moieties in cysteine-containing peptides, to give a variety of acyclic and cyclic 18F(SCF3)-labeled peptides, such as the dipeptide 5 and cyclic RGDFC peptides (6). The synthesis of the 18F-labeled Umemoto reagent 3 was achieved through nucleophilic 18 F[F]2 fluorination of 2-[(boromodifluoromethy)lthio]-1,10 -biphenyl (1), followed by m-chloroperoxybenzoic acid (mCPBA)-mediated oxidative cyclization (Fig. 69).41 6.3 Sharpless click reactions for positron emission tomography tracers Sharpless has developed convenient synthetic route for conjugating the terminal alkynes and azides through a modified Huisgen [3 1 2] cycloaddition reaction using Cu(I) catalysis, and these reactions are generally referred to as the “click chemistry” or “click reactions.” In the absence of the Cu(I) catalysis, the original Huisgen 1,3-dipolar cycloaddition requires elevated temperatures and long reaction times, and is not regioselective. The Cu(I)-catalyzed click reactions proceed under relatively mild conditions (in some cases at ambient temperatures), under physiological conditions, and also in the cellular environments, to give exclusively the 1,4-substituted [1,2,3]triazoles. Thus, the Sharpless click reaction can serve as a 226 Organofluorine Chemistry mCPBA/Tf2 O [18F]KF/diCy-18-Cr-6 SCF2 Br S SCF2 18F AgOTf (2 equiv) OTf CF2 18F DCE, 60 o C, 20 min 1 2 3 SH SCF2 18F (e.g., peptide-SH) 4 Selected examples: SCF2 [18F] O SCF2 18F O BocHN O N H CH3 O O HO NH N H O NH2 N NH H HN NH HN O O O cRGDFC[18F]CF3 ( 6) Boc-Gly-CysS-[18F]CF3-OMe ( 5) FIGURE 6–9 Electrophilic radiotrifluoromethylation of cysteine-containing peptides. DCE, 1,2-Dichloroethane; diCy18-cr-6, dicyclohexano-18-crown-6; mCPBA, m-chloroperoxybenzoic acid; Tf2O, triflic anhydride. bioorthogonal reaction (i.e., the reaction tolerates the presence of various otherwise reactive biomolecules, water, and oxygen) and is useful for the conjugation of 18F-labeled prosthetic groups with biomolecules such as peptides, carbohydrates, and nucleotides. This reaction has found wide applications in conjugation of various prosthetic organic moieties to the biologically interesting molecules, including peptides and proteins, and provides a convenient synthetic route for the preparation of the 18F-labeled biomolecules and pharmaceuticals (Fig. 610).42 H R Cu(II)SO 4 /Na-ascorbate + N N N R' or Cu(I)Br R' N N N R R, R' = e.g., peptide, nucleotide, and 18 Flabeled substituents and pharmaceuticals FIGURE 6–10 Sharpless click chemistry for 18F-labeling of biologically interesting molecules and pharmaceuticals. Efficient synthetic methods for a variety of 18F-labeled azides and alkynes have been developed, expanding the scope of the click chemistry for the preparation of various PET imaging agents (Fig. 611).42 Chapter 6 • Synthesis and applications of 18F-labeled compounds 227 N N N3 18 F O 18 F N3 N3 18 F n 3 18 F N3 N3 O N Si 18 18 18 CO2 H F F NH 2 F p -Azidophenylalanine O H N O N3 OH O NH2 N ε-Azidoethoxycarbonyl-lysine FIGURE 6–11 Examples of 18F-labeled azide and alkyne click-chemistry precursors for the synthesis of PET tracers. PET, Positron emission tomography. A triazole derivative, [18F]-TBD (Fig. 612), was synthesized from the click reaction of 2-[ F]fluoroethyl azide with the N-propargyl derivative of a caspase-3 substrate. This 18 F-labeled triazole derivative was used in the dynamic PET studies of the caspase-3 activation to probe pharmacodynamics of the apoptotic cell death in vivo, and thereby to probe the effectiveness of the apoptosis-targeted pharmaceuticals (such as cisplatin).43 18 H3 C O H N O O N H O N-propargyl derivative of a caspace-3 substrate CO 2 H H N H H 3C 18 O N3 Cu(I) O H N F O O N H O CO2H H N 18 N N F N O [18 F]-TBD FIGURE 6–12 Synthesis of 18F-TBD, used in the PET studies of caspase-3 activation. PET, Positron emission tomography. 6.3.1 Protein and oligonucleotide triazole positron emission tomography tracers The use of the oligomeric triazole, tris[(1-benzyl-1H-1,2,3-triazol-4-yl)-methyl]amine (TBTA) as Cu(I) chelator allows efficient coupling of alkyne or azide-substituted RNA oligonucleotides and human serum albumin to 18F-labeled alkynes or azides to give their corresponding PET tracers (Fig. 613).44 228 Organofluorine Chemistry O 5'-CCGCACCGCACAGCCG -3' N H N3 O S O + 18 F N CH3 RNA-oligonucleogide-N3 Ph N N N NN N N NN CuBr/TBTA/DMSO; 20 min, 10 °C Ph TBTA Ph O O S 18 F N N O N N N H 5'-CCGCACCGCACAGCCG-3' CH3 FIGURE 6–13 Preparation of 18F-labeled RNA oligonucleotides using click chemistry. 6.3.2 18 F-octreotate positron emission tomography tracers for tumor imaging Neuroendocrine tumors (also called neuroendocrine neoplasms, NENs), which occur mostly in the digestive tract and respiratory tract, are formed from the diffuse endocrine system cells. The NENs contain abundant SSTRs, and therefore somatostatin-based compounds are effective NEN antagonists and thus are attractive targets as PET imaging agents and also for internal radiotherapy. 177Lu- and 68Ga-based octreotate compounds, 177Lu-DOTA-TATE and 68 Ga-DOTA-TATE, are FDA-approved PET tracers as well as therapeutics (i.e., theranostics) for these tumors.45,46 The 18F-analogs of these compounds would provide safer and relatively more effective PET tracers. A click chemistry-derived PET tracer analog, 18F-fluoroethyl triazole 18 F-octreotate (a somatostatin analog, targeting somatostatin receptors), was synthesized through automated synthesizer from the corresponding cyclic-hexapeptide (somatostatin; c-CYHKTC)-derived terminal alkyne and [18F]fluoroethylazide. This octreotate-based PET tracer showed favorable safety, imaging, and dosimetric profile for PET imaging of neuroendocrine tumors in the human clinical trials, and further clinical trials are ongoing (Fig. 614).47 A related 18F-labeled fluoroborate analog, the cyclic peptide octreotate (a somatostatin analog, targeting somatostatin receptors) conjugated to 18F-labeled Ammoniomethyl-trifluoroborate, called [18F]AMBF3-TATE, was synthesized through the azidealkyne click chemistry (from the corresponding somatostatin-derived azide and trifluoroborate-derived terminal alkyne reactants), followed by [18F]fluoride exchange of the fluoroborate moiety using microscale methods (i.e., on microfluidic chips) (Fig. 615). This 18F-PET tracer was obtained in an overall decay-corrected radiochemical yield (RCY) of 16% in 40 min, with a radiochemical purity greater than 99% and with high molar activity. Preclinical PET evaluations in micebearing human SSTRs (SSTR2)-overexpressing xenografts showed favorable biodistribution, with the highest tracer accumulation in the bladder and gastrointestinal tissues.35 Chapter 6 • Synthesis and applications of 18F-labeled compounds 229 OH NH O O Ph H H N H N O O N H NH NH 2 O O 18 F N3 HN N H N H O O H N S N H S HN Cu(II)SO4, Na-ascorbate OH O (automated synthesizer) O OH H 3C OH O βAG-TOCA OH NH O 18 F Ph N H N H N N N O O O O O H N N H NH O O NH2 HN N H S N H N H S HN O OH H 3C OH OH O O 18 F-FET-βAG TOCA (18F-octreotate) FIGURE 6–14 Automated synthesis of 18F-octreotate, through click chemistry, as a PET tracer for neuroendocrine tumors. PET, Positron emission tomography. 6.3.3 Strain-promoted click chemistry Strain-promoted alkyneazide cycloaddition (SPAAC) is a variant of the Sharpless click reaction and takes advantage of the high reactivity of the strained cyclooctyne with azide moiety, even in the absence of any Cu(I) catalysis. These SPAAC reactions are bioorthogonal as they proceed at ambient temperature and under physiological conditions, and also in the cellular environments, tolerating a variety of functional groups of the proteins and carbohydrates.48 This reaction is invaluable in the design of the bioorthogonal 18F-labeled probes, such as 18 F-labeled cyclic RGD (arginineglycineaspartic acid)containing peptides for PET imaging.4952 A variety of 18F-labeled aza-dibenzocyclooctyne (ADIBO) amide derivatives, used as 18 F-labeled tagging agents, have been synthesized through conjugation of ADIBO with the 230 Organofluorine Chemistry OH NH O F N F B F O Ph O N N N N H NH O H N NH2 O O [18F]F– HN N H S N H OH N H S (isotopic exchange on a microfluidic chip) O OH HN H 3C OH Unlabeled AMBF3 -TATE (synthesized through click reaction) O O OH NH O 18 F F B F N Ph O N N N O O H N N H NH O O NH2 HN N H N H S N H S HN O OH H 3C [18F]AMBF3 -TATE OH OH O O FIGURE 6–15 Synthesis of 18F-labeled PET tracer [18F]AMBF3-TATE on microfluidic chips. PET, Positron emission tomography. 18 F-labeled carboxylic acids. The latter 18F-labeled fluoroalkyl or fluoroaryl carboxylic acid could be synthesized through the nucleophilic substitution reactions (SN2 or SNAr) using the carrier-free [18F]fluoride source. These strained azacyclooctyne compounds serve as precursors for the synthesis of numerous 18F-PET tracers via the SPAAC of azide-derivatized substrates (e.g., peptide or protein azides) (Fig. 616).42 As an example of this strategy for the incorporation of the PET label, a dimeric RGD conjugated with ADIBO was reacted with the 18F-labeled polyethyleneglycol azide at ambient temperature for 15 min (in aqueous ethanol) to afford the corresponding 1,2,3-triazole, di-cRGD-PEG5-ADIBOT-18F, with a decay-corrected RCY of 92%.49 Any unreacted ADIBO starting compound could be removed by a simple filtration through a terminal azidederivatized polystyrene resin (a process called chemoorthogonal scavenger-assisted purification), and thus this synthetic strategy does not involve a final high performance liquid chromatography (HPLC) purification unlike most other synthetic methods for the PET tracers (Fig. 617). 18 ADIBO-conjugated F-synthons: 18 N H F Synthesis of 18 18 N O F 18 5 O N O N H N O O F N 4H O F-PET tracers through SPAAC: N N N N3 18 (peptide or small molecule azides) N O N H F N O O 18 N H F O 18 FIGURE 6–16 F-labeled strained azacyclooctynes as bioorthogonal precursors of the PET tracers; these strained alkynes react at ambient temperature with azide derivatives of substrates (such as peptides, nucleotides, and proteins) in the absence of any metal catalysts to afford the 18F-labeled compounds. PET, Positron emission tomography; SPAAC, strain-promoted azidealkyne cycloaddition. O OH O OH O HN NH O HN NH H N O HN O O O HN N H NH2 OH HN O H N O N H N H N3 HN 18 4 H N HN O HN O F EtOH/H 2O (1/1); 25 °C, 15 min O 4 PS (ADIBO-scavenger resin); 20 min/25 °C (for removal of unreacted ADIBO) NH2 HN RGDYK O OH O OH O NH HN 18 F O O NH 4 HN HN O HN N H NH2 OH O H N O OH N H N N N O O HN N H N O O N3 O NH O ADIBO O O OH O N O O O O 4 N H O O HN NH O H N O RGDYK HN O HN NH2 HN di-cRGD-PEG 5 -ADIBOT- 18 F Decay corrected RCY: 92% FIGURE 6–17 Synthesis of a cyclic RGD-linked 18F-labeled PET probe. PET, Positron emission tomography. 232 Organofluorine Chemistry This RGD-linked 18F-tracer (di-cRGD-PEG5-ADIBOT-18F) was shown to selectively bind to the tumors in tumor-mice models with good tumor-to-background contrast, with a relatively high tumor uptake as compared to other major organs in PET imaging (Fig. 618).49 A blocking experiment involving the coinjection of the corresponding unlabeled compound showed significantly lower uptake of the labeled compound (90 min after postinjection), confirming the specific tumor uptake of the di-cRGD-PEG5-ADIBOT-18F (Fig. 618B). Further optimization of the structural features of the latter ADIBOT-derived PET tracers may lead to the design of the effective PET imaging agents. 6.4 Staudinger ligation reactions for positron emission tomography tracers Originally developed by Staudinger in 1919, the Staudinger reaction involves reduction of alkyl or aryl azides by triarylphosphine to give the corresponding amines. Bertozzi and coworkers, in 2000, adapted the Staudinger reaction for the bioorthogonal ligation of azides with arylphosphines to selectively form an amide bond between the reacting partners, in the presence of complex biological environment.53,54 The reaction sequence and an overview of the mechanism are given in Fig. 619. There are two versions of the Staudinger ligation reactions: one in which the phosphine oxide moiety is retained in the product amide (called nontraceless reaction; as shown in Fig. 619) and a variation in which the product amide does not incorporate the phosphine oxide (traceless reaction). The traceless Staudinger ligation reaction (Fig. 620) provides an alternative, and more efficient, approach (i.e., product does not contain the high molecular weight phosphine moiety) for the synthesis of 18F-labeled PET tracers. Gouverneur and coworkers synthesized β-[18F]fluoroethylamide derivatives of the N-acetylamino acids, as potential PET tracers, through the traceless Staudinger ligation of β-[18F] fluoroethyl azide with N-acetylamino acid thioesters in RCYs of over 95%.55 This traceless Staudinger reaction, unlike the Cu(I)-catalyzed azidealkyne cycloaddition reactions, does not involve the metal ion impurities, and thus the resulting PET tracers are easy to purify, although it requires relatively high temperatures (Fig. 621). Furthermore, the phosphine reagents used in these Staudinger ligation reactions are prone to oxidation under the reaction conditions, forming minor impurities that need to be removed by careful purification. The 2-[18F] fluoroethylazide used in the latter reactions can be synthesized through the reaction of 2-azidoethyl tosylate with [18F]F2 in the presence of Kryptofix2.2.2 (4,7,13,16,21,24-hexaoxa1,10,diazobicyclo[8.8.8]hexacosane) at 80 C110 C, in RCYs of about 55%.27 The 2-[18F]fluoroethylazide is also widely used for the synthesis of a variety of 18F-labeled compounds through Sharpless azidealkyne cycloaddition reactions. Traceless Staudinger ligation reaction of 18F-labeled 2-fluoroethylazide with quinolonebased thioesters afforded the 18F-labeled PET tracers for selective binding to GABAA receptors, with a nondecay corrected RCY of 7% and with a specific radioactivity of 0.9 GBq/μmol.56 These fluoroalkyl 4-quinolone derivatives exhibited nanomolar to subnanomolar affinity for the GABAA receptors (Fig. 622). Chapter 6 • Synthesis and applications of 18F-labeled compounds 233 FIGURE 6–18 (A) In vivo evaluation of di-cRGD-PEG5-ADIBOT-18F; 3D reconstruction (upper), coronal (middle), and transverse section (lower) combined PETCT images of the U87MG tumor-bearing mice at 30, 60, 90, and 120 min postinjection of dicRGD-PEG5-ADIBOT-18F (1.8 MBq). (B) “Blocking” images with a coinjection of nonradioactive di-cRGD-PEG5-ADIBOT-F (10 mg/kg). 3D, Three-dimensional; CT, computed tomography; K, kidney; PET, positron emission tomography; T, tumor. Adapted from Kim, H.L.; Sachin, K.; Jeong, H.J.; Choi, W.; Lee, H.S.; Kim, D.W. F-18 Labeled RGD Probes Based on Bioorthogonal Strain-Promoted Click Reaction for PET Imaging. ACS Med. Chem. Lett. 2015, 6, 402407, Copyright 2015, American Chemical Society. 234 Organofluorine Chemistry NHR F OR' N N N O P O RF O P R F = Fluoroalkyl/fluoroaryl N N N Mechanistic outline RF OR' OR' P OR' O N N N N R –N 2 P O P O N N RF RF N O O O H 2O P N P N O H RF RF HN P O RF R' O FIGURE 6–19 Staudinger ligation reaction (nontraceless) and an overview of its mechanism. O X R' N N N RF P XH O NHR F R' + P O 2. H 2 O X = O/S RF = Fluoroalkyl/fluoroaryl X = O/S N Mechanistic outline N N RF –N 2 H O O X P N RF X R' H2O P R' N RF O H H 2O FIGURE 6–20 Traceless Staudinger ligation reaction for the preparation of 18F-PET tracers. PET, Positron emission tomography. Chapter 6 • Synthesis and applications of 18F-labeled compounds O K+O O O O O N 18 F N3 80–110 °C, 2–15 min O R [18F]F (Kryptofix-2.2.2-K [18 F]F) OTs N3 N 235 N N N 18 F S O THF/H2 O PPh2 R 80 °C, 30 min HS Ph O P 18 F N H RCY > 95% Ph Selected examples: O O NH2 FIGURE 6–21 Synthesis of N H N 18 F NH 2 HN N H 18 F 18 F-labeled peptide analogs through traceless Staudinger reaction. O Ph 18F O S N H PPh 2 N3 CH 3CN, DMF 130 °C, 15 min O O 18 N H Ph F N H GABAA receptor binding PET tracer FIGURE 6–22 Synthesis of an 18F-labeled GABAA receptor antagonist through traceless Staudinger ligation reaction. 18 F-labeled amide derivatives of D-glucose and other alkyl amides could be synthesized using the Staudinger ligation under relatively mild condition. The precursor 18F-labeled triarylphosphine esters were synthesized in a one-step process from the corresponding tosylates and K[18F]F in the presence of polar protic solvents, such as tert-butyl alcohol, under phasetransfer conditions, in RCYs ranging from 2% to 65% (Fig. 623). 18F-labeled biotin derivative was obtained using a similar procedure from the biotin-derived azide in 12% RCY.57 6.5 Radiofluorination via aromatic nucleophilic substitution Aromatic nucleophilic substitution (SNAr) of aryl quaternary ammonium salts (7), with electron-withdrawing groups, such as carbonyl or sulfonyl groups, ortho- or para- to the ammonium moiety, with fluoride anion give the corresponding fluorinated compounds 236 Organofluorine Chemistry O O O TsO 18F [18F]F– , TBAOH 4 PPh2 N O 4 CH 3CN, t-BuOH N N O PPh2 18 N H 90 °C, 10 min F 5 100 °C, 10 min O HN NH H O O H S N 4 H N Biotin-azide N N O N N O N OO O 90 °C, 10 min 18 O O HN NH H O O H S 18 N 4 H N H O F HN O F 5 OO 5 Biotin-PET agent FIGURE 6–23 Staudinger ligation (traceless) of alkyl azides with 18F-labeled triarylphosphine esters. TBAOH, Tetrabutylammonium hydroxide. (Fig. 624). This reaction could be readily adapted to the synthesis of 18F-labeled aromatic compounds, which, through a sequence of reactions, can be transformed to the corresponding thiol-reactive maleimides (9).58,59 These thiol-reactive maleimides are useful radiolabeled precursors in the preparation of RGD peptide-based tracers (12) for imaging ανβ3 integrin proteins, the transmembrane cell adhesion receptor proteins that are overexpressed in tumor cells.60 This synthetic strategy, however, involves early-stage radiofluorination, and requires relatively high temperatures for the radiofluorination, thus lowering RCYs of the final PET tracers. 6.5.1 [18F]fluoro-(1)-biotin Ipso nucleophilic aromatic substitution (SNAr) of nitro-aromatics using carrier-free [18F]F2 sources gives the 18F-labeled aromatics, at relatively high temperatures. The scope of this radiofluorination reaction is limited to the compounds that are stable under high temperature conditions. Electron-withdrawing groups ortho- or para- to the nitro group facilitate the nucleophilic substitution reaction. Ortho-nitropyridyl compounds can also be used in these nucleophilic aromatic substitutions. A biotin-derived ortho-nitropyridyl ether, synthesized in four steps starting from biotin, was reacted with Kryptofix2.2.2-complexed K[18F]F2 in dimethyl sulfoxide at 160 C to give the 18F-labeled biotin derivative in high chemical- and radiochemical yields, with specific activity in the range of 153 GBq/μmol (Fig. 625).61 Through complexation of this 18F-labeled biotin to fluorescently-labeled avidin, the in vivo PET biodistribution was demonstrated in animal models, in tandem with fluorescence imaging. FIGURE 6–24 Synthesis of [18F]fluoroarylsulfonamido-maleimide and its reaction with glutathione and thiolfunctionalized cyclic RGD peptides to give the corresponding PET tracers. PET, Positron emission tomography. HN NH H H S O HN 1. EtOH/H2 SO4 , RT NH H 2. LiAlH 4 , DCM OH –78°C to RT 4 3. TsCl, py, RT O 2N H S 4 OTs Biotin O K[18F]F-Kryptofix-2.2.2 HN NH H DMSO, 160 °C, 5 min H S O 4 18 F N 18 Biotin- F PET tracer FIGURE 6–25 Synthesis of 18 O HO O O F-labeled biotin via SNAr reaction. DMF, 70°C, 2 h HN NH H H S O 4 O 2N N 238 Organofluorine Chemistry 6.5.2 L-3,4-Dihydroxy-6-[18F]fluorophenylalanine (6-[18F]L-DOPA) 6-[18F]L-DOPA is a clinically successful PET imaging agent for the neuroendocrine tumors (NETs).62 The synthesis of 18F-labeled DOPA was achieved through aromatic nucleophilic substitution (SNAr) reaction of nitroaromatic 13 (with an electron-withdrawing carbonyl group para to the NO2 moiety) using Kryptofix2.2.2-complexed K[18F]F in DMF solution, with radiochemical conversions of 76%82%. Transformation of the benzaldehyde moiety to the phenolic group through the BaeyerVilliger reaction, followed by deprotection of the methoxymethyl and N-trityl (triphenylmethyl) groups afforded the 18F-labeled dopamine (16) in high radiochemical conversion (Fig. 626).63 This synthetic method was shown to be amenable to automation. In the automated version the sequence of reactions proceed with an overall RCY of 20% and an enantiomeric excess of 96% of the 18F-labeled L-DOPA.63 FIGURE 6–26 Synthesis of 6-[18F]L-DOPA. 6.5.3 γ-Aminobutyric acid transporter positron emission tomography tracers γ-Aminobutyric acid (GABA) is one of the predominant inhibitory neurotransmitters, and dysregulation of the GABA system results in various neurological disorders such as epilepsy (seizures), schizophrenia, and autism spectrum disorder. Although benzodiazepines and other GABA system responsive drugs are widely used in clinical practice, there are currently no FDA-approved imaging agents for the presynaptic GABA-ergic neurons.64 Bloodbrain permeability and selective binding to the GABA receptors is a continuing challenge in this area. Toward developing GABA transporter type 1 (GAT-1) imaging agents, [18F]fluorinelabeled compound 18 was synthesized through aromatic nucleophilic substitution reaction of the p-chlorobenzophenone derivative 17 using carrier-free [18F]fluoride anions complexed to the Kryptofix2.2.2 with a RCY of 1% (Fig. 627).64 Hydrolysis of the Chapter 6 • Synthesis and applications of 18F-labeled compounds CO2Et CO2Et F F O N O [18F]KF, Kryptofix-2.2.2 O 239 N O DMF, 130 °C, 30 min 18 Cl F 17 18 1% RCY CO2H F O LiOH, 100 °C, 15 min N O 18 F 19 1% RCY FIGURE 6–27 Synthesis of a GABA transporter type 1 (GAT-1) PET imaging agent. GABA, γ-Aminobutyric acid; PET, Positron emission tomography. compound 18 gives the carboxylic acid analog 19. In vivo animal studies (rhesus monkey brain) showed that the ester 18 has significant bloodbrain permeability, whereas the carboxylic acid derivative is relatively impermeable to the brain, due to its predominant zwitterion character. However, the carboxylic acid moiety is necessary for the high affinity binding to the GAT-1, and further studies are needed to identify the optimal PET tracers for the GAT-1. 6.5.4 Radiofluorination of phenolic compounds Ritter and coworkers have achieved late-stage radiofluorination of phenols using a 1,3-diaryl-2-chloroimidazolium chloride and [18F]F2, eluted through an anion-exchange column. Otherwise tedious azeotropic drying of the 18F2 salts is not required for this radiofluorination, since the uronium salt 21 by itself, rather than the conventionally used aqueous alkaline solution, is the eluting agent for the 18F2 ions trapped on the anion-exchange cartridge; the 18 2 F anion readily exchanges with the Cl2 anion in 21. The mechanism of this deoxyfluorination is analogous to that of the deoxyfluorinations achieved using PhenoFluor reagent and may involve a concerted nucleophilic aromatic substitution (CSNAr).31 Thus, reaction of the phenolic substrates having electronwithdrawing substituents with N,N0 -1,3-bis(2,6-diisopropylphenyl)chloroimidazolium chloride (20; CsF complex of which is commercially available as PhenoFluorMix) resulted in the formation of the uronium salt 21. The latter uronium salt, upon passing through the 18F2 embedded anion-exchange column, undergoes Cl2 to 18F2 anion metathesis, and subsequent heating of the eluate-containing compound 22 (apparently an equilibrating mixture) at 130 C forms the 18F2-labeled aromatics in good to excellent radiochemical conversions (Fig. 628). 240 Organofluorine Chemistry FIGURE 6–28 Radiofluorination of phenolic compounds using PhenoFluor and [18F]F2. Although the originally developed deoxyradiofluorination of phenols is usually limited to aromatics with electron-withdrawing substituents, Ritter and coworkers later have demonstrated that phenolic compounds with electron-releasing substituents, when complexed with ruthenium(II), react with the N,N0 -1,3-bis(2,6-diisopropylphenyl)chloroimidazolium chloride (28) to give the corresponding uronium salt, which upon chloride/19F2 anion exchange on the anion-exchange column undergo deoxy-[18F]fluorination.65 In the latter case, the Ru(II)-complexed aryloxy uronium intermediate is sufficiently electron-deficient to stabilize the resulting Meisenheimer intermediate, even in the case of phenolic compounds with electron-donating substituents, so that the activation barrier for the deoxyfluorination is attenuated. A final rate-limiting decomplexation of the Ru(II) gives the radiofluorinated phenolic compounds in 10%99% radiochemical conversions. The ruthenium complexes are stable to moisture and ambient atmosphere, and the radiofluorinations have large substrate scope. This radiofluorination technique is fully automated, and a variety of biologically interesting compounds, such as 18F-labeled phenylalanine (34), and deoxy-fluorinated versions of the drug-candidates, such as ezetimibe (35) and estrone (36) were synthesized in micromolar scale using this technique (Fig. 629). These Chapter 6 • Synthesis and applications of 18F-labeled compounds Ru Cl OH R (28) R Cl N Ar + 18F Ar N OH 241 Cl Ru EtOH, 85 °C, 30 min CH 3CN:DMSO (1:1) 125 °C, 30 min 29 Ar N 18 F O Cl Ar N N R R Ar O 18 Ru Cl N Ar R SNAr mechanism (Fast) 18F Ru F Ru Ar N 30 N Ar 31 O 18 F Ru-decomplexation Slow (rate limiting step) R 32 Selected examples (with radiochemical conversions): 18F OH O O OMe 18 N F NHBoc 18F 33 F N O 34 98% 98% 99% Me O 18 F-ezetimibe (35; OH replaced by ezetimibe is a cholesterollowering drug. 18 F); H H F H 18F 88% F-estrone (36) FIGURE 6–29 Radiofluorination of phenols through Ru π-complexes. synthetic methods complement other synthetic methods based on the diaryliodonium salts and iodonium ylides (vide infra). The radiofluorination of Ru-complexed phenols (39) was adapted, in combination with solid-phase peptide synthesis, for the site-selective synthesis of 18F-labeled peptides on micromolar scale, and the radiofluorination was fully automated (Fig. 630).66 This radiofluorination methodology is amenable to the synthesis of a variety of small peptides, incorporating 18F-labeled phenylalanine (tyrosine analog; 43) either at the terminus or in the 242 Organofluorine Chemistry BF 4– Ru+ 1. HO 38 NH2 OH 37 HO 40 W blue LED O O 2. Fmoc-OSu, Na2 CO3 75% Ru NHFmoc O Ru O O 2 O O 2. Deprotection from the polymer resin 39 N O 1. SPPS Ru 18 F iPr iPr N N 40 iPr Cl Cl 18 F 42 iPr 41 CF3 CO2H, iPr 3SiH, H 2 O, DTT 50°C, 10 min 18 F 43 18F NH2 O O H 2N HN H N NH N H O H N O 18 HN O N H HO O O 44 F-c(RGDfk) FIGURE 6–30 Ru(II)-mediated solid-phase synthesis (SPSS) of 18F-labeled small peptides. c(RGDfk), Cyclic (ArgGlyAspPheLys peptide); DTT, dithiothreitol; Fmoc, 9-fluorenylmethoxycarbonyl; Fmoc-OSu, 9-Fluorenylmethoxycarbonyloxysuccinimide. middle of the peptide sequence. For example, a cyclic ArgGlyAsp (RGD) motifcontaining peptide analog, 18F-c(RGDfk) (44), was synthesized in 25% RCY. The RGD analogs could be used as PET tracers, for example, in the monitoring of angiogenesis of tumor cells.66 Chapter 6 • Synthesis and applications of 18F-labeled compounds 243 6.6 Transition metalmediated radiofluorination 6.6.1 Mn(III)-catalyzed radiofluorinations Groves and coworkers have demonstrated Mn(III)(salen)OTs-catalyzed late-stage 18F-fluorination of aliphatic CH bonds, using no-carrier-added [18F]fluoride, and used this facile-labeling synthetic strategy for the radiofluorination of a range of pharmaceutically active compounds, such as ibuprofen (a nonselective COX inhibitor), rasagiline (MAO-B inhibitor), dopamine (neurotransmitter), celecoxib (a COX-2 inhibitor), and enalaprilat (ACE inhibitor), in moderate to high RCYs (Fig. 631).67 The chiral Mn(salen) complexes were also shown to achieve enantioselective fluorination in some cases (up to 25% ee). N N Mn (III) O O Mn(III)(salen)OTs (10 mol%) R PhIO (1 equiv); [18F]F H 18 R K2 CO3 , acetone 50°C, 10 min t-Bu F TsO t-Bu RCC: 20%–72% t-Bu t-Bu Mn(III)(salen)OTs Selected examples: CO2 Me O N 18 F 18 CF3 18 F-Ibuprofen (COX inhibitor) F RCC: 51% 18 F-TFA-rasagiline (MAO-B inhibitor) EtO F3 C N N NHBoc AcO RCC: 65% RCC: 72% 18 F AcO 18 SO2 CH3 F-dopamine derivative (neurotransmitter) O H3 C F 18 O N COCF3 N CO2 Et CH 218F RCC: 46% RCC: 23% 18 F-celecoxib analog (COX-2 selective inhibitor) 18 F-enalaprilat (ACE inhibitor) derivative FIGURE 6–31 Mn(salen)-catalyzed radiofluorination of aliphatic CH bonds, with some illustrative applications in the preparation of 18F-labeled pharmaceuticals. 244 Organofluorine Chemistry The proposed mechanism (Fig. 632) involves 18F2 exchange of the tosylate 45, followed by oxidation of the Mn(salen) 46 by iodosobenzene to give the Mn(V) complex 47. Hydrogen atom abstraction of the aliphatic CH bond by the Mn(V) complex gives the alkyl free-radical species (RU) that abstracts the fluorine atom from the Mn(IV)18F N Mn III O O OTs t-Bu t-Bu N t-Bu t-Bu 45 18 F TsO N MnIII O O 18 F t-Bu t-Bu N t-Bu t-Bu PhI = O 46 18 F Ph-I R H N N OH N Mn III O O t-Bu t-Bu t-Bu O N Mn V O O 18 t-Bu t-Bu F t-Bu t-Bu 47 t-Bu 49 RH R 18 F R. R. t-Bu N OH N Mn IV O O 18F t-Bu t-Bu R t-Bu . 