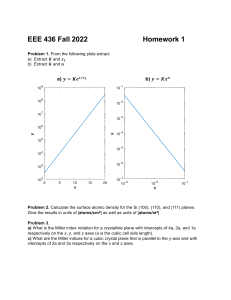
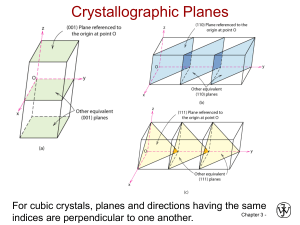
CHAPTER 3 ATOMIC STRUCTURE AND BONDING
3.1
Define a crystalline solid.
A crystalline solid is one which has a crystal structure in which atoms or ions are
arranged in a pattern that repeats itself in three dimensions.
3.2
Define a crystal structure. Give examples of materials which have crystal structures.
A crystal structure is identical to a crystalline solid, as defined by the solution of Problem
3.1. Examples include metals, ionic crystals and certain ceramic materials.
3.3
Define a space lattice.
A space lattice is an infinite three-dimensional array of points with each point having
identical surrounding points.
3.4
Define a unit cell of a space lattice. What lattice constants define a unit cell?
The unit cell of a space lattice represents a repeating unit of atomic spatial positions. The
cell is defined by the magnitudes and directions of three lattice vectors, a, b, and c: axial
lengths a, b, and c; interaxial angles α , β , and γ .
3.5
What are the 14 Bravais unit cells?
The fourteen Bravais lattices are: simple cubic, body-centered cubic, face-centered
cubic, simple tetragonal, body-centered tetragonal, simple orthorhombic, base-centered
orthorhombic, body-centered orthorhombic, face-centered orthorhombic, simple
rhombohedral, simple hexagonal, simple monoclinic, base-centered monoclinic, and
simple triclinic.
3.6
What are the three most common metal crystal structures? List five metals which have
each of these crystal structures.
The three most common crystal structures found in metals are: body-centered cubic
(BCC), face-centered cubic (FCC), and hexagonal close-packed (HCP). Examples of
metals having these structures include the following.
BCC: α − iron, vanadium, tungsten, niobium, and chromium.
FCC: copper, aluminum, lead, nickel, and silver.
HCP: magnesium, α − titanium, zinc, beryllium, and cadmium.
3.7
How many atoms per unit cell are there in the BCC crystal structure?
Smith
Foundations of Materials Science and Engineering Solution Manual
21
A BCC crystal structure has two atoms in each unit cell.
3.8
What is the coordination number for the atoms in the BCC crystal structure?
A BCC crystal structure has a coordination number of eight.
3.9
What is the relationship between the length of the side a of the BCC unit cell and the
radius of its atoms?
In a BCC unit cell, one complete atom and two atom eighths touch each other along the
cube diagonal. This geometry translates into the relationship 3a = 4 R.
3.10
Molybdenum at 20ºC is BCC and has an atomic radius of 0.140 nm. Calculate a value
for its lattice constant a in nanometers.
Letting a represent the edge length of the BCC unit cell and R the molybdenum atomic
radius,
3a = 4 R or a =
3.11
4
4
R=
(0.140 nm) = 0.323 nm
3
3
Niobium at 20ºC is BCC and has an atomic radius of 0.143 nm. Calculate a value for its
lattice constant a in nanometers.
For a BCC unit cell having an edge length a and containing niobium atoms,
3a = 4 R or a =
3.12
4
4
R=
(0.143 nm) = 0.330 nm
3
3
Lithium at 20ºC is BCC and has an atomic radius of 0.35092 nm. Calculate a value for
the atomic radius of a lithium atom in nanometers.
For the lithium BCC structure, which has a lattice constant of a = 0.35092 nm, the
atomic radius is,
R=
3.13
3
3
a=
(0.35092 nm) = 0.152 nm
4
4
Sodium at 20ºC is BCC and has an atomic radius of 0.42906 nm. Calculate a value for the
atomic radius of sodium atom in nanometers.
For the sodium BCC structure, with a lattice constant of a = 0.42906 nm, the atomic
radius is,
Smith
Foundations of Materials Science and Engineering Solution Manual
22
R=
3.14
3
3
a=
(0.42906 nm) = 0.186 nm
4
4
How many atoms per unit cell are there in the FCC crystal structure?
