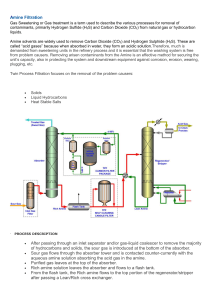
TOPIC III ACID GAS REMOVAL Course Contents 1) 2) Introduction on Acid Gases Various Types of Processes : a. Chemical Absorption I. Amine Solutions • • • • II. Potassium Carbonate Solutions • • b. Conventional Potassium Carbonate Process Improved Hot Carbonate Process Physical Absorption I. c. d. Applications of Amine Solutions Design & Operation of Amine Units Recommendations of Unit Design Other Types of Amines The IFPEXOL Process Adsorption Permeation ACID GASES Definition Acid Gas • is a gas containing acidic components such as CO2, H2S, COS (carbonyl sulphide), CS2 (carbon sulphide) RSH that can form acidic solutions when mixed with water. Composition • • mainly contains of CO2 and H2S gases. Both gases can cause corrosion. These gases are obtained after a sweetening process applied to a sour gas. accompanied by small quantities of CS2, COS and RSH Target i. Sales Gas: ii. Crygonic treatment: i. Gas transmission: H2S content 4 ppm H2S content 4 ppm CO2 content 100 ppm; to prevent CO2 fr. Freezing out at low T H2S content can be left as it is CO2 content can be left as it is Have to be extracted first if • to be used commercially, removed highly toxic H2S • to undergo cryogenic treatment, reduced CO2 and H2S • to be piped to a distant treatment plant, left CO2 and H2S during transportation. CHEMICAL ABSORPTION Definition: Chemical absorption • is a type of separation process. • absorbent solution (solvent) reacts chemically with acid gases presence in natural gas to produce compounds. • the compounds can be dissociated by heating or operating at low pressure of stripping. • Used solvent is regenerated and re-use again • example : gas sweetening process. Types of Adsorbents (solvent): • amine solutions – absorption at ambient temperature • potassium carbonate solutions – absorption at high temperature (100-110oC) ACID GASES REMOVAL Types of Process • Chemical Absorption of Acid Gases – using hot K2CO3 solution – using amine solution • Physical Absorption • Adsorption Characteristic of Process Types Highly depends on: • Concentration of acid gases • Specifications to be met for acid gases Technologies • • The carbon dioxide concentration must be removed to less than 2% Carbon dioxide must also be removed prior to low temperature processing for NGL recovery. • • Current facilities used is amine processing Carbon dioxide is removed through the use of amine based solvents. The challenge faced is from keeping the solvent clean and operating within the process constraints of the system. • • • • Membrane systems also have been used for carbon dioxide removal One challenge for membrane systems is reaching the low allowable carbon dioxide levels required by the pipeline system Using adsorbent (molecular sieve system) for carbon dioxide removal can, for certain applications, allow for CO2 removal without the operational challenges of amine based systems or the process limitations faced by the membrane systems. Generalized N2/CO2 Isotherms for Molecular Sieve Adsorbents Comparison: N2 / CO2 Removal with Molecular Gate adsorbents • In the removal of carbon dioxide from natural gas: – CO2 (3.3 Å) is both a smaller molecule than CH4 (3.8 Å) – One that is adsorbed more strongly • The combination of pore size optimization and adsorbent attraction results: – Ability to remove CO2 with minimal adsorbent inventories – High CH4 recoveries. • CO2 is strongly adsorbed, the adsorbent properties can be tailored so that it can also remove H2O vapor. • Elimination is for dehydration, as is the case with N2 rejection, providing an operational and cost benefit. Amine Solutions For industrial purposes, can use 3 types of amines: • Monoethanolamine (MEA) • Diethanolamine (DEA) • Methyldiethanolamine (MDEA) Properties MEA = 15-18 wt% (H2S, CO2) ; <10% of acid gases DEA = 15-20 wt% / 25-40 wt% (H2S, CO2) Gas MDEA = 35-50 wt% (H2S) DGA = 60-65 wt% (H2S, CO2) DIPA = 30-50 wt% (H2S, COS) SEPARATION DRUM Final separate NG and amine solution Acid gas CONDENSER Water Lean amine Tray = 4-6 P= change V= change T= water 35-50oC Tray = 20 P = Low Tray = 20-30 T = 120-140 C(160- 247-269oC) SEPARATOR P = high water P = change V = change N2 Remove loose H2O, liquid HC, solid particles Amine Solutions Process Description Acid Gases Removal in NG (Step 1-3) Step 1: i. Removal of loose water, liquid hydrocarbon, solid particles ii. In a separator (change in Pressure and Volume of NG) Step 2: i. Takes place in an absorber ii. Absorption of acid gases takes place by contact in absorber trays in the column (20-30 trays depending on the severity of the treatment) between ascending NG and descending