48 FIGURE 6–32 Aliphatic CH radiofluorination by the Mn(salen) complex: schematic outline of the reaction mechanism. Chapter 6 • Synthesis and applications of 18F-labeled compounds 245 complex 48 to give the corresponding 18F-labeled compound and the Mn(III) complex 49.67 This CH activation cycle is further propagated through fluoride exchange to give the 18 F-labeled Mn(III) complex 2. These radiofluorination reactions proceed under relatively mild conditions with a short reaction time of about 10 min to give moderate to high radiochemical conversions. Whereas the abovementioned CH radiofluorination using the Mn(salen) complexes is restricted to the relatively weaker benzylic CH bonds, CH radiofluorination using tetrakis (pentafluorophenyl)porphyrin Mn(III)tosylate (Mn(TPFPP)OTs) (50) proceeds at the most electron-rich methylene (CH2) and methine (CH) (but not at the methyl) CH bonds that are farther removed from the electron-withdrawing groups such as carbonyl groups.68 Thus, using Mn(TPFPP)OTs porphyrin (50)-based catalyst and iodosobenzene (PhIO) as the terminal oxidant, butyl benzoate is radiofluorinated at the distant methylene group, affording 3-[18F]fluorobutyl benzoate in about 39% radiochemical conversion. Groves and coworkers, using this approach, synthesized a variety of 18F-labeled pharmaceutical analogs, such as amantadine (antiparkinsonian and antiviral agent), ezetimibe (cholesterol-lowering drug), flutamide (prostate cancer), lyrica (anticonvulsant), and 18F-labeled amino acid derivatives (Fig. 633).68 Ar F N MnIII N N N Ar F 50 (10 mol%) R18 F R H PhIO (1.7 eq.), K2CO 3, 18F Ar F – F TsO Ar 50 (Mn(TPFPP)OTs; Ar F = pentafluorophenyl) acetone/ACN, 50 °C, 10 min OAc 18 O F BocHN 18F O N 18 O F RCC: 19% RCC: 39% RCC: 31% 18 F-N-Boc-amantadine (anti-parkinsonian and antiviral) F 18 F-ezetimibe analog (cholesterol-lowering drug) O O2 N O F3 C N H 18 F 18 NHBoc RCC: 32% 18 18 F-flutamide derivative (prostate cancer) NHBoc O F RCC: 22% F-lyrica Boc protected) (anticonvulsant) FIGURE 6–33 Mn(III)-catalyzed radiofluorination of pharmaceuticals. O 18 F 18 O RCC: 29% F-leucine derivative (amino acid transporter) 246 Organofluorine Chemistry 6.6.2 Pd-catalyzed radiofluorinations Ritter and coworkers have developed the late-stage electrophilic fluorination of aromatics to give the aryl[18F]fluorides using Pd catalysis. In this transformation the electrophilic Pd(IV)[18F]F (55), generated through ligand exchange with the carrier-free [18F]fluoride anion with the Pd(IV) complex 54, was allowed to react with the preformed arylpalladium(II) complexes (53) to give the corresponding aryl[18F]fluorides (56).34 The Pd(IV) complex 54, in which the polydentate tris(pyrazolyl)borate ligand helps prevent the reductive elimination in the high valent Pd(IV) complex, was synthesized through Selectfluor oxidation of the corresponding Pd (II) complex. Through this late-stage electrophilic fluorination, pharmaceutically interesting compounds, such as [18F]fluorodeoxyestrone (58), were synthesized in high RCYs (Fig. 634). Using nucleophilic radiofluorination strategy combined with Pd(0)-catalyzed fluoroalkylation of aryl halides (or tosylates), Ritter and coworkers have synthesized 18F-difluoromethylarenes (68) from aryl halides or tosylates (63) in three steps, involving Pd(0)-catalyzed fluoroacetophenonation of aryl halides (tosylates), followed by enolate bromination, [18F]fluoride anion exchange of the bromide (5 min at 100 C), and potassium hydroxide (KOH)-mediated debenzoylation (5 min at 100 C).69 A variety of functional groups on the aromatic ring, such as carbonyl, morpholine, ester, amino, and olefinic moieties are unaffected under these reaction conditions, and this synthetic method was adaptable to the synthesis of 18F-labeled pharmaceutically interesting compounds, such as fluoxetine (71; antidepressant), claritin (72; antiallergic), estrone (73; a hormone), fenofibrate (cholesterol-lowering drug), and ezetimibe analogs (cholesterol-lowering drug) (Fig. 635). 6.6.3 Au(III) catalysis for the synthesis of [18F]trifluoromethyl compounds Toste and coworkers have synthesized 18F-radiolabeled aliphatic trifluoromethyl compounds starting from the N-heterocyclic carbene (NHC)-stabilized alkylbis(trifluoromethyl)Au(III) complex (74) in three steps, with the final step being the radiofluorination.70 Reaction of the NHC-stabilized alkylbis(trifluoromethyl)Au(III) (74), synthesized via a multistep process, with tris(perfluorophenyl)boron in the presence of trimethylsilyl bromide results in the abstraction of the fluoride anion from the [Au]-CF3 (74) by the sterically crowded Lewis acid (C6F5)3B forming the transient [Au]CF21 cation (75), which undergoes migratory insertion of the [Au]R bond with concomitant bromide anion capture to give the [Au]CF2R(Br) (76). Anion exchange of the latter compound using silver acetate, followed by reaction with Kryptofix2.2.2-complexed [18F]fluoride anion, results in the outer-sphere (C6F5)3B-mediated CF218F bond formation, concomitant with reductive elimination to give the 18F-labeled aliphatic trifluoromethyl compounds and the [Au](I) complex.70 Stoichiometric amounts of the Au (III) catalyst is required for this radiofluorination, because the byproduct Au(I) cannot be retransformed to Au(III) under these reaction conditions. Using this synthetic procedure, a pharmaceutically interesting Bayer lead compound [18F]BAY 59-3074 was synthesized in 12% radiochemical conversion (Fig. 636). Chapter 6 • Synthesis and applications of 18F-labeled compounds 247 B(OH) 2 O O S Ar B(OH) 2 (OAc) N Pd N 52 N R (Ligand exchange) OAc 51 O O S Ar N Me 2+ N 2 – OTf 18 F Pd N + N – N N Pd B N N N OTf N N N N 18 – F Pd 18-Cr-6 KHCO3 /acetone N N B N N N H R Me O Acetone, 85 °C, 10 min 56 H 55 H F N N Me O H R N 55 54 18 (53) N H 18 H F [Pd] 58 57 RCY = 92% [Pd] 18 F 55 Acetone, 85 °C, 10 min OBn OBn 59 60 RCY = 93% H O BocHN 55 H H O BocHN Acetone, 85 °C, 10 min H [Pd] 18 F 62 61 RCY = 18% FIGURE 6–34 Pd(IV)-catalyzed radiofluorination of arylboronic acids. 6.6.4 Ni(II)-catalyzed radiofluorinations The Pd-catalyzed radiofluorinations, despite being efficient, require longer reaction time and high temperature for the radiofluorination and thus have limited scope in their automation. In order to shorten the reaction time and to reduce the number of steps, Ritter and 248 Organofluorine Chemistry 50%–82% yield ACN/PhCl (20 μL/0.2 mL) KOH/H2 O (45 wt.%, 40 μL) FIGURE 6–35 Pd(0)-mediated radiofluorination at the benzylic site for preparation of pharmaceutically active compounds, such as fluoxetine and estrone analogs. coworkers have developed a one-step oxidative radiofluorination of the ArNi(II), using aqueous 18F2 and a hypervalent iodonium oxidant. The intermediate Ar(18F)Ni(III) species formed in the latter step spontaneously undergoes reductive elimination at room temperature in less than 1 min to give high RCYs of the 18F-labeled compounds (Fig. 637).71 The air- and Chapter 6 • Synthesis and applications of 18F-labeled compounds 249 F B(C 6 F5) 3 IPr Au F3C CF3 Me3 SiBr/B(C 6F 5) 3 R IPr Au F 3C DCM or DCE RT - 80 °C F C R IPr F F3 C F C R Au F Me 3SiBr Me3 SiF – B(C 6 F5 )3 75 74 k[18 F]F/Kryptofix-2.2.2 IPr Au F3C CF2 R Br IPr AgOAc Au F 3C DCM/MeOH DCM; 8–25 min CF2R 18 F R OAc F F 76 IPr(CF3 )Au i-pr IPr = i-pr N N i-pr i-pr Selected examples: 18 F O O 18 O F F F F F CF3 CN O O S O O 18 F F F [18F] BAY 59-3074 RCC = 31% FIGURE 6–36 Au(III)-catalyzed synthesis of RCC = 27% RCC = 12% 18 F-labeled aliphatic trifluoromethyl compounds. FIGURE 6–37 Oxidative radiofluorination of ArNi(II) complexes. moisture-stable ArNi(II) complexes were synthesized through oxidative addition of aryl halides to bis(cyclooctadiene)Ni(0) (Ni(COD)2), followed by sulfonamide ligand exchange. Using this synthetic approach, Ritter and coworkers have synthesized human-injectable [18F] fluorouracil, a PET tracer for cancer diagnosis.72 250 Organofluorine Chemistry 6.6.5 Cu(I)-catalyzed radiofluorinations Cu(I)-catalyzed radiotrifluoromethylation, through the reaction of the in situ generated [18F] FCF2Cu with aryl and heteroaryl iodides, gives access to the [18F]trifluoromethyl aryl and heteroaryl PET tracers.73 The [18F]FCF2Cu is formed, in situ, through the reaction of methyl chlorodifluoroacetate (CF2ClCO2Me), [18F]F2 and CuI. Using this synthetic method, radiopharmaceuticals, such as [18F]fluoxetine (Prozac; antidepressant) and [18F]flutamide (Eulexin, prostate cancer therapeutic), could be synthesized from the corresponding aryl iodides with high radiochemical conversions and high radiochemical purity (Fig. 638). The two-step synthesis of [18F]fluoxetine, with a 37% RCY, was achieved in a total reaction time of 25 min, at 150 C. Under the similar reaction conditions, [18F]fluoxetine was synthesized in one step, with a RCY of 55%. [18F]fluoxetine (Prozac) [18F]flutamide (Eulexin) FIGURE 6–38 Cu(I)-catalyzed synthesis of [18F]fluoxetine and [18F]flutamide; TMEDA 5 tetramethylethylenediamine. 6.7 Radiofluorination via diaryliodonium salts Aromatic nucleophilic substitution reactions of diaryliodonium salts (e.g., 7779) using the [18F]fluoride anion give the corresponding [18F]fluoroaryl compounds. Electronreleasing groups ortho- or para- to the iodonium moiety disfavor the SNAr reactions, so the inert aryl group in the iodonium salts should consist of electron-releasing groups (e.g., OMe and Me) for optimal radiochemical conversions of the diaryliodonium salts to the desired PET tracers.74 For example, 4-[18F]fluorophenylalanne can be synthesized through the iodonium salts, in which one of the aryl rings is p-methoxyphenyl or 2,4,6-trimethylphenyl (mesityl). The latter reaction proceeds under relatively milder conditions using Cu (I) catalysis, and the radiofluorination does not require the use of the macrocyclic crown ether, Kryptofix2.2.2 (Fig. 639).75 The diaryliodonium strategy has been used for the Chapter 6 • Synthesis and applications of 18F-labeled compounds MeO OH Toluene, 150 °C, 4 min OMe N(Boc) 2 I O 1. [ 18F]KF/Kryptofix-K2.2.2 O 2. HI, 150 °C, 4 min OTf 77 251 NH2 18 F 4-[ 18F]fluorophenylalanine RCC: 40 % MeO I O O Me Me OMe NHBoc I Me 1. [ 18F]KF (eluted with K2 CO3/MeOH) OTf OH 2. MeOH evaporation 3. (CH 3 CN)4 CuOTf, DMF, 85 °C, 20 min 4. 12 M HCl/ 140 °C, 10 min 78 Me N O I OTs 79 F N N N Boc 1. n-Bu N[18 F]F, Bu NHCO , 4 4 3 DMF, 130 °C, 10 min 2. 2 N HCl, MeCN/H2O (3:1) 100 °C, 10 min NH2 18F 4-[ 18F]fluorophenylalanine RCY: 53%–66% (109 GBq/μmol) NH O 18 F 80 F N N N 5-HT 2C receptor selective PET tracer RCY: 7.8% (89 GBq/μmol) >99% radiochemical purity FIGURE 6–39 Radiofluorination of diaryliodonium salts for the synthesis of [18F]fluoroaryl PET tracers. PET, Positron emission tomography. synthesis of a pyrimidine derivative 80, which exhibited relatively high specific binding to 5-HT2C receptors in vivo in the rat brain, comparable with that of lorcaserin, a FDAapproved selective 5-HT2C antagonist.76 The abnormal functioning of the latter 5-HT2C receptors affects signaling mechanisms mediated by various neurotransmitters, including epinephrine, GABA, glutamate, and dopamine, and results in psychiatric disorders, such as schizophrenia, depression, and anxiety. The diaryliodonium strategy was used in the automated synthesis of the [18F]fluorodopamine in high specific yields.77 The diaryliodonium [18F]fluoride salt, formed after anion exchange of the corresponding triflate salt ([2-[2-[bis[(1,1-dimethylethoxy)carbonyl] amino] ethyl]-4,5-dimethoxyphenyl](4-methoxyphenyl) iodonium triflate; Fig. 640), with the [18F] fluoride anion, in the presence of the phase-transfer agent Kryptofix2.2.2, was thermolyzed at relatively high temperature to afford the radiofluorinated product. The OMe and the Boc protecting groups were removed after the radiofluorination using aqueous HI. With a slight modification of the latter synthetic procedure, using O-ethoxymethyl protecting groups, the radiofluorination proceeds under relatively mild conditions and facilitates the final 252 Organofluorine Chemistry O N(Boc)2 I+ O – OTf 1. Kryptofix-K2.2.2- 18F MeCN/Toluene, 150 °C 2. HI (7.6 M), 155 °C NH3+ HO HO 18F O O O O N(Boc)2 I+ O – 1. Kryptofix-K2.2.2-18F OTf MeCN/Toluene, 120 °C NH3+ HO HO 18 F 2. HCl (4 M), 95 °C O 18 FIGURE 6–40 Synthesis of [ F]fluorodopamine through nucleophilic aromatic substitution of aryliodonium salts. purification.78 Through this improved procedure, [18F]fluorodopamine was obtained in .99% radiochemical purity after recrystallization from methyl tert-butyl ether (Fig. 640). [18F]fluorodopamine PET tracer is transported via the norepinephrine transporter and thus is useful for the diagnostic imaging of the neuroblastoma. A pediatric neuroblastoma imaging (PETCT) trial of this PET tracer revealed high quality images within minutes after its injection.78 6.7.1 Cu(I)-catalyzed radiofluorination of diaryliodonium salts Cu(I) catalysis of the diaryliodonium salts provides a convenient method for the radiofluorination of the electron-rich aromatics. The diaryliodonium(III) salts (83) could be synthesized in situ from the corresponding electron-rich aromatics and mesityl iodonium(III) reagent under ambient conditions, through electrophilic aromatic substitution reaction. In this reaction, TMSOTf transforms the mesityl-iodo hydroxyl moiety in 81 into a better leaving group, forming the mesityl-iodonium triflate (82). This electrophilic iodonium cation undergoes FriedelCrafts reaction with the aromatics to give the diaryliodonium salt 83. A Cu(I)-catalyzed radiofluorination of the diaryliodonium salt 83 at 85 C provided the 18F-labeled aromatics in high radiochemical conversions and with high specific activities.30 This synthetic strategy is thus useful for the late-stage radiofluorination of aromatics, such as toluene, anisole, aniline, pyrrole, and thiophene derivatives, and it could be automated for the synthesis of pharmaceutically interesting compounds, such as nimesulide (an antiinflammatory agent), and propofol, an anesthetic compound (Fig. 641). Overall, this radiofluorination is an aryl CH activation, the reaction proceeding through the FriedelCrafts iodination to give the diaryliodonium(III) intermediate, which undergoes Cu(I)-catalyzed reductive elimination to give the radiolabeled aryl fluorides. Chapter 6 • Synthesis and applications of 18F-labeled compounds 253 FIGURE 6–41 Synthesis of [18F]fluoroarenes using aryliodonium reagents. TMSOTf, Trimethylsilyl triflate. 6.7.2 Radiofluorination via iodonium ylides The spirocyclic hypervalent iodonium (III) ylides (86) are bench-stable intermediates and provide an efficient radiofluorination of nonactivated aromatics.79 They can be synthesized from the aryl iodides in two short steps, involving the mCPBA (or oxone)-mediated oxidative iodo-acetoxylation (or iodo-trifluoroacetoxylation) of aryl iodides to give 84, followed by base-catalyzed condensation of 84 with the spirocyclic diester 85 to give the iodonium ylide 86. These spirocyclic iodonium ylides are resistant to decomposition and disproportionation pathways that are usually accompanied with the conventionally used nonspirocyclic iodonium ylides, such as those based on the Meldrum’s acid. Reaction of the spirocyclic iodonium ylides with no-carrier-added [18F]fluoride ion in the presence of quaternary ammonium salts, such as tetraethylammonium bicarbonate, proceeds in DMF at 120 C in 254 Organofluorine Chemistry 10 min to give the corresponding 18F-labeled aromatics in high RCYs. The radiofluorination of these ylides is tolerant to a variety of functional groups in the aryl ring, such as carbonyl, ether, amine, halogens, and nitro moieties. Aromatics with electron-withdrawing or electrondonating groups, as well as those with sterically crowded substituents, undergo radiofluorination under these conditions (Fig. 642). A possible mechanism of radiofluorination of the iodonium ylides involves nucleophilic addition of the fluoride anion to the ylide iodine(III), O I mCPBA or Selectfluor CH3CO 2H R O O OAc I OAc O O I O (85) O Na2CO3, 25 °C R 84 18 [ 18F]F O R 86 ~85% yield F R TEAB, DMF, 120 °C, 10 min O Mechanistic outline: O I O O (88) 18 O F O 18 [ 18F]F I O F I O O O R 18 O R O R 87 86 Selected examples: NHCbz 18 F 18 18 F O F CO2Me O 5-[ 18F]fluorouracil (anticancer pharmceutical) RCC: 11% Specific activity: 398 mCi/mmol 18 RCC: 65% H 3C O H OMe NHCO2Et F H 18 4-[ 18F]fluorophenylalanine (N,O-protected) RCC: 55% FIGURE 6–42 Radiofluorination via spirocyclic iodonium ylides. F N O NH N H F RCC: 77% RCC: 22% RCC: 44% 18 18 CO2Me H F [18F]fluoroestrone RCC: 23% F Chapter 6 • Synthesis and applications of 18F-labeled compounds 255 followed by reductive elimination of the aryl [18F]fluoride (with expulsion of the resonance stabilized α-iodoenolate). The spirocyclic iodonium ylides are relatively more stabilized toward the reductive elimination of the starting materials (due to the conformational enforcement of the stabilizing interactions between the carbonyls and iodine) as compared to those of Meldrum’s acid (2,2-dimethyl-1,3-dioxan-4,6-dione)-derived analogous nonspirocyclic iodonium ylides. This enhanced stabilization of the ylides prevents their degradation through reductive elimination, as is the case with Meldrum’s acid-derived (and related) iodonium ylides, and therefore relatively higher RCYs of the [18F]fluorinated products were realized through the use of spirocyclic iodonium ylide reagents. Furthermore, the spirocyclic iodonium ylides are crystalline salts that could be readily synthesized from the corresponding aryl iodides or (diacetoxyiodo)arenes in a one-step process. Pharmaceutically significant 18F-labeled PET tracers, such as [18F]fluorouracil and [18F] fluoroestrone, and [18F]phenylalanine, were synthesized in moderate radiochemical conversions using the latter spirocyclic iodonium ylide strategy (Fig. 642). Vasdev and coworkers have synthesized 18F-labeled lorlatinib, an anticancer drug to treat NSCLC, that is, in phase III clinical trials, using this strategy (Fig. 644; vide infra).80 Liang and coworkers have synthesized an 18F-labeled translocator protein 18 kDa (TSPO)-specific PET ligand, [ 18F]FDPA (93), via the spirocyclic iodonium ylide strategy, using no-carrier-added [18F]F2, in high yield and with high specific activity.81 They have demonstrated saturable specific binding of the [ 18F]FDPA to TSPO in the preclinical models of brain neuroinflammation that are associated with cerebral ischemia and AD (Fig. 643). O O N N I OCOCF3 N N Oxone TFA/CHCl3 O NEt2 O (91) I OCOCF3 O O 10% Na2CO3, EtOH RT, 70 min NEt2 90 89 O N N I O O N N O [18F]F – (eluted from QMA cartridge) O NEt2 92 34% yield for the combined two steps TBAOMs or TEAClO4 , DMF 120 ºC/15 min 18 F O NEt2 93 [18F]FDPA RCC: ~20–40% FIGURE 6–43 Synthesis of [18F]FDPA, a PET tracer for brain neuroinflammation in cases of Alzheimer’s disease and cerebral ischemia. PET, Positron emission tomography; TBAOMs 5 tetrabutylammonium methanesulfonate; TEAClO4 5 tetraethylammonium perchloroate. 256 Organofluorine Chemistry 6.8 Enzymatic fluorination reactions for [18F]-labeled positron emission tomography tracers 6.8.1 50 -Fluoro-50 -deoxyadenosine and 5-fluororibose O’Hagan and coworkers have pioneered the fluorinase-catalyzed synthesis of 50 -fluoro-50 deoxyadenosine (95) and extended this approach for the synthesis of 18F-labeled nucleosides and their derivatives, such as 50 -fluoro-50 -deoxyinosine, 50 -deoxy-50 -fluororibose, and fluoroacetate.82 Fluorinase enzyme can be obtained in multimilligram scale through overexpression of the protein in the bacterium Streptomyces cattleya. This enzyme-catalyzed fluorination reaction was earlier shown to proceed through a bimolecular nucleophilic substitution (SN2) mechanism with inversion of configuration at the reacting site.82,83 Singlecrystal X-ray structural studies revealed that the CS bond breakage is in concert with the CF bond formation in the enzyme active site.83 The fluorinase-catalyzed radiofluorination is reversible, and under optimal conditions, 18F-labeled 50 -fluoro-50 -deoxyadenosine was obtained in over 95% RCYs. Coupling of this reaction with adenylic acid deaminase enzyme helped shift the equilibrium toward the formation of the [18F]-5-fluoro-50 -deoxyinosine (96), whereas coupling of the fluorination reaction with purine nucleotide phosphorylase (to give the 18F-labeled D-ribose-1-phosphate), followed by phytase-catalyzed dephosphorylation, afforded [18F]-5-fluoro-5-deoxyribose (97) in high RCYs (Fig. 644). NH2 NH2 N CH3 S O –O NH3 + N N F O N 18 F 18 – N N O + NH3 Fluorinase + CH 3 L-methionine OH OH OH S –O O 18 – F N N 95 94 1. Purine nucleotide phosphorylase 2. Phytase-catalyzed dephosphorylation Adenylic acid deaminase OH N N 18 F O OH OH 96 N N 18 F O OH OH OH 97 FIGURE 6–44 Fluorinase-catalyzed synthesis of 50 -[18F]fluoro-50 -deoxyadenosine (95), 50 -[18F]fluoro-50 -deoxyinosine (96), and 5-[18F]fluororibose (97). Chapter 6 • Synthesis and applications of 18F-labeled compounds 257 6.8.2 Fluorinase-catalyzed synthesis of [18F]50 -deoxy-50 -fluoroadenosinebiotin conjugate Fluorinase enzyme is also effective in the transhalogenation reactions. Fluorinase-catalyzed transhalogenation of 50 -chloro-50 -deoxyadenosine-PEG-biotin conjugate (99) using [18F]F2 afforded the 18F-labeled biotin conjugate (100) that has potential applications in PET imaging and as a therapeutic due to the strong affinity of the biotin to the avidin/streptavidin (Fig. 645).84 NH2 O N N N HN O N Cl O O O O NH2 O H 98 DMF, RT, 24 h (+)-Biotin NH2 N N Cl O N HN N O O O O O H N NH H H 4 S F pH 7.8 NH2 O 18 – 99 N N Fluorinase, L-selenomethionine O OH OH PyBOP, DIPEA H HO 4 S OH OH 18F NH O N N HN O O O O H N H NH H 4 S O OH OH 100 FIGURE 6–45 Synthesis of biotin conjugate of the 50 -[18F]fluoro-50 -deoxydenosine-biotin conjugate. 6.8.3 50 -Fluoro-50 -deoxyadenosine-RGD conjugate in cancer detection Fluorinase-catalyzed radiofluorination of 50 -chloro-50 -deoxy-2-ethynyladenosine (101; 69% radiochemical conversion), followed by azidealkyne click reaction of the 18F-labeled 102 with a RGDFK cyclic peptide-derived terminal azide, 103 afforded the 18F-labeled RGDFK conjugate 104 (Fig. 646). The latter RGD cyclic peptide conjugate was shown to selectively bind to αvβ3 integrin, which is located primarily in the liver and intestinal walls, kidneys, and bladder. A PETCT image of this radiotracer was consistent with that of the natural biodistribution of the αvβ3 integrin, showing its potential applications in cancer detection and in developing effective therapeutics.85 258 Organofluorine Chemistry NH 2 NH2 N N N Cl O N N N Fluorinase/L-selenomethionine 18 [ F]F N 18 F N O – N N OH OH OH OH N 102 101 O O N H NH 2 NH O HO HN NH N H NH HN O O O 103 NH2 N 18 F N N N N N N O OH OH O O N H O HO NH NH NH 2 HN N H NH HN O O O 104 FIGURE 6–46 Fluorinase-catalyzed synthesis of 50 -fluoro-50 -deoxyadenosine and synthesis of its RGDFK conjugate through Cu(I)-catalyzed azidealkyne cycloaddition. 6.9 Positron emission tomography tracers in Alzheimer’s disease There are more than 5 million people living with AD in the United States, and there are about 50 million AD cases worldwide. It is expected that this number would reach 75 million by 2030 and to 132 million by 2050 worldwide as the aging population increases. It is estimated that the cost of caring for AD patients is $800 billion per year and would rise to $2 trillion by the year 2030, as the number of the AD cases continue to rise.86 AD is a devastating neurologic disorder and is characterized by the progressive accumulations of Aβ plaques and tau-protein aggregates, NFTs, in the brains of the affected individuals. Although the causative factors for the AD are not clearly established, the extent of the Aβ and NFT accumulations correlates with the progression of the AD and its associated dementia. The 11C-labeled PiB along with three other 18F-labeled compounds, florbetaben (Neuraceq), florbetapir (Amyvid), Chapter 6 • Synthesis and applications of 18F-labeled compounds 259 and flutemetamol (Vizamyl), are approved by the FDA for imaging Aβ plaques in the brains (Fig. 61).87,88 These PET tracers show high specificity for binding to the Aβ plaques (see Fig. 63 for the florbetapir PET imaging). Among the PET tracers for selective binding to NFTs, flortaucipir-18F (T807; AV-1451; LY 3191748; Fig. 640) has emerged as the leading candidate, after its phase III clinical trials (vide infra). Some of the quinoline-derived 18F-PET tracers, such as THK series (vide infra) and related isoquinoline-based 18F-PET imaging agent, 18F-MK6240, are currently at various stages of clinical trials for their selective NFT-binding.89 Eli Lilly’s flortaucipir- 18F (T807; AV-1451; LY 3191748), a selective NFT imaging agent, is a welcome addition for the precise diagnosis of the AD, although it is not yet approved by the FDA. Correlation between the obstructive sleep apnea syndrome and the extent of aggregation of the Aβ and NFTs was demonstrated through the combined use of [ 18F]Florbetaben and [18F]flortaucipir PET brain imaging.90 These PET imaging studies showed that the obstructive sleep apnea is correlated with AD pathology ant that this correlation is moderated by apolipoprotein E ε4 allele (APOE ε4) and body mass index. 6.9.1 [18F]Flortaucipir (a neurofibrillary tangle biomarker) Flortaucipir-18F (T807; AV-1451; LY 3191748; Fig. 647) shows substantial selectivity in its binding to NFTs (tau-protein aggregates) over Aβ peptide aggregates, and the PET brain scans are diagnostic of AD and to some extent at the prodromal stage of the AD, also called mild cognitive impairment (MCI). Through clinical trials consisting of 710 participants, it was demonstrated that [18F]flortaucipir PET was able to discriminate AD from other neurodegenerative diseases, such as behavioral variant frontotemporal dementia, nonfluent variant primary progressive aphasia, semantic variant primary progressive aphasia, dementia with Lewy bodies, progressive supranuclear palsy, corticobasal syndrome, Parkinson disease with or without cognitive impairment, and vascular dementia.91 In September 2018 Eli Lilly has successfully completed the Phase III clinical trials on the effectiveness of the Flortaucipir-18F (T807; AV-1451; LY-3191748) for PET imaging of the brain tau-NFTs, and pending FDA approval, this PET imaging agent would be a clinically useful biomarker for the tau-neuropathy, such as AD.92 [18F]flortaucipir PET is also diagnostic of the chronic traumatic encephalopathy (also called traumatic brain injury), as shown in a recent PET scan of a former football player with concussion symptoms.93 Although flortaucipir ([18F]AV-1451) is among the most widely used PET tracers for imaging NFTs, there is substantial evidence that flortaucipir has also off-target binding, that is, it also binds to regions other than NFTs, including monoamine oxidase A and B, pigmented cells in the central nervous system, and elevated iron levels.94 This off-target binding of flortaucipir, as demonstrated by the PET imaging of a normal control, is localized mostly to basal ganglia, choroid plexus (CP), and substantia nigra (SN). Similar PET imaging of AD 260 Organofluorine Chemistry (A) (B) FIGURE 6–47 Two slices of representative clinical [18F]AV-1451 PET scans of a 69-year-old normal control (A) and 71-year-old tau-positive AD patient (B). Both sets of images clearly show off-target binding in the BG, CP, and SN. The AD patient also has significant tau burden in the EC, TL, and OC. PET images are mean SUVR images 75105 min postinjection; MRI images are MP RAGE (T1 weighted) images. AD, Alzheimer’s disease; BG, basal ganglia; CP, choroid plexus; EC, entorhinal cortex; MRI, magnetic resonance imaging; OC, occipital cortex; PET, positron emission tomography; SUVR, standardized uptake value ratio; SN, substantia nigra; TL, lateral temporal lobe. Adapted from Drake, L.R.; Pham, J.M.; Desmond, T.J.; Mossine, A.V.; Lee, S.J.; Kilbourn, M.R.; Koeppe, R.A.; Brooks, A.F.; Scott, P.J.H. Identification of AV-1451 as a Weak, Nonselective Inhibitor of Monoamine Oxidase. ACS Chem. Neurosci. 2019, 10, 38393846, Copyright 2019, American Chemical Society. patient shows that flortaucipir has significant tau burden in the entorhinal cortex, lateral temporal lobe (TL), and occipital cortex, in addition to the off-target binding to the SN and CP (Fig. 647).94 Chapter 6 • Synthesis and applications of 18F-labeled compounds 261 6.9.1.1 Synthesis of [18F]flortaucipir Pd(0)-catalyzed Stille cross coupling of N-t-Boc protected 7-bromo-5H-pyrido[4,3-b]indole with 2-nitro-5-(trimethylstannyl)pyridine, followed by SNAr [18F]fluorination of the resulting T807P precursor compound (7-(6-nitropyridin-3-yl)-5H-pyrido[4,3-b]indole) with K[18F]F/ Kryptofix2.2.2 in dimethylsulfoxide (DMSO) gave the [18F]Flortaucipir in 5%10% decay corrected RCY (Fig. 648). This radiofluorination is fully automated.95 The SNAr radiofluorination was also accomplished using similar substrates with other leaving groups, such as trimethylammonium moiety (instead of NO2).96 [ 18F]flortaucipir; T807 5%–10% FIGURE 6–48 Synthesis of [ F]flortaucipir through end-stage SNAr radiofluorination. Boc, tert-Butyloxycarbonyl; DCM, dichloromethane; DMAP 5 4-(N,N-dimethylamino)pyridine; HPLCSPE 5 high performance liquid chromatography, combined with solid phase extraction. 18 6.9.2 2-(4-Aminoaryl)quinoline-based 18F-labeled positron emission tomography tracers (THK series) Okamura and coworkers have synthesized tau-protein aggregate (NFT) specific 18F-labeled 2-(4-aminoaryl)quinoline-based compounds [18F]-THK-5105, [18F]-THK-5117, [18F]-THK5116, and [18F]-THK-523.97 In vitro binding studies of these compounds through autoradiographic analyses of AD brain sections demonstrated the selective binding to the NFTs. Of these compounds, [18F]-THK-5105 and [18F]-THK-5117 are promising PET tracer candidates for tau imaging, as they do not have significant binding to other neuroreceptors, ion channels, and transporters at 1-μM concentration. The THK-series of compounds were synthesized from their corresponding tosylates using 18F-labeled KF in the presence of the Kryptofix2.2.2, and this procedure is amenable to the automated radiosynthesis. The optically pure (S)-[18F]-THK-5351 was also synthesized using this protocol in 46% 6 13% RCY (radiochemical purity: 99%; specific activity: 254 6 47 GBq/mmol) (Fig. 649).98 Clinical studies of the latter optically pure compound on AD patients, along with Pittsburg B compound (11C-PiB), showed promising results in the diagnosis of the disease progression and had higher retention in the TL, clearly distinguishing the AD patients from 262 Organofluorine Chemistry OH 18 OH O F 18 F O N N CH3 N CH3 [18 F]-THK-5105 H N CH3 [18 F]-THK-5117 OH 18 F O 18 F O N [18 F]-THK-5116 N H N H N H [18 F]-THK-523 18 F OH 18 F (S) H 2N O N N N 18 [ F]-THK-5351 H N N CH3 N [18F]-MK-6240 (Merck Laboratories) Synthesis of THK series of PET tracers: OH OTHP TsO H 18 F 18 O 1. K F N N R R O K2 CO3 , Kryptofix-2.2.2 DMSO, 110 °C, 10 min 2. 