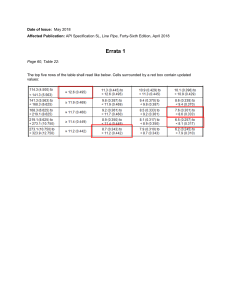
THERMOCHEMISTRY 5 • The Nature of Chemical Energy • The First Law of Thermodynamic • Enthalpy • Enthalpies of Reaction • Calorimetry • Hess’s Law • Enthalpies of Formation THERMITE REACTION. The reaction between aluminum metal and iron oxide produces aluminum oxide and iron metal. It also generates enough heat to melt the iron metal formed in the reaction. The thermite reaction is a dramatic illustration of how potential energy stored in chemical bonds can be converted to heat. M05_BROW4232_14_SE_C05_pp162-211.indd 162 • Bond Enthalpies 15/11/16 10:34 PM • Foods and Fuels Energy and thermodynamics • Energy is the ability to do work or transfer heat. • Thermodynamics is the study of energy and its transformations. • Specifically, thermochemistry is the study of chemical reactions and the energy changes that involve heat. • If we are to properly understand chemistry, we must understand the energy changes that accompany chemical reactions. © 2018 Pearson Education Ltd. Figure 5.1 Chemical reactions and energy. Chemical energy • With the exception of the energy from the Sun, most of the energy used in our daily lives comes from chemical reactions. • The combustion of gasoline, • the production of electricity from coal, • the heating of homes by natural gas, • the use of batteries to power electronic devices. • Chemical reactions provide the energy that sustains living systems. • Plants use solar energy to carry out photosynthesis, allowing them to grow. In the process, they store a portion of the Sun’s energy in the chemical bonds of the molecules that are produced during photosynthesis. • When animals eat and digest plants, they derive the energy needed to move, maintain body temperature, and carry out all other bodily functions from that same chemical energy. ⻄元1776年 英國⼈瓦特的蒸汽機 James Watt (1736-1819) Finally, in 1776, the first engines were installed and working in commercial enterprises. ⻄元1814年 英國⼈史蒂⽂⽣(George Stephenson) The steam locomotive operates by converting thermal energy into mechanical energy. The diesel locomotive converts chemical energy into electrical energy, then electrical energy into mechanical energy to generate motion. All these energy conversion processes are governed by thermodynamics. The global contribution to world's GDP by major economies from 1 AD to 2008 AD according to Angus Maddison's estimates. Chemical Energy is Mainly Potential Energy • The most important form of potential energy in molecules is electrostatic potential energy, Eel: • Reminder: the unit of energy commonly used is the Joule: Figure 5.2 Electrostatic potential energy. © 2018 Pearson Education Ltd. Attraction between Ions • Electrostatic attraction is seen between oppositely charged ions. • Energy is released when chemical bonds are formed; energy is consumed when chemical bonds are broken. Figure 5.3 Electrostatic potential energy and ionic bonding. © 2018 Pearson Education Ltd. First Law of Thermodynamics Energy can be converted from one form to another, but it is neither created nor destroyed. • To heat your home, chemical energy needs to be converted to heat. • Sunlight is converted to chemical energy in green plants. • There are MANY more examples of conversion of energy. © 2018 Pearson Education Ltd. Definitions: System and Surroundings • The portion of the universe that we single out to study is called the system (here, the hydrogen and oxygen molecules). • The surroundings are everything else (here, the cylinder, piston and everything beyond). Figure 5.4 A closed system. © 2018 Pearson Education Ltd. Types of Systems 1) Open System: a region of the universe being studied that can exchange heat AND mass with its surroundings. 2) Closed System: a region of the universe being studied that can ONLY exchange heat with its surroundings (NOT mass) 3) Isolated System: a region of the universe that can NOT exchange heat or mass with its surroundings Figure 5.4 A closed system. Note that this is a CLOSED system. © 2018 Pearson Education Ltd. Internal Energy • The internal energy of a system is the sum of all kinetic and potential energies of all components of the system; we use E to represent it. • We generally don’t know E, only how it CHANGES (ΔE). • By definition, the change in internal energy, ΔE, is the final energy of the system minus the initial energy of the system: ΔE = Efinal − Einitial © 2018 Pearson Education Ltd. Changes in Internal Energy IF: ΔE > 0, Efinal > Einitial