lean amine at T=35-50oC • T= lean amine inlet > T=NG to prevent condensation of hydrocarbon • Trays have to be kept far apart Step 3: • Takes place in a separation drum • Control entrainment of amine solution (impingement extractor) • Final separation between NG and amine solution (residual) Amine Solutions Process Description Regeneration of Rich Amine- NG+Acid Gases (Step 4-7) Step 4: i. 1st stage separation of rich amine solution and light hydrocarbon ii. Rich amine solution is flashed in a flash drum (change in Pressure and Volume) iii. Re-absorption using 4-6 trays Step 5: i. Takes place in an Regeneration Column ii. Preheated (heat exchanger) and fed to the top of regeneration column (20 trays) at low pressure iii. Absorption of acid gases takes place by contact in absorber trays in the column iv. Reboiling temperature must be carefully monitored to prevent thermal degradation of amine solution. Step 6: i. Takes place in a condenser ii. Acid gases and water vapour leave at the top of the regenerator; and enter a condenser where most of the water is condensed iii. The water is re-routed to the top of the regenerator to provide a reflux for the regeneration column Step 7: i. The hot lean (or regenerated) aminesolution leaves the bottom of the regenerator heats the rich amine ii. Lean amine is then cooled by air/water before being fed into amine surge tank iii. Amine tank has to be inerted (maintain under N2 pressure) to avoid any risk of amine oxidising with air Amine Solutions (4) With respect to H2S: - instantaneous reaction. - amine behaves like MEA. - examples of reaction 1) 2RNH2 +H2S 2) RNH2 + H2S (RNH3)2S RNH3HS With respect to CO2: - primary amines behave like MEA. - secondary amines behave like DEA. - rapid reaction. - both later form ethanolamine carbamate. - using hydrolysis of CO2 will form bicarbonate (slow reaction). - reactions occur: 1) 2R2NH + CO2 R2NCOOR2NH2 (carbamate) 2) R2NH + CO2 + H2O R2R’NH HCO3 (bicarbonate) Amine Solutions (5) Tertiary amine: MDEA - adsorbs H2S rapidly but CO2 much more slowly - selective function preferably adsorption of H2S - only forms bicarbonate but not carbamate - reaction occur: R2R’N + CO2 + H2O R2R’NH HCO3 (bicarbonate) Reactions: - allow determination of minimum flow rate of amine solution. - according to amine solution’s concentration. - the circulation flow rates used is higher than stoichiometric flow rate. Applications of Amine Solutions 1. MEA advantages: • • • • • • • • used in solution of 15-18 wt%, to avoid corrosion reacts strongly with CO2 and H2S easily regenerated easily degraded: - by oxidation - by reaction of COS and CS2 - by overheating the solution remove degradation product that can cause corrosion and foaming. high vapor pressure causes non negligible losses. recommended for treatment of natural gas with low acid gas content (<10%) enables compliance with strict acid gas specifications disadvantages: • • operation energy consuming. required very careful operation of unit. Applications of Amine Solutions (2) 2. DEA advantages: • • • • • • used in 15-20 wt% solution but upgraded from 25-40 wt% solution reduced in solution circulation flow rate and energy consumption greater resistance to degradation than MEA low in vapor pressure make DEA losses are reduced effective in simultaneously remove CO2,H2S preferable to replace MEA for treatment of natural gas with low acid gas content disadvantages: • • operation energy consuming. required very careful operation of unit. Applications of Amine Solutions (3) 3. MDEA advantages: • • • • • • • used in 35 –50 wt% solution for extraction of H2S and leaving some CO2 in treated gas for production of CO2 with little H2S to enhance oil recovery fluid. H2S enrichment of acid gas before treatment in Claus unit not behave in the same way as MEA and DEA rate of reaction for MDEA-H2S extremely fast rate of reaction for MDEA-CO2 involves formation of carbonic acid and slow disadvantages: • • difference rates of reaction - possible to simultaneously adsorbs H2S contact time is too short for adsorption of all CO2 Design And Operation of Amine Treating Units (1) Purposes: • to minimize investment and operating costs • to achieve optimum performance (meet specifications or stream factor) Control Methods: • optimization of process scheme – split flow scheme – two stage scheme – recovery of energy from rich amine expansion • reduction of foaming and corrosion problems – amine degradation – foaming – corrosion **(figure 2) **(figure 3) Design And Operation Of Amine Treating Units (2) • recommendation for unit design and operation – natural gas feed inlet separator – absorber – flash drum – amine