2M HCl, 3 min (deprotection of THP) N N R R [18F]-THK-5116 (R = H) [18F]-THK-5105 (R = Me) TsO O 18 F 1. K18F N N H H K2 CO3 , Kryptofix-2.2.2 O N DMSO, 110 °C, 10 min [18F]-THK-523 N H H 18 FIGURE 6–49 Structures of the quinoline- and isoquinoline-based F-labeled PET tracers and the synthesis of the THF series of compounds. PET, Positron emission tomography; THF, tetrahydrofuran. the healthy controls. The [18F]-THK-5351 also showed higher contrast and lower subcortical white matter retention that that for the [18F]-THK-5117 and is comparable to that of the 11 C-PiB.98 In a relatively larger clinical trial the compound [18F]-THK-5351 showed relatively higher retention in the frontal, lateral temporal, superior parietal, inferior parietal, anterior Chapter 6 • Synthesis and applications of 18F-labeled compounds 263 cingulate, hippocampus, and other regions in the brain, as compared to that of the control age-matched subjects.99 Furthermore, these PET studies showed that the retention of the [18F]-THK-5351 is negatively correlated with the cerebral glucose metabolism (i.e., the lower the cerebral glucose metabolism, the higher is the NFT aggregation), as monitored by [18F] FDG uptake, in AD and MCI cases. The preclinical studies demonstrated improved pharmacokinetic profiles for the S-enantiomer over the R-enantiomer for the [18F]-THK-5351 and related THK-series of compounds. [18F]-THK-5351 showed high selective binding to NFT, with low binding affinity for white matter and rapid pharmacokinetics, and the signal-to-background ratios of [18F]-THK5351were higher than those for the [18F]-THK-105 and [18F]-THK-5117. Autoradiography on human brain sections revealed that [18F]-THK-5351 binds with high specificity to NFTs, without any significant binding to Aβ, α-synuclein, and TDP43 (transactive response DNAbinding protein 43) deposits.98 6.9.3 Tropomyosin receptor kinase targeted 18F-positron emission tomography Tropomyosin receptor kinase A/B/C (TrkA/B/C) family of proteins is encoded by NTRK genes and is responsible for the human neuronal growth, survival, and differentiation. The downregulation of these kinases is responsible for neurological disorders, including AD, and cancers. Larotrectinib, a TrkA/B/C inhibitor, was approved by FDA in 2018 for the treatment of NTRK gene fusion-specific metastatic solid tumors. Bailey and coworkers have developed a pan-Trk selective 18F-PET tracer, [18F]TRACK for in vivo Trk PET imaging. The latter compound is in clinical development (Fig. 650).100 F N N O OH N N HN F N O F Larotrectinib (TrkA, TrkB, and TrkC inhibitor) N N H N N N 18 F [18F]TRACK Trk-targeted [18 F]-PET radiotracer FIGURE 6–50 Structures of an anticancer drug Larotrectinib, a pan-Trk inhibitor, and [18F]TRACK, a PET tracer for imaging human brains. PET, Positron emission tomography. [18F]TRACK, currently in preclinical studies, is permeable to the bloodbrain barrier and exhibits relatively faster and reversible kinetics as compared to the 11C-labeeled tradiotracer [11C-IPMICF16] (Fig. 651).100 264 Organofluorine Chemistry FIGURE 6–51 In vivo PET imaging of [18F]TRACK (A) and [11C]-(R)-IPMICF16 (B) in the human brain. Top row: T1MPRAGE MR images of the subject. Bottom rows: overlays of the 010, 1030, 3060, and 6090 min postinjection (p.i.) summed SUV PET images with the MR images. Scans with both radioligands were performed in the same healthy human subject. PET, Positron emission tomography; SUV, standardized uptake value. Adapted from Bailey, J.J.; Kaiser, L.; Lindner, S.; Wust, M.; Thiel, A.; Soucy, J.-P.; Rosa-Neto, P.; Scott, P.J.H.; Unterrainer, M.; Kaplan, D.R.; et al. First-in-Human Brain Imaging of [18F]TRACK, a PET Tracer for Tropomyosin Receptor Kinases. ACS Chem. Neurosci. 2019, 10, 26972702, Copyright 2019, American Chemical Society. 6.10 18 F-positron emission tomography tracers in cancer diagnosis 6.10.1 [18F]-(R)-lorlatinib Vasdev and coworkers have synthesized the 18F-labeled orphan receptor tyrosine kinase (c-ros oncogene 1 (ROS1)) inhibitor lorlatinib through their iodonium ylide strategy (Fig. 652).80 Reaction of compound 105 (synthesized in a multistep pathway) with Selectfluor and trimethylsilyl acetate (TMSOAc) afforded the diacetoxyiodo aryl compound 106, which was transformed into the corresponding spirocyclic iodonium ylide 108, in 36% overall yield for the two steps. Radiofluorination of 108 was achieved using 18F-labeled Chapter 6 • Synthesis and applications of 18F-labeled compounds H 3C O N N N CH3 N I H 3C O N AcO O Boc I OAc H3 C Boc N N CH3 O N Boc H 3C O N N I O N O ( 107 ) O O Na2 CO3 36% for two steps 106 H 3C O N O O O N Boc 105 O CH3 N Selectfluor/(Me) 3SiOAc N H3 C N N 265 Boc N 80 °C; 10 min O N Boc 18 F 2. HPLC purification N H3 C O Boc 108 2 mg in 400 μL of anhydrous DMF N Boc 109 H 3C O N 1. 4M HCl, 90 °C, 10 min (Boc deprotection) N N CH3 N 2. Neutralization to pH 5 18 (14% RCY for two steps) CH3 1. [18F]-Et 4 NF N H3 C N N F N H3 C O 110 H N H FIGURE 6–52 Synthesis of [18F]-(R)-lorlatinib through iodonium ylide route. tetraethylammonium fluoride in anhydrous DMF to give the 18F-labeled (R)-larlatinib (110), after deprotection of the Boc group, in 14% uncorrected RCY and with high radiochemical purity. The PET imaging of the C-11-labeled lorlatinib showed its high bloodbrain permeability. Lorlatinib is the next-generation small-molecule inhibitor of the ROS1 that is undergoing phase III clinical trials for NSCLC.101 The PET assays using 11C and 18F-labeled lorlatinib would help in establishing its biodistribution and whole-body dosimetry assessments in the clinical oncology. 6.10.2 Cyclic RGDYK (arginine-glycine-aspartic acid-tyrosine-lysine) dimer-derived positron emission tomography tracers 6.10.2.1 FPPRGD2 (dimeric cyclic RGDYK peptide) The integrin family of proteins, comprising 24 transmembrane receptors, helps in the integration of the cell adhesion and intracellular signaling, transmitting signals across the cell 266 Organofluorine Chemistry membranes upon ligand binding to the receptors. The integrin protein αvβ3, among other integrin proteins, serves as a receptor for the extracellular matrix proteins containing arginineglycineaspartic acid (RGD) sequence, and the αvb3 levels correlate with tumor metastasis and progression. The integrin αvβ3 is highly expressed in the epithelial cells of solid tumors, whereas it is expressed in low levels in the normal cells. Radiotracers consisting of RGD sequence-containing peptides uniquely are suited for probing the extent of tumor metastasis and for the development of antiangiogenic drugs for tumor suppression.102104 Angiogenesis is involved in the tumor growth and progression, and the expression of αvβ3 integrin and its interaction with matrix ligands play a crucial role in the tumor angiogenesis and metastasis. Thus, there is an emerging interest in the development of clinically useful RGD-PET tracers for targeting αvβ3 integrin proteins. A variety of 18F-labeled peptides incorporating RGD sequence have been synthesized and explored for their efficacy in PET-imaging and also as potential therapeutics for cancers.27 18 F-labeled FPRGD2 (2-fluoropropanoyl-labeled PEGylated dimeric cyclic RGDYK peptide) has been one such PET tracer that is approved by the FDA as an exploratory investigative new drug. This PET tracer is currently in clinical trials for predicting therapeutic efficiency of antiangiogenesis therapy in cancer patients.105,106 Synthesis of 18F-labeled FPRGD2 was achieved in a multistep synthetic strategy involving the radiofluorination as the early step through an automated radiosynthesis.107 Nucleophilic substitution of methyl 2-bromopropanoate using [18F]KF complexed to the phase-transfer agent 2,2,2-Kryptofix-(2.2.2), followed by alkaline hydrolysis gave potassium 2-[18]F-fluoropropanoate (112). Reaction of the latter 18F-labeled compound, 112, with di(4-nitrophenyl) carbonate (113), in acetonitrile at 110 oC, afforded the 4-nitrophenyl 2-[18]F-fluoropropanoate (114), which upon conjugation with the terminal amino group of the PEG-tethered dimeric version of the cyclic peptide—arginineglycineaspartic acid-tyrosine-lysine ((RGDYK)2PEG-NH2)—gave the 18F-labeled dimeric cyclic peptide FPPRGD2 (FPP (RGD)2).107 Under optimized conditions, this multistep process gave the PET-tracer in RCYs of 16.9% 6 2.7% with a specific radioactivity of 114 6 72 GBq/μmol (3.08 6 1.95 Ci/μmol) (Fig. 653). Clinical grade samples were obtained after a final HPLC purification. The FPPRGD2 PET tracer was shown to be superior to [18F]FDG or brain MRI for imaging glioblastoma multiforme (GBM).104 6.10.2.2 [18F]FAl-NOTA-PRGD2 (18F-alfatide) and [ 68Ga]-NOTA-PRGD2 Although the FDA-approved [18F]FPPRGD2 has favorable pharmacokinetic properties for its use as PET imaging agent to selectively bind to the αvβ3 integrin receptors, its synthesis involves several steps, with the [18F]fluoride incorporation being at the early stage of synthesis, and thus affords relatively low RCYs. One approach of synthesizing [18F]FPPRGD2 derivatives with high RCYs is based on the late-stage incorporation of the [18F]fluoride. It involves synthesis of the macrocyclic polyaminocarboxylates, such as NOTA (1,4,7-triazacyclononane1,4,7-triacetic acid) conjugates of the PPRGD2, followed by the complexation of the NOTA by [18F]AlF, using AlCl3 and no-carrier-added [18F]F2. This alternative approach involving latestate incorporation of the [18F]fluoride affords improved access to the 18F-labeled PET Chapter 6 • Synthesis and applications of 18F-labeled compounds 267 FIGURE 6–53 Synthesis the 18F-PET tracer FPPRGD2. PET, Positron emission tomography. tracers. The aluminum fluoride complex [18F]FAl-NOTA-PRGD2 (18F-alfatide) is an analog of the [18F]FPPRDG2, in which the 18F label was introduced at the end-stage of the synthesis through the complexation of the [18F]fluoride anion with the in situ generated Al(III) complex of the NOTA-PRGD2.108 The side chain NOTA was conjugated with the same PEGylated dimeric RGD peptide that was used in the synthesis of the original parent PET tracer [18F] FPPRGD2 so as to retain the pharmacokinetic properties (Fig. 654).109 A pilot clinical study 268 Organofluorine Chemistry O O O OH OH N H NH O HN H N NH O N H NH O O O HN RGD NH2OH HN O 18 NH O O F S O HN N Al N O N O O HN O O O OH 18 F-Al-NOTA NH HN OH O O NH O O HN H N HN NH2 NH O ( F-Alfatide; Alfatide I) O OH O O OH N H NH FAl-NOTA-PRGD2 18 O HN NH H N O O O HN O O NHO O O HN NH2 OH HN RGD H N O O O O O NH O O NH HN OH O NH O O O N H N O 18 Al N F NO O O 18 F-Al-NOTA O HN H N H N O HN NH2 NH O FAl-NOTA-E[PEG4-c(RGDFK)]2PRGD2 [18 F]Alfatide II) O OH O O OH N H NH O HN O N H NH HN O H N O O O O HN NH 2 OH HN RGD HN O O N H O S O HN O N 68 Ga O N O O O OH O NH O 18 F-Al-NOTA NH HN HN H N O O O HN NH2 NH [ 68 Ga]-NOTA-PRGD2 FIGURE 6–54 Structures of the PRGD2-based 18F- and 68Ga-PET tracers FAl-NOTA-PRGD2 (18F-alfatide), [18F]alfatide II, and [68Ga]NOTA-PRGD2; the [18F]fluoride and [68Ga] were introduced at the end-stage of the synthesis. PET, Positron emission tomography. Chapter 6 • Synthesis and applications of 18F-labeled compounds 269 of the 18F-alfatide in 13 patients with NSCLC demonstrated its high sensitivity, specificity, and accuracy for imaging the lymph node metastases.110 Due to the neighboring group participation of the thiourea moiety, alfatide is relatively unstable to hydrolytic degradation. In order to overcome this hydrolytic instability, a secondgeneration alfatide, called [18F]alfatide II (FAl-NOTA-E[PEG4-c(RGDFK)]2PRGD2), was developed.111 This PET tracer is well tolerated in healthy volunteers and was useful in the accurate diagnosis of brain metastatic lesions in the clinical trials. In contrast, methods using [18F]-FDG PET or CT were successful in about half the cases.111 A gallium analog of the alfatide I, [68Ga]-NOTA-PRGD2 (Fig. 654), in which Ga-68 was incorporated at the end-stage of the synthesis, as in the case of the alfatide, was investigated as a PET tracer in preclinical trials.105 The pharmacokinetic and imaging properties of the [18F]FPPRDG2, [18F]FAl-NOTA-PRGD2 ([18F]alfatide I), and [68Ga]-NOTA-PRGD2 in the U87MG glioblastoma xenograft models showed high tumor uptake with high target-tobackground ratios, for all three radiotracers. The latter readily synthesizable 18F-aluminumNOTA and 68Ga-NOTA PET tracers are promising PET tracers for monitoring angiogenesis in tumor cells through their selective binding to the αvβ3 integrin receptors. Cell-binding assays of these RGD-derived PET tracers in U87MG tumor cells revealed relatively improved half-maximal inhibitory concentrations (IC50) for the NOTA-derived PET tracers. The IC50 values for binding to these cells were determined as 175.4, 119.2, and 82.7 nM, respectively, for FPPRGD2, Al-NOTA-PRGD2, and Ga-NOTA-PRGD2, showing the improved efficacy for the AlF-NOTA-ORGD2 and 68Ga-NOTA-PRGD2 over the parent FPPRGD2.105 However, the final 18F labeling of the NOTA macrocyclic ligand proceeds at relatively high temperatures (100 C120 C for 10 min), and thus the applications of these tracers are limited to thermally stable peptides. 6.10.2.3 NOTA-conjugated linear peptides 18F-AlF-NOTA-IF7 and 18F-Al-NOTAMATBBN An 18F-complexed NOTA-derived polypeptide, 18F-AlF-NOTA-IF7 (Fig. 655; IF7 is a heptapeptide, IFLLWQR) was synthesized in two steps, involving peptide conjugation to the NOTA ligand, followed by complexation with AlCl3 in the presence of the [18F]F2 source. This 18F-PET imaging agent was found to target Anxal, a highly specific surface marker in tumor vasculature, with good tumor uptake, and is a promising PET tracer for imaging of cancers.112 A structurally similar linear peptide conjugated to the NOTA macrocyclic ligand, 18F-AlNOTA-MATBBN [(Fig. 655); MATBBN is a bombesin analog polypeptide], was found to selectively bind to the gastrin-releasing peptide receptor in prostate cancer, and its tumor uptake was relatively higher than that of 18F-FDG.113 6.10.2.4 Folate-NOTA-Al18F An 18F-labeled PET tracer for folate receptor (FR)-expressing cancers, folate-NOTA-[18F]AlF (Fig. 656), was synthesized by conjugating folate moiety to the macrocyclic ligand NOTA and then heating the derived NOTA-folate in the presence of 18F2 and AlCl3 at 100 C for 270 Organofluorine Chemistry O O N O N Al N HO HN NH O S NH H N O 18 18 NH 2 NH O N H O F O H N N H O NH O H N OH N H O F-Al-NOTA O O NH2 Ile-Phe-Leu-Leu-Trp-Gln-Arg 18 O 18 F NH O H2N O H N O 18 NH HN Al N NO F-AlF-NOTA-IF7 O H N H N N H S O N H O O O O H N N H OH O O NH2 H O N N H NH 2 O H N O O F-Al-NOTA H N N H N O O N H O O N H O N H N CH 3 NH NH 18 H N F-Al-NOTA-MATBBN FIGURE 6–55 Structures of 18F-AlF-NOTA-IF7 and 18F-AlNOTA-MATBBN PET tracers. O O N HN H2 N N CO2 H N H N H H N O O O N H O 18 F N N O O Al N N O Folate FAl-NOTA Folate-NOTA-Al18 F O FIGURE 6–56 Structure of folate- for FPPRGD2, Al-NOTA-PRGD2, and Ga-NOTA-PRGD2-Al18F. 15 min.114 The radiochemical synthesis and purification was achieved in a total time of 37 min to afford specific activity of 68.82 6 18.5 GBq/μmol, with a radiochemical purity of 98.3%. In vivo studies in tumor xenograft models showed that this PET tracer was comparable with the clinically established 99mTc-EC20 radiotracer. The folate-NOTA 18F-tracer was shown to selectively bind to the FR β in the atherosclerotic plaques in the tissue samples of mice, rabbits, and humans with ischemic symptoms and may thus be developed into clinically useful PET tracer for monitoring the atherosclerotic inflammation.3 Chapter 6 • Synthesis and applications of 18F-labeled compounds 6.10.2.5 271 18 F-fluciclovine (Axumin) 18 F-fluciclovine (Axumin), anti-1-amino-3-[18F]fluorocyclobutane-1-crboxylic acid (FACBC), is used as an FDA-approved PET tracer for imaging suspected prostate cancer in patients with elevated prostate-specific antigen (PSA).115 Although the 11C- or 18F-labeled choline provides an accurate estimate of the prostate cancer relapse, choline detects only half of the prostate cancer lesions due to the limited amount of choline uptake by the prostate cancer lesions. On the other hand, 18F-fluciclovine is transported by two different amino acid transporters and the biodistribution of this amino acid is more favorable than that of choline.116 6.10.2.5.1 Synthesis of 18F-fluciclovine The radiosynthesis of 18F-fluciclovine has been achieved using an automated radiosynthesis apparatus (Fig. 657).117 In this synthetic automated procedure, the triflate 115 was allowed to react with 18F-KF, complexed to cryptand, Kryptofix2.2.2, in acetonitrile at 85 C for 3 min to give the 18F-labeled 116. Hydrolysis of the ester moiety in 117 using aqueous NaOH (5 min at room temperature) and deprotection of the t-Boc using aqueous HCl (5 min at 60 C) gave the 18F-fluciclovine in an overall decay-corrected RCY of 62.5% 6 1.93%. FIGURE 6–57 Automated radiosynthesis of 18F-fluciclovine PET tracer. PET, Positron emission tomography. References 1. Weber, W. A. Use of PET for Monitoring Cancer Therapy and for Predicting Outcome. J. Nucl. Med. 2005, 46, 983995. 2. Choi, S. R.; Ploessl, K.; Zhu, L.; Kung, H. F. PET Imaging Agents for Alzheimer’s Disease. Top. Med. Chem 2017, 24, 117. 3. Silvola, J. M. U.; Li, X.-G.; Virta, J.; Marjamaki, P.; Liljenback, H.; Hytonen, J. P.; Tarkia, M.; Saunavaara, V.; Hurme, S.; Palani, S.; Hakovirta, H.; Yla-Herttuala, S.; Saukko, P.; Chen, Q.; Low, P. S.; Knuuti, J.; Saraste, A.; Roivainen, A. Aluminum Fluoride-18 Labeled Folate Enables in Vivo Detection of Atherosclerotic Plaque Inflammation by Positron Emission Tomography. Sci. Rep. 2018, 8, 115. 272 Organofluorine Chemistry 4. Faerber, S. F.; Wurzer, A.; Reichart, F.; Beck, R.; Kessler, H.; Wester, H.-J.; Notni, J. Therapeutic Radiopharmaceuticals Targeting Integrin αvβ6. ACS Omega 2018, 3, 24282436. 5. Baum, R. P.; Kulkarni, H. R. THERANOSTICS: From Molecular Imaging Using Ga-68 Labeled Tracers and PET/CT to Personalized Radionuclide Therapy The Bad Berka Experience. Theranostics 2012, 2, 437447. 6. Bateman, R. J.; Xiong, C.; Benzinger, T. L. S.; Fagan, A. M.; Goate, A.; Fox, N. C.; Marcus, D. S.; Cairns, N. J.; Xie, X.; Blazey, T. M.; Holtzman, D. M.; Santacruz, A.; Buckles, V.; Oliver, A.; Moulder, K.; Aisen, P. S.; Ghetti, B.; Klunk, W. E.; McDade, E.; Martins, R. N.; Masters, C. L.; Mayeux, R.; Ringman, J. M.; Rossor, M. N.; Schofield, P. R.; Sperling, R. A.; Salloway, S.; Morris, J. C. Clinical and Biomarker Changes in Dominantly Inherited Alzheimer’s Disease. N. Engl. J. Med. 2012, 367, 795804. 7. Klunk, W. E.; Engler, H.; Nordberg, A.; Wang, Y.; Blomqvist, G.; Holt, D. P.; Bergstrom, M.; Savitcheva, I.; Huang, G.-f; Estrada, S.; Ausen, B.; Debnath, M. L.; Barletta, J.; Price, J. C.; Sandell, J.; Lopresti, B. J.; Wall, A.; Koivisto, P.; Antoni, G.; Mathis, C. A.; Langstrom, B. Imaging Brain Amyloid in Alzheimer’s Disease with Pittsburgh Compound-B. Ann. Neurol. 2004, 55, 306319. 8. Villemagne, V. L.; Ong, K.; Mulligan, R. S.; Holl, G.; Pejoska, S.; Jones, G.; O’Keefe, G.; Ackerman, U.; Tochon-Danguy, H.; Chan, J. G.; Reininger, C. B.; Fels, L.; Putz, B.; Rohde, B.; Masters, C. L.; Rowe, C. C. Amyloid Imaging with 18F-Florbetaben in Alzheimer Disease and Other Dementias. J. Nucl. Med. 2011, 52, 12101217. 9. Barthel, H.; Gertz, H.-J.; Dresel, S.; Peters, O.; Bartenstein, P.; Buerger, K.; Hiemeyer, F.; Wittemer-Rump, S. M.; Seibyl, J.; Reininger, C.; Sabri, O. Cerebral Amyloid-β PET with Florbetaben (18F) in Patients with Alzheimer’s Disease and Healthy Controls: A Multicentre Phase 2 Diagnostic Study. Lancet Neurol. 2011, 10, 424435. 10. Vandenberghe, R.; Van, L. K.; Ivanoiu, A.; Salmon, E.; Bastin, C.; Triau, E.; Hasselbalch, S.; Law, I.; Andersen, A.; Korner, A.; Minthon, L.; Garraux, G.; Nelissen, N.; Bormans, G.; Buckley, C.; Owenius, R.; Thurfjell, L.; Farrar, G.; Brooks, D. J. 18F-Flutemetamol Amyloid Imaging in Alzheimer Disease and Mild Cognitive Impairment: A Phase 2 Trial. Ann. Neurol. 2010, 68, 319329. 11. Nelissen, N.; van Laere, K.; Thurfjell, L.; Owenius, R.; Vandenbulcke, M.; Koole, M.; Bormans, G.; Brooks, D. J.; Vandenberghe, R. Phase 1 Study of the Pittsburgh Compound B Derivative 18F-Flutemetamol in Healthy Volunteers and Patients with Probable Alzheimer Disease. J. Nucl. Med. 2009, 50, 12511259. 12. Kung, H. F. The β-Amyloid Hypothesis in Alzheimer’s Disease: Seeing Is Believing. ACS Med. Chem. Lett 2012, 3, 265267. 13. Yang, L.; Rieves, D.; Ganley, C. Brain Amyloid Imaging FDA Approval of Florbetapir F-18 Injection. N. Engl. J. Med. 2012, 367, 885887. 14. Clark, C. M.; Schneider, J. A.; Bedell, B. J.; Beach, T. C.; Bilker, W. B.; Mintun, M. A.; Pontecorvo, M. J.; Hefti, F.; Carpenter, A. P.; Flitter, M. L.; Krautkramer, M. J.; Kung, H. F.; Coleman, R. E.; Doraiswamy, P. M.; Fleisher, A. S.; Sabbagh, M. N.; Sadowsky, C. H.; Reiman, P. E. M.; Zehntner, S. P.; Skovronsky, D. M. Use of Florbetapir-PET for Imaging B-Amyloid Pathology. JAMA, J. Am. Med. Assoc. 2011, 305, 275283. 15. Miller, G. Alzheimer’s Biomarker Initiative Hits Its Stride. Science 2009, 326, 386389. 16. Gillies, R. J.; Robey, I.; Gatenby, R. A. Causes and Consequences of Increased Glucose Metabolism of Cancers. J. Nucl. Med. 2008, 49, 24S42S. 17. Plathow, C.; Weber, W. A. Tumor Cell Metabolism Imaging. J. Nucl. Med. 2008, 49, 43S63S. 18. Hu, Z.; Yang, W.; Liu, H.; Wang, K.; Bao, C.; Song, T.; Wang, J.; Tian, J. From PET/CT to PET/MRI: Advances in Instrumentation and Clinical Applications. Mol. Pharm. 2014, 11, 37983809. 19. Ganem, J.; Thureau, S.; Gardin, I.; Modzelewski, R.; Hapdey, S.; Vera, P. Delineation of Lung Cancer with FDG PET/CT During Radiation Therapy. Radiat. Oncol. 2018, 13, 219. Chapter 6 • Synthesis and applications of 18F-labeled compounds 273 20. Sunnetcioglu, A.; Arisoy, A.; Demir, Y.; Ekin, S.; Dogan, E. Associations Between the Standardized Uptake Value of 18F-FDG PET/CT and Demographic, Clinical, Pathological, Radiological Factors in Lung Cancer. Int. J. Clin. Exp. Med. 2015, 8, 1579415800. 21. Lopez, G. J. L.; Gladish, G.; Komaki, R.; Gomez, D.; Zhuang, Y.; Liao, Z. Large Decreases in Standardized Uptake Values After Definitive Radiation Are Associated with Better Survival of Patients with Locally Advanced Non-Small Cell Lung Cancer. J. Nucl. Med. 2012, 53, 225233. 22. Horti, A. G.; Chefer, S. I.; Mukhin, A. G.; Koren, A. O.; Gundisch, D.; Links, J. M.; Kurian, V.; Dannals, R. F.; London, E. D. 6-[18F]fluoro-A-85380, a Novel Radioligand for In Vivo Imaging of Central Nicotinic Acetylcholine Receptors. Life Sci. 2000, 67, 463469. 23. Bottlaender, M.; Valette, H.; Roumenov, D.; Dolle, F.; Coulon, C.; Ottaviani, M.; Hinnen, F.; Ricard, M. Biodistribution and Radiation Dosimetry of 18F-Fluoro-A-85380 in Healthy Volunteers. J. Nucl. Med. 2003, 44, 596601. 24. Mabondzo, A.; Bottlaender, M.; Guyot, A.-C.; Tsaouin, K.; Deverre, J. R.; Balimane, P. V. Validation of In Vitro Cell-Based Human Blood-Brain Barrier Model Using Clinical Positron Emission Tomography Radioligands to Predict In Vivo Human Brain Penetration. Mol. Pharm. 2010, 7, 18051815. 25. Hooshyar Yousefi, B.; Manook, A.; Grimmer, T.; Arzberger, T.; von Reutern, B.; Henriksen, G.; Drzezga, A.; Foerster, S.; Schwaiger, M.; Wester, H.-J. Characterization and First Human Investigation of FIBT, a Novel Fluorinated Aβ Plaque Neuroimaging PET Radioligand. ACS Chem. Neurosci. 2015, 6, 428437. 26. van der Born, D.; Pees, A.; Poot, A. J.; Orru, R. V. A.; Windhorst, A. D.; Vugts, D. J. Fluorine-18 Labelled Building Blocks for PET Tracer Synthesis. Chem. Soc. Rev. 2017, 46, 47094773. 27. Krishnan, H. S.; Ma, L.; Vasdev, N.; Liang, S. H. 18F-Labeling of Sensitive Biomolecules for Positron Emission Tomography. Chem.—Eur. J. 2017, 23, 1555315577. 28. Preshlock, S.; Tredwell, M.; Gouverneur, V. 18F-Labeling of Arenes and Heteroarenes for Applications in Positron Emission Tomography. Chem. Rev. (Washington, DC, U. S.) 2016, 116, 719766. 29. Gray, E. E.; Nielsen, M. K.; Choquette, K. A.; Kalow, J. A.; Graham, T. J. A.; Doyle, A. G. Nucleophilic (Radio)Fluorination of α-Diazocarbonyl Compounds Enabled by Copper-Catalyzed H-F Insertion. J. Am. Chem. Soc. 2016, 138, 1080210805. 30. McCammant, M. S.; Thompson, S.; Brooks, A. F.; Krska, S. W.; Scott, P. J. H.; Sanford, M. S.; CuMediated, C.-H. 18F-Fluorination of Electron-Rich (Hetero)arenes. Org. Lett. 2017, 19, 39393942. 31. Neumann, C. N.; Hooker, J. M.; Ritter, T. Concerted Nucleophilic Aromatic Substitution with 19F- and 18 F. Nature (London, UK) 2016, 534, 369373. 32. Neumann, C. N.; Ritter, T. Facile C-F Bond Formation Through a Concerted Nucleophilic Aromatic Substitution Mediated by the PhenoFluor Reagent. Acc. Chem. Res. 2017, 50, 28222833. 33. Huang, X.; Liu, W.; Ren, H.; Neelamegam, R.; Hooker, J. M.; Groves, J. T.; Late Stage.; Benzylic, C.-H. Fluorination with [18F]fluoride for PET Imaging. J. Am. Chem. Soc. 2014, 136, 68426845. 34. Lee, E.; Kamlet, A. S.; Powers, D. C.; Neumann, C. N.; Boursalian, G. B.; Furuya, T.; Choi, D. C.; Hooker, J. M.; Ritter, T. A Fluoride-Derived Electrophilic Late-Stage Fluorination Reagent for PET Imaging. Science (Washington, DC, U. S.) 2011, 334, 639642. 35. Lisova, K.; Sergeev, M.; Evans-Axelsson, S.; Stuparu, A. D.; Beykan, S.; Collins, J.; Jones, J.; Lassmann, M.; Herrmann, K.; Perrin, D.; Lee, J. T.; Slavik, R.; van Dam, R. M. Microscale Radiosynthesis, Preclinical Imaging and Dosimetry Study of [18F]AMBF3-TATE: A Potential PET Tracer for Clinical Imaging of Somatostatin Receptors. Nucl. Med. Biol., 61. ; 20183644. 36. Kim, D. W.; Ahn, D.-S.; Oh, Y.-H.; Lee, S.; Kil, H. S.; Oh, S. J.; Lee, S. J.; Kim, J. S.; Ryu, J. S.; Moon, D. H.; Chi, D. Y. A New Class of SN2 Reactions Catalyzed by Protic Solvents: Facile Fluorination for Isotopic Labeling of Diagnostic Molecules. J. Am. Chem. Soc. 2006, 128, 1639416397. 274 Organofluorine Chemistry 37. Hwang, D. R.; Eckelman, W. C.; Mathias, C. J.; Petrillo, E. W., Jr.; Lloyd, L.; Welch, M. J. Positron-Labeled Angiotensin-Converting Enzyme (ACE) Inhibitor: Fluorine-18-Fluorocaptopril. Probing the ACE Activity In Vivo by Positron Emission Tomography. J. Nucl. Med. 1991, 32, 17301737. 38. Nielsen, M. K.; Ugaz, C. R.; Li, W.; Doyle, A. G. PyFluor: A Low-Cost, Stable, and Selective Deoxyfluorination Reagent. J. Am. Chem. Soc. 2015, 137, 95719574. 39. Teare, H.; Robins, E. G.; Aarstad, E.; Luthra, S. K.; Gouverneur, V. Synthesis and Reactivity of [18F]-NFluorobenzenesulfonimide. Chem. Commun. (Cambridge, UK) 2007, 23302332. 40. Teare, H.; Robins, E. G.; Kirjavainen, A.; Forsback, S.; Sandford, G.; Solin, O.; Luthra, S. K.; Gouverneur, V. Radiosynthesis and Evaluation of [18F]Selectfluor Bis(triflate). Angew. Chem., Int. Ed. 2010, 49, 68216824. 41. Verhoog, S.; Kee, C. W.; Wang, Y.; Khotavivattana, T.; Wilson, T. C.; Kersemans, V.; Smart, S.; Tredwell, M.; Davis, B. G.; Gouverneur, V. 18F-Trifluoromethylation of Unmodified Peptides with 5-18F(Trifluoromethyl)dibenzothiophenium Trifluoromethanesulfonate. J. Am. Chem. Soc. 2018, 140, 15721575. 42. Meyer, J.-P.; Adumeau, P.; Lewis, J. S.; Zeglis, B. M. Click Chemistry and Radiochemistry: The First 10 Years. Bioconjug Chem. 2016, 27, 27912807. 43. Engel, B. J.; Gammon, S. T.; Chaudhari, R.; Lu, Z.; Pisaneschi, F.; Yang, H.; Ornelas, A.; Yan, V.; Kelderhouse, L.; Najjar, A. M.; Tong, W. P.; Zhang, S.; Piwnica-Worms, D.; Bast, R. C.; Millward, S. W. Caspase-3 Substrates for Noninvasive Pharmacodynamic Imaging of Apoptosis by PET/CT. Bioconjug Chem. 2018, 29, 31803195. 44. Ramenda, T.; Steinbach, J.; Wuest, F. 4-[18F]fluoro-N-methyl-N-(propyl-2-yn-1-yl)benzenesulfonamide ([18F]F-SA): A Versatile Building Block for Labeling of Peptides, Proteins and Oligonucleotides with Fluorine-18 via Cu(I)-Mediated Click Chemistry. Amino Acids 2013, 44, 11671180. 45. Wang, H.; Cheng, Y.; Zhang, J.; Zang, J.; Li, H.; Liu, Q.; Wang, J.; Jacobson, O.; Li, F.; Zhu, Z.; Chen, X. Response to Single Low-Dose 177Lu-DOTA-EB-TATE Treatment in Patients with Advanced Neuroendocrine Neoplasm: A Prospective Pilot Study. Theranostics 2018, 8, 33083316. 46. Zhang, L.; Vines, D. C.; Scollard, D. A.; McKee, T.; Komal, T.; Ganguly, M.; Do, T.; Wu, B.; Alexander, N.; Vali, R.; Shammas, A.; Besanger, T.; Baruchel, S. Correlation of Somatostatin Receptor-2 Expression with Gallium-68-DOTA-TATE Uptake in Neuroblastoma Xenograft Models. Contrast Media Mol. Imaging 2017, 9481276. 47. Dubash, S. R.; Keat, N.; Mapelli, P.; Twyman, F.; Carroll, L.; Kozlowski, K.; Al-Nahhas, A.; Saleem, A.; Huiban, M.; Janisch, R.; Frilling, A.; Sharma, R.; Aboagye, E. O. Clinical Translation of a Click-Labeled 18 F-Octreotate Radioligand for Imaging Neuroendocrine Tumors. J. Nucl. Med. 2016, 57, 12071213. 48. Baskin, J. M.; Bertozzi, C. R. Copper-Free Click Chemistry: Bio-Orthogonal Reagents for Tagging Azides. Aldrichim. Acta 2010, 43, 1523. 49. Kim, H. L.; Sachin, K.; Jeong, H. J.; Choi, W.; Lee, H. S.; Kim, D. W. F-18 Labeled RGD Probes Based on Bioorthogonal Strain-Promoted Click Reaction for PET Imaging. ACS Med. Chem. Lett. 2015, 6, 402407. 50. Bouvet, V.; Wuest, M.; Wuest, F. Copper-Free Click Chemistry with the Short-Lived Positron Emitter Fluorine-18. Org. Biomol. Chem. 2011, 9, 73937399. 51. Campbell-Verduyn, L. S.; Mirfeizi, L.; Schoonen, A. K.; Dierckx, R. A.; Elsinga, P. H.; Feringa, B. L. StrainPromoted Copper-Free “Click” Chemistry for 18F Radiolabeling of Bombesin. Angew. Chem., Int. Ed. 2011, 50, 1111711120. 52. Zhou, Z.; Chitneni, S. K.; Devoogdt, N.; Zalutsky, M. R.; Vaidyanathan, G. Fluorine-18 Labeling of an Anti-HER2 VHH Using a Residualizing Prosthetic Group via a Strain-Promoted Click Reaction: Chemistry and Preliminary Evaluation. Bioorg. Med. Chem. 2018, 26, 19391949. 53. Prescher, J. A.; Dube, D. H.; Bertozzi, C. R. Chemical Remodelling of Cell Surfaces in Living Animals. Nature (London, UK) 2004, 430, 873877. Chapter 6 • Synthesis and applications of 18F-labeled compounds 275 54. Saxon, E.; Armstrong, J. I.; Bertozzi, C. R. A “Traceless” Staudinger Ligation for the Chemoselective Synthesis of Amide Bonds. Org. Lett. 2000, 2, 21412143. 55. Carroll, L.; Boldon, S.; Bejot, R.; Moore, J. E.; Declerck, J.; Gouverneur, V. The Traceless Staudinger Ligation for Indirect 18F-Radiolabelling. Org. Biomol. Chem. 2011, 9, 136140. 56. Gaeta, A.; Woodcraft, J.; Plant, S.; Goggi, J.; Jones, P.; Battle, M.; Trigg, W.; Luthra, S. K.; Glaser, M. Use of 2-[(18)F]fluoroethylazide for the Staudinger Ligation Preparation and Characterisation of GABA(A) Receptor Binding 4-Quinolones. Bioorg. Med. Chem. Lett. 2010, 20, 46494652. 57. Pretze, M.; Wuest, F.; Peppel, T.; Koeckerling, M.; Mamat, C. The Traceless Staudinger Ligation with Fluorine-18: A Novel and Versatile Labeling Technique for the Synthesis of PET-Radiotracers. Tetrahedron Lett. 2010, 51, 64106414. 58. Fujita, Y.; Murakami, Y.; Noda, A.; Miyoshi, S. Design and Synthesis of an Easily Obtainable Maleimide Reagent N-[2-(4-[18F]fluoro-N-methylbenzenesulfonamido)ethyl]maleimide ([18F]FBSEM) to Radiolabel Thiols in Proteins. Bioconjug. Chem. 2017, 28, 642648. 59. Toyokuni, T.; Walsh, J. C.; Dominguez, A.; Phelps, M. E.; Barrio, J. R.; Gambhir, S. S.; Satyamurthy, N. Synthesis of a New Heterobifunctional Linker, N-[4-(Aminooxy)butyl]maleimide, for Facile Access to a Thiol-Reactive 18F-Labeling Agent. Bioconjug. Chem. 2003, 14, 12531259. 60. Cai, W.; Zhang, X.; Wu, Y.; Chen, X. A Thiol-Reactive 18F-Labeling Agent, N-[2-(4-18F-Fluorobenzamido) ethyl]maleimide, and Synthesis of RGD Peptide-Based Tracer for PET Imaging of αvβ3 Integrin Expression. J. Nucl. Med. 2006, 47, 11721180. 61. Damont, A.; Boisgard, R.; Dolle, F.; Hollocou, M.; Kuhnast, B. Avidin/Biotin Bioinspired Platform for Dual In Vivo 18F-PET/NIRF Molecular Imaging. Bioconjug. Chem. 2017, 28, 25242529. 62. Hoegerle, S.; Ghanem, N.; Altehoefer, C.; Schipper, J.; Brink, I.; Moser, E.; Neumann, H. P. H. 18F-DOPA Positron Emission Tomography for the Detection of Glomus Tumours. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 689694. 63. Pretze, M.; Franck, D.; Kunkel, F.; Fosshag, E.; Waengler, C.; Waengler, B. Evaluation of Two Nucleophilic Syntheses Routes for the Automated Synthesis of 6-[18F]fluoro-L-DOPA. Nucl. Med. Biol. 2017, 45, 3542. 64. Sowa, A. R.; Brooks, A. F.; Shao, X.; Henderson, B. D.; Sherman, P.; Arteaga, J.; Stauff, J.; Lee, A. C.; Koeppe, R. A.; Scott, P. J. H.; Kilbourn, M. R. Development of Positron Emission Tomography Radiotracers for the GABA Transporter 1. ACS Chem. Neurosci. 2018, 9, 27672773. 65. Beyzavi, M. H.; Mandal, D.; Strebl, M. G.; Neumann, C. N.; D’Amato, E. M.; Chen, J.; Hooker, J. M.; Ritter, T. 18 F-Deoxyfluorination of Phenols via Ru π-Complexes. ACS Cent. Sci. 2017, 3, 944948. 66. Rickmeier, J.; Ritter, T. Site-Specific Deoxyfluorination of Small Peptides with [18F]fluoride. Angew. Chem., Int. Ed. 2018, 57, 1420714211. 