the system absorbed energy from the surroundings. Figure 5.5 Changes in internal energy. © 2018 Pearson Education Ltd. Changes in Internal Energy IF: ΔE < 0, Efinal < Einitial the system released energy to the surroundings. Figure 5.5 Changes in internal energy. © 2018 Pearson Education Ltd. Thermodynamic Quantities Have Three Parts 1. A number 2. A unit 3. A sign Note about the sign: – A positive ΔE results when the system gains energy from the surroundings. – A negative ΔE results when the system loses energy to the surroundings. © 2018 Pearson Education Ltd. Energy Diagram • The energy diagram shows that the internal energy of the mixture of H2 and O2 is greater than that of the H2O produced in the reaction. Figure 5.6 Energy diagram for the reaction 2 H2(g) ☞ O2(g) → 2 H2O(I). © 2018 Pearson Education Ltd. Changes in Internal Energy • When energy is exchanged between the system and the surroundings, it is exchanged as either heat (q) or work (w). • That is, ΔE = q + w. Figure 5.7 Sign conventions for heat and work. © 2018 Pearson Education Ltd. ΔE, q, w, and Their Signs – Notice that any energy entering the system as either heat or work carries a positive sign. © 2018 Pearson Education Ltd. Exchange of Heat between System and Surroundings • When heat is absorbed by the system from the surroundings, the process is endothermic. © 2018 Pearson Education Ltd. Figure 5.8 (a) Endothermic reaction Exchange of Heat between System and Surroundings • When heat is released by the system into the surroundings, the process is exothermic. © 2018 Pearson Education Ltd. Figure 5.8 (b) Exothermic reaction State Functions • Usually we have no way of knowing the internal energy of a system; finding that value is simply too complex a problem. • However, we do know that the internal energy of a system is independent of the path by which the system achieved that state. – In the system below, the water could have reached room temperature from either direction. © 2018 Pearson Education Ltd. Figure 5.9 Internal energy, E, a state function. State Functions • Therefore, internal energy is a state function. • It depends only on the present state of the system, not on the path by which the system arrived at that state. • And so, ΔE depends only on Einitial and Efinal. © 2018 Pearson Education Ltd. State Functions • However, q and w are not state functions. • Whether the battery is shorted out or is discharged by running the fan, its ΔE is the same, but q and w are different in the two cases. Figure 5.10 Internal energy is a state function, but heat and work are not. © 2018 Pearson Education Ltd. Work • Usually the only work done by chemical or physical change is the mechanical work associated with a change in volume of gas. Figure 5.13 Pressure–volume work. © 2018 Pearson Education Ltd. Work • We can measure the work done by the gas if the reaction is done in a vessel that has been fitted with a piston: w = −PΔV • The work is NEGATIVE because it is done BY the system. Figure 5.11 A system that does work on its surroundings. © 2018 Pearson Education Ltd. Enthalpy • If a process takes place at constant pressure (and we usually work at atmospheric pressure) and the only work done is this pressure–volume work, we can account for heat flow during the process by measuring the enthalpy (H) of the system. • Enthalpy is the internal energy plus the product of pressure and volume: H = E + PV © 2018 Pearson Education Ltd. Enthalpy • When the system changes at constant pressure, the change in enthalpy, ΔH, is ΔH = Δ (E + PV) • This can be written ΔH = ΔE + PΔV © 2018 Pearson Education Ltd. Enthalpy • Since ΔE = q + w and w = −PΔV, we can substitute these into the enthalpy expression: ΔH = ΔE + PΔV ΔH = (q + w) − w ΔH = q • So, at constant pressure, the change in enthalpy is the heat gained or lost. © 2018 Pearson Education Ltd. Endothermic and Exothermic • A process is endothermic when ΔrH is positive. • A process is exothermic when ΔrH is negative. Figure 5.12 Endothermic and exothermic processes. © 2018 Pearson Education Ltd. Enthalpy of Reaction • The change in enthalpy, ΔrH, is the enthalpy of the products minus the enthalpy of the reactants: ΔrH = ΔHrxn = Hproducts − Hreactants • This quantity, ΔrH, is called the enthalpy of reaction, or the heat of reaction. Figure 5.14 Exothermic reaction of hydrogen with oxygen. © 2018 Pearson Education Ltd. Enthalpy of Reaction • The exothermic nature of this reaction is also shown in the enthalpy diagram in the Figure 5.14. Figure 5.14 Exothermic reaction of hydrogen with oxygen. © 2018 Pearson Education Ltd. The Truth about Enthalpy 1. Enthalpy is an extensive property. 