Each unit cell of the FCC crystal structure contains four atoms.
3.15
What is the coordination number for the atoms in the FCC crystal structure?
The FCC crystal structure has a coordination number of twelve.
3.16
Gold is FCC and has a lattice constant of 0.40788 nm. Calculate a value for the atomic
radius of a gold atom in nanometers.
For the gold FCC structure, which has a lattice constant of a = 0.40788 nm, the atomic
radius is,
R=
3.17
2
2
a=
(0.40788 nm) = 0.144 nm
4
4
Platinum is FCC and has a lattice constant of 0.39239 nm. Calculate a value for the
atomic radius of a platinum atom in nanometers.
For the platinum FCC structure, with a lattice constant of a = 0.39239 nm, the atomic
radius is,
R=
3.18
2
2
a=
(0.39239 nm) = 0.139 nm
4
4
Palladium is FCC and has an atomic radius of 0.137 nm. Calculate a value for its lattice
constant a in nanometers.
Letting a represent the FCC unit cell edge length and R the palladium atomic radius,
2a = 4 R or a =
3.19
4
4
R=
(0.137 nm) = 0.387 nm
2
2
Strontium is FCC and has an atomic radius of 0.215 nm. Calculate a value for its lattice
constant a in nanometers.
For an FCC unit cell having an edge length a an containing strontium atoms,
2a = 4 R or a =
Smith
4
4
R=
(0.215 nm) = 0.608 nm
2
2
Foundations of Materials Science and Engineering Solution Manual
23
3.20
Calculate the atomic packing factor for the FCC structure.
By definition, the atomic packing factor is given as:
Atomic packing factor =
volume of atoms in FCC unit cell
volume of the FCC unit cell
These volumes, associated with the four-atom FCC unit cell, are
ª4
º 16
Vatoms = 4 « π R3 » = π R3
¬3
¼ 3
and
Vunit cell = a3
where a represents the lattice constant. Substituting a =
Vunit cell
4R
,
2
64 R3
=a =
2 2
3
The atomic packing factor then becomes,
§ 16π R3 · § 1 2 · π 2
APF (FCC unit cell) = ¨
¸ ¨¨
3¸
¸ = 6 = 0.74
3
32
R
©
¹©
¹
3.21
How many atoms per unit cell are there in the HCP crystal structure?
The hexagonal prism contains six atoms.
3.22
What is the coordination number for the atoms in the HCP crystal structure?
The coordination number associated with the HCP crystal structure is twelve.
3.23 What is the ideal c/a ratio for HCP metals?
The ideal c/a ratio for HCP metals is 1.633; however, the actual ratios may deviate
significantly from this value.
3.24 Of the following HCP metals, which have higher or lower c/a ratios than the ideal ratio:
Zr, Ti, Zn, Mg, Co, Cd and Be?
Cadmium and zinc have significantly higher c/a ratios while zirconium, titanium,
magnesium, cobalt and beryllium have slightly lower ratios.
3.25 Calculate the volume in cubic nanometers of the titanium crystal structure unit cell.
Titanium is HCP at 20ºC with a = 0.29504 nm and c = 0.46833 nm.
For a hexagonal prism, of height c and side length a, the volume is given by:
Smith
Foundations of Materials Science and Engineering Solution Manual
24
V = (Area of Base)(Height) = [(6 × Equilateral Triangle Area)(Height)]
= (3a 2 sin 60 )(c)
= 3(0.29504 nm)2 (sin 60 )(0.46833 nm)
=0.106 nm 3
3.26 Rhenium at 20ºC is HCP. The height c of its unit cell is 0.44583 nm and its c/a ratio is
1.633 nm. Calculate a value for its lattice constant a in nanometers.
The rhenium lattice constant a is calculated as,
a=
c
0.44583 nm
=
= 0.273 nm
c/a
1.633
3.27 Osmium at 20ºC is HCP. Using a value of 0.135 nm for the atomic radius of osmium
atoms, calculate a value for its unit-cell volume. Assume a packing factor of 0.74.
From the definition of the atomic packing factor,
HCP unit cell volume =
volume of atoms in HCP unit cell
APF
Since there are six atoms in the HCP unit cell, the volume of atoms is:
§4
·
Vatoms = 6 ¨ π R3 ¸ = 8π (0.135)3 = 0.0618 nm3
©3
¹
The unit cell volume thus becomes,
0.0618 nm3
HCP unit cell volume =
= 0.084 nm 3
0.74
3.28
How are atomic positions located in cubic unit cells?