67. Liu, W.; Groves, J. T. Manganese Catalyzed C-H Halogenation. Acc. Chem. Res. 2015, 48, 17271735. 68. Liu, W.; Huang, X.; Placzek, M. S.; Krska, S. W.; McQuade, P.; Hooker, J. M.; Groves, J. T. Site-Selective 18 F Fluorination of Unactivated C-H Bonds Mediated by a Manganese Porphyrin. Chem. Sci. 2018, 9, 11681172. 69. Shi, H.; Braun, A.; Wang, L.; Liang, S. H.; Vasdev, N.; Ritter, T. Synthesis of 18F-Difluoromethylarenes From Aryl (Pseudo) Halides. Angew. Chem., Int. Ed. 2016, 55, 1078610790. 70. Levin, M. D.; Chen, T. Q.; Neubig, M. E.; Hong, C. M.; Theulier, C. A.; Kobylianskii, I. J.; Toste, F. D.; Levin, M. D.; Hong, C. M.; Toste, F. D.; Janabi, M.; O’Neil, J. P. A Catalytic Fluoride-Rebound Mechanism for C(sp3)-CF3 Bond Formation. Science 2017, 356, 12721276. 71. Lee, E.; Hooker, J. M.; Ritter, T. Nickel-Mediated Oxidative Fluorination for PET with Aqueous [18F] Fluoride. J. Am. Chem. Soc. 2012, 134, 1745617458. 276 Organofluorine Chemistry 72. Hoover, A. J.; Lazari, M.; Ren, H.; Narayanam, M. K.; Murphy, J. M.; van Dam, R. M.; Hooker, J. M.; Ritter, T. A Transmetalation Reaction Enables the Synthesis of [18F]5-Fluorouracil From [18F]fluoride for Human PET Imaging. Organometallics 2016, 35, 10081014. 73. Huiban, M.; Tredwell, M.; Mizuta, S.; Wan, Z.; Zhang, X.; Collier, T. L.; Gouverneur, V.; Passchier, J. A Broadly Applicable [18F]trifluoromethylation of Aryl and Heteroaryl Iodides for PET Imaging. Nat. Chem. 2013, 5, 941944. 74. Chun, J.-H.; Pike, V. W. Single-Step Syntheses of No-Carrier-Added Functionalized [18F]fluoroarenes as Labeling Synthons From Diaryliodonium Salts. Org. Biomol. Chem. 2013, 11, 63006306. 75. Zlatopolskiy, B. D.; Zischler, J.; Krapf, P.; Zarrad, F.; Urusova, E. A.; Kordys, E.; Endepols, H.; Neumaier, B. Copper-Mediated Aromatic Radiofluorination Revisited: Efficient Production of PET Tracers on a Preparative Scale. Chem.—Eur. J. 2015, 21, 59725979. 76. Kim, J.; Moon, B. S.; Lee, B. C.; Lee, H.-Y.; Kim, H.-J.; Choo, H.; Pae, A. N.; Cho, Y. S.; Min, S.-J. A Potential PET Radiotracer for the 5-HT2C Receptor: Synthesis and in Vivo Evaluation of 4-(3-[18F]fluorophenethoxy)pyrimidine. ACS Chem. Neurosci. 2017, 8, 9961003. 77. Neumann, K. D.; Qin, L.; Vavere, A. L.; Shen, B.; Miao, Z.; Chin, F. T.; Shulkin, B. L.; Snyder, S. E.; Di Magno, S. G. Efficient Automated Syntheses of High Specific Activity 6-[18F]fluorodopamine Using a Diaryliodonium Salt Precursor. J. Labelled Compd. Radiopharm. 2016, 59, 3034. 78. Vavere, A. L.; Neumann, K. D.; Butch, E. R.; Hu, B.; DiMagno, S. G.; Snyder, S. E. Improved, One-Pot Synthesis of 6-[18F]fluorodopamine and Quality Control Testing for Use in Patients with Neuroblastoma. J. Labelled Compd. Radiopharm. 2018, 61, 10691080. 79. Rotstein, B. H.; Stephenson, N. A.; Vasdev, N.; Liang, S. H. Spirocyclic Hypervalent Iodine(III)-Mediated Radiofluorination of Non-Activated and Hindered Aromatics. Nat. Commun. 2014, 5, 4365. 80. Collier, T. L.; Normandin, M. D.; Stephenson, N. A.; Livni, E.; Liang, S. H.; Wooten, D. W.; Esfahani, S. A.; Stabin, M. G.; Mahmood, U.; Chen, J.; Wang, W.; Maresca, K.; Waterhouse, R. N.; El Fakhri, G.; Richardson, P.; Vasdev, N. Synthesis and Preliminary PET Imaging of 11C and 18F Isotopologues of the ROS1/ALK Inhibitor Lorlatinib. Nat. Commun. 2017, 8, 15761. 81. Wang, L.; Cheng, R.; Fujinaga, M.; Yang, J.; Zhang, Y.; Hatori, A.; Kumata, K.; Yang, J.; Vasdev, N.; Du, Y.; Ran, C.; Zhang, M.-R.; Liang, S. H. A Facile Radiolabeling of [18F]FDPA via Spirocyclic Iodonium Ylides: Preliminary PET Imaging Studies in Preclinical Models of Neuroinflammation. J. Med. Chem. 2017, 60, 52225227. 82. Deng, H.; Cobb, S. L.; Gee, A. D.; Lockhart, A.; Martarello, L.; McGlinchey, R. P.; O’Hagan, D.; Onega, M. Fluorinase Mediated C-18F Bond Formation, an Enzymatic Tool for PET Labelling. Chem. Commun. (Cambridge, UK) 2006, 652654. 83. Deng, H.; McMahon, S. A.; Eustaquio, A. S.; Moore, B. S.; Naismith, J. H.; O’Hagan, D. Mechanistic Insights Into Water Activation in SAM Hydroxide Adenosyltransferase (duf-62). ChemBioChem 2009, 10, 24552459. 84. Lowe, P. T.; Dall’Angelo, S.; Devine, A.; Zanda, M.; O’Hagan, D. Enzymatic Fluorination of Biotin and Tetrazine Conjugates for Pretargeting Approaches to Positron Emission Tomography Imaging. ChemBioChem 2018, 19, 19691978. 85. Thompson, S.; Onega, M.; Ashworth, S.; Fleming, I. N.; Passchier, J.; O’Hagan, D. A Two-Step Fluorinase Enzyme Mediated 18F Labelling of an RGD Peptide for Positron Emission Tomography. Chem. Commun. (Cambridge, UK) 2015, 51, 1354213545. 86. Frankish, H.; Horton, R. Prevention and Management of Dementia: A Priority for Public Health. Lancet 2017, 390, 26142615. 87. Reddy, V. P. Organofluorine Compounds in Biology and Medicine; Elsevier, 2015. 88. Heurling, K.; Leuzy, A.; Zimmer, E. R.; Lubberink, M.; Nordberg, A. Imaging β-Amyloid Using [18F]flutemetamol Positron Emission Tomography: From Dosimetry to Clinical Diagnosis. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 362373. Chapter 6 • Synthesis and applications of 18F-labeled compounds 277 89. Murugan, N. A.; Nordberg, A.; Aagren, H. Different Positron Emission Tomography Tau Tracers Bind to Multiple Binding Sites on the Tau Fibril: Insight From Computational Modeling. ACS Chem. Neurosci. 2018, 9, 17571767. 90. Elias, A.; Cummins, T.; Tyrrell, R.; Lamb, F.; Dore, V.; Williams, R.; Rosenfeld, J. V.; Hopwood, M.; Villemagne, V. L.; Rowe, C. C. Risk of Alzheimer’s Disease in Obstructive Sleep Apnea Syndrome: Amyloid-β and Tau Imaging. J. Alzheimer’s Dis 2018, 66, 733741. 91. Ossenkoppele, R.; Rabinovici, G. D.; Smith, R.; Cho, H.; Schoell, M.; Strandberg, O.; Palmqvist, S.; Mattsson, N.; Janelidze, S.; Santillo, A.; Ohlsson, T.; Joegi, J.; Tsai, R.; La Joie, R.; Kramer, J.; Boxer, A. L.; Gorno-Tempini, M. L.; Miller, B. L.; Choi, J. Y.; Ryu, Y. H.; Lyoo, C. H.; Hansson, O. Discriminative Accuracy of [18F]flortaucipir Positron Emission Tomography for Alzheimer Disease vs Other Neurodegenerative Disorders. JAMA, J. Am. Med. Assoc. 2018, 320, 11511162. 92. Lilly Announces Positive Phase 3 Results in Study of Flortaucipir PET Imaging Agent, Web content current as of: 10/29/2019; ,https://www.prnewswire.com/news-releases/lilly-announces-positive-phase-3results-in-study-of-flortaucipir-pet-imaging-agent-300707314.html.. 93. Underwood, E. NEUROSCIENCE. Can Brain Scans Reveal Concussion-Linked Disease? Science 2016, 352, 881. 94. Drake, L. R.; Pham, J. M.; Desmond, T. J.; Mossine, A. V.; Lee, S. J.; Kilbourn, M. R.; Koeppe, R. A.; Brooks, A. F.; Scott, P. J. H. Identification of AV-1451 as a Weak, Nonselective Inhibitor of Monoamine Oxidase. ACS Chem. Neurosci. 2019, 10, 38393846. 95. Gao, M.; Wang, M.; Zheng, Q.-H. Fully Automated Synthesis of [18F]T807, a PET Tau Tracer for Alzheimer’s Disease. Bioorg. Med. Chem. Lett. 2015, 25, 29532957. 96. Holt, D. P.; Ravert, H. T.; Dannals, R. F. Synthesis and Quality Control of [18F]T807 for Tau PET Imaging. J. Labelled Compd. Radiopharm. 2016, 59, 411415. 97. Okamura, N.; Furumoto, S.; Harada, R.; Tago, T.; Yoshikawa, T.; Fodero-Tavoletti, M.; Mulligan, R. S.; Villemagne, V. L.; Akatsu, H.; Yamamoto, T.; Arai, H.; Iwata, R.; Yanai, K.; Kudo, Y. Novel 18F-Labeled Arylquinoline Derivatives for Noninvasive Imaging of Tau Pathology in Alzheimer Disease. J. Nucl. Med. 2013, 54, 14201427. 98. Harada, R.; Okamura, N.; Furumoto, S.; Furukawa, K.; Ishiki, A.; Tomita, N.; Tago, T.; Hiraoka, K.; Watanuki, S.; Shidahara, M.; Miyake, M.; Ishikawa, Y.; Matsuda, R.; Inami, A.; Yoshikawa, T.; Funaki, Y.; Iwata, R.; Tashiro, M.; Yanai, K.; Arai, H.; Kudo, Y. 18F-THK5351: A Novel PET Radiotracer for Imaging Neurofibrillary Pathology in Alzheimer Disease. J. Nucl. Med. 2016, 57, 208214. 99. Kang, J. M.; Lee, S.-Y.; Seo, S.; Jeong, H. J.; Woo, S.-H.; Lee, H.; Lee, Y.-B.; Yeon, B. K.; Shin, D. H.; Park, K. H.; Kang, H.; Okamura, N.; Furumoto, S.; Yanai, K.; Villemagne, V. L.; Seong, J.-K.; Na, D. L.; Ido, T.; Cho, J.; Lee, K.-M.; Noh, Y. Tau Positron Emission Tomography Using [18F]THK5351 and Cerebral Glucose Hypometabolism in Alzheimer’s Disease. Neurobiol. Aging 2017, 59, 210219. 100. Bailey, J. J.; Kaiser, L.; Lindner, S.; Wust, M.; Thiel, A.; Soucy, J.-P.; Rosa-Neto, P.; Scott, P. J. H.; Unterrainer, M.; Kaplan, D. R.; Wangler, C.; Wangler, B.; Bartenstein, P.; Bernard-Gauthier, V.; Schirrmacher, R. First-in-Human Brain Imaging of [18F]TRACK, a PET Tracer for Tropomyosin Receptor Kinases. ACS Chem. Neurosci. 2019, 10, 26972702. 101. Collier, T. L.; Normandin, M. D.; Liang, S. H.; Vasdev, N.; Collier, T. L.; Maresca, K. P.; McCarthy, T. J.; Waterhouse, R. N.; Richardson, P.; Waterhouse, R. N. Brain Penetration of the ROS1/ALK Inhibitor Lorlatinib Confirmed by PET. Mol Imaging 2017, 16 1536012117736669. 102. Shi, J.; Wang, F.; Liu, S. Radiolabeled Cyclic RGD Peptides as Radiotracers for Tumor Imaging. Biophys. Rep. 2016, 2, 120. 103. Mittra, E. S.; Goris, M. L.; Iagaru, A. H.; Kardan, A.; Burton, L.; Berganos, R.; Chang, E.; Liu, S.; Shen, B.; Chin, F. T.; Chen, X.; Gambhir, S. S. Pilot Pharmacokinetic and Dosimetric Studies of 18F-FPPRGD2: A PET Radiopharmaceutical Agent for Imaging αvβ3 Integrin Levels. Radiology 2011, 260, 182191. 278 Organofluorine Chemistry 104. Debordeaux, F.; Chansel-Debordeaux, L.; Pinaquy, J.-B.; Fernandez, P.; Schulz, J. What About αvβ3 Integrins in Molecular Imaging in Oncology? Nucl. Med. Biol. 2018, 6263, 3146. 105. Lang, L.; Li, W.; Guo, N.; Ma, Y.; Zhu, L.; Kiesewetter, D. O.; Shen, B.; Niu, G.; Chen, X. Comparison Study of [18F]FAl-NOTA-PRGD2, [18F]FPPRGD2, and [68Ga]Ga-NOTA-PRGD2 for PET Imaging of U87MG Tumors in Mice. Bioconjug. Chem. 2011, 22, 24152422. 106. ClinicalTrials.gov; Web content current as of 10/29/2019; ,https://clinicaltrials.gov/ct2/show/ NCT01806675.. 107. Chin, F. T.; Shen, B.; Liu, S.; Berganos, R. A.; Chang, E.; Mittra, E.; Chen, X.; Gambhir, S. S. First Experience with Clinical-Grade ([18F]FPP(RGD2)): An Automated Multi-Step Radiosynthesis for Clinical PET Studies. Mol Imaging Biol 2012, 14, 8895. 108. Liu, S.; Liu, H.; Jiang, H.; Xu, Y.; Zhang, H.; Cheng, Z. One-Step Radiosynthesis of 18F-AlF-NOTA-RGD2 for Tumor Angiogenesis PET Imaging. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 17321741. 109. Wan, W.; Guo, N.; Pan, D.; Yu, C.; Weng, Y.; Luo, S.; Ding, H.; Xu, Y.; Wang, L.; Lang, L.; Xie, Q.; Yang, M.; Chen, X. First Experience of 18F-Alfatide in Lung Cancer Patients Using a New Lyophilized Kit for Rapid Radiofluorination. J. Nucl. Med. 2013, 54, 691698. 110. Zhou, Y.; Gao, S.; Huang, Y.; Zheng, J.; Dong, Y.; Zhang, B.; Zhao, S.; Lu, H.; Liu, Z.; Yu, J.; Yuan, S. A Pilot Study of 18F-Alfatide PET/CT Imaging for Detecting Lymph Node Metastases in Patients with NonSmall Cell Lung Cancer. Sci. Rep. 2017, 7, 17. 111. Yu, C.; Pan, D.; Mi, B.; Xu, Y.; Lang, L.; Niu, G.; Yang, M.; Wan, W.; Chen, X. 18 F-Alfatide II PET/CT in Healthy Human Volunteers and Patients with Brain Metastases. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 20212028. 112. Gu, X.; Jiang, M.; Pan, D.; Cai, G.; Zhang, R.; Zhou, Y.; Ding, Y.; Zhu, B.; Lin, X. Preliminary Evaluation of Novel 18F-AlF-NOTA-IF7 as a Tumor Imaging Agent. J. Radioanal. Nucl. Chem. 2016, 308, 851856. 113. Pan, D.; Yan, Y.; Yang, R.; Xu, Y. P.; Chen, F.; Wang, L.; Luo, S.; Yang, M. PET Imaging of Prostate Tumors with 18F-Al-NOTA-MATBBN. Contrast Media Mol. Imaging 2014, 9, 342348. 114. Chen, Q.; Meng, X.; McQuade, P.; Rubins, D.; Lin, S.-A.; Zeng, Z.; Haley, H.; Miller, P.; Gonzalez Trotter, D.; Low, P. S. Synthesis and Preclinical Evaluation of Folate-NOTA-Al18F for PET Imaging of FolateReceptor-Positive Tumors. Mol. Pharm. 2016, 13, 15201527. 115. Parent, E. E.; Schuster, D. M. Update on 18F-Fluciclovine PET for Prostate Cancer Imaging. J. Nucl. Med. 2018, 59, 733739. 116. Schiavina, R.; Brunocilla, E.; Martorana, G. The New Promise of FACBC Position Emission Tomography/Computed Tomography in the Localization of Disease Relapse After Radical Treatment for Prostate Cancer: Are We Turning to the Right Radiotracer? Eur. Urol. 2014, 65, 255256. 117. Svadberg, A.; Ryan, O.; Smeets, R. WO2014023775A1; Preparation of [18F]-Fluciclovine PET Tracer Using an Automated Radiofluorination Apparatus; GE Healthcare Limited, 2014. 7 Materials applications of organofluorine compounds Chapter Outline 7.1 Introduction ............................................................................................................................... 280 7.2 Fluorinated surfactants............................................................................................................. 280 7.2.1 Perfluorocarbon nanomaterials ..................................................................................... 282 7.2.2 Fluorous catalysis ............................................................................................................. 285 7.2.3 Environmentally benign perfluorosurfactants.............................................................. 285 7.3 Fluoropolymers.......................................................................................................................... 286 7.3.1 Poly(tetrafluoroethylene) ............................................................................................... 287 7.3.2 Poly(vinylidene fluoride) ................................................................................................. 287 7.4 Fluorinated π-conjugated polymeric materials in photovoltaic devices ............................. 289 7.4.1 ππ Stacking interactions in polyfluoroaromatics ....................................................... 289 7.4.2 π-Conjugated polymers................................................................................................... 289 7.4.3 Synthesis of the fluorinated donoracceptor polymers for fullerenepolymer solar cells .......................................................................................................................... 293 7.4.4 π-Conjugated benzodithiophenequinoxaline copolymers ....................................... 296 7.4.5 Fluorinated polymers in fullerene-free, all-polymer (organic) solar cells .................. 296 7.5 Fluorinated poly(aryl thioethers) in organic electronic materials ........................................ 298 7.6 Polymer electrolytes.................................................................................................................. 300 7.7 Fluorinated ionomers as proton-exchange membranes in fuel cells ................................... 304 7.8 Fluorinated carbon nanoparticles and nonaqueous electrolytes in lithium- and lithium-ion batteries ................................................................................................................. 307 7.9 Fluorinated hyperbranched dendrimers: synthesis and applications .................................. 308 7.10 Fluorinated compounds in drug delivery and magnetic resonance imaging ..................... 310 7.10.1 Fluorinated curcumin analogs as 19F MRI agents ....................................................... 310 7.10.2 Polyfluorinated dendrimer amphiphiles as 19F MRI probes and drug delivery agents .... 311 7.11 Organofluorine liquid crystal materials .................................................................................. 313 7.11.1 Fluorinated dendrimer-based liquid crystals............................................................... 313 7.12 Organofluorine compounds in high-energy materials.......................................................... 313 7.12.1 N,N-Difluoramine (NF2) compounds ............................................................................ 314 7.12.2 Pentafluorosulfanyl (SF5) compounds.......................................................................... 318 References........................................................................................................................................... 321 Organofluorine Chemistry. DOI: https://doi.org/10.1016/B978-0-12-813286-9.00007-9 © 2020 Elsevier Inc. All rights reserved. 279 280 Organofluorine Chemistry 7.1 Introduction Organofluorine compounds are ubiquitously found in a wide variety of materials, including biomaterials, smart materials, liquid crystal displays (LCDs), solar cells, electrode and electrolyte materials in lithium-ion batteries, fuel cell membranes, and as components in numerous consumer goods. Organofluorine compounds play a key role in almost all of the modern LCDs (vide infra). The relatively strong CF bond strength, low polarizability of the CF bonds, and extremely low reactivity of CF bonds toward oxidizing and reducing reagents, acids, and bases are responsible for the unique characteristics of organofluorine compounds and fluoropolymers. Fluorinated materials are of emerging interest in the biomedical area. Perfluorinated hydrocarbon-based nanomaterials, because of their high oxygen solubility, are used in the photodynamic therapy (PDT). The inadequate oxygen supply in the tumor cells hampers the PDT. However, the oxygen-enriched perfluorocarbon-based nanomaterials, loaded with near-infrared photosensitizers, significantly enhance the PDT efficiency, in treating cancers (vide infra). Fluorinated polymers are indispensable in the modern world. Fluoropolymers, such as poly(vinylidene fluoride) (PVDF), and poly(tetrafluoroethylene) (PTFE), are ubiquitously found in numerous consumer goods, high-tech materials, and electronics. The electron-poor aromatic rings in polyfluoroaromatics exhibit strong ππ stacking interactions with nonfluorinated aromatic rings, forming donoracceptor (DA) complexes that are exploited in the design of functional materials, such as solar cells. Solar cells designed from organofluorine compounds and fluoropolymers provide high power conversion efficiencies (PCE) (vide infra). This area is exponentially progressing and may eventually become cost-effective so that organo-based solar cells could replace the fossil fuelbased energy sources. Fluorinated compounds such as difluoramines and pentafluorosulfanyl compounds exhibit high energy densities, while having relatively low shock sensitivities, and therefore are of emerging interest in the area of high-energy oxidizers and materials. Efficient synthetic methods for these high-energy fluorine-containing compounds are therefore of considerable interest (vide infra). 7.2 Fluorinated surfactants Surfactant molecules with perfluoroalkyl chains are biologically inert, exhibit enhanced hydrophobicity, and dissolve oxygen in relatively high concentrations. The perfluoroalkyl group is hydrophobic as well as lipophobic, and thereby the perfluoroalkylated surfactants are among the most effective surface-active agents, lowering the surface tension of the water more effectively than the corresponding hydrocarbon analogs. In some cases, mixtures of the fluorinated and nonfluorinated surfactants could be used to make them cost-effective. The mixtures of fluorinated and nonfluorinated surfactants also have the dual advantage of Chapter 7 • Materials applications of organofluorine compounds 281 attenuated surface tension (owing to the fluorosurfactants), and diminished interfacial tension between the water and oil (owing to the hydrocarbon surfactants). A variety of fluorinated zwitterionic, nonionic, and anionic surfactants derived from naturally occurring amino acids, carbohydrates, and lipids, with varying lengths of perfluoroalkyl chain length (Fig. 71A), have found a range of biomedical applications, including in ultrasound imaging, oxygen transport, blood substitutes, tissue engineering, and drug delivery.1 As an alternative to the use of pre-existing surfactants, Swager and coworkers elegantly demonstrated the rapid production of surfactants and double emulsions through reversible, spontaneous imine formation of the amines (e.g., primary amino groups of lysine residues of proteins or poly(ethylene glycol)-derived terminal amines) and perfluoroalkyl aldehydes at the oilwater interface. This in situ imine surfactant formation with biologically interesting molecules, such as antibodies, is potentially useful for the biosensing applications (Fig. 71B).2 Industrially used fluorinated surfactants, perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS), contain hydrophilic head groups and lipophobic perfluoroalkyl moieties as tails, and are used in the stabilization of various emulsions and vesicles (Fig. 72). Because of their excellent performance as emulsifiers, the perfluorosurfactants PFOA and PFOS have found numerous applications in consumer goods and pharmaceutical industries. For example, the PFOA and PFOS are components of food packaging materials, nonstick cookware, fire extinguishing materials, coatings, and drug delivery agents. However, because of the slow degradation of these fluorosurfactants in the environment, they are persistent in the environment for long durations and pose toxicity hazards in humans and animals. The long-chain perfluorinated compounds were shown to have endocrine disrupting effects. Therefore the use of the long-chain perfluorosurfactants is discontinued in the United States, and there is an urgent need to develop alternative natural productderived surfactants to replace the PFOA and PFOS.3 Emulsion of perfluorotributylamine or perfluorodecalin in albumin was originally developed as a blood substitute and was sold under the trade name “Fluosol.” Although Fluosol was approved by FDA, its use in medical applications was halted due to the technical difficulties of handling this emulsified blood substitute. The efficient oxygen transport achieved by the perfluorocarbon emulsions prompted the development of perfluorocarbon emulsionbased tissue engineering, which is useful, especially in the cardiac tissue engineering. Similarly, oxygent an emulsified form of perfluorooctyl bromide, when added to the tissue culture media provides enhanced oxygen transport.4,5 Early preclinical and clinical trials of Oxygent showed that a relatively low dose of 1.35 g/kg of Oxygent, used as an alternative to the intraoperative blood transfusion, was able to compensate the ongoing blood loss.6 Oxygent is approved in Russia as an oxygen carrier for hemorrhagic shock and perfusion of human organs. Furthermore, fluorinated surfactants solubilize the organofluorine compounds as emulsions and thereby find applications in biomedical areas, including in drug delivery and tissue engineering. Biocompatible fluorosurfactants could be readily synthesized from the naturally occurring carbohydrates, amino acids, and lipids, and their physiochemical and biochemical characteristics could be modulated by the nature and number of the fluoroalkyl moieties. 282 Organofluorine Chemistry (A) Surfactants based on naturally occurring compounds: O RF O NH 3 + O N(CH 3 )3 O O R F = e.g., C 9 F19 R F-lysine R F-phosphocholine O P O O RF n O O OH R F n O O O O O HO HO RF OH O P R F = C 8 F17 RF O O NH 3 + R F-glycine OH RF O O R F = e.g., C 4F 9 HO HO O H N RF OH O P O O O O N(CH 3 )3 RF R F = e.g., C 8F 17 n RF = e.g., C6 F13 RF-D-glucose n = e.g., 5; R F = e.g., C 4 F9 RF-D-glucophospholipid R F-phosphotidylcholine (B) In situ formation of the fluorocarbon surfactants: H O O n NH 2 + H F + F O F F F F O F F F F F F H 3C F F F F F F F H 3C O F F F F F F O n HF H 3C O H F NH 2 F F F F F O N n F F F F F F Fluorocarbon–imine surfactant FIGURE 7–1 Structures of typical perfluoroalkyl-derived surfactants based on α-amino acids, carbohydrates, and phosphatidylcholine (A); and in situ formation of the imine surfactants (B). 7.2.1 Perfluorocarbon nanomaterials Perfluorinated hydrocarbon nanomaterials have found applications in the PDT. In PDT, cancer cells are destroyed by reactive, singlet oxygen (1O2) species that is produced upon photoirradiation in the presence of a photosensitizer. However, due to the inadequate oxygen Chapter 7 • Materials applications of organofluorine compounds F F F F F F F F F F F F F O F Br F F F F F F F F F OH F F F F F F F F F F F F F F F F Perfluorooctyl bromide (Oxygent) 283 SO 3H F F F F F F F F PFOA PFOS FIGURE 7–2 Structures of perfluorooctyl bromide, a constituent of the oxygen-carrier biomaterial, Oxygent, and the widely used, but now phased out perfluorinated surfactants, PFOA, and PFOS. PFOA, Perfluorooctanoic acid; PFOS, perfluorooctanesulfonic acid. supply (hypoxic), the efficacy of the PDT is significantly hampered. Cheng and coworkers have designed an oxygen self-enriching PDT, by loading the near-infrared photosensitizer, IR780, and the perfluorocarbon nanodroplets into the lipid vesicles. Due to the relatively high concentration of oxygen in the perfluorocarbon nanoparticles, and because of the relatively longer lifetime of the singlet oxygen (1O2) in perfluorocarbons, the photodynamic efficiencies of this novel system are enhanced, resulting in elevated cytotoxicity to tumor cells (Fig. 73).7 IR780 Laser irradiation 1 O2-enriched perfluorocarbon O2 Tumor cell apoptosis (singlet oxygen) N Cl N I IR780; photosensitizer FIGURE 7–3 Schematic illustration of the photodynamic therapy using the oxygen-enriched perfluorocarbon materials; laser irradiation of the coencapsulated oxygen-enriched perfluorocarbon materials and the IR780 photosensitizer generates the cytotoxic singlet oxygen in relatively high concentrations and thereby results in the tumor suppression. Because of the high oxygen solubility and transport properties of the fluorinated amphiphilic micelles, fluorinated nanoplatforms offer unique opportunities in designing therapeutics for the PDT.8,9 For example, the fluorinated nanoplatforms of spherical micelles, 284 Organofluorine Chemistry consisting of copolymers of poly(ethylene glycol) (PEG) and perfluorophenyl methacrylate, tethered to the amino-porphyrins (as amide derivatives), such as tetrakis(4-aminophenyl)porphyrin (PEG-b-PPFMA/porphyrin), have high oxygen solubility. In these fluorinated nanomaterials, conjugated to the porphyrin photosensitizers, the production efficacy of the singlet oxygen was enhanced with increase in the ratio of the pentafluorophenyl to the porphyrin moieties.10 In vitro photocytotoxic experiments revealed high efficacy of the porphyrin-conjugated fluorinated nanoplatforms. NH2 Me CN PEG O Me Me x O O z O F F O NH N N H NH2 N HN F F F NH 2 porphyrin PEG-b-PPFMA/porphyrin Nanoparticulate perfluorooctyl bromide, stabilized by albumin, is an effective oxygen carrier and could be used in the tumor-specific delivery of oxygen, thereby leading to the effective cancer therapeutics.11 The hypoxic microenvironment of the solid tumors limits the therapeutic effectiveness of the anticancer drugs and radiotherapy. In radiotherapy, abundant oxygen supply is essential for the production of reactive oxygen species that promotes the cancer cell destruction. Furthermore, the perfluorocarbon-based nanoparticles, such as those derived from perfluorotributylamine, have platelet inhibition capability, and thereby increase tumor blood vessel permeability to red blood cells and promote excessive oxygen delivery at the tumor sites.12,13 On the other hand, the perfluorinated nanoparticles, because of their high affinity for oxygen, deplete oxygen concentrations in the tumor cells with high efficiency. Furthermore, fluorocarbons, such as perfluorotributylamine, form spherical nanoparticles when ultrasonically emulsified with human serum albumin and IR780, a near-infrared photosensitizer. These nanoparticles, because of their high oxygen affinity, enhance hypoxic environment in the tumor cells and also have enhanced tumor permeability. The perfluorinated nanoparticles, when used in combination with hypoxiabased agents, such as anaerobic bacteria and bioreductive prodrugs, promote hypoxic environment in the tumor cells, upon irradiation with an 808 nm laser, and thereby enhance the efficacy of hypoxia-based bacterial cancer therapies.13 Small molecule fluorocarbon-based microbubbles are produced through ultrasonication in the presence of perfluorosurfactants. These microbubbles, consisting of fluorinated inner gas surrounded by the perfluorosurfactants (or other naturally occurring emulsifying agents), Chapter 7 • Materials applications of organofluorine compounds 285 have found therapeutic applications, primarily in the cardiovascular diagnosis, tumor diagnosis, tissue engineering, and also as ultrasound-targeted drug and gene delivery agents.14,15 7.2.2 Fluorous catalysis Perfluorosurfactants also can be used to catalyze a variety of organic reactions. The perfluorosurfactants enable the reactions in water or in supercritical CO2 through their propensity for the formation of emulsion and thus serve as green catalysts, minimizing the production of the environmental waste. The perfluorosurfactant-based catalysts can be used in aqueous or supercritical CO2 media, replacing the conventionally used organic solvents, and thus would lead to a substantial decrease in the environmental E factor (kg of waste produced/kg of products formed).16 As an illustrative example, the copolymerization of carbon monoxide and ethylene in supercritical CO2 medium, in the presence of a fluorous Pd(II) catalyst PdClMe(dfppp) and silver tetrafluoroborate (AgBF4), gives the poly(ethylene ketone), in the absence of any organic solvents (Fig. 74).17 P Cl H H + CO H H P Pd C 6 F13 Me C 6 F13 O (PdClMe(dfppp) n Supercritical CO 2 , AgBF4 FIGURE 7–4 Fluorous Pd(II) catalysis in supercritical CO2 for the copolymerization of ethylene and carbon monoxide. 