2. The enthalpy change for a reaction is equal in magnitude, but opposite in sign, to ΔrH for the reverse reaction. 3. The enthalpy change for a reaction depends on the states of the reactants and the products. Figure 5.15 ΔH for a reverse reaction. © 2018 Pearson Education Ltd. Using Enthalpy as a Guide • Chemical reactions that are highly exothermic (ΔH much less than 0) usually occur spontaneously. • But often need some energy to get the process started. Figure 5.16 Exothermic reactions and spontaneity. © 2018 Pearson Education Ltd. Calorimetry • Since we cannot know the exact enthalpy of the reactants and products, we measure ΔrH through calorimetry, the measurement of heat flow. • The instrument used to measure heat flow is called a calorimeter. Figure 5.18 Coffee-cup calorimeter. © 2018 Pearson Education Ltd. Heat Capacity and Specific Heat • The amount of energy required to raise the temperature of a Q substance by 1 K (1 °C) is its heat capacity, C = . ΔT (extensive) • If the amount of the substance heated is one gram, it is the 1 Q specific heat, Cs = . (intensive) m ΔT • If the amount is one mole, it is the molar heat capacity , 1 Q Cm = . (intensive) n ΔT © 2018 Pearson Education Ltd. Constant volume vs constant pressure heat capacity • Heat is not a state function. • Heat absorbed by the system depends on the path. Q . • Heat capacity is a path function! C = ΔT • Heat capacity measured at constant volume, Q ΔE CV = = ( ΔT ) ( ΔT ) V V QV = ΔE • Heat capacity measured at constant pressure, QP Q ΔH CP = = ( ΔT ) ( ΔT ) P P • In general, CP © 2018 Pearson Education Ltd. ≠ CV = ΔH Constant volume vs constant pressure heat capacity • Consider infinitesimal temperature change, ΔT ∂E Constant volume heat capacity CV = • ( ∂T ) V →0 ∂H Constant pressure heat capacity CP = • ( ∂T ) P © 2018 Pearson Education Ltd. Thermodynamic properties (variables) • A thermodynamic property is any macroscopic property that is measurable, whose value describes a state of a physical system. • Any variable that can be used to characterize the system is called a variable of state, a state variable, or a state function. • Important variables: • Extensive ( • Intensive ( ∝ n): n, V, E, H, CV, CP, … ∝ n 0 = 1): P, T, CV,m, CP,m, … • System consisting of single component, single phase: only three are independent: (n, V, T), or (n, p, T), or (n, V, p)… • The choice of independent variables are predetermined by researchers. • The other thermodynamic variables are functions of these independent variables. E(V, T ), E(P, T ), H(P, T ), … Heat Capacity and Specific Heat • For example, 209 J is required to increase the temperature of 50.0 g of water by 1.00 K. • Thus, the specific heat of water is Figure 5.17 Specific heat of water. © 2018 Pearson Education Ltd. Heat Capacity and Specific Heat • Notice that the specific heat of liquid water is higher than those of the other substances listed. • The high specific heat of water affects Earth’s climate because it makes the temperatures of the oceans relatively resistant to change. © 2018 Pearson Education Ltd. Constant-Pressure Calorimetry • By carrying out a reaction in aqueous solution in a simple calorimeter, the heat change for the system can be found by measuring the heat change for the water in the calorimeter. • The specific heat for water is 4.184 J/g·K. We use this value for dilute solutions. • We can calculate ΔH for the reaction with this equation: qsoln = Cs × msoln × ΔT = –qrxn Figure 5.18 Coffee-cup calorimeter. © 2018 Pearson Education Ltd. Bomb Calorimetry • Reactions can be carried out in a sealed “bomb” such as this one. • The heat absorbed (or released) by the water is a very good approximation of the enthalpy change for the reaction. qrxn = – Ccal × ΔT Figure 5.19 Bomb calorimeter. © 2018 Pearson Education Ltd. Bomb Calorimetry • Because the volume in the bomb calorimeter is constant, what is measured is really the change in internal energy, ΔE, not ΔH. • For most reactions, the difference is very small. Figure 5.19 Bomb calorimeter. © 2018 Pearson Education Ltd. Sample Exercise 5.7 Measuring qrxn Using a Bomb Calorimeter The combustion of methylhydrazine (CH6N2), a liquid rocket fuel, produces N2(g), CO2(g), and H2O(l): 2 CH6N2(l) + 5 O2(g) 2 N2(g) + 2 CO2(g) + 6 H2O(l) When 4.00 g of methylhydrazine