regeneration – re-claimer – rich amine- lean amine heat exchanger – filtration – injection of an foaming agent – corrosion of equipment Process Schemes Split Flow (Fig 2) • flow of regenerated amine was split into 2 streams • first stream is fed in to the absorber a little less than half way up • second stream is fed into the top of the absorber • complementary cooling the amine means that a stricter specification can be achieved for gas treated • diameter of upper part of absorber will be reduced. Two Stage (Fig 3) • two different amine solutions fed into absorber at different levels. • semi regenerated solution withdrawn from the middle of regenerator. • it is cooled and fed into absorber halfway up • regenerated solution from bottom of regenerator is cooled • it is fed into top of absorber to meet acid gas specifications • more complex Recovery of Energy • less capital investment • reduces energy consumption in regenerator reboiler • from rich amine expansion • for absorber operated at high pressure and high amine circulation flow rate • rich amine leave absorber in an expansion turbine before fed to flash drum • energy thus recovered can supply part of energy required for pumping lean amine that feeds the absorber Foaming and Corrosion Problems (1) Amine Degradation (amine loss) • thermal degradation of amine excess rise in temperature in regenerator reboiler. • imperative not to exceed the maximum operating temperature for amine. • slow oxidation of amine by oxygen in air gives formation of corrosive products. • amine storage tanks must be inerted with N2. • MEA reacts with COS and CS2 to form non-regenerated compounds. Foaming (low amine performance) • reduced efficiency, decreased flow of treated gas and amine loss • caused by presence of: • liquid HC • solid particles present in the feed and produced by corrosion • amine degradation products / present in solution • corrosion inhibitor for instance Corrosion (erosion problem) • in the presence of acid gases in the unit • amine degradation products also lead to cause corrosion • for equipment contain highest concentration of acid gases at high T • if care not taken, presence of solid particles will lead to erosion • examples of unit:1) rich amine and lean amine heat exchanger 2) regenerator 3) reboiler 4) associated lines and valves Recommendations of Unit Design (1) Natural Gas Feed Inlet Separator • • • • • • very important function separate liquid HC and solid particles that could promote foaming water, contain salt and chemical products which can cause foaming,corrosion salt will deposit on reboiler tube the unit should be carefully designed and sized factor not to be overlooked is liquid flow often irregular (slug flow) Flash Drum • • • • to recover light HC dissolved in amine solution. light HC contain acid gases reduced during regeneration of solution. degassed HC can be used in fuel gas network. if HC contain of acid gases, later re-absorbed by washing with lean amine Recommendations of Unit Design (2) Absorber • • • a place where acid gases were removed by chemical reactions minimized the amine circulation flow rate gives reason of economy avoid corrosion by following two constraints: • Maximum amine concentration in the solution • Maximum acid gas concentration in the amine solution • • • • • • • amine solution enters top of absorber at high temperature gas fed into bottom of the absorber, to avoid risk of heavy HC condensed absorber trays should be spaced sufficiently wide apart. foaming will increase pressure drop hence delta P in column should regularly being monitored droplet separator is placed at top of column to limit entrainment of amine can be reduced by treated gas and by washing the gas with water make up distilled water is injected into washing loop Recommendations of Unit Design (3) Amine Regeneration • • has high concentration of acid gases at high temperature reboiler tubes must be spaced far apart in square pitch just to: • facilitate cleaning of tube bundle and flow of liquid • evacuate the vapor phase at reasonable rate • • • • advisable to raise tube bundle higher in the shell to facilitate flow of liquid saturated low pressure steam is reboiled far to avoid thermal degradation of amine and limit corrosion of reboiler the tube bundle constantly submerged with liquid to prevent tubes drying out and formation of hot spots make sure that the levels of liquid is 15 –20 cm above upper tubes of bundle Recommendations of Unit Design (4) Reclaimer • • • • • • • • • • used when justified by production of non-regenerable (chemical reaction) a systematically in MEA units operation is carried out at T= 125oC with