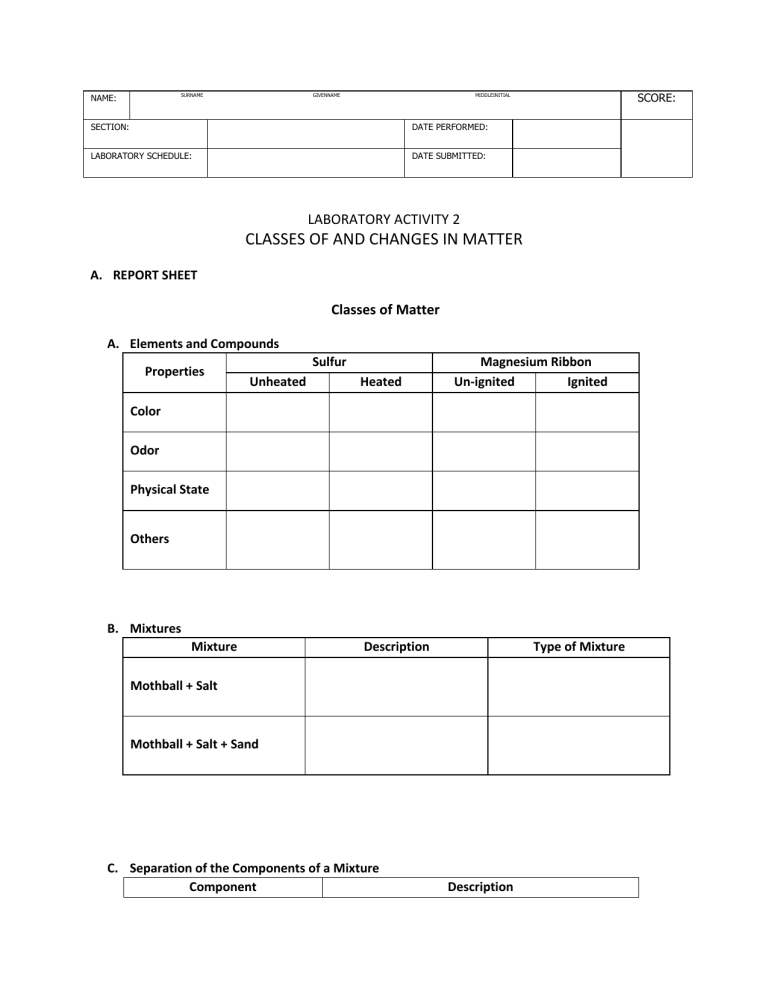
SURNAME NAME: GIVENNAME SCORE: MIDDLEINITIAL SECTION: DATE PERFORMED: LABORATORY SCHEDULE: DATE SUBMITTED: LABORATORY ACTIVITY 2 CLASSES OF AND CHANGES IN MATTER A. REPORT SHEET Classes of Matter A. Elements and Compounds Properties Sulfur Unheated Heated Magnesium Ribbon Un-ignited Ignited Color Odor Physical State Others B. Mixtures Mixture Description Type of Mixture Mothball + Salt Mothball + Salt + Sand C. Separation of the Components of a Mixture Component Description The mixture after mixing with water Decantate Residue on the side of funnel Residue on the evaporating dish Substance Physical and Chemical Change Type of Change Justification Table Salt Table Sugar Iodine crystal NaHCO3 Types of Chemical Change Type of Chemical Change Observation Chemical Product Synthesis Decomposition Displacement Metathesis B. QUESTIONS 1. Give 3 other examples of homogeneous and heterogeneous mixture. 2. Give some differences between chemical and physical changes. 3. Classify the following changes (Write Physical change or Chemical change) a. Rusting of iron b. Yellowing of leaves c. Milling of palay d. Roasting of meat e. Water cycle f. Washing of dishes g. Burning of wood h. Melting of candle i. Dyeing of hair j. Decoloration of stain with bleach C. CONCLUSION



