Physics of VLSI Devices –
MVLD501L (Module 1)
Dr. Rajan Pandey
Associate Professor, SENSE
Semiconductors
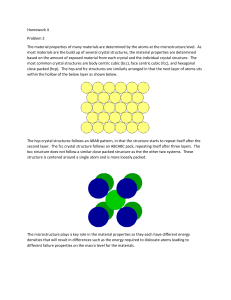
Cubic Lattices
Unit cells for three types of cubic lattice structures
Nearest neighbours
6
Simple Cubic (SC)
8
Body-Centred Cubic (BCC)
12
Face-Centred Cubic (FCC)
How many atoms per unit cell in each unit cell type?
Types of Cubic Cells
𝑃𝐹 =
𝐴𝑡𝑜𝑚𝑠 𝑝𝑒𝑟 𝐶𝑒𝑙𝑙 . 4/3. 𝜋𝑟
𝐿𝑎𝑡𝑡𝑖𝑐𝑒 𝑐𝑜𝑛𝑠𝑡𝑎𝑛𝑡
Different cubic cells result from different packing of atoms
First nearest
neighbours
Cubic Lattices
Reference
atom
Nearest neighbours
8x1=8
First nearest
neighbours 6
Second nearest
neighbours
Reference
atom
Body-Centred
Cubic (BCC)
Third nearest
neighbour
Simple Cubic (SC)
Reference
atom
Face-Centred
Cubic (FCC)
First nearest neighbours
6 x 2 = 12
Face Centered Cubic (FCC)
Atomic packing pactor (APF): 0.74
Number of atoms per unit cell: 4
• The face is a square of side a and the
diagonal is 𝑎 + 𝑎 or 𝑎 2.
• The diagonal has one atom at the center of
diameter 2R, which touches two atoms
centered at the corners.
• The diagonal, going from corner to corner, is
therefore R + 2R + R.
• Thus, 4R = 𝑎 2 and a = 4R∕ 2 = 2R 2.
The FCC unit cell. The atomic radius is R and the lattice parameter is a
Types of Solids
• In the basic building blocks of todays integrated circuits, there are all kinds of
materials: metals, alloys, semiconductors, low, ultra-low, and high-k insulators.
• Also, these materials have various physical forms or states, namely crystalline,
amorphous, and polycrystalline.
• Amorphous solids lack a regular three-dimensional arrangement of atoms. They
lack long-range structural order.
• Crystalline solids have atoms/ions/molecules arranged in regular, repeating
patterns. They possess long-range periodicity.
• Polycrystals contain multiple grains of crystallites. They have short range order.
Crystalline
Polycrystalline
Amorphous Polycrystalline
Amorphous Crystalline
Silicon Crystal Structure
• Unit cell of silicon crystal is
cubic. 8 atoms in the unit
cell.
Average bond length = 2.35 Å
Conventional unit cell
Electron wavefunction in a
periodic crystal:
• Each Si atom has 4 nearest
neighbors.
• Cell volume = (5.43 Å)3
= 1.6 X 10-22 cm3
• Density of Si atoms:
= 8/1.6 X 10-22 = 5 X 1022 cm-3
Lattice parameter = 5.43 Å
2.35 Å
uk has the same periodicity
as the crystal.
k = 2π/a, a = lattice parameter
Similar to k = 2π/λ for a wave
Diamond structure:
Two interpenetrating FCC lattices 1/4, 1/4, 1/4 along the body diagonal.
FCC Face Centred Cubic Lattice (there are 14 Bravais lattices)
Silicon Wafers and Crystal Planes
z
z
z
(100) plane
x [100] direction
y
x
(110) plane
y
[110] direction
Si (111) plane
x
[111]
direction
(111) plane
y
Si (100)
Si (110)
• The standard notation for
crystal planes is based on the
cubic unit cell.
• Silicon wafers are usually cut
along the (100) plane with a
flat or notch to help orient the
wafer during IC fabrication.
Si (111)
• As electrons move along (100) direction, they see a
particular arrangement of Si atom.
• If they move along (110) or (111) direction, they will see a
very different arrangements of Si atoms in space.
• The electron transport in (100) or (110) or (111) orientations,
will be different from each other.
Directions in the cubic crystal system
100 [100], [010], [001], [ 1 00], [0 1 0], [00 1 ]
<100> - Cube edges
<110> - Face diagonals
<111> - Body diagonals
Properties of crystals may
be different along different
directions because atomic
periodicities are different.
Wafer Type and Orientation
Wafer flats: Indicates the
type and orientation of the
crystal.