reaction of COS and CS2 to form non-regenerable compounds impose purification of MEA solution to avoid accumulation of contaminants a type of kettle placed parallel to regenerator reboiler perform batch distillation of small proportion of lean amine (1-3%) degradation products, non-volatile salts and solid particles accumulates in bottom of shell focus on sized up the unit with exception of distance between lower part of bundle and bottom of shell is greater (25-40 cm). this to avoid disrupting flow of liquid around tubes requires the use of stainless steel for the tubes of bundle Recommendations of Unit Design (5) Filtration • • • • • important in amine unit perform in two stages 1) Cartridge type filter -to remove solid particles 2) activated carbon bed -to remove HC and contaminants in amine solution is located at point where lean amine leaves amine surge drum solution flow through filtration section about 10-20% of amount solution flow comes directly from discharge of main pump sending lean amine to absorber Injection of Anti-foaming Agent • • • inject small amount of anti forming agent to reduce foaming need to test first: 1) to ensure its efficiency 2) to make sure there is no undesirable secondary effect particularly to observe manufacturer’s instruction with regard to dose Recommendations of Unit Design (6) Rich amine/Lean amine Heat Exchanger • • • • • • rich amine solution circulate on the tube side has two stacked shells rich amine is fed in from bottom minimize the degassing effect by control valve of rich amine that located on the outlet of exchanger using stainless steel for the exchanger current trend used removable stainless steel plates with high transfer coefficient, the area exchange is smaller and used low delta T to recover more heat from lean amine Recommendations of Unit Design (7) Corrosion of Equipment • to minimize the risk, must follow the imperative: • not to exceed the recommended concentration of the amine solution • to limit the acid gas content of the amine solution to the prescribed value by ensure the required amine circulation flow rate • • • • • • • • basic material used is carbon steel but some used of stainless steel corrosion much more severe in processing rich CO2 unit used of copper alloy is strictly prohibited in amine units expansion valves on rich amine line and piping immediately sensitive points with respect to erosion corrosion used of ‘stellite’ expansion valve and large size piping can minimize corrosion caused by flow velocity and degassing of acid gases flow velocity should be limited to around 1m/s and bends with wide radius anneal all the welded areas in order to relieve stresses caused by welding develop several corrosion inhibitors where the effectiveness depends on number of factors Other Types of Amines Diglycolamine (DGA) • • • • primary amine, hydroxyethanolamine. performance in quality close to MEA. solution contain is up to 60-65 wt% DGA. DGA solution circulation flow rate lower and amount of energy required is reduced. Diissopropanolamine (DIPA) • • • • • • used in aqueous solution in concentration of 30-50% DIPA selectivity absorbs H2S rather than CO2 performance is similar to DEA more expensive than DEA but require less energy for regeneration more efficient where COS is concerned used in Sulfinol process mixed with physical solvent (sulfolane) Chemical Absorption -Using Potassium Carbonate Potassium Carbonate Solutions • specific feature is absorption takes place at high temperature (110-120oC) • frequent use of term is ‘hot carbonate’ • same operating principle as amine process • absorber and regenerator at similar temperatures • has different flow scheme where heat recovery is concerned • performance of conventional process with respect to sales gas specifications greatly improves by used of additives • known commercial processes are: – Catacarb – Benfield – Giammarco-Vetrocoke Conventional Potassium Carbonate Process • • • • • • Absorber and regenerator operate at same temperature No rich solution-lean solution heat exchanger as in amine processes Treated gas leaving the absorber at high temperature Preheated feed gas entering the bottom of absorber Operate at T=110-120oC make it possible to increase solubility of potassium carbonate in water and amount used is 30-40 wt% K2CO3 Carried out in two stages of reactions 1) K2CO3 + H2O KOH + KHCO3 ( hydrolysis of K2CO3) 2) KOH +CO2 KHCO3 (bicarbonate) 3) KOH +H2S KHS +H2O • • • • • • • • • • carbonate solution hydrolyses COS and CS2 into CO2 ,and H2S are absorbed by solution RSH are partially removed from feed gas process is well suited to feed gases containing high proportion of CO2 