Atomic positions are located in cubic unit cells using rectangular x, y, and z axes and unit
distances along the respective axes. The directions of these axes are shown below.
+z
-x
-y
+y
+x
-z
Smith
Foundations of Materials Science and Engineering Solution Manual
25
3.29
List the atom positions for the eight corner and six face-centered atoms of the FCC unit
cell.
The atom positions at the corners of an FCC unit cell are:
(0, 0, 0), (1, 0, 0), ( 1, 1, 0), (0, 1, 0), (0, 0, 1), (1, 0, 1), (1, 1, 1), (0, 1, 1)
On the faces of the FCC unit cell, atoms are located at:
(½, ½, 0), (½, 0, ½), (0, ½, ½), (½, ½, 1), (1, ½, ½), (½, 1, ½)
3.30
How are the indices for a crystallographic direction in a cubic unit cell determined?
For cubic crystals, the crystallographic direction indices are the components of the
direction vector, resolved along each of the coordinate axes and reduced to the smallest
integers. These indices are designated as [uvw].
3.31
Draw the following directions in a BCC unit cell and list the position coordinates of the
atoms whose centers are intersected by the direction vector:
(a) [100]
(b) [110]
(c) [111]
z
(1, 1, 1)
y
(0, 0, 0)
(0, 0, 0)
x
(0, 0, 0)
(1, 1, 0)
(1, 0, 0)
(a) Position Coordinates:
(0, 0, 0), (1, 0, 0)
3.32
(b) Position Coordinates:
(0, 0, 0), (1, 1, 0)
(c) Position Coordinates:
(0, 0, 0), (1, 1, 1)
Draw direction vectors in unit cubes for the following cubic directions:
(a) [11 1]
(b) [11 0]
(c) [1 21]
(d) [1 1 3]
(a)
z
(b)
y
x
Smith
x = +1
y = -1
z = -1
[1 1 1]
x = +1
y = -1
z=0
[1 1 0]
Foundations of Materials Science and Engineering Solution Manual
26
(d)
(c)
[1 1 3]
½
½
Dividing by 2,
x = -½
y=1
z = -½
⅓
Dividing by 3,
x=–⅓
y=–⅓
z=1
[12 1]
⅓
3.33
Draw direction vectors in unit cubes for the following cubic directions:
(a) [11 2]
(d) [0 21]
(g) [1 01]
(j) [10 3]
z
(b) [12 3]
(e) [2 1 2]
(h) [12 1]
(k) [12 2]
(c) [331]
(f) [2 33]
(i) [3 21]
y
(l) [2 23]
x
½
½
1
1
⅓
⅓
1
⅔
(a) Dividing [11 2] by 2,
1
1
x = , y = − , z = −1
2
2
1
(b) Dividing [123] by 3,
(c) Dividing [3 31] by 3,
1
2
x = , y = − , z =1
3
3
x = −1, y = 1, z =
1
3
½
1
1
⅔
1
½
1
(d) Dividing [0 21] by 2,
1
x = 0, y = −1, z =
2
Smith
(e) Dividing [21 2] by 2,
1
x = 1, y = − , z = 1
2
(f) Dividing [233] by 3,
2
x = , y = −1, z = 1
3
Foundations of Materials Science and Engineering Solution Manual
27
½
1
½
1
1
⅓
⅔
(h) Dividing [121] by 2,
1
1
x = , y = 1, z = −
2
2
(g) For [1 01],
x = −1, y = 0, z = 1
(i) Dividing [321] by 3,
2
1
x = 1, y = , z =
3
3
1
1
1
1
⅓
½
⅔
⅔
(j) Dividing [103] by 3,
1
x = , y = 0, z = −1
3
3.34
(l) Dividing [2 23] by 3,
(k) Dividing [12 2] by 2,
1
x = , y = −1, z = −1
2
2
2
x = − , y = − , z =1
3
3
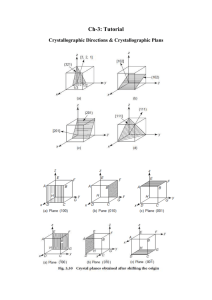
What are the indices of the directions shown in the unit cubes of Fig. P3.34?