Primary flat (P): The flat of
the longest length located in
the circumference of the
wafer. The primary flat has a
specific crystal orientation
relative to the wafer surface
(major flat).
Secondary flat (S): Indicates
the crystal orientation and
doping of the wafer.
Questions
• Why the device fabrication has to be in a certain crystallographic
orientation?
• Why some specific types of devices are fabricated in a certain
crystal orientation e.g. CMOS along <100> and <110>?
• Whereas certain other type of devices are fabricated in some
other crystal orientation e.g. BJT along <111>?
Wafer Type and Orientation
Processed wafer
with identical chips.
• The crystal is usually doped p- or n-type while grown.
• Only a thin layer of the wafer, approximately 1-μm to 15- μm deep, is used to
define the device structure (out of 600 – 800 μm).
• The remainder of the wafer acts as a mechanical support.
• Several process parameters and device characteristics are sensitive to wafer
orientation.
• These include rate of crystal growth, rate of thermal oxidation, diffusivity of
impurities, electronic band structure, electronic transport etc.
• Since the {111} planes have the smallest separation, silicon grows faster along
a <111> direction than along a <110> or <100> direction.
• Also, since {111} planes have the highest density of atoms, the dissolution of
silicon in an etching solution is slowest in the <111> direction.
• The oxidation rate of silicon is largest in the <111> direction and smallest in
the <100> direction.
• Electronic traps created at the silicon surface increase the surface charge and
alter device parameters. The density of these interface traps depends on the
crystallographic orientation.
• It is highest in <111> oriented wafers and lowest in <100> oriented wafers. This
is one reason for choosing <100> oriented wafers for MOS devices fabrication.
• The current flow is higher along <110> compared to <100>.
Questions
• Why the device fabrication has to be in a certain crystallographic orientation?
• Why some specific type of devices are fabricated in a certain crystal orientation?
• Whereas the certain other type of devices are fabricated in some other crystal
orientation?
• We have got the answers of the above questions. But we just figured out that
the density of interface traps are highest along <111> oriented wafers and
lowest along <100> oriented wafers. So then why Bipolar devices are fabricated
along <111> oriented wafers, whereas MOS devices are not?
• The answer lies in the way of functioning of these devices. Whereas MOS
devices are sensitive to the interface traps, the bipolar devices may not be.
Interplanar spacing
The interplanar spacing is given by
A more generalized form is
d
hkl
1
h2
k 2
l2
2
2
2
a
b
c
Example: The lattice constant for aluminum is 4.041 angstroms. What is d between
adjacent (220) planes, i.e. d220?
Aluminum has fcc structure, so a = b = c = 4.041 Å
d hkl
1
h2 k2
a2
1
22 22
4 . 041 2
1 . 43 angstroms
Angles between planes
The angle θ between two directions
[h1 k1 l1] and [h2 k2 l2] is given by
the relation:
As an example the angle between
(111) and (100) planes will be
°
The angle between (111) and (100) surface is 54.74 degrees.
Elemental and Compound Semiconductors
Elemental
IV Compound
III-V Compound II-VI Compound
Si
SiC
AlP
ZnS
Ge
SiGe
AlAs
GaP
ZnSe
ZnTe
GaAs
InAs
CdS
CdSe
InSb
InP
HgS
ZnO
GaN
HgSe
• Most semiconductors have diamond or zinc blende crystal structures.
• What is crystal structure? What is diamond or zinc blende crystal structure?
Effective Mass
Ex
An external force Fext applied to an
electron in a vacuum results in an
acceleration avac = Fext /me
Ex
An external force Fext applied to an
electron in a crystal results in an
acceleration acryst = Fext /me*
Electrons in solids under electric force field behave as if they have effective
mass rather than free electron mass, due to crystal potential.
Effective Mass
In an electric field, E, an electron or a hole accelerates.
∗
𝒆
Electrons,
∗
holes
Electrons and holes in solids under electric force field behave
as if they have effective mass rather than free electron mass,
due to crystal potential.
=
∗
∗
∗
Effective mass is a measure of the curvature of the band.
Larger the curvature, smaller the effective mass and vice versa.
k = 2π/a,
a = lattice parameter
Electron and hole effective masses
In semiconductors or metals
we are only concerned about
the regions very close to the
band edges for transport or
optical properties.
m*e / m0
m*h / m0
Si
Ge
GaAs
InAs
0.2
0.49
0.08
0.33
0.067
0.51
0.026
0.42
Free hole band will also be parabolic close to the
band edge but the curvature will be opposite.
The bands are parabolic in
the close vicinity of the
band edges.