is combusted in a bomb calorimeter, the temperature of the calorimeter increases from 25.00 to 39.50 °C. In a separate experiment the heat capacity of the calorimeter is measured to be 7.794 kJ/°C. Calculate the heat of reaction for the combustion of a mole of CH6N2. Solution Analyze We are given a temperature change and the total heat capacity of the calorimeter. We are also given the amount of reactant combusted. Our goal is to calculate the enthalpy change per mole for combustion of the reactant. Plan We will first calculate the heat evolved for the combustion of the 4.00-g sample. We will then convert this heat to a molar quantity. Solve For combustion of the 4.00-g sample of methylhydrazine, the temperature change of the calorimeter is: ΔT = (39.50 °C – 25.00 °C) = 14.50 °C We can use ΔT and the value for Ccal to calculate the heat of reaction (Equation 5.23): © 2018 Pearson Education Ltd. Sample Exercise 5.7 Measuring qrxn Using a Bomb Calorimeter Continued We can readily convert this value to the heat of reaction for a mole of CH6N2: Check The units cancel properly, and the sign of the answer is negative as it should be for an exothermic reaction. The magnitude of the answer seems reasonable. Practice Exercise 1 The combustion of exactly 1.000 g of benzoic acid in a bomb calorimeter releases 26.38 kJ of heat. If the combustion of 0.550 g of benzoic acid causes the temperature of the calorimeter to increase from 22.01 to 24.27 °C, calculate the heat capacity of the calorimeter. (a) 0.660 kJ/°C (b) 6.42 kJ/°C (c) 14.5 kJ/°C (d) 21.2 kJ/g-°C (e) 32.7 kJ/°C Practice Exercise 2 A 0.5865-g sample of lactic acid (HC3H5O3) reacts with oxygen in a calorimeter whose heat capacity is 4.812 kJ/°C. The temperature increases from 23.10 to 24.95 °C. Calculate the heat of combustion of lactic acid (a) per gram and (b) per mole. © 2018 Pearson Education Ltd. The Regulation of Body Temperature • The body increases its internal energy content by ingesting foods. Δr H = − 2803 kJ/mol • Heat is removed from the body as the perspiration evaporates into the surroundings. Δr H = 44 kJ/mol Figure 5.20 Perspiration. © 2018 Pearson Education Ltd. • Macroscopic variables: Δ vs Δr • Physical variables: T, V, p, … • Chemical variables: ni, ξ, … • Two kind of deltas, Δ vs Δr: • Physical changes: ΔT ΔE = E2 − E1… = T2 − T1, ΔV = V2 − V1, • Chemical reaction aA + bB → cC + dD: Δr E = cEC + dED − aEA − bEB, Δr H = cHC + dHD − aHA − bHB • More generally, for a chemical reaction, 0 Δr H = ∑ i νiHi = ∑ i νiAi: Hess’s Law • ΔrH is well known for many reactions, and it is inconvenient to measure ΔrH for every reaction in which we are interested. • However, we can calculate ΔrH using published ΔrH values and the properties of enthalpy. • Hess’s law states that if a reaction is carried out in a series of steps, ΔrH for the overall reaction equals the sum of the enthalpy changes for the individual steps. © 2018 Pearson Education Ltd. Hess’s Law /mol /mol /mol /mol Figure 5.21 Enthalpy diagram for combustion of 1 mol of methane. © 2018 Pearson Education Ltd. Hess’s Law • Because H is a state function, for a particular set of reactants and products, ΔrH is the same whether the reaction takes place in one step or in a series of steps. /mol /mol Figure 5.22 Enthalpy diagram illustrating Hess’s law. © 2018 Pearson Education Ltd. Enthalpies of Formation • An enthalpy of formation, ΔHf (ΔfH), is defined as the enthalpy change for the reaction in which a compound is made from its constituent elements in their elemental forms. © 2018 Pearson Education Ltd. Standard Enthalpies of Formation • Standard enthalpies of formation, ΔHf° (ΔfH°), are measured under standard conditions (25 °C and 1.00 atm pressure). © 2018 Pearson Education Ltd. Calculation of ΔrH • We can write this equation as the sum of three formation equations: Δf H ∘[CO2(g)] • Use values from Table 5.3 to calculate ΔH°rxn © 2018 Pearson Education Ltd. Using Enthalpies of Formation to Calculate Enthalpies of Reaction Calculation of ΔrH Figure 5.23 Enthalpy diagram for propane combustion. © 2018 Pearson Education Ltd. Calculation of ΔrH • We can use Hess’s law in this way: where n and m are the stoichiometric coefficients. © 2018 Pearson Education Ltd. Bond Enthalpy • The enthalpy associated with breaking one mole of a particular bond in a gaseous substance. Cl2(g) → 2Cl(g) ΔrH = 242 kJ/mol CH4(g) → C(g) + 4H(g) © 2018 Pearson Education Ltd. ΔrH = 242 kJ/mol Bond Enthalpy • The bond enthalpy