z
(a)
(b)
a
¾
d
½
⅓
¼
⅔
y
¼
Smith
h ¾
½
⅓
f
g
½
x
z
e
c
b
¼
¼
y
¾
x
Foundations of Materials Science and Engineering Solution Manual
28
⅓
a
1
/6
c
¼
b
New 0
½
¼
½
New 0
a. Vector components:
x = -1, y = 1, z = 0
Direction indices: [110]
b. Moving direction vector
down ¼ , vector components
are: x = 1, y = -1, z = ¼
Direction indices: [4 41]
c. Moving direction vector forward ½, vector components
1
are: x = - /6 , y = 1, z = 1
Direction indices: [1 66]
¼
½ ¾
-⅓
New 0
d
⅔
e
⅓
f
¼
New 0
d. Moving direction vector
left ¼, vector components
are: x = 1, y = ½ , z = 1
Direction indices: [212]
e. Vector components are:
x = -¾ , y = -1, z = 1
Direction indices: [34 4]
f. Moving direction vector up
⅓, vector components are:
x = -1, y = 1, z = -⅓
Direction indices: [331]
New 0
New 0
¾
½
¾
g
-¾
¼
¾
g. Moving direction vector
up ½, vector components
are: x = 1, y = -1, z = -¼
Direction indices: [4 41]
Smith
h
h. Moving direction vector
up ¼, vector components
are: x = ¾ , y = -1, z = -¾
Direction indices: [343]
Foundations of Materials Science and Engineering Solution Manual
29
3.35
A direction vector passes through a unit cube from the ( ¾, 0, ¼ ) to the ( ½, 1, 0 )
positions. What are its direction indices?
The starting point coordinates, subtracted from the end point, give the vector
components:
x=
1 3
1
− =−
2 4
4
y = 1− 0 = 1
z = 0−
1
1
=−
4
4
The fractions can then be cleared through multiplication by 4, giving
x = −1, y = 4, z = −1. The direction indices are therefore [1 4 1].
3.36
A direction vector passes through a unit cube from the ( 1, 0, ¾ ) to the ( ¼ , 1, ¼ )
positions. What are its direction indices?
Subtracting coordinates, the vector components are:
x=
1
3
−1 = −
4
4
y = 1− 0 = 1
z=
1 3
1
− =−
4 4
2
Clearing fractions through multiplication by 4, gives x = −3, y = 4 , z = −2.
The direction indices are therefore [3 4 2].
3.37
What are the crystallographic directions of a family or form? What generalized notation
is used to indicate them?
A family or form has equivalent crystallographic directions; the atom spacing along each
direction is identical. These directions are indicated by uvw .
3.38
What are the directions of the 10 0 family or form for a unit cube?
[10 0] , [010] , [0 01] , [1 0 0] , [01 0] , [0 01]
3.39
What are the directions of the 11 1 family or form for a unit cube?
[111] , [1 1 1] , [111] , [111] ,
[111] , [11 1] , [111] , [1 11]
3.40
[1 0 1]
[0 1 1]
What 11 0 -type directions lie on the (111)
[0 1 1]
plane of a cubic unit cell?
[0 11] , [011] , [110] , [11 0] , [1 01] , [10 1]
Smith
[1 1 0]
[1 0 1]
[1 1 0]
Foundations of Materials Science and Engineering Solution Manual
30
3.41
What 11 1 -type directions lie on the (110)
[111]
plane of a cubic unit cell?
[111]
[111] , [111] , [11 1] , [111]
[111]
[111]
3.42
How are the Miller indices for a crystallographic plane in a cubic unit cell determined?
What generalized notation is used to indicate them?
The Miller indices are determined by first identifying the fractional intercepts which the
plane makes with the crystallographic x, y, and z axes of the cubic unit cell. Then all
fractions must be cleared such that the smallest set of whole numbers is attained. The
general notation used to indicate these indices is (hkl), where h, k, and l correspond to the
x, y and z axes, respectively.