high H2S with little CO2, make solution difficult to regenerate CO2 contributes to extraction of H2S from solution during regeneration involves lower capital investment and operating costs Does not meet severe specifications for the sales gas level of performance can be improved by using split / 2-stage scheme advisable to use stainless steel for the boiler tubes and expansion valves annealing is good to relieve stresses cause by welding. K2CO3 = remove RSH (partially) = hydrolysis of COS and CS2 T = high T = 110-120 oC T = 110-120 oC K2CO3 = 30-40 wt% ‘Improved’ Hot Carbonate Process Processes include: Benfield Process -hot carbonate solution is activated by DEA and other additives Catacarb Process -incorporates amine borate and other additives into carbonate solution Giammarco-Vetrocoke Process -arsenic salts are added to the potassium carbonate solution Why? To give solution a certain degree of selectivity with respect to H2S and CO2: • by improving sales gas specifications • by reducing energy required to regenerate solution • by choosing and dosage of additives Performance achieved by conventional process: • improved reactivity of carbonate solutions to meet specifications for sales gas • facilitated the absorption and desorption of acid gas • reduced risk of corrosion PHYSICAL ABSORPTION PHYSICAL ABSORPTION • Definition: – Is a physical absorption whereby acid gases present in natural gas is removed by absorbing the acid gases physically under prescribed operating conditions (T & P) • Characteristics – Acid gases dissolve in the solution under pressure and temperature – Favoured by high acid gas partial pressure concentrations (contributed by both pressure of CO2 and/or H2S) and low temperature – Use solvent is an operating parameter that will dictate the degree of efficiency of absorption – Solvent is regenerated by low pressure expansion of solution rich in dissolved acid gases – Involves no chemical reaction between acid gases and the solvent Description of process Principle Based on the solubility variation of the acid gases in the solvent as a function of the partial pressure The process flow diagram does not change whatever the solvent used, with the exception of the final solvent regeneration Process Description Acid Gases Removal in NG (Step 1-2) Step 1: i. Separator Unit ii. Removal of loose water, liquid hydrocarbon, solid particles iii. Principle of operation: change in Pressure and Volume of NG Step 2: i. Absorber Unit ii. Lean solvent is fed into the top absorber; contact with feed gas flowing counter-currently in the column iii. Absorption of acid gases takes place by contact in absorber trays in the column (20-30 trays depending on the severity of the treatment) between ascending NG and descending lean solvent • Absorption pressure = high; temperature= low • Example of Solvent = methanol, dimethyl ether, propylene carbonate, n-methyl pyrrolidine Process Description (cont’d) Process Description Solvent Regeneration (Step 3 - ???) Step 3: i. Separator Unit ii. Removal of loose water, liquid hydrocarbon, solid particles iii. Principle of operation: change in Pressure and Volume of NG Step 4: i. Absorber Unit ii. Lean solvent is fed into the top absorber; contact with feed gas flowing counter-currently in the column iii. Absorption of acid gases takes place by contact in absorber trays in the column (20-30 trays depending on the severity of the treatment) between ascending NG and descending lean solvent • Absorption pressure = high; temperature= low • Example of Solvent = methanol, dimethyl ether, propylene carbonate, n-methyl pyrrolidine PHYSICAL ABSORPTION PROCESSES (5) IFPEXOL Process(cold Methanol; IFP ) Selexol Process (DMPEG, dimethylether of polyethylene glycol; ) Rectisol Process (cold methanol) Purisol Process (NMP, n-methyl pyrrolidone) Sulfinol Process (DIPA-sulfolane di-isopropanolamine; SHELL) PHYSICAL ABSORPTION • • Definition: – Is a physical absorption whereby acid gases present in natural gas is removed by absorbing the acid gases physically under prescribed operating conditions (T & P) Characteristics: – Acid gases dissolve in the solution under pressure and temperature – Favored by high acid gas partial pressure concentrations (contributed by both pressure of CO2 and/or H2S) and low temperature – Use solvent is an operating parameter that will dictate the degree of efficiency of absorption – Solvent is regenerated by low pressure expansion of solution rich in dissolved acid gases Description of process Principle • • Based on the solubility variation of the acid gases in the solvent as a function of the partial pressure The process flow diagram does not