7.2.3 Environmentally benign perfluorosurfactants The use of environmentally less hazardous perfluorosurfactants, those with short-chain perfluoroalkyl moieties (C4C6) and perfluoroalkoxy ethers that are more easily degraded, are potentially useful alternatives to the long-chain perfluorosurfactants,18 although they are relatively less effective as emulsifying agents as compared to PFOA and PFOS. Sodium salts of (trifluoromethoxy)alkyl sulfonic acids (1), (p-trifluoromethylphenoxy)alkyl sulfonic acids (2), and N,N-bis(trifluoromethyl)aminoalkyl sulfonic acids (3) exhibit relatively low surface tension, comparable to that for the PFOA.18,19 Oligomers of hexafluoropropylene oxide (4) are also potential alternative biodegradable surfactants (Fig. 75).20 The biodegradation studies of the 10-(trifluoromethoxy)decyl sulfonate (5) shows the formation of the intermediate degradation products, 10-(trifluoromethoxy)decanoic acid (6), (trifluoromethoxy)acetic acid (7), and the transiently formed unstable product, trifluoromethanol. A minor pathway involving the formation of β-keto-10-trifluoromethoxy)decanesulfonic acid was also detected, showing the involvement of the β-oxidation pathways in the biodegradation of these alternative fluoroalkyl surfactants (Fig. 74).19 286 Organofluorine Chemistry F3 C O SO3 – Na+ n F 3C O SO3– Na + n F3C CF3 N SO3 – Na+ F F F F F n F n = 8–12 1 F3 C 2 – O + SO3 Na 10 O CO2H 9 CF3 OH n O 4 3 Desulfonative oxidation of terminal carbon O F3 C β-Oxidation Observed by LC/MS 5 F3 C n = 8–12 n = 8–12 O 6 CO2H CF3OH 7 (Observed by LC/MS) FIGURE 7–5 Structures of some of the potentially biodegradable alternatives to the PFOA and PFOS surfactants, and proposed biodegradation pathway for the ω-(trifluoromethoxy)decylsulfonate salt.19 PFOA, Perfluorooctanoic acid; PFOS, perfluorooctanesulfonic acid. 7.3 Fluoropolymers Fluoropolymers revolutionized the polymer industry since the beginning of the early 20th century. The fluoropolymers that have found wide industrial applications include PTFE (Teflon), PVDF, poly(vinyl fluoride), poly(chlorotrifluoroethylene) (PCTFE), poly(chlorotrifluoroethyleneco-ethylene), poly(tetrafluoroethylene-co-ethylene), and Nafion (a perfluorinated ion-exchange membrane, vide infra). Fluoroalkyl-derived copolymers were shown to be ideal photoresist materials, especially in the 157 nm lithography, as the fluorinated polymers are transparent at 157 nm. Due to the acidity enhancing effect of the perfluoroalkyl moieties, α-trifluoromethyl alcohols are as acidic as phenolic compounds and are soluble in mild aqueous bases, so that they could be easily washed out with aqueous bases after the photochemical irradiation. A variety of fluoropolymers were designed for the photoresist lithography.21 The trifluoromethylated photoresist material 12 is one such example. Poly(vinyl alcohol-co-trifluoromethyl vinyl alcohol), 11, was synthesized through azobis(isobutyronitrile) (AIBN)-initiated free-radical copolymerization of vinyl acetate (8) and α-trifluoromethylvinyl acetate (9), followed by hydrolysis. Protection of the alcohol moieties to tetrahydropyranyl ether then gives the photoresist material 12, which is acid-cleavable under photoresist lithography conditions to give the polymer 11 (Fig. 76). The α-trifluoromethyl alcohol moiety in the polymer 11, being as acidic as phenolic compounds, reacts with mild bases, such as tetramethylammonium Chapter 7 • Materials applications of organofluorine compounds CF3 + OAc AIBN n n H OAc F 3C OAc OAc 8 9 O 10 H3O + 287 n H OHn F3C OH 11 n H O n F C O 3 O O 12 FIGURE 7–6 Synthesis of a photoresist material, the copolymer of vinyl acetate and α-trifluoromethylvinyl acetate. hydroxide, to give the water-soluble salts. Thus, the polymer 11 is readily removed by 0.26N aqueous tetramethylammonium hydroxide during the subsequent development stage.21,22 Furthermore, fluoropolymers have high solubility in supercritical CO2. Carbon dioxide is relatively nontoxic and nonflammable and is abundantly available. CO2 is also fluorophilic and, therefore, supercritical CO2 can be used in the polymerization of fluoroolefins. Thus, the supercritical CO2 serves as a green solvent for the polymerization of fluoroolefins, such as trifluoroethylene, vinylidene fluoride, and fluoroalkyl acrylates.2325 Composite materials of poly(propylene) and PTFE, foamed with supercritical CO2, are superhydrophobic and are potentially suitable for a large scale oil recovery from the contaminated water.26 7.3.1 Poly(tetrafluoroethylene) PTFE (Fig. 77) is synthesized through free-radical polymerization of tetrafluoroethylene. A range of free-radical initiators, such as ammonium persulfate, benzoyl peroxide, trimethylene oxide, and di-tert-butyl peroxide, were used in the polymerization of the tetrafluoroethylene to give the PTFE. This polymer is chemically inert to strong acids, and has exceptionally high thermal stability, and thereby has found numerous applications, for example, in the manufacture of electric insulators and dielectrics, pyrotechnics, lubricants, hydrophobic coatings, and coated fabrics. The biomedical applications of PTFE include vascular grafts, stents, and coatings on surgical devices. Unfortunately, PTFE polymeric materials are not recyclable, unlike other polymeric materials, and therefore have large environmental impact. Despite the recent trend in replacing the fluoropolymers by environmentally sustainable polymers, some of the PTFE polymers are hard to replace.27 7.3.2 Poly(vinylidene fluoride) PVDF is an industrially produced polymer and has numerous materials- and biomaterials applications. This polymer exhibits piezoelectric properties, converts electrical energy into 288 Organofluorine Chemistry Free-radical polymerization F F F F F n F F F PTFE Tetrafluoroethylene Free-radical polymerization F H F F F n H PVDF Vinylidene fluoride F F H H F Vinylidene fluoride Trifluoroethylene F + H Vinylidene fluoride F F F n Poly(VDF–trifluoroethylene) Free-radical polymerization F F F F H F F H H F + Free-radical polymerization F CF3 Hexafluoropropene F F F F F CF3 n Poly(VDF–hexafluoropropene) FIGURE 7–7 Synthesis and structures of PTFE, PVDF, and some of its industrially significant copolymers. PTFE, Poly (tetrafluoroethylene); PVDF, poly(vinylidene fluoride). mechanical energy and vice versa, and thus has found extensive industrial applications in the manufacture of smart materials. The PVDF also exhibits ferroelectric, hydrophobic, and oleophobic properties and is chemically inert to strong acids. It has found, therefore, numerous industrial applications, for example, in coatings, functional membranes for water treatment, aeronautics, and biomedical applications.28 As smart materials, the PVDF and its copolymers have the ability to change their shape and size when subjected to the electric fields, reversibly converting mechanical strain into electrical signals.29 Copolymerization of trifluoroethylene with vinylidene fluoride gives poly(VDF-trifluoroethylene), and the latter Chapter 7 • Materials applications of organofluorine compounds 289 copolymer is one of the major commercially viable smart materials, along with PVDF. The copolymer of vinylidene fluoride and hexafluoropropene (poly(VDFhexafluoropropene) shows improved hydrophobic and mechanical properties as compared to PVDF. The latter copolymer exhibits lower crystallinity than that of PVDF and is also used as a membrane separator in lithium-ion batteries (Fig. 77). 7.4 Fluorinated π-conjugated polymeric materials in photovoltaic devices 7.4.1 ππ Stacking interactions in polyfluoroaromatics Polyfluoroaromatics as composites with nonfluorinated aromatics exhibit improved thermal and chemical stabilities because of the high CF bond strengths and also due to the electrostatic ππ stacking interactions of the perfluoroaryl groups with the nonfluorinated aromatics.30,31 The existence of ππ stacking interactions of the fluorinated and nonfluorinated aromatics (as in the case of aggregate 15) is unambiguously evident from the markedly increased melting point of 250 C252 C for the 1:1 molar mixture of perfluorotriphenylene (14, mp 109 C) and triphenylene (13, 199 C) (Fig. 78).32 These arylfluoroaryl ππ stacking interactions often result in improved physicochemical and thermochemical characteristics for the polymeric materials. For example, Grubbs and coworkers have confirmed the existence of these π_π stacked complexes, through differential scanning calorimetry (DSC), using the polynorbornene polymer 16, having the sidechain triphenylene moieties. A 1:1 mixture of the latter polynorbornene polymer and perfluorotriphenylene (14) exhibits a Tg of 123 C, whereas the polymer 16 alone, in the absence of the perfluorotriphenylene (14), exhibits a glass transition temperature of 41 C.33 Thus, through addition of small perfluorinated molecules, such as 14, polymers displaying only a glass transition could be transformed into crystalline materials. This ππ stacking interactions in fluoroaromatics plays an important role in the organic solar cells (vide infra). 7.4.2 π-Conjugated polymers Fluoride-initiated copolymerization of 1,4-phenylene bis(trimethylsilylethyne) (17) with perfluorobenzene gives the corresponding copolymer 18, with high regioselectivity; the reaction proceeds through the aromatic nucleophilic substitution (SNAr) of the fluorines at the 1,4positions in the perfluorobenzene (Fig. 79).34 The number average molecular weights (Mn) of these polymeric materials are in the range of 34153 kDa. These poly(arylene ethynylene) materials are commonly used as active components in various materials applications, such as solar cells, sensors, organic light-emitting diodes, and field effect transistors.35,36 Fluorinated poly(thienothiophene (TT)-co-benzodithophene (BnDT)) polymers (e.g., PTBF1, PTBF2; Fig. 7-10), when used as composite materials with fullerenes, exhibit semiconducting properties, and their photovoltaic efficiencies could be tailored by the 290 Organofluorine Chemistry F F F F H H H H π–π Stacking interactions of polyfluoroaryl–aryl rings F F F F F F F F + F F F 1:1 F F F F 15 14 13 F mp 250–252 °C mp 109 °C O F n O F F F F F 1:1 T g = 123 °C + F F F F F 14 F F F F F F F mp 199 °C F F 16 T g = 41 °C FIGURE 7–8 ππ Stacking interactions of polyfluorinated aryl rings with nonfluorinated aromatics and its usefulness in the design of task-specific polymeric materials. Chapter 7 • Materials applications of organofluorine compounds F OR RO F SiMe 3 Me 3Si F F TBAF or CsF F –Me 3SiF F OR RO 291 17 RO OR F F RO OR F F n 18 FIGURE 7–9 Transition metalfree copolymerization in the synthesis of fluorinated poly(arylene ethynylene) copolymers. stereoelectronic effect of the substituents on the polymer backbone.37 A vast majority of the polymeric materials used in the solar cell applications have alternating DA moieties. In most cases, electron-withdrawing moieties, such as fluorines, are attached to the acceptor moieties. The electron-withdrawing effect of the fluorine(s) lowers the HOMO as well as LUMO energy levels of the polymers, with HOMO lowering being relatively greater than for the LUMO. This relatively greater energy-lowering effect of the HOMO, as compared to the LUMO, results in the increase of the polymer HOMOLUMO gap, as well as increase in the HOMO (polymer)LUMO (fullerene) energy difference, thereby increasing the solar cell open-circuit voltage (Voc). This increase in the solar cell open-circuit voltage (Voc), due to the enhanced HOMO (polymer)LUMO (fullerene) energy difference, translates into the increased PCE of the solar cells.38 Thus, fluorination of the π-conjugated polymers offers a viable strategy for the design of the polymer solar cells that can deliver up to .7% (and even up to 13% in some all-polymer solar cells) efficiency (vide infra).36,39 Fluorine has obvious advantages in the solar cell applications, as it has highest electronegativity of all the elements (Pauling EN of fluorine 5 3.98) and its van der Waals radius is only 20% greater than for the hydrogen. It is also electron-donating through resonance, somewhat counteracting the electronegative effects. Noncovalent interactions involving hydrogen bonds of fluorine to neighboring nitrogen, oxygen, and sulfur atoms can alter the physicochemical properties of the polymeric materials, especially in thin-film-based devices used in organic solar cells. The DA polymers are typically used as electron-donating components along with fullerene-based compounds as electron-accepting components in the bulk heterojunction (BHJ) solar power cells. However, there is also increasing interest in developing all-polymer solar power cells that can rival in their PCE.40 As described earlier, in most of the fluorinated polymers used in the organic solar cells to date, fluorines are placed on the electron-acceptor unit of the DA polymers. You and coworkers broadly classified these fluorinated acceptor units 292 Organofluorine Chemistry N S N N F F R N F Benzothiadiazole (BT) N F Benzotriazole (TAZ) R R N N F F R O O F S S Quinoxaline (Qx) Thienothiophene (TT) Illustrative polymers consisting of the above moieties: R R S S R F F F F S R S n R = 3-butylnonyl R1 = 2-butyloctyl n PBnDT-DTffBT R1 S S N S N S R R1 N N N S R PBnDT-XTAZ R2 O F S O OR 2 N S OR N O S S S F S F S S S OR F n R1 n PTBFF1 (R = 2-ethylhexyl) PBQ-4 O O F S PTBF1 S S S S S OMe OMe S S OMe OMe F OMe OR F OMe F n n PTBF2 FIGURE 7–10 Donoracceptor (DA) polymers with fluorinated acceptor moieties; one example of a polymer with a fluorinated donor moiety, PTBF2, is also shown. Chapter 7 • Materials applications of organofluorine compounds 293 into four classes: those based on benzothiadiazole (BT), benzotriazole (TAZ), quinoxaline, and thienothiophene moieties (Fig. 710).36 Among these polymeric materials, BT acceptor-based DA copolymers are widely used in solar cell applications.39 In addition to the energy lowering of the HOMO and LUMO levels, fluorine atoms also increase the planarity of the polymer chains, thereby increasing maximum absorption coefficient relative to the nonfluorinated polymers, due to the hydrogen bonding and electrostatic ππ stacking interactions. Furthermore, fluorination of the DA polymers enhances their miscibility with the widely used electron-acceptor fullerene, phenyl-C61-butyric acid methyl ester (PC61BM; PCBM, Fig. 712). When nonfullerene acceptors (as in the case of the allpolymer solar cells) are used in conjunction with the π-conjugated fluorinated DA polymers, PCE of up to 13.1% could be achieved (vide infra).40 Fluorination of the donor moieties in the DA polymers (which are used in the fullerene-based BHJ solar cells), as in the case of the fluorination of the acceptor moieties, also results in the lowering of the HOMO and LUMO energy levels and in the enhancement of the crystallinity and backbone planarity of the polymeric materials. However, the beneficial effects of the fluorination of the donor moieties, unlike that for the acceptor moieties, are not quite generally observed.36 The DA polymers most widely used in these applications consist of benzodithiophene (BnDT), thiophene, and benzene moieties. Some of the examples of these polymers are shown in Fig. 711. The fluorines in the PTBF3 polymers have detrimental effects on the polymer photochemical stability. The fluorine-containing PTBF3 polymers also have decreased compatibility with the fullerene-based electron acceptor, phenyl-C71-butyric acid methyl ester, PC71BM, as these polymers show phase separation in their BHJ solar cells.37 On the other hand, copolymerization of 2,3-difluorothiophene with the electron-rich BnDT-derived monomer affords the copolymer P2FT, which showed improved open-circuit voltage (Voc) and other favorable characteristics in its fullerenebased BHJ.41 When fluorinated benzene was used as a π-conjugated linker connecting the thiophene donor moieties, as in the case of PDTBz-4F, the performance of the fullerenebased BHJ was dependent on the number of the fluorines on the benzene moiety. For example, the mono-, and difluorinated analogs show favorable attributes to the solar cell performance, while the tetrafluorinated derivative PDTBz-4F has adverse effects on the morphology of the active layer and exhibits decreased solubility and phase separation with PC71BM.42 7.4.3 Synthesis of the fluorinated donoracceptor polymers for fullerenepolymer solar cells Stille-coupling condensation polymerization of the distannylated BnDT 19 and the 2,5-dibromo-3,4-difluorobenzo-2,1,3-thiadiazole 20 gave high yields of the difluorinated polymer PBnDTDTffBT (Fig. 712).39 The HOMO and LUMO energy levels of this polymer (25.54 and 23.33 eV, respectively) are lower than that of the nonfluorinated analog (25.40 and 23.13 eV, respectively). The BHJ solar cells made of PBnDtDTffBT and [6,6]-phenyl C61-butyric acid methyl ester, PC61BM, (PCBM), as the electron acceptor, performed superior 294 Organofluorine Chemistry R F F F S S F F F S F R Thiophene Benzodithiophene (BnDT) n-Bu O O O F F S S S S S S n O S OR n-Bu R F OR F F n F n-Bu n-Bu PTBF3 R R′ P2FT R′ R O O F S n F S S F N N S F PDTBTBz-4F FIGURE 7–11 Structures of fluorinated benzodithiophene, thiophene, and benzene-derived donor moieties in DA polymers, used in the BHJ solar cells. BHJ, Bulk heterojunction; DA, donoracceptor. to those of the corresponding nonfluorinated analog with a PCE of 7.2% (Voc 5 0.91 V) as compared to 5.0% (Voc 5 0.87) for the nonfluorinated analog. The PCE of 7.2% achieved using this polymer/P61BM BHJ device is in the top range of PCE reported for this type of fullerenepolymer-based BHJ solar cells. A fluorinated polymer, PBnDTXTAZ (X 5 F; 24), consisting of BnDT moieties as donor moieties and difluorinated TAZ moieties as acceptor moieties, when blended with the PCBM electron acceptor, affords high photovoltaic conversion efficiency of 7.1%, as compared to the corresponding nonfluorinated analog, 23, which affords a PCE of 4.3% (Fig. 713).38 This polymer was synthesized through Stille-coupling polycondensation polymerization of stannylated BnDT (21) and the brominated TAZ derivative (22) in high yields. Chapter 7 • Materials applications of organofluorine compounds S N R Pd 2(dba) 3 P(o-tolyl) 3 , o-xylene microwave, 150 ˚C 20 min N S Me 3 Sn SnMe3 + S S S Br 19 Br F F R 295 20 R O R S N S N S S R F CH3 O S F n PBnDT–DTffBT PCBM FIGURE 7–12 Synthesis of the DA polymer PBnDTDTffBT, used in the fullerene-based BHJ solar cells. BHJ, Bulk heterojunction; DA, donoracceptor. R1 R N N Pd 2(dba) 3 P(o-tol) 3 o-Xylene N S (H3 C)3 Sn Sn(CH 3 ) 3 S Br R R = 3-butylnonyl S S X X Br 95%–96% yield R 1 = 2-butyloctyl 21 X = H/F 22 R R1 N N N S S S R X X R = 3-butylnonyl R 1 = 2-butyloctyl S n PBnDT–XTAZ; X = H (23); X = F (24) FIGURE 7–13 Synthesis of the DA polymer PBnDTXTAZ, used in the BHJ solar cells. BHJ, Bulk heterojunction; DA, donoracceptor. 296 Organofluorine Chemistry 7.4.4 π-Conjugated benzodithiophenequinoxaline copolymers Fluorinated π-conjugated polymers serve as efficient semiconducting materials, and, in fact, some of the best performing semiconducting materials used in solar cell applications are fluorinated π-conjugated polymers.43 Polymer solar cells consisting of solution phase BHJ structure currently are capable of yielding PCE of about 10%. These polymeric materials are prepared through roll-to-roll solution coating, and in view of their ease of synthesis and high PCE, there is ever-increasing interest in the structural modification of the polymeric backbone for enhancing their optoelectronic properties. In particular, the PCE of these solar cells is directly correlated with the HOMO (donor)LUMO (acceptor) gap. Fluorination of the polymeric backbones of the conjugated polymers, in general, increases this HOMOLUMO gap, and thereby the fluorinated polymeric materials exhibit enhanced PCE. For example, the nonfluorinated analog of the copolymer PBQ-4, consisting of benzo[1,2-b:4,5-b0 ]dithiophene (BDT) as donor moiety and 2,3-diaryl-5,8-di(thiophen-2-yl)quinoxaline (DTQ) as acceptor unit exhibits an open-circuit voltage (Voc) of 0.64 V and a PCE of 5.63%. The corresponding fluorinated copolymer PBQ-4, on the other hand, exhibits a relatively higher Voc of 0.90 V and an improved PCE of 8.55%.44 The synthesis of the fluorinated polymer PBQ-4 was achieved through Pd(0)-catalyzed Stille-coupling polycondensation of the difluoro-BDT (25) and the difluoro-DTQ (26) (Fig. 714). 7.4.5 Fluorinated polymers in fullerene-free, all-polymer (organic) solar cells An all-polymer solar cell, consisting of a fluorinated acceptor polymer 27 (called PFBDTIDTIC) and a fluorinated donor polymer 28 (called PM6) provides high PCE of 10.3% (Fig. 715).45 The exceptionally high PCE achieved using this polymer composite was attributed to the favorable absorption property and high electron mobility of the polymer 27. A fullerene-free, all-organic solar cell consisting of a donor polymer, PBDB-T-SF, and a small molecule acceptor, IT-4F, with a 100200 nm thickness, exhibits a PCE of 13.1%. The electrondonor polymer PBDB-T-SF was synthesized through Pd(0)-catalyzed Stillecross coupling condensation polymerization of the distannylated thiazole derivative, BDT, and the brominated thiazole derivative, BDD (Fig. 716A). The small molecule electron-acceptor molecule IT-4F was synthesized through the aldol condensation reaction of the activated ketone EG-2F using pyridine as a mild base (Fig. 716B).40 The fluorinated small molecule acceptor IT-4F, in comparison with its nonfluorinated version, exhibits slightly enhanced absorption coefficient in its UVvis spectrum, and also its absorption maximum is red-shifted by 17 nm, indicating an enhanced intramolecular charge transfer, and thereby increased harvesting of solar photons, by this compound. Furthermore, the fluorinated versions of the donor and acceptor components exhibit downshifted HOMO and LUMO energy levels as compared to the corresponding nonfluorinated compounds. The active layer of organic solar cell, consisting of blended components of the donor polymer PBDB-T-SF and the acceptor small molecule IT-4F, shows more ordered Chapter 7 • Materials applications of organofluorine compounds 297 R2 O R1 S OR 2 N S N S S S S R1 n PBQ-1; nonfluorinated version of PBQ-4 Open-circuit voltage (Voc) = 0.64 V Power conversion efficiency (PCE) = 5.63% R1 R2 O F S OR 2 N N S Me 3 Sn SnMe 3 + Br S S F F S F Br R1 26 25 R1 S R2 O F S OR 2 N S N S S F F S F S R1 n D A PBQ-4 Open-circuit voltage (Voc) = 0.90 V Power conversion efficiency (PCE) = 8.55% FIGURE 7–14 Synthesis of fluorinated benzodithiophenequinoxaline copolymers (bottom) and the structure of the corresponding nonfluorinated copolymer (top). 298 Organofluorine Chemistry CN NC S O F R F R n F R S R F S O R1 S S O S S S NC S CN S S S R1 R1 R1 R 1 = 2-ethylhexyl R = C 16H 33 R1 S R1 O n R1 = 2-ethylhexyl PFBDT–IDTIC (27) PM6 (28) FIGURE 7–15 Structure of a fluorinated polymer composite of PFBDTIDTIC (27) and PM6 (28) for solar cell applications. intermolecular arrangements (i.e., increase in crystallinity), and the hole mobility (μh) of the PBDB-T-SF and electron mobility (μe) of the IT-4F are slightly improved over that of the nonfluorinated version of the molecular blend.40 7.5 Fluorinated poly(aryl thioethers) in organic electronic materials Fluorinated poly(aryl ethers) and poly(aryl thioethers) are high-performance polymeric materials with desirable characteristics, such as high thermal stability and enhanced chemical stabilities, and are hydrophobic materials. Polymer composites of these materials with nonfluorinated aryl polymers would be expected to have enhanced thermal and chemical stabilities and are useful for applications in a variety of applications, including in the area of organic electronic materials. Practical and convenient synthetic approaches for the polymeric materials consisting of thiolated polyfluoroaryl copolymers are of substantial interest in the area of organic electronic materials.34,46 Toward that goal, synthesis of alternating thiophene and perfluoroarene copolymers 30 and 31 was achieved through fluoride-activated copolymerization of silylated thiophene derivatives (e.g., 29) with perfluorinated aromatics (Fig. 717). The volatile byproduct trimethylsilyl fluoride (bp 16 C) could be easily removed from the polymeric materials, and thus this synthetic route is amenable for the industrial scale synthesis of perfluoroarylthiophene copolymers.46 (A) F SR S R R S O + S Me3 Sn SnMe 3 S S Br S Br S S BDD BDT F Pd(0) O SR F SR S R S R O S O S S S S S n F SR PBDB-T-SF (electron-donor polymer) R (B) R O S + F NC S F Pyridine, CHCl3 O S CN NC F CN F reflux S O R EG-2F R DTIDT–CHO R R F CN O S CN CN F F NC S S S F O R R IT-4F (electron-acceptor compound) FIGURE 7–16 Synthesis of the donor polymer PBDB-T-SF (A) and a small molecule electron-acceptor IT-4F (B), used in an all-organic solar cell that gives a power conversion efficiency of 13.1%. 300 Organofluorine Chemistry F F F F F F F F F F S Bun -O F SiMe 3 O-Bu n F F F O-Bu n F S n Bun -O 30 F F F F 29 F F 0.1 equiv CsF, 18-crown-6 toluene, heat Me 3Si F F F F 0.1 equiv CsF, 18-crown-6 toluene, heat F S n Bun -O F F O-Bu n 31 FIGURE 7–17 Synthesis of polyfluoroarylthiophene copolymers through fluoride-initiated copolymerization. A convenient synthesis of poly(fluoroaryl thioethers) involves reaction of the fluorinated aromatics, such as perfluorobenzene and perfluorobiphenyl (34), with S,S-bis(trimethylsilyl) dithiols (32) in the presence of mild organobases, such as 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD), at room temperature (Fig. 718).30 This synthetic strategy could be used for the polymerization of a variety of polyfluorinated and other activated aromatics and is useful in the processing and fabrication of fluorinated polymers, as there are no extra purification steps for the removal of the volatile organocatalysts and trimethylsilyl fluoride byproducts. These reactions proceed through aromatic nucleophilic substitution mechanism (SNAr), as substantiated by ab initio calculations. 7.6 Polymer electrolytes A solid polymer electrolyte material, with propylene carbonate side chains, poly(vinylidene fluoride-co-(2-oxo-1,3-dioxolan-4-yl)methyl 2-(trifluoromethacrylate) (39), was synthesized by the copolymerization of the trifluoromethacrylate ester 38 and vinyldine fluoride (Fig. 719).47 This random copolymer with added LiClO4 exhibits ionic conductivity values as high as 2 3 1024 S/cm at room temperature and shows high lithium-ion transference number and relatively large electrochemical window, from 1.4 to 4.9 V versus Li/Li1. Because of the PVDF nano-domains, this polymer has high thermal and mechanical stability and thus is a potentially next-generation solid polymer electrolyte for the solid-state lithium-ion batteries. Polymer electrolytes, with ionic liquid moieties (such as imidazolium moiety) incorporated into the side chains, afford relatively high ionic conductivities. These solid polymer Chapter 7 • Materials applications of organofluorine compounds 301 F F F F F F TBD, DMF F F S S F n F Me3 Si S S 33 SiMe 3 Aryl poly(thioether) 5 32 TBD, DMF F F F F F F F N S F F F H N F F F F S F F F F 34 n N Aryl poly(thioether) 35 (TBD) FIGURE 7–18 Synthesis of poly(fluoroaryl thioethers) through fluoride-initiated copolymerization of perfluoroaromatics with silylated dithiols. CF3 CF3 Cl O HO O + O 37 36 O F Pyridine O O O O O 38 F TAPE 74°C, 24 h CF3 F F O O n O 39 O O FIGURE 7–19 Synthesis of a PVDF copolymer with lithium-ion conducting propylene carbonate moieties as side chains. PVDF, Poly(vinylidene fluoride); TAPE, tert-amyl peroxy-2-ethylhexanoate free-radical initiator. 302 Organofluorine Chemistry electrolytes, also called poly(ionic liquids), are usually synthesized by controlled radical polymerization reactions (in the case of polyacrylates and polyvinyl polymers), or through ringopening metathesis polymerization as in the case of the polynorbornene polymers (e.g., 44).48 A variety of these poly(ionic liquids) can be assembled using the corresponding imidazolium, pyrrolidinium, and ammonium cations, associated with the relatively non2 2 2 nucleophilic counteranions, such as BF2 4 , PF6 , (CF3 SO 2 ) 2N , and CF3 SO3 . Poly(methyl methacrylate)-based poly(ionic liquids) consisting of tetraalkylammonium (40), pyrrolidinium (41), and imidazolium cation (42) side chains along with bis(trifluoromethylsulfonyl)imide [TFSI; (CF3SO2)2N2)] anions, and other poly(ionic liquids) with a poly (ethylene) backbone (43) typically have conductivities ranging from 10210 to 1025 S/cm, depending on the alkyl substituents on the cationic nitrogen (Fig. 720). The counteranions play a significant role in modulating the ionic conductivities of these polymer electrolytes, and the strongly delocalized TFSI anion exhibits typically enhanced ionic conductivity as compared to the other fluorinated counteranions, such as tetrafluoroborate, hexafluorophosphate, and trifluoromethanesulfinate anions. For example, poly(1-(2methacryloyloxy)ethyl)-3-butylimidazoluim salts (42) show the following conductivities (S/cm) at 110 C as a function of the counteranion: 4.0 3 1024 (TFSI); 1.5 3 1025 26 26 48 (CF3 SO2 (BF2 (PF2 Similarly enhanced conductivities for the 3 ); 6.4 3 10 4 ); 3.8 3 10 6 ). CH 3 n O CH3 CH3 O n O n O O N O CH3 H 3C CH3 N H3 C N(SO2 CF3) 2 N N(SO 2CF3 )2 41 40 H3 C N N CH 3 N(SO 2CF3 )2 N CH 2CH3 N(SO 2CF3 )2 42 43 n O O H3 C N CH 3 N N(SO 2CF3 )2 44 FIGURE 7–20 Structures of selected poly(ionic liquid) electrolytes for applications as solid polymer electrolytes in lithium-ion batteries. Chapter 7 • Materials applications of organofluorine compounds 303 TFSI counteranions were found in other polymer electrolytes, such as those derived from the poly(norbornenes) (44).49 Gel polymer electrolytes, formed by mixing the polymer electrolyte with an ionic liquid, exhibit improved lithium-ion conductivities and other favorable electrochemical properties, such as decreased operational temperature for the lithium-ion batteries. Yin and coworkers have developed a new kind of polymer gel electrolyte that consists of a composite of the dicationic ionic liquid 48 and an ionic liquid 49 (in Fig. 721) in the presence of excess LiTFSI.50 The increased charge carriers in this polymeric gel electrolytes allowed assembly of Li/LiPO4 cells that afforded relatively high discharge capacities (160 (A h)/g at 40 C). The synthesis of this dicationic polymer electrolyte, as shown in Fig. 721, involves AIBN-catalyzed radical polymerization of N-vinylimidazole, followed by N-alkylation using 2-bromo-(N,N,N-trimethyl)ethanamine and anion metathesis with LiTFSI salt. AIBN N n N N Br N + Br– n 2 Br– N LiN(SO2CF3 )2 N N 45 46 N+ 47 CH 3 n N O N N N + CH 3 N(SO2CF3 )2 N CH3 2 N(SO2CF3 )2 48 49 FIGURE 7–21 Synthesis of a dicationic polymer electrolyte and the structure of the coadditive ionic liquid electrolyte 49. A copolymer of VDF with polyethylene trifluoromethacrylate 52, with side-chain poly(ethylene oxide) (PEO) groups [poly(VDF-MAFTEG)] was synthesized through radical copolymerization (Fig. 722).51 This polymeric material with grafted PEO moieties could be used as a gel polymer electrolyte, when mixed with an ionic liquid electrolyte, 1-propyl-1methylpyrrolidinium bis(fluorosulfanyl)imide and lithium bis(trifluoromethylsulfonyl)imide (LiTFSI). This electrolyte material shows ambient conductivity in the range of 0.2 mS/cm, and a relatively high electrochemical window of 1.54.1 V versus Li/Li1. 304 Organofluorine Chemistry F CF 3 CF 3 Cl O HO + O O O n 51 F O n (VDF) Radical polymerization 52 50 CF 3 n O F F O O n Poly(VDF-co-MAFTEG) 53 FIGURE 7–22 Synthesis of poly(VDF-co-MAFTEG) gel polymer electrolyte. 