3.43
Draw in unit cubes the crystal planes that have the following Miller indices:
z
(a) (1 1 1)
(d) (213)
(g) (2 0 1)
(j) (133)
(b) (10 2)
(e) (3 21)
(h) (212)
(k) (31 2)
(c) (1 2 1)
(f) (30 2)
(i) (2 32)
(l) (331)
-½ (0, 0, 0)
•
•
+1
x
(0, 0, 0)
(0, 0, 0)
-1
y
•
+1
+1
-½
(102)
(1 1 1)
(1 2 1)
-1
-1
a. For (1 1 1) reciprocals
are: x = 1, y = -1, z = -1
(0, 0, 0)
•
+½
-⅓
b. For (10 2) reciprocals
are: x = 1, y = ∞, z = -½
c. For (1 2 1) reciprocals
are: x = 1, y = -½ , z = -1
(0, 0, 0)
+1
+1
+⅓
•
(213)
-½
(30 2)
-½
(321)
d. For (213) reciprocals
are: x = ½ , y = 1, z = -⅓
Smith
•(0, 0, 0)
+⅓
e. For (321) reciprocals
are: x = ⅓ , y = -½ , z = 1
f. For (302) reciprocals
are: x = ⅓ , y = ∞, z = -½
Foundations of Materials Science and Engineering Solution Manual
31
(0, 0, 0)
•
+½
-½
•
(0, 0, 0)
+1
(232)
-½
-1
+½
(201)
-½
(212)
(0, 0, 0)
h. For ( 212) reciprocals
are: x =-½ , y = 1, z = -½
g. For (201) reciprocals
are: x = ½ , y = ∞, z = -1
•
+⅓
i. For ( 232) reciprocals are:
x =-½ , y = ⅓, z = ½
(0, 0, 0) +⅓
•
-⅓
+1
+½
+⅓
•
•(0, 0, 0)
-1
+⅓
(31 2)
(133)
-⅓
(0, 0, 0)
(331)
-1
3.44
k. For (331) reciprocals
are: x = -⅓ , y = ⅓, z = -1
k. For (31 2) reciprocals
are: x = ⅓ , y = -1, z = ½
j. For (133) reciprocals
are: x = 1 , y = ⅓, z = -⅓
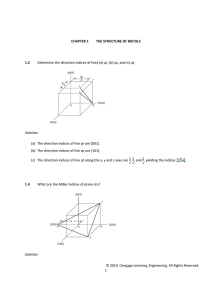
What are the Miller indices of the cubic crystallographic planes shown in Fig. P3.44?
z
(a)
d
¾
⅔
b
¾
⅓
a
¼
z
(b)
½
a
⅓
½
c
⅓
⅔
⅓
y
y
d
c
x
Smith
b
x
⅔
Foundations of Materials Science and Engineering Solution Manual
32
Miller Indices for Figure P3.44(a)
Plane a based on (0, 1, 1) as origin
Planar
Intercepts
x=∞
y = -1
z=−
1
4
Reciprocals
of Intercepts
1
=0
x
1
= −1
y
1
= −4
z
Plane b based on (1, 1, 0) as origin
Planar
Intercepts
x = -1
y=
−5
12
z=∞
Reciprocals
of Intercepts
1
= −1
x
1 −12
=
y
5
1
=0
z
The Miller indices of plane a are ( 0 1 4).
The Miller indices of plane b are ( 5 12 0).
Plane c based on (1, 1, 0) as origin
Plane d based on (0, 0, 0) as origin
Planar
Intercepts
x=∞
y = -1
z=
1
3
Reciprocals
of Intercepts
1
=0
x
1
= −1
y
1
=3
z
The Miller indices of plane c are ( 0 1 3).
Planar
Intercepts
x=1
y=1
z=
2
3
Reciprocals
of Intercepts
1
=1
x
1
=1
y
1 3
=
z 2
The Miller indices of plane d are (2 2 3).
Miller Indices for Figure P3.44(b)
Plane a based on (1, 0, 1) as origin
Planar
Intercepts
x = -1
y=∞
z=−
1
3
Reciprocals
of Intercepts
1
= −1
x
1
=0
y
1
= −3
z
The Miller indices of plane a are ( 1 0 3).
Smith
Plane b based on (0, 1, 1) as origin
Planar
Intercepts
x=1
y = −1
z=−
2
3
Reciprocals
of Intercepts
1
=1
x
1
= −1
y
1
3
=−
2
z
The Miller indices of plane b are (2 2 3).