is always positive because energy is required to break chemical bonds. • Energy is always released when a bond forms between gaseous fragments. • The greater the bond enthalpy, the stronger the bond. © 2018 Pearson Education Ltd. Bond Enthalpy • A molecule with strong chemical bonds is generally less likely to undergo chemical change than one with weak bonds. © 2018 Pearson Education Ltd. Bond Enthalpies and Enthalpy of Reaction • ADD bond energy for ALL bonds made (+) • SUBTRACT bond energy for ALL bonds broken (–) • The result is an estimate of ΔrH. ΔrH = Σ (bond enthalpies – Σ (bond enthalpies of bonds broken) of bonds formed) © 2018 Pearson Education Ltd. Bond Enthalpies and Enthalpy of Reaction • So, we can predict whether a chemical reaction will be endothermic or exothermic using bond energies. © 2018 Pearson Education Ltd. Figure 5.24 Using bond enthalpies to estimate ΔHrxn. Energy in Foods • The energy released when one gram of food is combusted is its fuel value. © 2018 Pearson Education Ltd. Energy in Foods • Most of the energy in foods comes from carbohydrates, fats, and proteins. • Carbohydrates (17 kJ/g): C6H12O6(s) + 6 O2(g) 6 CO2(g) + 6 H2O(l) ΔrH° = –2803 kJ/mol • Fats (38 kJ/g): 2 C57H110O6(s) + 163 O2(g) ΔrH° = –71609 kJ/mol 114 CO2(g) + 110 H2O(l) 71609/163 = 439 kJ/mol • Proteins produce 17 kJ/g (same as carbohydrates): Their chemical reaction in the body is NOT the same as in a calorimeter. © 2018 Pearson Education Ltd. Energy in Fuels © 2018 Pearson Education Ltd. Energy in Fuels • The vast majority of the energy consumed in the United States comes from fossil fuels. • Nuclear fission produces 8.6% of the U.S. energy needs. • Renewable energy sources, like solar, wind, geothermal, hydroelectric, and biomass sources produce 9.9% of the U.S. energy needs. Figure 5.26 Energy consumption in the United States. Figure 5.27 Sugarcane can be converted to a sustainable bioethanol product. © 2018 Pearson Education Ltd. Why Combustions Are Always Exothermic? • The double bond in O2 is much weaker than other double bonds or pairs of single bonds, and therefore the formation of the stronger bonds in CO2 and H2O results in the release of energy, which is given off as heat or increases thermal motion. • This explains why fire is hot regardless of fuel composition. • The bond energies in the fuel play only a minor role; for example, the total bond energy of CH4 is nearly the same as that of CO2. • A careful analysis in terms of bond enthalpies, counting double bonds as two bonds to keep the total number of bonds unchanged, gives the heat of combustion close to −418 kJ/mol (i.e., −100 kcal/mol) for each mole of O2, in good agreement (±3.1%) with data for >500 organic compounds; the heat of condensation of H2O, −44 kJ/mol, is also included in the analysis. For 268 molecules with ≥ 8 carbon atoms, the standard deviation from the predicted value is even smaller, 2.1%. This enables an instant estimate of the heat of combustion simply from the elemental composition of the fuel, even for a complex mixture or unknown molecular structure, and explains principles of biofuels production. • The analysis indicates that O2, rather than fuels like octane, H2, ethanol, or glucose, is the crucial “energy-rich” molecule; we briefly explain why O2 is abundant in air despite its high enthalpy. Why Combustions Are Always Exothermic, Yielding About 418 kJ per Mole of O2 Klaus Schmidt-Rohr* J. Chem. Educ. 2015, 92, 12, 2094–2099 Why Combustions Are Always Exothermic? Why Combustions Are Always Exothermic, Yielding About 418 kJ per Mole of O2 Klaus Schmidt-Rohr* J. Chem. Educ. 2015, 92, 12, 2094–2099 • Hydrocarbons combusted in air react with O2 to form CO2 and H2O.* The number of molecules of O2 required and the number of molecules of CO2 and H2O formed depend on the composition of the hydrocarbon, which acts as the fuel in the reaction. • Many substances, fuels for example, release energy when they react. • The chemical energy of a fuel is due to the potential energy stored in the arrangements of its atoms. • Chemical energy is released when bonds between atoms are formed, and consumed when bonds between atoms are broken. When a fuel burns, some bonds are broken and others are formed, but the net effect is to convert chemical potential energy to thermal energy, the energy associated with temperature. The increase in thermal energy arises from increased molecular motion and hence increased kinetic energy at the molecular level.