7.7 Fluorinated ionomers as proton-exchange membranes in fuel cells Nafion-H is the state-of-the-art proton-exchange membrane (PEM) and is widely used in the fuel cell applications.52 Nafion-H has a linear perfluoroalkyl moiety as the backbone with flexible perfluoroalkylsulfonic acid moieties as side chains. Several variations of the Nafion-H involve subtle differences in the side-chain sulfonic acid moieties. The structure of the widely used Nafion 117 is shown in Fig. 723. In the direct methanol fuel cells (DMFCs), chemical energy stored in methanol is converted into the electrical energy. In this electrochemical fuel cell, methanol is oxidized at the anode and the resulting protons diffuse toward the cathode through a PEM, such as Nafion-H, where they combine with electrons to generate electrical energy and release water and CO2 as byproducts. F F F F F F y xF O F F CF F 3 F O F SO3H n F F FIGURE 7–23 Structure of Nafion-H (Nafion 117). Although, in theory, DMFCs provide substantially higher power densities as compared to that of the state-of-the-art lithium-ion batteries, the currently attainable overall efficiencies are in the order of only about 25%, limiting their practical applications. Among the most important factors contributing to these limitations are the reaction kinetics at the anode and the unwanted methanol crossover through the PEMs. Thus, there are numerous attempts of improving the thermochemical, electrochemical, and chemical stability of the PEM ionomers. Chapter 7 • Materials applications of organofluorine compounds 305 The success of the widely used Nafion-H membranes lies in their strongly hydrophobic polymer backbone and the hydrophilic sulfonic acid side chains. Among various ionomers developed as alternatives to the Nafion-H, the poly(ether sulfone) and poly(imide) materials with appropriately functionalized perfluoroalkylsulfonic acid side chains are widely used in the fuel cell applications.5364 Illustrative examples of these ionomer membranes are outlined next. A poly(ether sulfone) ionomer (56 in Fig. 724) with the perfluoroalkylsulfonic acid side chain, 2 CF2CF2OCF2CF2SO3H, was synthesized through electrophilic bromination of the poly(ether sulfone) 54, followed by Cu(0)-catalyzed coupling of the resulting aryl bromide 55 with the potassium 1,1,2,2-tetrafluoro-2-(1,1,2,2-tetrafluoro-2-iodoethoxy)ethanesulfonate and subsequent neutralization with HCl. This poly(ether sulfone)-based ionomer (56) has relatively high proton conductivity of 0.12 S/cm at 80 C under 90% relative humidity and has relatively higher flexibility as compared to the nonfluorinated sulfonated poly(ether sulfone).53 O O Br2/DCM, 0°C to RT Br S O O S O O n n 54 55 O F F 1. Cu/DMSO, 120 °C F F F F 2. I SO 3– K + O F F F F S O O O F F F F F F n SO3 H DMSO, 120 °C 3. HCl 56 FIGURE 7–24 Synthesis of poly(ether sulfone)-based perfluoroalkylsulfonic acid for applications as proton-exchange membrane in direct methanol fuel cells. Zheng and coworkers have synthesized a poly(ether sulfone) ionomer (61) in a three-step synthetic procedure involving step-growth condensation polymerization of 4,40 -bis(difluorophenyl)sulfone with the imide 57, followed by the BBr3-mediated deprotection of the methyl ether and subsequent ring-opening perfluoroalkylation reaction using the perfluoroalkylsulfonic acid lactone 60 (Fig. 725).56 This ionomer has relatively high mechanical and thermal stability and exhibited relatively low methanol permeability and conductivity of up to 0.083 S/cm, comparable to the state-of-the-art Nafion 117 membrane. Saito and coworkers have synthesized a poly(imide) copolymer with pendant perfluoroalkylsulfonic acid moieties (65) in a one-step condensation copolymerization of 1,4,5,8naphthalenetetracarboxylic acid dianhydride (62), aryloxyperfluoroalkylsulfonic acid 63 and 306 Organofluorine Chemistry O S OH F F N K2CO3 /DMSO, 140 ˚C N OCH 3 O O O S O O O HO O 57 n OCH3 58 O F O O S O O S O CF3 O BBr3 /DCM (60) N n O F F DMSO, RT to 110 ˚C OH 59 O O S O O N n F F O O SO 3H F CF3 61 FIGURE 7–25 Synthesis of a poly(ether sulfone) ionomer for applications as a proton-exchange membrane in fuel cells. the triazole 64 (Fig. 726). This ionomer was soluble in polar organic solvents, was stable up to about 180 C, and exhibited a relatively high proton conductivity of 6.6 3 1024 S/cm at 80 C, comparable with the proton conductivity of the conventional sulfonated poly(arylene ether) ionomer membranes.55 A composite membrane, synthesized through radical polymerization of styrene and divinylbenzene (as a cross-linker), admixed with PVDF, (Fig. 727) proved to be superior to the state-of-the-art Nafion-H membranes in terms of performance characteristics, such as decreased methanol crossover and water management, inDMFCs.65 Chapter 7 • Materials applications of organofluorine compounds NH2 O O H 2N N O O NH2 + O O 307 + H2 N OCF2 CF2SO 3H 62 m-Cresol, TEA, benzoic acid N N H 175°C to 195°C 64 63 O N O O N O O N N O O N O F F O HO3S F F 50 N N H 50 65 FIGURE 7–26 Synthesis of the poly(imide)-based ionomers for applications as a proton-exchange membrane in the fuel cells. TEA, Triethylamine. F F F 1. Styrene/DVB/AIBN n PVDF matrix 2. ClSO3H/CHCl3 3. H2O, 60°C F n HO3S n SO3 H FIGURE 7–27 Synthesis of PVDFpolystyrenesulfonic acid composite material as proton-exchange membrane for fuel cell applications. PVDF, Poly(vinylidene fluoride). 7.8 Fluorinated carbon nanoparticles and nonaqueous electrolytes in lithium- and lithium-ion batteries Fluorinated graphite (CFx)n (also called CFx) materials are used as positive electrodes in the primary lithium batteries as they provide high specific charge densities compared to the conventionally used metal-oxide cathodes. The CFx electrodes exhibit relatively low electrical conductivity with increase in the fluorine content, because the CF bonds are sp3-hybridized, and therefore optimal carbon-to-fluorine ratio is necessary for efficient performance of the lithium batteries. The CFx electrodes can be used over a wide temperature range, from 260 C to 190 C, and exhibit theoretical discharge capacity of about 870 (A h)/kg. 308 Organofluorine Chemistry Fluorinated carbon nanoparticles (F-CNPs) were shown to have improved discharge capacities as compared to the state-of-the-art CFx cathodes that are widely used in primary lithium batteries, although these materials are not commercialized to date.66 The F-CNPbased electrodes have intrinsic properties of the CFx and those of the nanoscopic materials. The carbon nanoparticles are prepared by electrochemical reduction of the fused Li2CO3Na2CO3K2CO3 at about 600 C, with nickel sheet as the working electrode and graphite rods as counter-electrodes. Thus obtained carbon nanoparticles are fluorinated using fluorine gas, as dilute solution in argon, to give the F-CNPs. High thermal stabilities of the lithium-ion batteries are required for their use in electric and hybrid electric vehicles. To enhance thermal stabilities, inorganic phosphates are used as additives, although these additives lower the battery performance. Thermal stabilities of the electrolytes could also be enhanced by using fluoroalkyl-derived dialkyl ethers (e.g., 66) and carbonate-based solvents (e.g., 67). Organofluorine electrolyte solvents, including fluoroalkyl ethers [e.g., 3-(1,1,2,2-tetrafluoroethoxy)-1,1,2,2-tetrafluoropropane, 66], fluoroalkylsubstituted propylene oxide [e.g., 4-(2,2,3,3,3-pentafluoropropoxymethyl)-1,3-dioxolan-2one, 67], and fluorinated esters (e.g., methyl difluoroacetate), afford enhanced thermochemical stabilities to the lithium-ion batteries.67 Fluorinated ionic liquid electrolytes have potential advantages toward enhanced thermochemical and electrochemical stabilities, although there remain challenges in improving their ionic conductivities and viscosity effects. Fluoroalkyl-derived imidazolium-based ionic liquids (e.g., 68), due to their enhanced thermal stability, ionic conductivity, and electrochemical stability, are potentially useful as nonaqueous electrolytes in the lithium-ion and lithium batteries (Fig. 728).6870 O F F HF2C O O O CF2 H F F O CF3 F 66 67 F RF N R N X– X– = e.g., BF4–, (CF3 SO2 )2 N– 68 FIGURE 7–28 Structures of some fluorinated nonaqueous electrolytes. 7.9 Fluorinated hyperbranched dendrimers: synthesis and applications Wooley and coworkers have developed synthetic methods for the amphiphilic polyfluorianted hyperbranched homopolymers and copolymers through living radical polymerization. Thus, the living atom transfer radical polymerization (ATRP) of pentafluorostyrene (69) and 4-[oxy(tri(ethylene glycol)bromoisobutyryl]-2,3,5,6-tetrafluorostyrene inimer (70) gave the hyperbranched polymer 71.7173 The bifunctional monomer 70 is called inimer (initiator Chapter 7 • Materials applications of organofluorine compounds 309 monomer) because it consists of dual polymerization-initiating functional groups, at either end of the monomer: styrene and α-bromo ester moieties. The polymerization achieved by such inimers is also called self-condensing vinyl copolymerization, as originally introduced by Frechet and coworkers.74,75 This living radical polymerization was achieved using CuCl/ CuCl2 as the catalyst in the presence of 2,20 -bipyridine as the deactivating ligand (Fig. 729). The homopolymers (72), synthesized from the inimer 70 alone (i.e., in the absence of x F F F F F y FF F FF F F F F F F O F F O O O 3 3 O O x F F F F F F F F F F O O O 3 O O F 72 homopolymer F CuCl/CuCl2 N (Bpy) F F F CuCl/CuCl2 N F F 71 copolymer F 69 N N (Bpy) F O O F F O 3 F 3 O Br 70 FIGURE 7–29 Synthesis of the fluorinated hyperbranched dendrimers 71 and 72, through the ATRP of the bifunctional inimer 70. 310 Organofluorine Chemistry monomer 69), as well as copolymer 71, have high thermal stabilities up to 175 C210 C and were soluble in a broad range of organic solvents. The linker tri(ethylene glycol) moieties facilitate formation of the water-dispersible micelles of these hyperbranched polymers. These hyperbranched fluoropolymers can be used as antibiofouling materials. The hyperbranched polymers 71 and 72 exhibit anti-icing characteristics and therefore would be suitable as anti-icing coatings for applications in extreme environments.76 Furthermore, these polymeric materials are amenable for structural modifications for applications in drug delivery and in magnetic resonance imaging (MRI) (vide infra). 7.10 Fluorinated compounds in drug delivery and magnetic resonance imaging Fluorinated compounds are increasingly explored as MRI agents, because unlike other NMR imaging approaches, 19F NMR is not affected by extraneous signals arising from the biological environments. 19F NMR has 100% natural abundance and is nearly as sensitive as 1H NMR, and more importantly, there are no endogenous sources of fluorinated compounds in the biological media. The fluorinated MRI agents can be designed such that they can be conjugated to the pharmaceutically active drug candidates and thereby can be used as the drug delivery agents. 7.10.1 Fluorinated curcumin analogs as 19F MRI agents Curcumin is an effective antioxidant and exhibits antitumor and antiamyloid effects. It can permeate the bloodbrain barrier, and therefore curcumin and its derivatives are potential therapeutic targets for Alzheimer’s disease (AD) and other neurological disorders. 19F NMR of the 19F-labeled curcumin derivatives would be useful as probes to monitor the amyloid-β plaques in the brain, as curcumin binds to Aβ plaques. Derivatives of curcumin with trifluoromethoxy moieties (Fig. 730) were synthesized and used as probes for monitoring Aβ formation in the mouse AD models.77 Through 19F MRI studies on amyloid-β overexpressing Tg2576 mouse AD models, it was shown that a trifluoromethoxy derivative of curcumin is effective in binding to the amyloid-β plaques.77 O O O F3CO OCF3 OH HO O OCH3 H 3CO O OCH3 OH HO Curcumin Trifluoromethyl analog of curcumin FIGURE 7–30 Structures of curcumin and a trifluoromethyl analog for 19F MRI studies. MRI, Magnetic resonance imaging. Chapter 7 • Materials applications of organofluorine compounds 311 7.10.2 Polyfluorinated dendrimer amphiphiles as 19F MRI probes and drug delivery agents A polyfluorinated dendrimer amphiphile decorated with perfluoro-tert-butyloxy moieties, with 81 fluorines for each of the dendrimer molecules (Fig. 731), was shown to be a potential 19F MRI probe in drug delivery applications. This dendritic amphiphile, when incorporated into liposome nanoparticles along with drug candidates of interest, such as doxorubicin, could be used as a 19F MRI probe for tracing drug distribution.78 The pseudosymmetric environment of the fluorines in this compound results in a single 19F NMR signal and thus affords high sensitivity of the MRI. MeO MeO n n F3C CF3 OMe F C CF3 F3C 3 CF3 n O O O O O O O F3C O O CF3 CF3 O NH HN HN O F3C F3C F3C F3C O O CF3 CF3 O H N O N H O F3 C F 3C CF3 O H N H N O O O n O O O O n MeO n OMe O OMe n O O NH O O n OMe HN NH MeO OMe n O HN O O NH O F3 C O F 3C CF O 3 CF3 CF3 O CF3 CF3 CF3 CF3 FIGURE 7–31 Structure of a fluorinated dendrimer, with peripheral perfluoro-tertiary-butyl ether moieties. A dendrimer decorated with 540 pseudosymmetrical fluorines was synthesized through a convergent synthetic route involving sequential deoxybromination reactions and Williamson ether synthesis (Fig. 732).79 The benzylic hydroxy moiety is relatively more acidic than the primary hydroxy moiety due to the electron-withdrawing inductive effect of the adjacent 312 Organofluorine Chemistry Me HO CF3 F 3C F3C Me CF3 O F 3C F 3C O Me CF3 Me CF3 O F 3C O F3C PBr3 OH F 3C OH CF3 CF 3 CF3 O F 3C OH CF3 OH CF 3 CF3 F 3C O CF 3 K2 CO3, acetone, 18-crown-6 reflux Br OH O CF3 Me F 3C O F 3C Me DMF, 100 o C F3C OH 1. repeat the steps H 3CO OH K2 CO3, acetone, 18-crown-6 2. Final step CF 3 CF 3 OCH 3 CF3 CF 3 CF3 CF3 CF 3 CF3 CF3 CF3 H 3CO CF 3 CF 3 CF3 OCH 3 CF3OCH 3 F 3C O F 3C CF 3 CF3 CF3 CF3 CF 3 O CF3 CF 3 CF3 O F 3C O CF3 CF 3 OCH3 CF 3 CF 3 OCH 3 O OCH 3 F 3C CF3 CF3 CF 3 CF3 O CF 3 O CF3 CF 3 O CF 3 O CF3 F 3C CF3 O O O CF 3 CF3 CF3 O O CF 3 CF 3 CF3 OCH 3 CF 3 H 3CO CF3 CF3 O F 3C F3C O F 3C CF3 H 3CO F 3C CF3 O CF3 CF 3 CF3 O CF3 O CF3 CF3 O CF 3 H 3CO CF 3 CF 3 CF 3 O CF 3 CF 3 CF3 F 3C O CF3 CF 3 O CF 3 CF3 CF 3 CF3 H3CO H CO CF3 3 CF 3 OCH 3 CF3 CF3 CF 3 H3CO OCH 3 O CF3 OCH3 CF 3 O CF3 O CF 3 CF3 CF 3 H 3CO CF3 CF 3 CF 3 CF3 F3C O CF 3 F 3C O F 3C OCH3 CF 3 F3C O CF 3 CF 3 CF3 O H 3CO CF3 CF 3 CF 3 OCH3 O CF3 CF3 CF3 CF3 CF3 OCH 3 CF3 CF 3 CF3 CF 3 O CF 3 3 CF 3 O CF 3 CF3 CF3 H 3CO CF 3 CF 3OCH O H 3CO H 3CO CF3 CF3 CF3 O CF3 O CF3 OH CF 3 H 3CO CF3 CF3 OCH 3 CF3 CF 3 H 3CO HO CF 3 H 3CO CF3 OCH 3 H 3CO CF3 H 3CO H 3CO CF3 CF 3 CF3 O CF 3 CF 3 OCH 3 CF 3 CF 3 CF3 CF 3 CF 3 CF3 CF 3 OCH 3 F 3C H3CO OCH3 CF 3 OCH 3 CF 3 CF3 FIGURE 7–32 Polyfluorinated pseudosymmetric dendrimer decorated with surface trifluoromethyl groups. Chapter 7 • Materials applications of organofluorine compounds 313 trifluoromethyl moieties, and therefore the Williamson reaction occurs exclusively at the benzylic hydroxylic moiety under the reaction conditions. Because of the pseudosymmetry of this dendrimer, it shows a single δ19F NMR signal for all the trifluoromethyl groups and therefore exhibits relatively high sensitivity in the 19F MRI. This fluorinated dendrimer shows relatively low spinlattice (T1) and spinspin relaxation times (T2) of 366 and 122 ms, as compared to that of trifluoroethanol with a T1 and T2 of 2379 and 266 ms, respectively. Thus, the T1 and T2 values in the polyfluorinated dendrimer are dramatically reduced because of its large molecular size. The short relaxation times, combined with high fluorine content, thus significantly contribute to the high MRI sensitivity of the fluorinated dendrimers. Using the above dendrimer, 19F MRI could be performed at as low concentration as 18.5 μM. The dendrimer-based fluorinated molecules thus would be potentially useful as drug delivery agents as well as for monitoring the biodistribution of the drug candidates. 7.11 Organofluorine liquid crystal materials Fluorinated molecules are widely used as LCDs, including everyday electronics and flat panel TVs. Fluorine or fluoroalkyl groups, when used as terminal groups in place of the terminal cyano groups of cyanobiphenyl- and related aryl cyanide-based liquid crystals (e.g., 73 and 74) that were widely used prior to the 1990s, afford relatively high-voltage holding ratio (VHR) and provide nematic liquid crystals suitable for applications in active matrix LCDs. A range of terminally substituted fluoroalkyl-, fluoroalkoxy-, and fluorosulfanyl-aromatics (e.g., 7583 in Fig. 733) provide nematic liquid crystals with high VHR and strong dielectric anisotropy and find numerous applications in LCDs and other optoelectronics (Fig. 733).80,81 7.11.1 Fluorinated dendrimer-based liquid crystals Carbosilane dendrimers, with terminal perfluoroalkylthio groups, were synthesized by freeradical addition of the perfluoroalkyl mercaptan to the allyl-terminated carbosilane dendrimer, in the presence of AIBN as the initiator (Fig. 734). The first-generation dendrimer (G1) formed a mesophase at 215 C to 239 C, whereas the second- and third-generation dendrimers (G2 and G3) exhibited hexagonally ordered array of columns.82 Other carbosilane dendrimers, functionalized with terminal perfluoroalkyl groups, also exhibit liquid crystalline properties.83 The PAMAM [poly(amidoamine)] dendrimers (Fig. 735), as well as the [poly(propyleneimine)] dendrimers, when functionalized as ammonium carboxylates using perfluorononanoic acid or 2H,2H,3H,3H-perfluoroundecanoic acid, exhibit liquid crystalline behavior.83 7.12 Organofluorine compounds in high-energy materials There is an emerging interest in developing fluorinated compounds as high-energy materials. Pentafluorosulfanyl (SF5) compounds and gem-difluoramine (NF2) compounds exhibit high energy densities with relatively low shock sensitivity and thus find applications in highenergy materials.84 314 Organofluorine Chemistry N R N RO 74 73 F C 3H 7 O CHF 2 C 3H 7 75 76 O CF 3 C 3H 7 CF3 C 3H 7 78 77 O CF 2 CF3 C 3H 7 SF 5 C 3H 7 80 79 F F F C 3H 7 F 81 C3 H 7 F 82 F F F F O 83 F F F C 2H 5 F F F FIGURE 7–33 Structures of illustrative cyanoaryl- and fluoro-, fluoroalkyl-, and pentafluorosulfanyl-based liquid crystals. 7.12.1 N,N-Difluoramine (NF2) compounds NF2-containing compounds, because of their relatively high energy densities and low shock sensitivities, are potentially useful as high energy materials, or can be used as additives to the conventionally used high-energy compounds, such as RDX (1,3,5-trinitroperhydro-1,3,5triazine), HMX (octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine), and TNT (2,4,6-trinitrotoluene). The N,N-difluoramine analogs of HMX are also of potential use as defensive agents against biological weapons. 3,3,7,7-Tetrakis(difluoramino)octahydro-1,5-dinitro-1,5-diazocine (HNFX), a NF2 analog of the HMX (Fig. 7-36) is therefore of interest both as a high-energy compound as well as in the decontamination of the bacterial spores; the detonation products of HNFX—HF and fluorine gas—destroy anthrax Bacillus spores with an exposure time of Chapter 7 • Materials applications of organofluorine compounds 315 Si Si Si Si Si Si HS(CH2)2C6F13 Si Si Si Si Si Si FS SRR F AIBN, 70°C RF S Si Si RFS Si Si SR F SR F SR F Si RFS RFS Si Si Si SR F SRF RF S SRF Si R FS Si R FS Si RFS RFS R FS SR F SR F Si Si Si Si Si Si SRF Si RFS SRF Si RF S SR F SRF R FS Si RFS RFS Si Si SR F SR F SR F SR F SR F SRF SRF RF = e.g., –CH2CH2C6F13 FIGURE 7–34 Synthesis of a G2terminally fluoroalkyl-substituted dendrimer (36 terminal perfluoroalkyl moieties) through the AIBN-catalyzed hydro-thiolation reaction. AIBN, Azobis(isobutyronitrile). about 0.4 h.85 HNFX has a density of 1.807 g/cm3, which is comparable to that of HMX (1.91 g/cm3) and relatively higher than that of TNT (1.65 g/cm3). The higher densities of the materials translate into their higher detonation pressures and detonation speeds.86 7.12.1.1 Synthesis of HNFX Chapman and coworkers synthesized HNFX in a multistep process. The key steps of the conversion include conversion of the carbonyl moiety of the diazocine-diketone 84 to the gem-difluoramine derivative (85), and desulfonative-N-nitration of compound 85 (Fig. 736).8691 The diketone 84 was reacted with a mixture of difluoramine (HNF2) and concentrated sulfuric acid in a low-boiling CFCl3 solvent (bp 23.7 C) to give the gem-difluoramine derivative 85 in moderate yields. The N,N-difluorosulfamic acid (NF2SO3H), formed in situ, formed through the reaction of HNF2 with H2SO4, is the de facto gem-difluoramination reagent in this reaction. The use of the low-boiling CFCl3 solvent prevents the possible detonations that would otherwise result from the shock-sensitive difluoramine compounds. The N-nitration of the sulfonamide derivative 85 was accomplished using a mixture of nitric acid in triflic acid (CF3SO3H) to give HNFX in a moderate yield of 65%. 316 Organofluorine Chemistry RFCO2– RFCO2– NH3 + + +H 3N – 2 N RFCO O HN 3N H3 N – RFCO2 N O O NH3+ N N NH 3+ HN N NH O NH3 + N NH HN N RFCO2– O O NH O H N N NH3 + O RFCO2– RFCO2– NH3 + NH3+ RFCO2– RFCO2– O H N N NH 3N HN O O RFCO +H NH N N +H N 3 – 2 RFCO2– N RFCO2– + NH3 + N O HN +H RFCO2– H3 N RFCO2– N + H 3N RFCO2– NH 3+ RFCO2– RF = e.g., CF3(CF2)7– FIGURE 7–35 Structure of a PAMAM dendrimer, terminally derivatized as the perfluoroalkylammonium salt to render liquid crystal characteristics. PAMAM, [Poly(amidoamine)]. 7.12.1.2 Synthesis of RNFX RNFX is a gem-difluoramine analog of the RDX, as the gem-difluoramine moiety is isosteric and isoelectronic with respect to the N-nitro moiety. Chapman and coworkers synthesized RNFX using a procedure analogous to that of the synthesis of HNFX, involving gem-difluoramination of the ketone 86 as a key step. The gem-difluoramination was achieved through the reaction of compound 86 with the in situ formed NF2SO3H (from the reaction of HNF2 with concentrated H2SO4), in a low-boiling CFCl3 solvent, to prevent detonation of the product. The final N-nitration of 87 with concomitant deprotection of the sulfonamide moiety, using nitric acid, affords the RNFX (Fig. 737).92,93 7.12.1.3 Synthetic methods for the gem-difluoramination Difluoramine (HNF2) is a low-boiling liquid, with a bp of 223 C and is an extremely shocksensitive compound, similar to that of nitroglycerin.9497 Operationally convenient reagents for the gem-difluoramination therefore are being sought in this area. The trityldifluoramine (Ph3CNF2) is a stable solid and forms HNF2, in situ, in reaction with H2SO4. Reaction of Chapter 7 • Materials applications of organofluorine compounds NO2 N NO2 N O2 N N N O 2N N CH3 O2 N NO2 N NO2 N NO2 NO2 RDX 317 NO2 HMX TNT O O S N O O2 N O N HNF2 + H2 SO4 /CFCl3 NO2 S O –15 °C to 0 °C; 15 days O 84 O S N O 2N F2N NF2 F2N O F2 N O N S O NF2 NO2 HNO3 /CF3SO 3H 55 °C, 40 h NF2 O2 N N N NO2 F2 N NF2 HNFX (density = 1.806 g cm–3) 85 60% 65% FIGURE 7–36 Structures of RDX, HMX, and TNT, and synthesis of HNFX. O HNO3 HNF2 /H2 SO4 /CFCl3 Nos N N 86 F2 N NF2 F2 N NF2 Nos –15 °C Nos N N O2 N Nos 87 SO 3H N N NO 2 RNFX O2 N FIGURE 7–37 Synthesis of RNFX, a gem-difluoramine analog of RDX; Nos 5 4-nitrobenzenesulfonyl. trityldifluoramine with carbonyl compounds in 30% oleum gives high yields of the corresponding gem-difluoramine derivatives (Fig. 738).98 A relatively more convenient synthetic method for the gem-difluoramination is provided by Prakash and coworkers using the reaction of sodium salt of N,N-difluorosulfamic acid (NF2SO3Na; 90) with the carbonyl compounds. The latter reaction affords the 318 Organofluorine Chemistry O Ph3 C–NF2 R R R F2 N NF2 30% oleum/CH 2Cl2 R 88 80%–90% O Na O H S N H O + –O 1. 2F2/H2O; 0 °C Na + O F O S N F O – 2. –H2O/HF R R H 2SO 4 /oleum R 100% by NMR 94% O Ph3 C–NF2 F2 N NF2 R R 30% oleum/CH2Cl2 R 88 90 89 R F2N NF2 R 88 80%–90% O Na + – O H O S N H O 89 1. 2F2/H2O; 0 °C Na + 2. –H2O/HF – O F O S N F O R R H 2SO 4 /oleum 90 94% F2N NF2 R R 88 100% by NMR FIGURE 7–38 Convenient synthetic methods for gem-difluoramination of carbonyl compounds. gem-difluoramine products in quantitative yields, under mild conditions (Fig. 738). The NF2SO3Na reagent can be synthesized through direct fluorination of sodium sulfamate (89), using elemental fluorine gas in aqueous solutions.99 7.12.2 Pentafluorosulfanyl (SF5) compounds SF5-containing organic compounds are of potential interest as oxidizers and high-energy materials, as they exhibit relatively higher densities, higher thermal and chemical stabilities, and relatively lower impact sensitivities.100102 For example, pentafluorosufanylnitramide salts 2 (NF1 4 SF5 NNO2 ) exhibit comparable oxidizer capacity with that of ammonium dinitramide 2 1 [NH4 NðNO2 Þ2 ], a nonchlorine-containing oxidizer.103 The oxidation products of SFs compounds, COS and HF, are environmentally benign, unlike those derived from the perchlorate explosive compounds. High-energy compounds, with high nitrogen content, such as triazoles, furazans, and tetrazoles, when derivatized with fluorinated substituents, such as SF5, significantly enhances their performance. For example, SF5-substituted furazans possess higher density and detonation properties (calculated values), as compared to the unsubstituted furazans.102 However, the SF5containing compounds have not found practical applications as high-energy materials to date. Chapter 7 • Materials applications of organofluorine compounds 319 Shreeve and coworkers synthesized the pentafluorosulfanyl 1,2,3-triazoles through Cu(I)catalyzed cycloaddition reactions of the (pentafluorosulfanyl)acetylene with various azides and showed that these compounds have relatively higher densities than the corresponding trifluoromethyl compounds, which, in turn, have relatively higher densities than the nonfluorinated analogs (Fig. 739).104 The higher densities are translated into the higher H3 C N3 N H3 C F5 S H H 3C CuI (10 mol%), 2,6-lutidine 6 h, RT 91 CH3 N N N N (70%) SF5 92 d = 1.61 g cm–3 N3 N3 N3 N3 F5 S N N H F5 S CuI (10 mol%), 2,6-lutidine 6 h, RT N N N NN F5 S N N N N SF5 N (73%) 93 SF5 94 Tm = 292 ˚C F5 S OH N3 N3 H CuI (10 mol%), 2,6-lutidine 6 h, RT F 5S N N N 96 d = 1.90 g cm–3 N N3 F5 S H N3 N3 CuI (10 mol%), 2,6-lutidine 6 h, RT F5 S N N N N N N F5S 97 SF5 (55%) 95 N N N N OH N N N SF5 ( 67%) 98 Tm = 169.3 ˚C FIGURE 7–39 Synthesis of SF5-triazoles through Cu(I)-catalyzed azidealkyne click reactions. 320 Organofluorine Chemistry O O 1. SF5 Cl, Et3B, –45 ˚C CH3 2. MeOH, 50 ˚C 99 HO OMe F5 S OMe F5 S Ph F5S OH O O 101 93% 71% O Cl OH Ph Cl F5S O O 103 102 42% 88% H 2N NH2 N N O O H 2N (104) pyridine/THF Cl F5 S HN SF5 NH N O N SF5 –furazan N N N O H 2N (105) 103 OMe F5 S 100 H OH O MeOH O H 2 O/NaOH then HCl O S O F5 S pyridine/THF HN N N N N H SF5 –tetrazole Cl Cl N N Cl CH 2N 2 N N CHN2 N N Cl Cl 106 107 NaN 3 N3 CHN2 N N N N3 108 N NH F5 S H N3 N N N SF5 N3 SF5 –pyrazole FIGURE 7–40 Synthesis of SF5-containing high-energy materials, furazan, tetrazole, and pyrazole derivatives. Chapter 7 • Materials applications of organofluorine compounds 321 detonation properties for these high-energy materials. The thermal melting temperatures (Tm) of these SF5-derived triazoles ranged from 120 C to 312 C and are insensitive to impact. The CF3- and SF5-substituted triazoles, in general, exhibited higher densities, and both series of compounds are of potential interest as high-energy materials. The SF5-triazole 92, for example, has a density of 1.61 g/cm3, which is substantially higher than that for nitrobenzene (1.2 g/cm3) or trifluorotoluene (1.19 g/cm3). The dimeric SF5-triazole 96 has even higher density of 1.90 g/cm3. Various derivatives of SF5-containing heterocyclic compounds, such as furazans,102 tetrazoles,102 and pyrazoless,105 were synthesized for their potential applications as high-energy materials (Fig. 740). These materials exhibit substantially higher densities than that of the analogous organic compounds, in the range of 1.802.08 g/cm3, and may find applications as alternatives to the conventionally used high-energy materials, such as RDX, HMX, and TNT. Dolbier and coworkers have developed synthetic methods for the free-radical pentafluorosulfanylation of alkenes, using pentafluorosulfanyl chloride (SF5Cl) and triethylborane (Et3B), the latter serving as a free-radical initiator.102,106 Thus, the reaction of vinyl acetate (99) with SF5Cl and Et3B, followed by methanolysis, gives the (pentafluorosulfanyl)ethanal dimethyl acetal (100). The acetal 100 could be transformed, in a series of reactions, to the (pentafluorosulfanyl)acetyl chloride 103 and (pentafluorosulfanyl)acetic acid (102). Either the acid chloride 103 or the carboxylic acid 102 can be used in the acylation of furazans (e.g., 104) or tetrazoles (105) to afford the corresponding SF5-containing high-energy compounds. SF5-derived pyrazoles are also thermally stable, high energy compounds. Shreeve and coworkers have synthesized a high-energy SF5-pyrazole compound, through the reaction of the (pentafluorosulfanyl)acetylene with 2-(diazomethyl)-4,6-diazidotriazole, 108. The latter compound has relatively higher density (1.85 g/cm3) and thereby enhanced detonation performance (detonation pressure 5 20 GPa; detonation velocity 5 7464 m s21), which is comparable to that of TNT.105 References 1. Riess, J. G.; Krafft, M. P. Fluorinated Materials for In Vivo Oxygen Transport (Blood Substitutes), Diagnosis and Drug Delivery. Biomaterials 1998, 19, 15291539. 2. Zentner, C. A.; Anson, F.; Thayumanavan, S.; Swager, T. M. Dynamic Imine Chemistry at Complex Double Emulsion Interfaces. J. Am. Chem. Soc. 2019, 141, 1804818055. 3. Dichiarante, V.; Milani, R.; Metrangolo, P. Natural Surfactants Towards a More Sustainable Fluorine Chemistry. Green Chem. 2018, 20, 1327. 4. Tan, Q.; El-Badry, A. M.; Contaldo, C.; Steiner, R.; Hillinger, S.; Welti, M.; Hilbe, M.; Spahn, D. R.; Jaussi, R.; Higuera, G.; van Blitterswijk, C. A.; Luo, Q.; Weder, W. The Effect of Perfluorocarbon-Based Artificial Oxygen Carriers on Tissue-Engineered Trachea. Tissue Eng. A 2009, 15, 24712480. 5. Iyer, R. K.; Radisic, M.; Cannizzaro, C.; Vunjak-Novakovic, G. Synthetic Oxygen Carriers in Cardiac Tissue Engineering. Artif. Cells, Blood Substitutes, Biotechnol. 2007, 35, 135148. 6. Keipert, P. E. Use of Oxygent, a Perfluorochemical-Based Oxygen Carrier, As an Alternative to Intraoperative Blood Transfusion. Artif. Cells, Blood Substitutes, Immobilization Biotechnol. 1995, 23, 381394. 322 Organofluorine Chemistry 7. Cheng, Y.; Cheng, H.; Jiang, C.; Qiu, X.; Wang, K.; Huan, W.; Yuan, A.; Wu, J.; Hu, Y. Perfluorocarbon Nanoparticles Enhance Reactive Oxygen Levels and Tumour Growth Inhibition in Photodynamic Therapy. Nat. Commun. 2015, 6, 8785. 8. Ma, S.; Zhou, J.; Zhang, Y.; Yang, B.; He, Y.; Tian, C.; Xu, X.; Gu, Z. An Oxygen Self-Sufficient Fluorinated Nanoplatform for Relieved Tumor Hypoxia and Enhanced Photodynamic Therapy of Cancers. ACS Appl. Mater. Interfaces 2019, 11, 77317742. 9. Dai, Y.; Xu, C.; Sun, X.; Chen, X. Nanoparticle Design Strategies for Enhanced Anticancer Therapy by Exploiting the Tumour Microenvironment. Chem. Soc. Rev. 2017, 46, 38303852. 10. Que, Y.; Liu, Y.; Tan, W.; Feng, C.; Shi, P.; Li, Y.; Huang, X. Enhancing Photodynamic Therapy Efficacy by Using Fluorinated Nanoplatform. ACS Macro Lett. 2016, 5, 168173. 11. Song, X.; Feng, L.; Liang, C.; Yang, K.; Liu, Z. Ultrasound Triggered Tumor Oxygenation with OxygenShuttle Nanoperfluorocarbon to Overcome Hypoxia-Associated Resistance in Cancer Therapies. Nano Lett. 2016, 16, 61456153. 12. Zhou, Z.; Zhang, B.; Wang, H.; Yuan, A.; Hu, Y.; Wu, J. Two-Stage Oxygen Delivery for Enhanced Radiotherapy by Perfluorocarbon Nanoparticles. Theranostics 2018, 8, 48984911. 