Foundations of Materials Science and Engineering Solution Manual
33
Plane c based on (0, 1, 0) as origin
Planar
Intercepts
Reciprocals
of Intercepts
1
=1
x
1 −12
=
y
5
1
=0
z
x=1
y=
−5
12
z=∞
The Miller indices of plane c are ( 5 12 0).
3.45
Plane d based on (0, 1, 0) as origin
Planar
Intercepts
Reciprocals
of Intercepts
1
=1
x
1
= −1
y
1
=2
z
x=1
y = -1
z=
1
2
The Miller indices of plane d are (1 1 2).
What is the notation used to indicate a family or form of cubic crystallographic planes?
A family or form of a cubic crystallographic plane is indicated using the notation {hkl}.
3.46
What are the {100} family of planes of the cubic system?
(10 0) , (010) , (0 01) , ( 1 0 0) , (01 0) , (0 01 )
3.47
Draw the following crystallographic planes in a BCC unit cell and list the position of the
atoms whose centers are intersected by each of the planes:
(a) (100)
(b) (110)
(c) (111)
x
•
•
•
•
a. (1, 0, 0), (1, 0, 1),
(1, 1, 0), (1, 1, 1)
3.48
•
z
z
•
y
x
•
•
•
z
•
y
x
b. (1, 0, 0), (1, 0, 1),
(0, 1, 0), (0, 1, 1), (½, ½, ½)
•
•
y
c. (1, 0, 0), (0, 0, 1),
(0, 1, 0)
Draw the following crystallographic planes in an FCC unit cell and list the position
coordinates of the atoms whose centers are intersected by each of the planes:
(a) (100)
(b) (110)
(c) (111)
Smith
Foundations of Materials Science and Engineering Solution Manual
34
z
z
•
•
x
•
•
•
a. (1, 0, 0), (1, 0, 1),
(1, 1, 0), (1, 1, 1)
(1, ½, ½)
3.49
•
•
y
x
•
•
•
•
b. (1, 0, 0), (1, 0, 1),
(0, 1, 0), (0, 1, 1),
(½, ½, 0), ( ½, ½, 1)
z
•
•
y
x
•
•
•
•
y
c. (1, 0, 0), (0, 0, 1),
(0, 1, 0), (½, 0, ½)
(½, ½, 0), (0, ½, ½)
A cubic plane has the following axial intercepts: a = ⅓, b = -⅔, c = ½ . What are the
Miller indices of this plane?
Given the axial intercepts of (⅓, -⅔, ½), the reciprocal intercepts are:
1
1
3 1
= 3,
=− ,
= 2. Multiplying by 2 to clear the fraction, the Miller indices are
x
y
2 z
( 6 3 4).
3.50
A cubic plane has the following axial intercepts: a = -½, b = -½, c = ⅔. What are the
Miller indices of this plane?
Given the axial intercepts of (-½, -½, ⅔), the reciprocal intercepts are:
1
1
1 3
= −2,
= −2,
= . Multiplying by 2, the Miller indices are ( 4 4 3).
x
y
z 2
3.51
A cubic plane has the following axial intercepts: a = 1, b = ⅔, c = -½. What are the Miller
indices of this plane?
Given the axial intercepts of (1, ⅔, -½), the reciprocal intercepts are:
1
1 3 1
= 1,
= ,
= −2. Multiplying by 2, the Miller indices are ( 2 3 4).
x
y 2 z
3.52
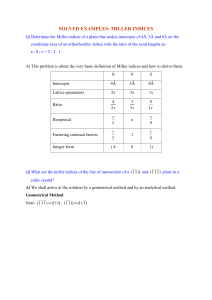
Determine the Miller indices of the cubic crystal plane that intersects the following
position coordinates: (1, 0, 0 ); ( 1, ½, ¼ ); ( ½, ½, 0 ).
First locate the three position coordinates as shown. Next, connect points a and b,
extending the line to point d and connect a to c and extend to e. Complete the plane by
Smith
Foundations of Materials Science and Engineering Solution Manual
35
connecting point d to e. Using (1, 1, 0) as the plane origin, x = -1, y = -1 and z = ½. The
1
1
1
intercept reciprocals are thus = −1, = −1, = 2. The Miller indices are ( 1 1 2).
x
y
z
(0, 0, 0)
••
(1, 0, 0)
3.53
• •e
d
• •c
b
(½, ½, 0)
a
(1, ½, ¼)
•
•
(1, 1, 0)
Determine the Miller indices of the cubic crystal plane that intersects the following
position coordinates: ( ½, 0, ½ ); ( 0, 0, 1 ); ( 1, 1, 1 ).
