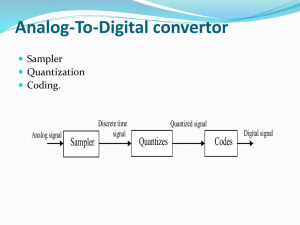
Energy Quantization in Physics Introduction: Energy quantization is a fundamental concept in physics that describes the discrete and quantized nature of energy levels in certain physical systems. This note provides a detailed explanation of energy quantization, supported by illustrations and examples. 1. The Wave-Particle Duality: The wave-particle duality is a fundamental principle in quantum mechanics. It states that particles, such as electrons or photons, exhibit both wave-like and particle-like properties. This duality is represented by wavefunctions, mathematical functions that describe the probability distribution of finding a particle in a particular state. 2. The Schrödinger Equation: The Schrödinger equation is a cornerstone of quantum mechanics. It describes the behavior of quantum systems and provides information about the energy states of particles. The equation is typically represented as follows: Hψ = Eψ Where: - H represents the Hamiltonian operator, which represents the total energy of the system. - ψ is the wavefunction of the particle. - E is the energy of the particle. Solving the Schrödinger equation yields a set of allowed energy levels, or eigenvalues, for the system. These energy levels are discrete and quantized, meaning they can only take specific values. 3. Energy Quantization in Atomic Systems: One of the most well-known examples of energy quantization is observed in atomic systems. Electrons in an atom occupy specific energy levels known as electron shells or orbitals. Each shell is associated with a particular energy value, and electrons can transition between these levels by absorbing or emitting energy. The diagram below illustrates the quantized energy levels of electrons in a hydrogen atom: As shown, the energy levels are labeled with quantum numbers, such as n=1, n=2, n=3, etc. Electrons can occupy these discrete energy levels, and when transitioning between levels, they emit or absorb photons with energies corresponding to the energy difference between the levels. 4. Discrete Absorption and Emission Spectra: The concept of energy quantization is directly linked to the observation of discrete absorption and emission spectra in atoms and molecules. When electromagnetic radiation, such as light, interacts with atoms or molecules, certain wavelengths are absorbed or emitted, producing characteristic spectral lines. For example, when white light passes through a gas composed of specific elements, such as hydrogen or sodium, it produces a spectrum with distinct colored lines. These lines correspond to the energy differences between different energy levels in the atoms of the gas. The diagram below demonstrates the discrete absorption and emission spectra of hydrogen: As depicted, the absorption spectrum shows dark lines corresponding to the absorbed wavelengths, while the emission spectrum displays bright lines representing the emitted wavelengths. 5. Examples from Particle Physics: Energy quantization is not limited to atomic systems but extends to elementary particles in particle physics. Particles, such as electrons, protons, and photons, also exhibit quantized energy levels. For instance, in the case of electrons, they occupy discrete energy levels within an atom. However, when these electrons are completely removed from the atom, they form a continuum of energy levels, as illustrated in the diagram below: The diagram demonstrates the quantized energy levels within an atom (discrete) and the continuum of energy levels when the electron is free from the atomic system. Conclusion: Energy quantization is a fundamental concept in physics that reveals the discrete nature of energy levels in quantum systems. The wave-particle duality and the Schrödinger equation explain energy quantization, and it is particularly evident in atomic systems. The discrete absorption and emission spectra observed in atoms and molecules are direct consequences of energy quantization. Furthermore, energy quantization applies to elementary particles in particle physics, where energy levels are also quantized. The concept of energy quantization provides a deeper understanding of the behavior of matter and the fundamental forces of nature.