13. Wang, W.; Cheng, Y.; Yu, P.; Wang, H.; Zhang, Y.; Xu, H.; Ye, Q.; Yuan, A.; Hu, Y.; Wu, J. Perfluorocarbon Regulates the Intratumoural Environment to Enhance Hypoxia-Based Agent Efficacy. Nat. Commun. 2019, 10, 111. 14. Nakata, M.; Tanimura, N.; Koyama, D.; Krafft, M. P. Adsorption and Desorption of a Phospholipid From Single Microbubbles Under Pulsed Ultrasound Irradiation for Ultrasound-Triggered Drug Delivery. Langmuir 2019, 35, 1000710013. 15. Krafft, M. P. Fluorine in Medical Microbubbles Methodologies Implemented for Engineering and Investigating Fluorocarbon-Based Microbubbles. J. Fluorine Chem. 2015, 177, 1928. 16. Jochyms, Q.; Mignard, E.; Vincent, J.-M. Fluorosurfactants for Applications in Catalysis. J. Fluorine Chem. 2015, 177, 1118. 17. Berven, B. M.; Koutsantonis, G. A.; Skelton, B. W.; Trengove, R. D.; White, A. H. Highly Fluorous Complexes of Nickel, Palladium and Platinum: Solubility and Catalysis in High Pressure CO2. Dalton Trans. 2011, 40, 41674174. 18. Kostov, G.; Boschet, F.; Ameduri, B. Original Fluorinated Surfactants Potentially Non-Bioaccumulable. J. Fluorine Chem. 2009, 130, 11921199. 19. Peschka, M.; Fichtner, N.; Hierse, W.; Kirsch, P.; Montenegro, E.; Seidel, M.; Wilken, R. D.; Knepper, T. P. Synthesis and Analytical Follow-Up of the Mineralization of a New Fluorosurfactant Prototype. Chemosphere 2008, 72, 15341540. 20. Kostjuk, S. V.; Ortega, E.; Ganachaud, F.; Ameduri, B.; Boutevin, B. Anionic Ring-Opening Polymerization of Hexafluoropropylene Oxide Using Alkali Metal Fluorides as Catalysts: A Mechanistic Study. Macromolecules (Washington, DC, U.S.) 2009, 42, 612619. 21. Krishnan, S.; Kwark, Y.-J.; Ober, C. K. Fluorinated Polymers: Liquid Crystalline Properties and Applications in Lithography. Chem. Rec. 2004, 4, 315330. 22. Schmaljohann, D.; Hamad, A. H.; Weibel, G. L.; Ober, C. K. Fluorinated Polyvinylalcohols as a Photoresist Platform for 157 nm Lithography. Polym. Mater. Sci. Eng. 2000, 83, 445446. 23. Du, L.; Kelly, J. Y.; Roberts, G. W.; De Simone, J. M. Fluoropolymer Synthesis in Supercritical Carbon Dioxide. J. Supercrit. Fluids 2009, 47, 447457. 24. Ahmed, T. S.; DeSimone, J. M.; Roberts, G. W. Kinetics of the Homopolymerization of Vinylidene Fluoride and Its Copolymerization with Hexafluoropropylene in Supercritical Carbon Dioxide: The Locus of Polymerization. Macromolecules (Washington, DC, U.S.) 2009, 42, 148155. 25. Romack, T. J.; DeSimone, J. M.; Treat, T. A. Synthesis of Tetrafluoroethylene-Based, Nonaqueous Fluoropolymers in Supercritical Carbon Dioxide. Macromolecules 1995, 28, 84298431. Chapter 7 • Materials applications of organofluorine compounds 323 26. Mi, H.-Y.; Jing, X.; Liu, Y.; Li, L.; Li, H.; Peng, X.-F.; Zhou, H. Highly Durable Superhydrophobic Polymer Foams Fabricated by Extrusion and Supercritical CO2 Foaming for Selective Oil Absorption. ACS Appl. Mater. Interfaces 2019, 11, 74797487. 27. Puts, G. J.; Crouse, P.; Ameduri, B. M. Polytetrafluoroethylene: Synthesis and Characterization of the Original Extreme Polymer. Chem. Rev. (Washington, DC, U.S.) 2019, 119, 17631805. 28. Falireas, P. G.; Ladmiral, V.; Debuigne, A.; Detrembleur, C.; Poli, R.; Ameduri, B. Straightforward Synthesis of Well-Defined Poly(Vinylidene Fluoride) and Its Block Copolymers by Cobalt-Mediated Radical Polymerization. Macromolecules (Washington, DC, U.S.) 2019, 52, 12661276. 29. Cardoso, V. F.; Correia, D. M.; Ribeiro, C.; Fernandes, M. M.; Lanceros-Mendez, S. Fluorinated Polymers as Smart Materials for Advanced Biomedical Applications. Polymers (Basel, Switz.), 10. ; 2018161161/161161/126. 30. Park, N. H.; Gomes, G. d P.; Fevre, M.; Jones, G. O.; Alabugin, I. V.; Hedrick, J. L. Organocatalyzed Synthesis of Fluorinated Poly(Aryl Thioethers). Nat. Commun. 2017, 8, 17. 31. Mugemana, C.; Almahdali, S.; Rodionov, V. O. Sequence-Controlled Polymerization Guided by ArylFluoroaryl π-π-Stacking. ACS Symp. Ser. 2014, 1170, 235253. 32. Smith, V. B.; Massey, A. G. Perfluorophenyl Derivatives of the Elements. XXII. Perfluorotriphenylene. Tetrahedron 1969, 25, 54955501. 33. Weck, M.; Dunn, A. R.; Matsumoto, K.; Coates, G. W.; Lobkovsky, E. B.; Grubbs, R. H. Influence of Perfluoroarene-Arene Interactions on the Phase Behavior of Liquid Crystalline and Polymeric Materials. Angew. Chem. Int. Ed. 1999, 38, 27412745. 34. Dutta, T.; Woody, K. B.; Watson, M. D. Transition-Metal-Free Synthesis of Poly(Phenylene Ethynylene)s with Alternating Aryl-Perfluoroaryl Units. J. Am. Chem. Soc. 2008, 130, 452453. 35. Nketia-Yawson, B.; Lee, H.-S.; Seo, D.; Yoon, Y.; Park, W.-T.; Kwak, K.; Son, H. J.; Kim, B.-S.; Noh, Y.-Y. Highly Planar Fluorinated Benzothiadiazole-Based Conjugated Polymer for High-Performance Organic Thin-Film Transistors. Adv. Mater. (Weinheim, Ger.) 2015, 27, 30453052. 36. Zhang, Q.; Kelly, M. A.; Bauer, N.; You, W. The Curious Case of Fluorination of Conjugated Polymers for Solar Cells. Acc. Chem. Res. 2017, 50, 24012409. 37. Son, H. J.; Wang, W.; Xu, T.; Liang, Y.; Wu, Y.; Li, G.; Yu, L. Synthesis of Fluorinated Polythienothiophene-co-Benzodithiophenes and Effect of Fluorination on the Photovoltaic Properties. J. Am. Chem. Soc. 2011, 133, 18851894. 38. Price, S. C.; Stuart, A. C.; Yang, L.; Zhou, H.; You, W. Fluorine Substituted Conjugated Polymer of Medium Band Gap Yields 7% Efficiency in Polymer-Fullerene Solar Cells. J. Am. Chem. Soc. 2011, 133, 46254631. 39. Zhou, H.; Yang, L.; Stuart, A. C.; Price, S. C.; Liu, S.; You, W. Development of Fluorinated Benzothiadiazole as a Structural Unit for a Polymer Solar Cell of 7% Efficiency. Angew. Chem. Int. Ed. 2011, 50, 29952998 S2995/2991-S2995/2916. 40. Zhao, W.; Li, S.; Yao, H.; Zhang, S.; Zhang, Y.; Yang, B.; Hou, J. Molecular Optimization Enables over 13% Efficiency in Organic Solar Cells. J. Am. Chem. Soc. 2017, 139, 71487151. 41. Wolf, J.; Cruciani, F.; El Labban, A.; Beaujuge, P. M. Wide Band-Gap 3,4-Difluorothiophene-Based Polymer with 7% Solar Cell Efficiency: An Alternative to P3HT. Chem. Mater. 2015, 27, 41844187. 42. Ko, S.-J.; Hoang, Q. V.; Song, C. E.; Uddin, M. A.; Lim, E.; Park, S. Y.; Lee, B. H.; Song, S.; Moon, S.-J.; Hwang, S.; Morin, P.-O.; Leclerc, M.; Su, G. M.; Chabinyc, M. L.; Woo, H. Y.; Shin, W. S.; Kim, J. Y. HighEfficiency Photovoltaic Cells with Wide Optical Band Gap Polymers Based on Fluorinated PhenyleneAlkoxybenzothiadiazole. Energy Environ. Sci. 2017, 10, 14431455. 43. Leclerc, N.; Chavez, P.; Ibraikulov, O. A.; Heiser, T.; Leveque, P. Impact of Backbone Fluorination on π-Conjugated. Polymers (Basel, Switz.), 8. ; 2016127. 324 Organofluorine Chemistry 44. Liu, D.; Zhao, W.; Zhang, S.; Ye, L.; Zheng, Z.; Cui, Y.; Chen, Y.; Hou, J. Highly Efficient Photovoltaic Polymers Based on Benzodithiophene and Quinoxaline with Deeper HOMO Levels. Macromolecules (Washington, DC, U.S.) 2015, 48, 51725178. 45. Yao, H.; Bai, F.; Hu, H.; Arunagiri, L.; Zhang, J.; Chen, Y.; Yu, H.; Chen, S.; Liu, T.; Lai, J. Y. L.; Zou, Y.; Ade, H.; Yan, H. Efficient All-Polymer Solar Cells Based on a New Polymer Acceptor Achieving 10.3% Power Conversion Efficiency. ACS Energy Lett. 2019, 4, 417422. 46. Wang, Y.; Watson, M. D. Transition-Metal-Free Synthesis of Alternating Thiophene-Perfluoroarene Copolymers. J. Am. Chem. Soc. 2006, 128, 25362537. 47. Boujioui, F.; Zhuge, F.; Damerow, H.; Wehbi, M.; Ameduri, B.; Gohy, J.-F. Solid Polymer Electrolytes From a Fluorinated Copolymer Bearing Cyclic Carbonate Pendant Groups. J. Mater. Chem. A 2018, 6, 85148522. 48. Shaplov, A. S.; Marcilla, R.; Mecerreyes, D. Recent Advances in Innovative Polymer Electrolytes Based on Poly(Ionic Liquid)s. Electrochim. Acta 2015, 175, 1834. 49. Vygodskii, Y. S.; Shaplov, A. S.; Lozinskaya, E. I.; Vlasov, P. S.; Malyshkina, I. A.; Gavrilova, N. D.; Kumar, P. S.; Buchmeiser, M. R. Cyclopolymerization of N,N-Dipropargylamines and N,N-Dipropargyl Ammonium Salts. Macromolecules (Washington, DC, U.S.) 2008, 41, 19191928. 50. Yin, K.; Zhang, Z.; Yang, L.; Hirano, S.-I. An Imidazolium-Based Polymerized Ionic Liquid via Novel Synthetic Strategy as Polymer Electrolytes for Lithium Ion Batteries. J. Power Sources 2014, 258, 150154. 51. Alaaeddine, A.; Vergnaud, J.; Rolland, J.; Vlad, A.; Gohy, J.-F.; Ameduri, B. Synthesis of an Original Fluorinated Triethylene Glycol Methacrylate Monomer and Its Radical Copolymerisation with Vinylidene Fluoride. Its Application as a Gel Polymer Electrolyte for Li-Ion Batteries. Polym. Chem. 2015, 6, 60216028. 52. Deluca, N. W.; Elabd, Y. A. Polymer Electrolyte Membranes for the Direct Methanol Fuel Cell: A Review. J. Polym. Sci. B: Polym. Phys. 2006, 44, 22012225. 53. Yoshimura, K.; Iwasaki, K. Aromatic Polymer with Pendant Perfluoroalkyl Sulfonic Acid for Fuel Cell Applications. Macromolecules (Washington, DC, U.S.) 2009, 42, 93029306. 54. Miyatake, K.; Zhou, H.; Watanabe, M. Proton Conductive Polyimide Electrolytes Containing Fluorenyl Groups: Synthesis, Properties, and Branching Effect. Macromolecules 2004, 37, 49564960. 55. Saito, J.; Tanaka, M.; Hirai, M.; Nanasawa, M.; Miyatake, K.; Watanabe, M. Polyimide Ionomer Containing Superacid Groups. Polym. Adv. Technol. 2011, 22, 13051310. 56. Zheng, J.; He, Q.; Gao, N.; Yuan, T.; Zhang, S.; Yang, H. Novel Proton Exchange Membranes Based on Cardo Poly(Arylene Ether Sulfone/Nitrile)s with Perfluoroalkyl Sulfonic Acid Moieties for Passive Direct Methanol Fuel Cells. J. Power Sources 2014, 261, 3845. 57. Danyliv, O.; Iojoiu, C.; Barbier, V.; Martin, V.; Sanchez, J.-Y. Aromatic Ionic Monomer Bearing Perfluorosulfonate Moiety and Its Polycondensation Toward High Performance Superacid Ionomers. J. Fluorine Chem. 2016, 189, 4350. 58. Li, H.; Jackson, A. B.; Kirk, N. J.; Mauritz, K. A.; Storey, R. F. Poly(Arylene Ether Sulfone) Statistical Copolymers Bearing Perfluoroalkylsulfonic Acid Moieties. Macromolecules (Washington, DC, U.S.) 2011, 44, 694702. 59. Shimura, T.; Watanabe, M.; Miyatake, K. Synthesis of Superacid-Modified Poly(Arylene Ether Sulfone)s via Post-Bromination. RSC Adv. 2012, 2, 51995204. 60. Nakabayashi, K.; Higashihara, T.; Ueda, M. Polymer Electrolyte Membranes Based on Poly(Phenylene Ether)s with Pendant Perfluoroalkyl Sulfonic Acids. Macromolecules (Washington, DC, U.S.) 2011, 44, 16031609. 61. Chang, Y.; Mohanty, A. D.; Smedley, S. B.; Abu-Hakmeh, K.; Lee, Y. H.; Morgan, J. E.; Hickner, M. A.; Jang, S. S.; Ryu, C. Y.; Bae, C. Effect of Superacidic Side Chain Structures on High Conductivity Aromatic Polymer Fuel Cell Membranes. Macromolecules (Washington, DC, U.S.) 2015, 48, 71177126. Chapter 7 • Materials applications of organofluorine compounds 325 62. Ghassemi, H.; Schiraldi, D. A.; Zawodzinski, T. A.; Hamrock, S. Poly(Arylene Ether)s with Pendant Perfluoroalkyl Sulfonic Acid Groups as Proton-Exchange Membrane Materials. Macromol. Chem. Phys. 2011, 212, 673678. 63. Liu, S.; Luo, W.; Zhang, H.; Li, X.; Hu, W.; Guiver, M. D.; Liu, B. Novel Iodo-Containing Poly(Arylene Ether Ketone)s as Intermediates for Grafting Perfluoroalkyl Sulfonic Acid Groups. React. Funct. Polym. 2017, 111, 713. 64. Zeng, Y.; Gu, L.; Zhang, L.; Cheng, Z.; Zhu, X. Synthesis of Highly Proton-Conductive Poly(Arylene Ether Sulfone) Bearing Perfluoroalkyl Sulfonic Acids via Polymer Post-Modification. Polymer 2017, 123, 345354. 65. Prakash, G. K. S.; Smart, M. C.; Wang, Q.-J.; Atti, A.; Pleynet, V.; Yang, B.; McGrath, K.; Olah, G. A.; Narayanan, S. R.; Chun, W.; Valdez, T.; Surampudi, S. High Efficiency Direct Methanol Fuel Cell Based on Poly(Styrenesulfonic) Acid (PSSA)-Poly(Vinylidene Fluoride) (PVDF) Composite Membranes. J. Fluorine Chem. 2004, 125, 12171230. 66. Groult, H.; Tressaud, A. Use of Inorganic Fluorinated Materials in Lithium Batteries and in Energy Conversion Systems. Chem. Commun. (Cambridge, U.K.) 2018, 54, 1137511382. 67. Nakajima, T. Fluorine Compounds as Energy Conversion Materials. J. Fluorine Chem. 2013, 149, 104111. 68. Zama, I.; Gorni, G.; Borzatta, V.; Cassani, M. C.; Crupi, C.; Di Marco, G. Fluorinated Imidazolium Salts Having Liquid Crystal Characteristics. J. Mol. Liq. 2016, 223, 749753. 69. Madria, N.; Arunkumar, T. A.; Nair, N. G.; Vadapalli, A.; Huang, Y.-W.; Jones, S. C.; Reddy, V. P. Ionic Liquid Electrolytes for Lithium Batteries: Synthesis, Electrochemical, and Cytotoxicity Studies. J. Power Sources 2013, 234, 277284. 70. Men, F.; Yang, Y.; Shang, Y.; Zhang, H.; Song, Z.; Zhou, Y.; Zhou, X.; Zhan, H. Fluorine-Substituted Ionic Liquid for Si Anode in Li-Ion Battery. J. Power Sources 2018, 401, 354361. 71. Powell, K. T.; Cheng, C.; Wooley, K. L. Complex Amphiphilic Hyperbranched Fluoropolymers by Atom Transfer Radical Self-Condensing Vinyl (Co)polymerization. Macromolecules (Washington, DC, U.S.) 2007, 40, 45094515. 72. Powell, K. T.; Cheng, C.; Du, W.; Wooley, K. L. Hyperbranched Fluoropolymers (HBFP(III)), Designed as Complex Nanostructures for Potential Imaging and Therapeutic Delivery. PMSE Prepr. 2007, 96, 334. 73. Pollack, K. A.; Imbesi, P. M.; Raymond, J. E.; Wooley, K. L. Hyperbranched FluoropolymerPolydimethylsiloxane-Poly(Ethylene Glycol) Cross-Linked Terpolymer Networks Designed for Marine and Biomedical Applications: Heterogeneous Nontoxic Antibiofouling Surfaces. ACS Appl. Mater. Interfaces 2014, 6, 1926519274. 74. Weimer, M. W.; Frechet, J. M.; Gitsov, I. Importance of Active-Site Reactivity and Reaction Conditions in the Preparation of Hyperbranched Polymers by Self-Condensing Vinyl Polymerization: Highly Branched vs. Linear Poly[4-(Chloromethyl)Styrene] by Metal-Catalyzed "Living" Radical Polymerization. J. Polym. Sci. A: Polym. Chem. 1998, 36, 955970. 75. Frechet, J. M. J.; Henmi, M.; Gitsov, I.; Aoshima, S.; Leduc, M. R.; Grubbs, R. B. Self-Condensing Vinyl Polymerization: An Approach to Dendritic Materials. Science (Washington, D. C.) 1995, 269, 10801083. 76. Zigmond, J. S.; Pollack, K. A.; Smedley, S.; Raymond, J. E.; Link, L. A.; Pavia-Sanders, A.; Hickner, M. A.; Wooley, K. L. Investigation of Intricate, Amphiphilic Crosslinked Hyperbranched Fluoropolymers as AntiIcing Coatings for Extreme Environments. J. Polym. Sci. A: Polym. Chem. 2016, 54, 238244. 77. Yanagisawa, D.; Amatsubo, T.; Morikawa, S.; Taguchi, H.; Urushitani, M.; Shirai, N.; Hirao, K.; Shiino, A.; Inubushi, T.; Tooyama, I. In Vivo Detection of Amyloid β Deposition Using 19F Magnetic Resonance Imaging with a 19F-Containing Curcumin Derivative in a Mouse Model of Alzheimer’s Disease. Neuroscience (Amsterdam, Neth.) 2011, 184, 120127. 78. Bo, S.; Yuan, Y.; Chen, Y.; Yang, Z.; Chen, S.; Zhou, X.; Jiang, Z.-X. In Vivo Drug Tracking with 19F MRI at Therapeutic Dose. Chem. Commun. (Cambridge, U.K.) 2018, 54, 38753878. 326 Organofluorine Chemistry 79. Yu, W.; Yang, Y.; Bo, S.; Li, Y.; Chen, S.; Yang, Z.; Zheng, X.; Jiang, Z.-X.; Zhou, X. Design and Synthesis of Fluorinated Dendrimers for Sensitive 19F MRI. J. Org. Chem. 2015, 80, 44434449. 80. Kirsch, P. Fluorine in Liquid Crystal Design for Display Applications. J. Fluorine Chem. 2015, 177, 2936. 81. Dabrowski, R.; Dziaduszek, J.; Bozetka, J.; Piecek, W.; Mazur, R.; Chrunik, M.; Perkowski, P.; Mrukiewicz, M.; Zurowska, M.; Weglowska, D. Fluorinated Smectics New Liquid Crystalline Medium for Smart Windows and Memory Displays. J. Mol. Liq. 2018, 267, 415427. 82. Lorenz, K.; Frey, H.; Stuehn, B.; Muelhaupt, R.; Carbosilane Dendrimers with Perfluoroalkyl End Groups. Core-Shell Macromolecules with Generation-Dependent Order. Macromolecules 1997, 30, 68606868. 83. Hernandez-Ainsa, S.; Barbera, J. Fluorinated Liquid Crystalline Dendrimers. J. Fluorine Chem. 2015, 177, 3745. 84. Reddy, V. P. Synthetic Methods for High Energy Organofluorine Compounds. In Energetic Materials: Advanced Processing Technologies for Next-Generation Materials; Mezger, M. J., Tindle, Kay J., Pantoya, Michelle, Groven, Lori J., Kalyon, Dilhan, Eds.; CRC Press: Boca Raton, FL, 2017; p 290. and references therein. 85. Chapman, R. D.; Thompson, D.; Ooi, G.; Wooldridge, D.; Cash, P. N.; Hollins, R. A. N,N-Dihaloamine Explosives to Defeat Biological Weapons; Chem Abstr.: 2010:1486774, American Chemical Society: New Orleans, LA, 2010, SESW-607. 86. Chapman, R. D.; Gilardi, R. D.; Welker, M. F.; Kreutzberger, C. B. Nitrolysis of a Highly Deactivated Amide by Protonitronium. Synthesis and Structure of HNFX. J. Org. Chem. 1999, 64, 960965. 87. Chapman, R.D.; Groshens, T.J. 3,3,7,7-Tetrakis(Difluoramino)Octahydro-1,5-Diazocinium Salts, US8444783B1; 2013. 88. Chapman, R.D.; Groshens, T.J. 3,3,7,7,-Tetrakis(Difluoramino)Octahydro-1,5-Diazocinium Salts and Method for Making the Same, US7563889B1; The United States of America as Represented by the Secretary of the Navy, 2009. 89. Chapman, R. D.; Welker, M. F.; Kreutzberger, C. B. Difluoramination of Heterocyclic Ketones: Control of Microbasicity. J. Org. Chem. 1998, 63, 15661570. 90. Chapman, R.D.; Nguyen, B.V. 5,5-Bis(Difluoramino)Hexahydro-1,3-Dinitropyrimidine (RNFX) and Electronegatively Substituted Pyrimidines as Explosive or Propellant Components, US6310204B1; United States of America as Represented by the Secretary of the Navy, 2001. 91. Axenrod, T.; Guan, X. P.; Sun, J.; Qi, L.; Chapman, R. D.; Gilardi, R. D. Synthesis of 3,3-Bis(Difluoramino) Octahydro-1,5,7,7-Tetranitro-1,5-Diazocine (TNFX), a Diversified Energetic Heterocycle. Tetrahedron Lett. 2001, 42, 26212623. 92. Chapman, R.D.; Nguyen, B.V. 5,5-Bis(Difluoramino)Hexahydro-1,3-Dinitropyrimidine (RNFX) and Electronegatively Substituted Pyrimidines as Explosive or Propellant Components, US6310204B1; United States of America as Represented by the Secretary of the Navy, 2001. Chem. Abstr.: 135:346536. 93. Chapman, R. D. Organic Difluoramine Derivatives. Struct. Bonding (Berlin, Ger.) 2007, 125, 123151. 94. Lawton, E. A.; Weber, J. Q. Direct Fluorination of Urea: Synthesis and Properties of Difluoramine. J. Am. Chem. Soc. 1959, 81, 4755. 95. Lawton, E. A.; Weber, J. Q. The Synthesis and Reactions of Difluoramine. J. Am. Chem. Soc. 1963, 85, 35953597. 96. Baum, K. Reaction of Acetylenes with Difluoramine. J. Am. Chem. Soc. 1968, 90, 70897091. 97. Baum, K. Reactions of Carbonyl Compounds with Difluoramine. J. Am. Chem. Soc. 1968, 90, 70837089. 98. Prakash, G. K. S.; Etzkorn, M.; Olah, G. A.; Christe, K. O.; Schneider, S.; Vij, A. Triphenylmethyldifluoramine: A Stable Reagent for the Synthesis of Gem-Bis(Difluoramines). Chem. Commun. (Cambridge, U.K.) 2002, 17121713. Chapter 7 • Materials applications of organofluorine compounds 327 99. Haiges, R.; Wagner, R.; Boatz, J. A.; Yousufuddin, M.; Etzkorn, M.; Prakash, G. K. S.; Christe, K. O.; Chapman, R. D.; Welker, M. F.; Kreutzberger, C. B. Preparation, Characterization, and Crystal Structures of the SO3NHF2 and SO3NF22 ions. Angew. Chem. Int. Ed. 2006, 45, 51795184. 100. Sitzmann, M. E.; Gilligan, W. H.; Ornellas, D. L.; Thrasher, J. S. Polynitroaliphatic Explosives Containing Pentafluorosulfanyl (SF5) Group: The Selection and Study of a Model Compound. J. Energ. Mater. 1990, 8, 352374. 101. Sitzmann, M. E. N-Pentafluorosulfanyl-N-Nitro Carbamates. J. Fluorine Chem. 1991, 52, 195207. 102. Martinez, H.; Zheng, Z.; Dolbier, W. R. Energetic Materials Containing Fluorine. Design, Synthesis and Testing of Furazan-Containing Energetic Materials Bearing a Pentafluorosulfanyl Group. J. Fluorine Chem. 2012, 143, 112122. 103. Sitzmann, M. E.; Gilardi, R.; Butcher, R. J.; Koppes, W. M.; Stern, A. G.; Thrasher, J. S.; Trivedi, N. J.; Yang, Z.-Y. Pentafluorosulfanylnitramide Salts. Inorg. Chem. 2000, 39, 843850. 104. Garg, S.; Shreeve, J. M. Trifluoromethyl- or Pentafluorosulfanyl-Substituted Poly-1,2,3-Triazole Compounds as Dense Stable Energetic Materials. J. Mater. Chem. 2011, 21, 47874795. 105. Ye, C.; Gard, G. L.; Winter, R. W.; Syvret, R. G.; Twamley, B.; Shreeve, J. M. Synthesis of Pentafluorosulfanylpyrazole and Pentafluorosulfanyl-1,2,3-Triazole and their Derivatives as Energetic Materials by Click Chemistry. Org. Lett. 2007, 9, 38413844. 106. Dolbier, W. R.; Ait-Mohand, S.; Schertz, T. D.; Sergeeva, T. A.; Cradlebaugh, J. A.; Mitani, A.; Gard, G. L.; Winter, R. W.; Thrasher, J. S. A Convenient and Efficient Method for Incorporation of Pentafluorosulfanyl (SF5) Substituents Into Aliphatic Compounds. J. Fluorine Chem. 2006, 127, 13021310. Index Note: Page numbers followed by “f” refer to figures. A Abemaciclib, 172, 173f Accufluor, 44 Acquired immune deficiency syndrome (AIDS) disease, 163 Acyl fluorides, 8 Afatinib, 167, 169f Ag(I)-catalyzed decarboxylative fluorination, 123, 124f Ag(II)-catalyzed oxidative ring-opening fluorination of cyclic amines, 121 123, 122f, 123f Aliphatic aldehydes, 111 Alkyl azides with 18F-labeled triarylphosphine esters, 238f Alkyltrifluoroborates, 54 Allylsilanes and enolsilyl ethers, 50 51, 51f α,α-difluoromethylated phosphonate esters, 46 47 α-fluorinated amino acids, synthesis of, 53 54, 53f, 54f, 55f α-fluorination of aldehydes, 48 49, 49f α-fluorination of amide, 50, 50f α-fluorination of ketones and 1,3-dicarbonyl compounds, 51 52 α-fluorophosphonate esters, 47, 48f α7 nicotinic acetylcholine receptor (nAChR) agonist, 136 137, 137f α-tocopherol, 105 Alzheimer disease (AD), 152 155, 152f, 157 158, 218, 257f, 296 Amantadine, 247 Amcinonide (Cyclocort), 201 Amidinate salt, 18 Amino-trifluoromethylation of styrenes, 92f Amitriptyline, 26 27 Amyloid beta (Aβ) plaques, 153 155, 221, 260 261 Amyloid plaques, 152 153, 218 219 Amyloid precursor protein (APP), 153 154 Angiogenesis, 267 268 Angiotensin-converting enzyme (ACE) inhibitor, 223 225, 225f Anti-Alzheimer pharmaceuticals, 152 163, 154f BACE-1 inhibitors, 153 159, 155f, 156f γ-secretase inhibitors and modulators, 159 163 Antibacterial pharmaceuticals, 141 146 erythromycin, 141 142, 142f fluoroquinolone, 142 145, 143f flurithromycin, 141 142, 142f tetracyclines, 145 146, 146f Anticancer pharmaceuticals, 167 185 abemaciclib, 172, 173f BRAF and mitogen-activated protein kinase kinase enzyme inhibitors, 183 185 cobimetinib, 171 172, 172f dacomitinib, 167 169, 168f enasidenib, 178 181, 180f fulvestrant, 177 178, 178f lorlatinib, 169 171, 171f nonsteroidal antiandrogens, 181 183 PARP inhibitors, 172, 173f taxoid anticancer agents, 173 177 Antidepressant, 202 204 Antidiabetic pharmaceuticals, 146 152 canagliflozin, 151 152, 151f carmegliptin, 150 151, 150f sitagliptin, 146 149, 147f, 148f Anti-HIV pharmaceuticals, 163 165, 164f bictegravir, 163 165, 164f doravirine, 165, 166f Antiinflammatory pharmaceuticals celecoxib, 200, 200f, 201f corticosteroids, 200 202, 202f, 203f nonsteroidal antiinflammatory agents, 199 Antimalarial pharmaceuticals, 165 167 329 330 Index Antimalarial pharmaceuticals (Continued) mefloquine, 166 167, 168f tafenoquine, 165 166, 167f Anti-Markovnikov hydro-trifluoromethylation of alkenes, 86, 87f Antiplatelet drugs cangrelor, 138 139 riociguat, 198, 199f Antiviral pharmaceuticals, 185 195 antiplatelet drugs, 189 190 glecaprevir, 190 194, 192f ledipasvir, 189 190 letermovir, 194 195, 195f pibrentasvir, 190 194, 192f sofosbuvir, 187 188, 188f, 189f tecovirimat, 185 187, 185f voxilaprevir, 194, 194f Aortic dissections, 142 144 Apalutamide (Erleada), 181 182, 182f Aromatic trifluoromethylation, 84 86, 85f Artemether, 167f Artemisinin, 165, 167f Artesunate, 167f Aryl(alkyl)sulfenyl chlorides, 31 Arylboronic acids and esters, fluoroalkylation of, 125 126 copper-mediated trifluoromethylation, 125, 125f Cu(I)-catalyzed trifluoromethylation of arylboronate esters, 126, 126f Pd(0)-catalyzed difluoroalkylation of arylboronic acids, 126, 127f Aryl difluoromethylation, 118 121, 120f, 121f Aryl fluorination, 106 108, 107f Aryl thiocyanates, 31 Aspirin, 199 Asymmetric trifluoromethylthiolation, 64 65, 65f Atabecestat, 154 155, 156f Atorvastatin (Lipitor), 104, 104f, 135 136, 135f, 136f, 195 196, 196f Au(I)-catalyzed hydrofluorination of alkenes and alkynes, 114 115, 114f, 115f, 116f, 117f Au(I)-catalyzed photoredox reactions, 113, 113f Au(III) catalysis for [18F]trifluoromethyl compounds synthesis, 248, 251f Avagacestat, 159, 161f Aza-dibenzocyclooctyne (ADIBO) amide derivatives, 231 232 Azetidines, 121 Azido-fluoroalkylation of alkenes, 88 90, 89f, 90f, 91f B BACE-1 inhibitors, 153 159, 155f, 156f atabecestat, structure of, 156f Aβ oligomers and Aβ aggregates, formation of, 155f CNP520, 156 157, 158f lanabecestat, structure of, 156f verubecestat, 157 159 structure of, 156f, 159f X-ray structure of, 159, 160f Baeyer Villiger reaction, 240 Baloxavir marboxil (Xofluza), 140, 141f Balz Schiemann reaction, 105 Benzophenone, 23 25 Benzylic fluorination, 108 111 Mn(III)-catalyzed benzylic fluorination, 108, 109f Pd(II)-catalyzed benzylic fluorination, 108 111, 110f Benzylic hydrogens, 108 β-amino-fluoroalkylation of alkenes, 80 82 Cu(I)-catalyzed amino-fluoroalkylation, 80 81, 81f Fe(II)-catalyzed azido- and aminotrifluoromethylation, 81, 82f Ru(II)-catalyzed amino-fluoroalkylation, 81 82, 83f β-(azido)fluoroalkylation of alkenes, 90f β-(azido)sulfones, 90f β-(azido)trifluoromethyl compounds, 88 90 β-carbonyl esters, 52 β-naphthols, 66 67, 69f β-secretase-I (BACE-1) inhibitor, 75 77 β-(trifluoromethyl)amines, 88 89, 89f Bicalutamide (Casodex), 181 182, 182f Bictegravir, 163 165, 164f Billard’s reagents, 63, 65 66, 66f synthesis of, 65, 67f Index trifluoromethylthiolation of alkynes and Grignard reagents, 65 66, 68f Binimetinib, 183, 184f Biotin, 174 176, 259, 259f Bis-cinchona alkaloids, 50 51 Blood brain barrier (BBB) permeability, 136 137, 138f, 220 221 BMS-932481, 161, 161f Borazine-mediated gem-difluroalkylation, 23 25, 26f Borazine-mediated trifluoromethylation, 23 25, 25f BPN-15606, 161 162, 161f BRAF and mitogen-activated protein kinase kinase enzyme inhibitors, 183 185 Bristol Myers Squibb, 161, 163, 164f Bronsted acids, 115 Buclizine (antihistamine), 118 121 Butyl ciprofibrate, 106 108 C Canagliflozin (Invokana), 151 152, 151f Cangrelor, 138 139, 198f Carbapenems, 145 Carbotegravir, 163 165, 164f Carmegliptin, 150 151, 150f structure of, 150f synthesis of, 151, 151f X-ray crystal structure of, 150, 150f Celecoxib, 108, 162, 199 200, 200f, 201f, 245 Celestolide, 108 Cell-cycle hypothesis, 153 Cephalosporins, 145 Ceritinib, 169 170 Cerivastatin, 135 136, 135f Chemoorthogonal scavenger-assisted purification, 232 Chichibabin reaction, 9 10 Chiral imidazolidinone catalyst, 48 49 Chiral N-acyl oxazolidinones, 96 Chlorodiphenhydramine, 118 121 Chloroquine, 165, 167f Chlorotetracycline, 145 Cholinesterase inhibitors, 154f Chronic obstructive pulmonary disorders, 45 46 331 Cinchona alkaloids, 51 Cinchonidine catalyst, 14 15 Ciprofloxacin (Cipro), 104, 104f, 142 144, 143f Cisplatin, 229 Citalopram, 202 204, 203f Claritin, 248 Click chemistry, 227 228, 230f strain-promoted, 231 234, 233f, 235f Clofibrate, 118 121 Clomipramine, 118 121 Clopidogrel (Plavix), 198 Coadditive ionic liquid electrolyte, 303f Cobimetinib, 171 172, 172f Copper/iridium catalysis, 77f, 78 Copper-mediated trifluoromethylation, 125, 125f Corticosteroids, 45 46, 200 202, 202f, 203f Crizotinib, 169 170 Cu(I)-catalyzed α-trifluoromethylation of hydrazones, 114f Cu(I)-catalyzed amino-fluoroalkylation, 80 81, 81f Cu(I)-catalyzed fluoroalkylation of aryl halides, 126 127, 128f Cu(I)-catalyzed radiofluorinations, 252, 254, 255f Cu(I)-catalyzed trifluoromethylation of arylboronate esters, 126, 126f Cu(I)-catalyzed trifluoromethylation of vinyl (or aryl) trifluoroborates, 89f Cu(I)-mediated dediazoniative difluoromethylation, 124, 125f Cu(II)-catalyzed trifluoromethylation of arylboronic acids, 125 Cyanuric fluoride, 2 3 Cyclic RGDYK (arginine-glycine-aspartic acidtyrosine-lysine) dimer-derived positron emission tomography tracers, 267 273 [18F]FAl-NOTA-PRGD2 (18F-alfatide), 268 271, 270f 18 F-fluciclovine (Axumin), 273, 273f folate-NOTA-Al18F, 271 272, 272f FPPRGD2 (dimeric cyclic RGDYK peptide), 267 268, 269f [68Ga]-NOTA-PRGD2, 268 271, 270f NOTA-conjugated linear peptides 18F-AlFNOTA-IF7 and 18F-Al-NOTAMATBBN, 271, 272f 332 Index CYP450 enzymes, 139 140 Cystic fibrosis drugs, 139 140 Cystic fibrosis transmembrane conductance regulator (CFTR) protein, 139 140 D Dacomitinib, 104, 104f, 167 169, 168f Dapagliflozin, 151 152, 151f Decarboxylative fluoroalkylation decarboxylate difluoromethylation, 78 79, 80f decarboxylative trifluoromethylation, 78 Deconstructive ring-opening fluorination of Nacyl cyclic amines, 123f Delafloxacin (Baxdela), 144, 144f δ-tocopherol, 108 Deoxo-Fluor [bis(2-methoxyethyl)aminosulfur trifluoride], 2 4, 6 8, 10 Desoximetasone (Topisolone), 201 Dexamethasone, 200 Diastereoselective trifluoromethylation of Nsulfinylimines, 24f Dicationic polymer electrolyte, 303f Diethylaminosulfur trifluoride (DAST), 2 4, 6 8, 10, 65 DAST mediated trifluoromethylthiolation of silylenol ethers, 66 67, 69f Difluoramines, 280 Difluoroalkylation of hydrazones, 111 113, 112f Difluoroenolates, 19 Difluoromethylation, 44 of pyrazole aldehyde, 13f Difluoromethylthiolation, 68 70, 69f, 70f Dimethylformamide (DMF), 12 13 5,5-dimethyl-1-pyrroline N-oxide (DMPO), 91 93 Dipeptidyl peptidase-4 (DPP-4), 75 77 Direct methanol fuel cells (DMFCs), 304 305 Diversinates, 82 84 DNA aptamer drug conjugates, 177 Docetaxel, 175f Dolutegravir, 163 165, 164f Donepezil, 153, 154f Dopamine, 108, 245 Doravirine, 165, 166f Doxycycline, 145 E Efavirenz, 15, 16f, 54 55, 163, 164f, 165 Electrochemical oxy-trifluoromethylation, 92f Electrophilic difluoromethylation, 57 60, 60f Electrophilic fluorinations α-fluorinated amino acids, synthesis of, 53 54, 53f, 54f, 55f enantioselective, 48 52 allylsilanes and enolsilyl ethers, 50 51, 51f α-fluorination of aldehydes, 48 49, 49f α-fluorination of amide, 50, 50f α-fluorination of ketones and 1,3-dicarbonyl compounds, 51 52 β-carbonyl esters, 52 of tetralones, 52f reagents for, 44 47, 45f fluorinated bioisosteres of phosphate esters, 46 47, 48f NFPy reagent, 44 Selectfluor, 44 46, 46f stereoselective electrophilic fluorination of β-diketone, 52f Electrophilic fluoroalkylation, 54 60 electrophilic difluoromethylation, 57 60, 60f NHC-catalyzed electrophilic trifluoromethylation, 57, 59f reagents for, 55 56 trifluoromethylated pharmaceuticals and herbicide, structure of, 56f Electrophilic O-difluoromethylation of alcohols, 60f Electrophilic radiotrifluoromethylation, 227, 228f Electrophilic trifluoromethoxylation, 60f, 61 62, 61f, 62f electrophilic O-difluoromethylation of alcohols, 60f O-trifluoromethylation, synthetic methods for, 61 62, 62f, 63f SCF3-containing pharmaceuticals and veterinary medicines, 61f Electrophilic trifluoromethylation electrophilic difluoromethylation, 57 60, 60f NHC-catalyzed electrophilic trifluoromethylation, 57, 59f reagents for, 55 56 Index trifluoromethylated pharmaceuticals and herbicide, structure of, 56f Electrophilic trifluoromethylthiolation, 60f, 61 62, 61f, 62f electrophilic O-difluoromethylation of alcohols, 60f O-trifluoromethylation, synthetic methods for, 61 62, 62f, 63f reagents, 127 SCF3-containing pharmaceuticals and veterinary medicines, 61f Elvitegravir, 163 165, 164f Empagliflozin (treatment of type II diabetes), 118 121 Emtricitabine, 163, 164f Enalaprilat, 108, 245 Enantiomeric fluorination of β-amido esters, 53f Enantioselective fluorinations, 