First locate the three position coordinates as shown. Next, connect points a and b and
extend the line to point d. Complete the plane by connecting point d to c and point c to b.
Using (1, 0, 1) as the plane origin, x = -1, y = 1 and z = –1. The intercept reciprocals are
1
1
1
thus = −1, = 1, = −1. The Miller indices are ( 1 1 1).
x
y
z
b
(0, 0, 1)
(1, 1, 1)
•
(0, 0, 0)
(1, 0, 1)
•
a
c
•
(½, 0, ½ )
d
3.54
•
Determine the Miller indices of the cubic crystal plane that intersects the following
position coordinates: (1, ½, 1 ); (½, 0, ¾ ); ( 1, 0, ½ ).
After locating the three position coordinates, connect points b and c and extend the line to
point d. Complete the plane by connecting point d to a and a to c. Using
(1, 0, 1) as the plane origin, x = -1, y = ½ and z = -½. The intercept reciprocals then
1
1
1
become = −1, = 2, = −2. The Miller indices are ( 1 2 2).
x
y
z
Smith
Foundations of Materials Science and Engineering Solution Manual
36
d
(½, 0, ¾)
• •
(1, 0, ½,)
3.55
b
(1, 0, 1)
(0, 0, 0)
•
c
• •a
•
(1, ½, 1)
Determine the Miller indices of the cubic crystal plane that intersects the following
position coordinates: ( 0, 0, ½ ); ( 1, 0, 0 ); ( ½, ¼, 0 ).
After locating the three position coordinates, connect points b and c and extend the line to
point d. Complete the plane by connecting point d to a and a to b. Using
(0, 0, 0) as the plane origin, x = 1, y = ½ and z = ½. The intercept reciprocals are thus
1
1
1
= 1, = 2, = 2. The Miller indices are therefore (1 2 2).
x
y
z
•
•
(0, 0, 0)
(0, 0, ½,)
d
•c
•
(1, 0, 0)
3.56
a
•
(0, ½, 0)
b
(½, ¼, 0)
Rodium is FCC and has a lattice constant a of 0.38044 nm. Calculate the following
interplanar spacings:
(a) d111
(b) d200
(c) d220
(a) d111 =
(b) d 200 =
(c) d 220 =
Smith
0.38044 nm
12 + 12 + 12
=
0.38044 nm
2
2
2 +0 +0
2
0.38044 nm
22 + 22 + 02
0.38044 nm
= 0.220 nm
3
=
0.38044 nm
= 0.190 nm
4
=
0.38044 nm
= 0.135 nm
8
Foundations of Materials Science and Engineering Solution Manual
37
3.57
Tungsten is BCC and has a lattice constant a of 0.31648 nm. Calculate the following
interplanar spacings:
(b) d220
(c) d310
(a) d110
0.31648 nm
(a) d110 =
12 + 12 + 02
(b) d 220 =
(c) d310 =
3.58
=
0.31648 nm
22 + 22 + 02
0.31648 nm
2
2
3 +1 + 0
2
0.31648 nm
= 0.224 nm
2
=
=
0.31648 nm
= 0.112 nm
8
0.31648 nm
= 0.100 nm
10
The d310 interplanar spacing in a BCC element is 0.1587 nm. (a) What is its lattice
constant a? (b) What is the atomic radius of the element? (c) What could this element
be?
(a) a = d310 h2 + k 2 + l 2 = (0.1587 nm) 32 + 12 + 02 = 0.502 nm
(b) R =
3a
3(0.502 nm)
=
= 0.217 nm
4
4
(c) The element is barium (Ba).
3.59
The d422 interplanar spacing in a FCC metal is 0.083397 nm. (a) What is its lattice
constant a? (b) What is the atomic radius of the metal? (c) What could this metal be?
(a) a = d 422 h2 + k 2 + l 2 = (0.083397 nm) 42 + 22 + 22 = 0.408 nm
(b) R =
2a
2(0.408 nm)
=
= 0.144 nm
4
4
(c) The element is gold (Au).
Smith
Foundations of Materials Science and Engineering Solution Manual
38