48 52 allylsilanes and enolsilyl ethers, 50 51, 51f α-fluorination of aldehydes, 48 49, 49f α-fluorination of amide, 50, 50f α-fluorination of ketones and 1,3-dicarbonyl compounds, 51 52 β-carbonyl esters, 52 of tetralones, 52f Enantioselective trifluoromethylation, 14 15, 15f, 16f Enasidenib, 178 181, 180f synthesis of, 181f Encorafenib (Braftovi), 183, 184f Enolsilyl ethers of tetralones, 66 67 Environmentally benign perfluorosurfactants, 285 Enzalutamide (Xtandi), 182 183, 182f, 183f, 184f Epi-androsterone, 4 Eravacycline (Xerava), 145 146, 146f, 147f Erythromycin, 141 142, 142f Escitalopram, 202 204, 203f Estradiol, structures of, 178f Estrone, 105, 128, 248 Ethyl bromodifluoroacetate, 112f EVP-0015962, 162 163 Ezetimibe, 116 117, 196, 197f, 242 243, 247 248 F 18 Fdifluoromethylarenes, 248 333 Fe(II)-catalyzed azido- and aminotrifluoromethylation, 81, 82f Fenbufen, 78 Fenofibrate, 118 121, 248 [18F]Florbetaben, 261 [18F]flortaucipir, 261 18 F-fluciclovine (Axumin), 273, 273f [18F]fluoroarylsulfonamido-maleimide, 239f [18F]fluorodopamine, 253 254, 254f [18F]fluoxetine, 252, 252f [18F]flutamide, 252, 252f 18 F-labeled aliphatic trifluoromethyl compounds, synthesis of, 251f 18 F-labeled aza-dibenzocyclooctyne (ADIBO) amide derivatives, 231 232 18 F-labeled captopril, 223 225, 225f 18 F-labeled compounds, 2, 219f, 222f enzymatic fluorination reactions for [18F]labeled PET tracers, 258 259 [18F]5ʹ-deoxy-5ʹ-fluoroadenosinebiotin conjugate, 259, 259f 5ʹFluoro-5ʹ-deoxyadenosine and 5fluororibose, 258, 258f 5ʹ-Fluoro-5ʹ-deoxyadenosine-RGD conjugate in cancer detection, 259, 260f [18F]FIBT, 221 222, 223f 18 F-labeled PET imaging agents, 218 18 F-labeled reagents, synthesis of, 223 227, 226f, 227f florbetapir, 221 PET tracers in Alzheimer’s disease, 260 265 2-(4-Aminoaryl)quinoline-based 18F-labeled PET tracers (THK series), 263 265, 264f, 265f flortaucipir-18F, 261 263, 262f, 263f tropomyosin receptor kinase, 265, 266f PET tracers in cancer diagnosis, 266 273 cyclic RGDYK dimer-derived PET tracers, 267 273 [18F]-(R)-lorlatinib, 266 267, 267f radiofluorination, synthetic methods for, 222 227, 224f radiofluorination via aromatic nucleophilic substitution, 237 244, 239f 334 18 Index F-labeled compounds (Continued) L-3,4-Dihydroxy-6-[18F]fluorophenylalanine (6-[18F]L-DOPA), 240, 240f [18F]fluoro-(1)-biotin, 238 239, 239f γ-Aminobutyric acid transporter positron emission tomography tracers, 240 241 phenolic compounds, 241 244, 242f, 243f, 244f radiofluorination via diaryliodonium salts, 252 257, 253f, 254f Cu(I)-catalyzed radiofluorination, 254, 255f iodonium ylides, 255 257, 256f, 257f Sharpless click reactions for PET tracers, 227 234, 228f, 229f 18 F-octreotate PET tracers for tumor imaging, 230, 231f, 232f protein and oligonucleotide triazole, 229, 230f Staudinger ligation reactions for, 234 237 strain-promoted click chemistry, 231 234, 233f, 235f Staudinger ligation reactions for PET tracers, 234 237, 236f, 237f alkyl azides with 18F-labeled triarylphosphine esters, 238f 18 F-labeled GABAA receptor antagonist, 237f 18 F-labeled peptide analogs, synthesis of, 237f traceless Staudinger ligation reaction, 236f transition metal-mediated radiofluorination, 245 252 Au(III) catalysis for [18F]trifluoromethyl compounds synthesis, 248, 251f Cu(I)-catalyzed radiofluorinations, 252 Mn(III)-catalyzed radiofluorinations, 245 247, 245f, 246f, 247f Ni(II)-catalyzed radiofluorinations, 249 251 Pd-catalyzed radiofluorinations, 248, 249f, 250f 18 F-labeled RNA oligonucleotides, 230f 18 F-labeled trifluoromethylthiolation of aromatics, 31f Flomoxef, 68 Florbetaben (Neuraceq), 218 219, 260 261 Florbetapir (Amyvid), 218 219, 221, 260 261 [18F]-(R)-lorlatinib, 266 267, 267f Flortaucipir-18F, 261 263, 262f, 263f Flucarbazone, 33, 61 Fluciclovine (Axumin), 218 Flunisolide (AeroBid), 201 FluoLead (4-tert-butyl-2,2-dimethylphenylsulfur trifluoride), 2 3, 12 Fluorinated acceptor polymer, 296, 298f Fluorinated bioisosteres of phosphate esters, 46 47, 48f Fluorinated carbon nanoparticles (F-CNPs), 307 308, 308f Fluorinated compounds in drug delivery and magnetic resonance imaging, 310 313 curcumin analogs, 310, 310f polyfluorinated dendrimer amphiphiles, 311 313, 311f, 312f Fluorinated dendrimer-based liquid crystals, 313, 315f Fluorinated donor acceptor polymers, synthesis of for fullerene polymer solar cells, 293 295, 295f Fluorinated donor polymer, 296, 298f Fluorinated graphite (CFx)n, 307 Fluorinated hyperbranched dendrimers, 308 310, 309f Fluorinated ionic liquid electrolytes, 308 Fluorinated ionomers as proton-exchange membranes in fuel cells, 304 306, 304f, 305f, 306f, 307f Fluorinated π-conjugated polymeric materials in photovoltaic devices, 289 298 fluorinated polymers in fullerene-free, allpolymer (organic) solar cells, 296 298, 299f π-conjugated benzodithiophene quinoxaline copolymers, 296, 297f π-conjugated polymers, 289 293, 291f, 292f, 294f π π stacking interactions in polyfluoroaromatics, 289, 290f synthesis of fluorinated donor acceptor polymers for fullerene polymer solar cells, 293 295, 295f Index Fluorinated pharmaceuticals for cardiovascular diseases, 195 198 ezetimibe, 196, 197f nebivolol, 196 197, 197f statin drugs, 195 196, 196f Fluorinated poly(arylene ethynylene) copolymers, 291f Fluorinated poly(aryl ethers), 298 Fluorinated poly(aryl thioethers) in organic electronic materials, 298 300, 300f, 301f Fluorinated polymers in fullerene-free, allpolymer (organic) solar cells, 296 298, 299f Fluorinated poly(thienothiophene-cobenzothophene)s, 292f Fluorinated surfactants, 280 285, 282f environmentally benign perfluorosurfactants, 285 fluorous catalysis, 285, 285f Oxygent, 281 perfluoroalkyl-derived surfactants, 282f perfluorocarbon nanomaterials, 282 285 perfluorooctyl bromide, 283f Fluorinated taxoids, tumor-targeted drug delivery of, 174 176, 176f Fluorine, 134 138, 145, 192, 291 293 Fluoroacetone hydrates for nucleophilic fluoroalkylations, 18 19, 19f Fluoroalkene peptide bioisosteres, 114, 114f Fluoroalkyl(aryl) moieties, 134 135 Fluoroalkylations, 12 30 of arylsilanes, 116 117, 118f, 119f fluoroacetone hydrates for nucleophilic fluoroalkylations, 18 19, 19f of hydrazones, 111 113 tetrakis(dimethylamino)ethylene-mediated fluoroalkylations, 28 30, 28f Fluoroalkyl-derived copolymers, 286 287 Fluoroalkyl-derived imidazolium-based ionic liquids, 308 Fluoroalkyl radicals, 81f Fluorocyclines, 145 Fluoroform (CHF3), 19 25, 20f, 22f, 23f, 24f, 127 Fluorohydrin, 121 122 Fluorometholone, 201 335 Fluoropolymers, 280, 286 289, 287f fluoroalkyl-derived copolymers, 286 287 photoresist material, synthesis of, 287f poly(tetrafluoroethylene), 287, 288f poly(vinylidene fluoride), 287 289, 288f 2-fluoropropanoyl-labeled PEGylated dimeric cyclic RGDYK peptide (FPRGD2), 267 268, 269f Fluoroquinolones, 142 145, 143f, 165 delafloxacin, 144, 144f FDA-approved, 143f levofloxacin, 144 mechanism of action, 145, 145f 5-fluorouracil, 177 Fluorouracil-phosphoramidite module, 177, 177f Fluorous catalysis, 285, 285f Fluorous Pd(I) catalysis, 285f Fluosol, 281 Fluoxetine, 104, 202 204, 248 Flurbiprofen, 49, 49f, 104, 104f, 162 163, 200f Flurithromycin, 141 142, 142f Flurprimidol, 33, 61 Flutamide (Eulexin), 181 182, 182f, 247 Flutemetamol (Vizamyl), 218 219, 260 261 Fluticasone, 43 46, 200 Fluticasone furoate, 45 46 Fluticasone propionate (Flonase), 46, 124f, 201 Fluvastatin (Lescol), 135 136, 135f, 195, 196f Fluvoxamine (Luvox), 202 204, 203f 18 F-octreotate PET tracers for tumor imaging, 230, 231f, 232f Fosnetupitant, 142f Fostamatinib (Tavalisse), 140, 141f [18F]phenylalanine, 257 Free-radical reactions β-amino-fluoroalkylation of alkenes, 80 82 Cu(I)-catalyzed amino-fluoroalkylation, 80 81, 81f Fe(II)-catalyzed azido- and aminotrifluoromethylation, 81, 82f Ru(II)-catalyzed amino-fluoroalkylation, 81 82, 83f CF3/CF2H substituents, pharmaceuticals containing, 76f decarboxylative fluoroalkylation 336 Index Free-radical reactions (Continued) decarboxylate difluoromethylation, 78 79, 80f decarboxylative trifluoromethylation, 78 fluoroalkylation, sodium triflinate, 82 94 aromatic trifluoromethylation, 84 86, 85f azido-fluoroalkylation of alkenes, 88 90, 89f, 90f, 91f functional group transformations, 84f hydro-trifluoromethylation of alkenes, 86 trifluoromethylation of arylboronic acids, 86 88, 88f, 89f trifluoromethylation of proteins, 93 94, 93f, 94f free-radical trifluoromethylation, reagents for, 77 78, 77f photoredox-catalyzed S-fluoroalkylation and arylation, 94 95, 95f radical fluoroalkylation of enolates, 96 97, 96f, 97f Togni’s and Umemoto’s reagents, structures of, 77f Free-radical S-fluoroalkylation, 95f Free-radical trifluoromethylation, reagents for, 77 78, 77f Friedel Crafts reactions, 56, 254 Friedel Crafts trifluoromethylation of aromatics, 56 Fulvestrant (Faslodex), 177 178, 178f structures of, 178f synthesis of, 178, 179f Furazans, 320f, 321 G Galantamine, 4, 153, 154f γ-Aminobutyric acid (GABA) transporter positron emission tomography tracers, 240 241 γ-Aminobutyric acid (GABA) transporter type 1 (GAT-1) imaging agents, 240 241, 241f γ-secretase inhibitors (GSIs), 153, 159 163 γ-secretase modulators (GSMs), 153, 159 163 BMS-932481, 161 162 BPN-15606, 161 162 nonsteroidal antiinflammatory drugs, 162 163, 162f GDC-0994 and its analog, 185f Gefitinib, 169f Gel polymer electrolytes, 303 gem-difluoramination, synthetic methods for, 316 318, 318f gem-difluorination of carbonyl compounds, 12f, 13f gem-difluoromethylation, 2 3, 3f, 77 78 gem-difluoromethylene phosphonate derivative, 47 gem-(difluoromethyl)thioethers, synthesis of, 30, 30f, 31f Gemifloxacin (Factive), 142 144, 143f Glecaprevir, 75 77, 190 194, 192f Glucocorticosterone drugs, 45 46 Grignard reactions of ethyl trifluoroacetate, 16 17, 17f Grignard reagents, 65 66 H Haas’s reagent, 63 Hepatitis C virus (HCV), 187 Hexafluoroacetone, 18, 19f HIV, 163 Homophenylalanine, 108 Human neuronal nitric oxide synthase (hnNOS) inhibitor, 138f Human type II topoisomerases, 145 Hydrazones, fluoroalkylation of difluoroalkylation of hydrazones, 111 113, 112f trifluoromethylation of hydrazones, 113 Hydrofluoric acid, 18f Hydro-trifluoromethylation of alkenes, 86 Hypercholesterolemia, 195 Hypervalent iodonium ylide reagent, 63 I Ibuprofen, 8, 108, 162, 245 Imidazole, 190 Imipramine, 65 Indoxacarb, 33, 61 Inimer (initiator monomer), 308 310 Integrin, 267 268 Interferon, 190 192 Iodosobenzene, 108 Index Ionazolac, 78 Iridium photoredox catalysis, 86 [Ir(III)] photoredox catalyzed difluoroalkylation of hydrazones, 112f Ishikawa’s reagent, 2 3 Isocitrate dehydrogenase-2 (IDH2) enzyme, 135 136 Isoxepac, 78 Itanapraced, 162f Ivacaftor, 139 140 Ivosidenib (Tibsovo), 171, 171f K Ketoprofen, 8 L Lamivudine, 165 Lanabecestat, 154 155, 156f Langlois reagent, 23 25, 25f, 63, 77 78, 82 84 aromatic trifluoromethylation, 84 86, 85f azido-fluoroalkylation of alkenes, 88 90, 89f, 90f, 91f functional group transformations, 84f hydro-trifluoromethylation of alkenes, 86 selective trifluoromethylation of proteins, 93 94, 93f, 94f trifluoromethylation of arylboronic acids, 86 88, 88f, 89f Larotrectinib (Vitrakvi), 171, 171f, 265f Ledipasvir, 189 190 structure of, 189f synthesis of, 190, 191f Letermovir (prevymis), 194 195, 195f Levofloxacin (Levaquin), 142 144, 143f Lewis acids, 91 93, 115 Lithium- and lithium-ion batteries fluorinated carbon nanoparticles and nonaqueous electrolytes in, 307 308 Lithium hexamethyldisilazide (LiHMDS), 64 66 Loratadine, 118 121 Lorlatinib, 167, 169 171, 171f, 257 Lovastatin (Mevacor), 195 177 Lu DOTA-TATE (Lutathera), 218, 230 Lumacaftor, 139 140, 140f Lung cancer, diagnosis of, 220, 221f Lyrica (anticonvulsant), 247 337 M Malaria, 165 Maraviroc, 104 Mavyret, 190 Mefloquine, 166 167, 167f, 168f Meisenheimer complex, 108, 242 243 Meldrum’s acid, 255 257 Memantine, 153 Mericitabine, 188f 9-Mesitylacridinium salt, 86 Methicillin-resistant Staphylococcus aureus, 68, 144 Microfluidic-based continuous flow reactor techniques, 43 44 Mild cognitive impairment (MCI), 261 Mitogen-activated protein kinase (MAPK), 171 172 Mn(III)-catalyzed benzylic fluorination, 108, 109f Mn(III)-catalyzed radiofluorinations, 245 247, 245f, 246f, 247f Mn(salen)-catalyzed radiofluorination of aliphatic C H bonds, 245f Monepantel, 44, 61 Monotrifluoromethylation, 17 18 Morphine, 4 Morpho-DAST (morpholinosulfur trifluoride), 2 3 Morpholino hydrazones, 111 Moxifloxacin (Avelox), 142 145, 143f, 145f Munavalli’s reagent, 63 64, 64f, 68 70 N Nafion-H, 304 306, 304f Naftifine, 26 27 Nanoparticulate perfluorooctyl bromide, 284 Naproxen, 8, 162 N-benzoylazacycloalkanes, 121 N-3,5-Bis(trifluoromethyl)benzylcarboxamides, 140 141 N-(difluoromethyl)phthalimide, 70f Nebivolol, 196 197, 197f Neuroendocrine neoplasms (NENs), 230 N-fluoroalkyl phthalimide reagents, 44 N-fluoroalkyl sulfenamide reagents, 44 N-fluorobenzenesulfonimide (NFSI), 43 45, 50 51, 108, 225 226 338 Index N-fluoropyridinium salts, 43 44 N-fluoro-2,4,6-trimethylpyridinium tetrafluoroborate (NFPy), 44 N-Heterocyclic carbenes (NHC)-catalyzed electrophilic trifluoromethylation, 57, 59f Ni(0)-catalyzed difluoromethylation of aryl chlorides, 121f Ni(0)-catalyzed difluoromethylation of chloroarenes, 120f Ni-catalyzed fluoroalkylation of aromatics, 116 121 aryl difluoromethylation, 118 121, 120f 121f fluoroalkylation of arylsilanes, 116 117, 118f, 119f Ni(II)-catalyzed radiofluorinations, 249 251 Ni-catalyzed trifluoromethylthiolation, 127 128 Ni-catalyzed trifluorothiomethylation of aryl triflates, 129f Nicotinic acetylcholine receptors (nAChRs), 220 Nimesulide, 254 NMDA receptor antagonist memantine, 154f N,N-difluoramines (NF2 compounds), 314 318 Nonaqueous electrolytes, in lithium- and lithiumion batteries, 307 308 Nonmetastatic castration-resistant prostate cancer (CRPC) (NM-CRPC), 181 182 Nonsmall-cell lung carcinoma (NSCLC), 167, 169 170, 220 Nonsteroidal antiandrogens, 181 183 Nonsteroidal antiinflammatory drug (NSAID), 49, 161 162, 199 Nonstructural protein 5A (NS5A) inhibitor, 190 192 Norfloxacin, 143f Novartis, 75 77, 156 N-protected α-fluoro-β-bromo glycine ester, synthesis of, 54f N-tosyl-p-chlorobenzene-sulfonimidoyl fluoride (SulfoxFluor), 6, 7f N-trifluoromethylation of amines, 26 28, 27f Nucleophilic deoxyfluorination, 3 8, 4f, 5f, 7f, 8f, 9f Nucleophilic difluoromethylation of aldehydes, 12 13, 13f Nucleophilic fluorination of pyridines and diazines, 8 10, 10f Nucleophilic fluoroalkylations, 2 Nucleophilic gem-difluorination of carbonyl compounds, 10 12, 11f, 12f, 13f Nucleophilic radiofluorination strategy, 248 Nucleophilic reactions, 2 deoxyfluorination, 3 8, 4f, 5f, 7f, 8f, 9f fluorination of pyridines and diazines, 8 10, 10f fluoroalkylations, 12 30 borazine-mediated gem-difluroalkylation, 23 25, 26f borazine-mediated trifluoromethylation, 23 25, 25f difluoromethylation of aldehydes, 12 13, 13f fluoroacetone hydrates for nucleophilic fluoroalkylations, 18 19, 19f fluoroform (CHF3), 19 25, 20f, 22f, 23f, 24f N-trifluoromethylation of amines, 26 28, 27f Ruppert Prakash reagent (CF3SiMe3) for, 13 18, 14f tetrakis(dimethylamino)ethylene-mediated fluoroalkylations, 28 30, 28f gem-difluorination of carbonyl compounds, 10 12, 11f, 12f, 13f reagents for, 2 3, 3f trifluoromethoxylations, 31 35, 33f trifluoromethylthiolation, 31, 32f Nucleophilic trifluoromethoxide anion, 35f O Ofloxacin, 142 144, 143f Olah’s reagent, 2, 53 54 Olaparib (Lynparza), 172, 173f Organoarsenicals, 141 Organofluorine compounds in high-energy materials, 313 321 N,N-difluoramines (NF2 compounds), 314 318 pentafluorosulfanyl (SF5) compounds, 318 321, 319f, 320f Organofluorine liquid crystal materials, 313, 314f fluorinated dendrimer-based liquid crystals, 313, 315f Organometallic approach, 128 Index Organotransition metal catalysis, 105 Ag(I)-catalyzed decarboxylative fluorination, 123, 124f Ag(II)-catalyzed oxidative ring-opening fluorination of cyclic amines, 121 123, 122f, 123f Au(I)-catalyzed hydrofluorination of alkenes and alkynes, 114 115, 114f, 115f, 116f, 117f Cu(I)-catalyzed fluoroalkylation of aryl halides, 126 127, 128f Cu(I)-mediated dediazoniative difluoromethylation, 124, 125f fluoroalkylation of arylboronic acids and esters, 125 126 copper-mediated trifluoromethylation, 125, 125f Cu(I)-catalyzed trifluoromethylation of arylboronate esters, 126, 126f Pd(0)-catalyzed difluoroalkylation of arylboronic acids, 126, 127f Ni-catalyzed fluoroalkylation of aromatics, 116 121 aryl difluoromethylation, 118 121, 120f, 121f fluoroalkylation of arylsilanes, 116 117, 118f, 119f Ni-catalyzed trifluoromethylthiolation, 127 128 Pd-catalyzed fluorination of aryl halides and triflates, 105, 106f Pd(II)-catalyzed (amino)trifluoromethoxylation, 129 130, 130f transition metal catalyzed C H fluorination, 106 113 aryl fluorination, 106 108, 107f benzylic fluorination, 108 111 fluoroalkylation of hydrazones, 111 113 Orkambi, 139 140 O-trifluoromethylation, synthetic methods for, 61 62, 62f, 63f Oxidative radiofluorination of Ar Ni(II) complexes, 251f Oxygent, 281, 283f P Paclitaxel (Taxol), 173 174, 175f Palbociclib, 172 339 PAMAM [poly(amidoamine)] dendrimers, 313, 316f Pantoprazole, 104 Parallel artificial membrane permeability for BBB (PAMPA-BBB) assay, 137 Parkinson’s disease, 104, 140, 218 219, 261 Paroxetine, 202 204, 203f PARP inhibitors, 172, 173f Pd-catalyzed electrophilic aromatic C H fluorination, 107f Pd-catalyzed fluorination of aryl halides and triflates, 105, 106f Pd-catalyzed radiofluorinations, 248, 249f, 250f Pd(II)-catalyzed (amino)trifluoromethoxylation, 129 130, 130f Pd(II)-catalyzed benzylic fluorination, 108 111, 110f Pd(II)-catalyzed enantioselective benzylic C H fluorination 2-alkylbenzaldehydes, 108 109 Pd(0)-catalyzed difluoroalkylation of arylboronic acids, 126, 127f Pd(0)-catalyzed ipso-fluorination of aryl bromides/triflates, 105 Pd(0)-catalyzed Suzuki reactions, 126 Penicillins, 141, 145 Pentafluoroethylation of aryl halides, 128f Pentafluoroethylation of aryl iodides, 126 127 Pentafluorosulfanyl (SF5) compounds, 280, 318 321, 319f, 320f Perfluorinated hydrocarbon-based nanomaterials, 280 Perfluorinated nanoparticles, 284 Perfluoroalkylated surfactants, 280 281 Perfluoroalkyl-derived surfactants, 282f Perfluoroalkylsulfonyl chloride, 81f Perfluorocarbon-based nanoparticles, 284 Perfluorocarbon nanomaterials, 282 285 Perfluorooctanesulfonic acid (PFOS), 281, 285, 286f Perfluorooctanoic acid (PFOA), 281, 285, 286f Perfluorooctyl bromide, 283f Perfluorotributylamine forms spherical nanoparticles, 284 Petrov reagent, 2 3 340 Index Pfizer’s neurokinin-receptor antagonist, 14 15 Pharmaceutical applications of organofluorine compounds anti-Alzheimer pharmaceuticals, 152 163, 154f BACE-1 inhibitors, 153 159, 155f, 156f γ-secretase inhibitors and modulators, 159 163 antibacterial pharmaceuticals, 141 146 erythromycin, 141 142, 142f fluoroquinolone, 142 145, 143f flurithromycin, 141 142, 142f tetracyclines, 145 146, 146f anticancer pharmaceuticals, 167 185 abemaciclib, 172, 173f BRAF and mitogen-activated protein kinase kinase enzyme inhibitors, 183 185 cobimetinib, 171 172, 172f dacomitinib, 167 169, 168f enasidenib, 178 181, 180f fulvestrant, 177 178, 178f lorlatinib, 169 171, 171f nonsteroidal antiandrogens, 181 183 PARP inhibitors, 172, 173f taxoid anticancer agents, 173 177 antidepressant, 202 204 antidiabetic pharmaceuticals, 146 152 canagliflozin, 151 152, 151f carmegliptin, 150 151, 150f sitagliptin, 146 149, 147f, 148f anti-HIV pharmaceuticals, 163 165, 164f bictegravir, 163 165, 164f doravirine, 165, 166f antiinflammatory pharmaceuticals celecoxib, 200, 200f, 201f corticosteroids, 200 202, 202f, 203f nonsteroidal antiinflammatory agents, 199 antimalarial pharmaceuticals, 165 167 mefloquine, 166 167, 168f tafenoquine, 165 166, 167f antiviral pharmaceuticals, 185 195 antiplatelet drugs, 189 190 glecaprevir, 190 194, 192f ledipasvir, 189 190 letermovir, 194 195, 195f pibrentasvir, 190 194, 192f sofosbuvir, 187 188, 188f, 189f tecovirimat, 185 187, 185f voxilaprevir, 194, 194f blood brain barrier (BBB) permeability, 136 137, 138f fluorinated pharmaceuticals for cardiovascular diseases, 195 198 ezetimibe, 196, 197f nebivolol, 196 197, 197f statin drugs, 195 196, 196f metabolic stability and bioavailability, 137 140 π π stacking interactions, 140 141, 142f PhenoFluor [1,3-bis(2,6-diisoproylphenyl)-2,2difluoro-4-imidazoline], 2 4 PhenoFluorMix, 241 Phenols, 4, 4f Phosphazene superbase (P4-tBu), 23, 24f Photodynamic therapy (PDT), 280, 282 283, 283f Photoredox catalysis, 55f, 77 78, 79f, 94, 111 Photoredox-catalyzed fluoroalkylation of dehydroalanine, 54 Photoredox-catalyzed S-fluoroalkylation and arylation, 94 95, 95f Phthalimido-Au(I) complex, 115 Phthalimido esters of carboxylic acids, 78 79 π-conjugated benzodithiophene quinoxaline copolymers, 296, 297f π-conjugated polymers, 289 293, 291f, 292f, 294f Pibrentasvir, 190 194, 192f, 193f Piperidines, 121 π π stacking interactions, 140 141, 142f in polyfluoroaromatics, 289, 290f Pitavastatin (Livalo), 195 Poly(chlorotrifluoroethylene) (PCTFE), 286 Poly(chlorotrifluoroethylene-co-ethylene), 286 Poly(ether sulfone) ionomer, 305, 305f, 306f Polyfluorinated compounds, 2 Polyfluoroaromatics, 289 Poly(fluoroaryl thioethers), synthesis of, 301f Polyfluoroaryl thiophene copolymers, 300f Poly(imide)-based ionomers, 307f Poly(imide) copolymer with pendant perfluoroalkylsulfonic acid moieties, 305 306 Poly(ionic liquids), 300 303, 302f Index Polymer electrolytes, 300 303, 301f, 302f, 303f, 304f gel polymer electrolytes, 303 poly(ionic liquid) electrolytes, 302f poly(VDF-co-MAFTEG) gel polymer electrolyte, 304f Polynorbornene polymer, 289 Poly(tetrafluoroethylene) (PTFE), 280, 286 287, 288f Poly(tetrafluoroethylene-co-ethylene), 286 Poly(VDF-co-MAFTEG) gel polymer electrolyte, 304f Poly(vinyl fluoride), 286 Poly(vinylidene fluoride) (PVDF), 280, 286 289, 288f, 301f, 306, 307f Positron emission tomography (PET) tracers in Alzheimer’s disease, 260 265 2-(4-Aminoaryl)quinoline-based 18F-labeled positron emission tomography tracers (THK series), 263 265, 264f, 265f flortaucipir-18F, 261 263, 262f, 263f tropomyosin receptor kinase, 265, 266f in cancer diagnosis, 266 273 cyclic RGDYK dimer-derived positron emission tomography tracers, 267 273 [18F]-(R)-lorlatinib, 266 267, 267f Sharpless click reactions for, 227 234, 228f, 229f 18 F-octreotate PET tracers for tumor imaging, 230, 231f, 232f protein and oligonucleotide triazole, 229, 230f Staudinger ligation reactions for, 234 237 strain-promoted click chemistry, 231 234, 233f, 235f Staudinger ligation reactions for, 234 237, 236f, 237f alkyl azides with 18F-labeled triarylphosphine esters, 238f 18 F-labeled GABAA receptor antagonist, 237f 18 F-labeled peptide analogs, synthesis of, 237f traceless Staudinger ligation reaction, 236f Pravastatin (Pravachol), 195 Primaquine, 165 166, 167f 341 Procymidone, 106 108 Protein and oligonucleotide triazole, 229, 230f Pyrazole, 320f, 321 2-pyridinesulfonyl fluoride (PyFluor), 2 3, 5f, 6, 7f, 225, 226f Pyriprole, 68 Pyrroles, 121 Q Quinine, 166 167 Quinoline-based drugs, 165 Quinolone, 142 145 Quinuclidine, 136 R Radical fluoroalkylation of enolates, 96 97, 96f, 97f Radiofluorination via aromatic nucleophilic substitution, 237 244, 239f [18F]fluoro-(1)-biotin, 238 239, 239f γ-Aminobutyric acid transporter positron emission tomography tracers, 240 241 L-3,4-Dihydroxy-6-[18F]fluorophenylalanine (6-[18F]L-DOPA), 240, 240f phenolic compounds, radiofluorination of, 241 244, 242f, 243f, 244f via diaryliodonium salts, 252 257, 253f, 254f Cu(I)-catalyzed radiofluorination, 254, 255f iodonium ylides, radiofluorination, 255 257, 256f, 257f synthetic methods for, 222 227, 224f Radiofluorination of enolsilanes and arylstannes, 45f Raltegravir, 163 165, 164f Rasagiline, 108, 245 Ribavirin, 190 192 Ribociclib, 172 Rifluzole, 33 Riluzole, 61 Riociguat, 198, 199f Rivastigmine, 153, 154f RNFX, synthesis of, 316, 317f Rofecoxib, 162, 199 200 Roflumilast, 75 77, 104, 200f 342 Index Rosuvastatin (Crestor), 104, 104f, 135 136, 135f, 195, 196f Rucaparib (Rubraca), 172, 173f, 174f Ru(II)-catalyzed amino-fluoroalkylation, 81 82, 83f Ru(II)-catalyzed redox reactions, 97f Ru(II)-mediated solid-phase synthesis (SPSS), 244f Ruppert Prakash reagent, 13 18, 14f, 28 29, 77 78 enantioselective trifluoromethylation, 14 15, 15f, 16f trifluoromethylation of imines, 18 trifluoromethyl ketones, synthesis of, 16 18, 17f S Safinamide (Xadago), 104, 104f, 140, 141f Sandmeyer-type of dediazoniative difluoromethylation of arylamines, 124, 125f SCF3-containing pharmaceuticals and veterinary medicines, 61f S-(difluoromethyl)diarylsulfonium tetrafluoroborate, 57 58 Selectfluor (1-chloromethyl-4-fluoro-1,4diazoniabicyclo[2.2.2]octane bis (tetrafluoroborate)), 43 46, 45f, 46f, 47f, 48 49, 52f, 54, 108, 121 123, 129 130, 130f, 225 226, 266 267 Selective serotonin reuptake inhibitors (SSRIs), 202 204, 203f Self-condensing vinyl copolymerization, 308 310 Semagacestat, 159 S-(fluoromethyl)diarylsulfonium tetrafluoroborate, 57 58 Shen’s reagent, 63 Silylenol ethers, 50 51, 66 67, 69f Simvastatin (Zocor), 195 196 Sitagliptin (Januvia), 75 77, 137 139, 139f, 146 149, 147f, 148f GLP-1, 146 148 structure of, 147f synthesis of, 148 149, 149f X-ray crystal structure, 148, 148f Sodium glucose cotransporter 2 (SGLT-2) inhibitors, 151 152, 151f Sodium triflinate, fluoroalkylation using, 82 94 aromatic trifluoromethylation, 84 86, 85f azido-fluoroalkylation of alkenes, 88 90, 89f, 90f, 91f functional group transformations, 84f hydro-trifluoromethylation of alkenes, 86 trifluoromethylation of arylboronic acids, 86 88, 88f, 89f trifluoromethylation of proteins, 93 94, 93f, 94f Sofosbuvir, 187 188, 188f, 189f, 190 192, 194 Sonidegib, 33, 61 Sparfloxacin, 142 144 S-perfluoroalkylation, 94 Spleen tyrosine kinase (SYK) inhibitor, 52, 53f Squamous- and basal-cell skin cancers, 159 Statins, 135 136, 135f, 195 196, 196f Staudinger ligation reactions for positron emission tomography tracers, 234 237, 236f, 237f alkyl azides with 18F-labeled triarylphosphine esters, 238f 18 F-labeled GABAA receptor antagonist, 237f 18 F-labeled peptide analogs, synthesis of, 237f traceless Staudinger ligation reaction, 236f Stereoselective electrophilic fluorination of β-diketone, 52f Stille-coupling condensation polymerization, 293 294 Strain-promoted alkyne azide cycloaddition (SPAAC), 231 Strain-promoted click chemistry, 231 234, 233f, 235f Streptomycin, 141 S-trifluoromethylated cysteine, 31, 32f S-trifluoromethylation, 94 Sulfa drugs, 141 Sulfonamide reagent, 65 66 Sulfuryl fluoride (SO2F2), 6, 7f, 12, 12f Suzuki Miyaura reaction, 190 T Tafenoquine (Krintafel), 165 166, 167f Tafluprost, 104 Index TAK-637, 140 141, 142f Talazoparib (Talzenna), 171, 171f Tarenflurbil, 161 162, 162f Taxoid anticancer agents, 173 177 drug delivery through aptamer, drug conjugates, 177, 177f paclitaxel (Taxol), 173 174, 175f tumor-targeted drug delivery of the fluorinated taxoids, 174 176, 176f Tebufenpyrad, 12 13 Tecovirimat, 185 187, 185f structure of, 185f synthesis of, 186 187, 186f Tenofovir alafenamide fumarate, 163, 164f Tenofovir disoproxil fumarate, 165 Testosterone, 4 Tetracaine, 26 27 Tetracyclines, 145 146, 146f Tetraethylammonium bicarbonate, 255 257 3,3,7,7-Tetrakis(difluoramino)octahydro-1,5dinitro-1,5-diazocine (HNFX), 314 315, 317f Tetrakis(dimethylamino)ethylene (TDAE), 28 30 Tetrakis(dimethylamino)ethylene (TDAE)mediated nucleophilic fluoroalkylation method, 28 30, 28f gem-(difluoromethyl)thioethers, synthesis of, 30, 30f, 31f TMSCF3 reagent, 28 29 trifluoromethylation of acyl chlorides, 29, 29f Tetralones, 52f Tetramethylammonium fluoride (TBAF), 6, 7f, 12, 15, 17 18 Tetrazole, 320f, 321 Tezacaftor, 139 140, 140f Thrope Ingold effect, 129 Ticagrelor (Brillinta), 198, 198f Tiflorex (anorectic), 44, 61 Togni’s reagents, 23 25, 25f, 44, 55 57, 61, 77 78, 80 81, 113 Cu(I)-catalyzed α-trifluoromethylation of hydrazones, 114f Cu(I)-catalyzed asymmetric aminotrifluoromethylation reactions, 81f electrophilic trifluoromethylations, 58f 343 Fe(II)-catalyzed vicinal azidotrifluoromethylation of alkenes, 82f structures of, 56f, 77f Toltrazuril, 44, 61 Tolvaptan, 118 121 Transition metal-mediated radiofluorination, 245 252 Au(III) catalysis for [18F]trifluoromethyl compounds synthesis, 248, 251f Cu(I)-catalyzed radiofluorinations, 252 Mn(III)-catalyzed radiofluorinations, 245 247, 245f, 246f, 247f Ni(II)-catalyzed radiofluorinations, 249 251 Pd-catalyzed radiofluorinations, 248, 249f, 250f Triamcinolone (Kenalog), 201 Triflumuron, 33, 61 Trifluoroacetaldehyde hydrate, 19 Trifluoroethanol (TFE), 86 Trifluoromethanol, 285 Trifluoromethoxylations, 31 35, 33f, 35f flucarbazone, 33 flurprimidol, 33 of glycosyl halides, 63f indoxacarb, 33 rifluzole, 33 sonidegib, 33 triflumuron, 33 trifluoromethyl benzenesulfonate mediated vicinal (bromo) trifluoromethoxylation, 33, 34f trifluoromethyl benzoate mediated trifluoromethoxylation, 34 35, 35f Trifluoromethyl anion, 21, 22f, 23 25, 23f Trifluoromethylation, 2, 12 30, 44 of acyl chlorides, 29, 29f of arylboronic acids, 86 88, 88f, 89f borazine-mediated gem-difluroalkylation, 23 25, 26f borazine-mediated trifluoromethylation, 23 25, 25f diaryldisulfides, 30 fluoroacetone hydrates for nucleophilic fluoroalkylations, 18 19, 19f fluoroform (CHF3), 19 25, 20f, 22f, 23f, 24f of hydrazones, 113 344 Index Trifluoromethylation (Continued) of imines, 18 N-trifluoromethylation of amines, 26 28, 27f of proteins, 93 94, 93f, 94f Ruppert Prakash reagent for, 13 18, 14f Trifluoromethyl benzenesulfonate (TFMS), 33 35, 34f Trifluoromethyl benzoate, 34 35, 35f Trifluoromethyl ketones, synthesis of, 16 18, 17f Trifluoromethyl phenylsulfonate (TFMS), 34f Trifluoromethyl radicals, 82f Trifluoromethylthiolation, 31, 32f of carbonyl compounds, amines, alkynes and arylboronic acids, 64f diethylaminosulfur trifluoride mediated trifluoromethylthiolation of silylenol ethers and β-naphthols, 66 67, 69f difluoromethylthiolation, 68 70, 69f, 70f reagents for, 63 65, 63f asymmetric trifluoromethylthiolation, 64 65, 65f Billard’s reagents, 65 66, 66f Munavalli’s reagent, 64, 64f, 68 70 trifluoromethylthiolating reagents, commercially available, 63, 63f Trifluoromethyltrimethylsilane (CF3TMS), 3, 13 14, 17 18, 17f, 20f, 23 25, 25f Trimethylsilyl azide (TMSN3), 81 82 1,3,5-trinitroperhydro-1,3,5-triazine (RDX), 314 316, 317f 2,4,6-trinitrotoluene (TNT), 314 315, 317f Tris(dimethylamino) sulfonium difluorotrimethylsilicate (TASF), 13 14 Tropomyosin receptor kinase, 265, 266f Type 2 diabetes, 146 148, 151 152, 195 196 U Umemoto’s reagents, 44, 55 56, 61, 77 78, 81 82, 227 electrophilic trifluoromethylations, 57f structures of, 56f, 77f V Vancomycin, 144 Velpatasvir (Vosevi), 194 Vemurafenib, 171 172, 172f Verubecestat, structure of, 156f Vicinal (bromo)trifluoromethoxylation, 33, 34f Vitamin E, 105 Vorapaxar (Zontivity), 197 198, 198f Voxilaprevir, 75 77, 194, 194f W Weinreb amides, 17 18, 17f X Xenon difluoride (XeF2), 43 44, 61 XtalFluor reagents, 8f XtalFluor-E [(diethylamino)difluorosulfinium tetrafluorobrate], 2 4, 6 8, 10 XtalFluor-M (morpholinodifluorosulfinium tetrafluoroborate), 2 4, 6 8, 10