change whatever the solvent used, with the exception of the final solvent regeneration Process Description Acid Gases Removal in NG (Step 1-2) Step 1: i. Separator Unit ii. Removal of loose water, liquid hydrocarbon, solid particles iii. Principle of operation: change in Pressure and Volume of NG Step 2: i. Absorber Unit ii. Lean solvent is fed into the top absorber; contact with feed gas flowing counter-currently in the column iii. Absorption of acid gases takes place by contact in absorber trays in the column (20-30 trays depending on the severity of the treatment) between ascending NG and descending lean solvent • Absorption pressure = high; temperature= low • Example of Solvent = methanol, dimethyl ether, propylene carbonate, n-methyl pyrrolidine Process Description (cont’d) Process Description Solvent Regeneration (Step 3-5) i. ii. iii. Successive stages (3 and 4) Rich solvent expands until reach pressure level close to atmospheric pressure Energy is recovered in hydraulic turbines to recycle lean solution available at low Pressure Step 3: i. First expansion phase ii. Releases dissolved constituents that not soluble in solvent iii. Applies to light HC and CH4 iv. Gas routed to fuel gas network v. Large amount gas released due to high solvent flow rate, then will be compressed and recycled to absorber Step 4: i. Second expansion phase ii. Released CO2 less strongly absorbed by physical solvent than H2S and RSH Step 5: i. Third phase (low pressure expansion) ii. Solution virtually be bring to atmospheric pressure Lean solvent P = high T = low Tray = 20-30 Solvent = CH3OH, DME 1st phase: Release light HC and CH4 SOLVENT REGENERATION 2nd phase: Release CO2 by physical solvent EXPANSION 3rd phase: SEPARATOR P = change V = change Remove loose water, liquid HC and solid particles P= 1 atm Utilization • Solubility of acid gases in physical solvent is proportional to partial pressure of acid gas • Solubility curves remain the same although used solvent with different gases • CO2 is less adsorbed than H2S where solubility is 3-9 times higher than CO2 • CH4 has very low solubility in physical solvent due to high concentration in feed gas and non-negligible quantity may absorbed • Physical solvents easily absorb heavy hydrocarbon (aromatics) • Low Temperature, gives high solubility of acid gas like hydrocarbon and solvent loss can be reduced at same time • Solubility of CO2 and H2S in CH3OH is high at -30oC to -10oC • For absorption capacity limitation, the solubility increase regularly with partial pressure in physical absorption Utilization (2) • Physical absorption effectively for high pressure processing of natural gas containing large amount of acid gases and traces amount of heavy HC • Attractive process when acid gas partial pressure is high and large flow of gas to be processed • Adsorbent solution is regenerated by successive phases of expansion without supply of any heat • In the last phase, energy consumption of processes is much lower than chemical absorption processes • Higher solubility of H2S in physical solvent compared to CO2 allow selective removal of H2S with respect to CO2 • For economic analysis, more advantageous if process run at acid gas partial pressure exceeding 4-5 bar Principle Physical Solvents For industrial used, properties of solvents should have: • high solubility with respect to acid gases • low viscosity at operating temperature to facilitate solvent circulation • resistivity to corrosion of carbon steel • high thermal stability, i.e. not degraded under operating conditions (T, P, reaction) • low vapor pressure at operating conditions to minimize solvent loss • reasonable price Solvent Specifications Envisaged: • Heating of the solution before the last expansion unit • stripping with air or N2 or reboiling the re-heated solution • flashing below atmospheric pressure by means of a compressor that compresses gases released under vacuum conditions Example: THE IFPEXOL PROCESS • • licensed by Institute Francais du Petrole used only one solvent Purposes: • dehydration of natural gas • removal of heavy hydrocarbons (C2+ cut, NGL) • acid gas removal with single solvent methanol Process Characteristics: • perform several functions with same solvent • save in capital investment because require less equipment, compactness of installation and reduced in operating cost. • perform selective purification treatment since H2S more soluble in CH3OH than CO2 THE IFPEXOL PROCESS (2) Process Description: • raw NG mixed with CH3OH from top of stripping column to inlet of separator • it is cooled to remove H2O and heavy HC in cold separator • aqueous phase (H2O +CH3OH) is fed to top of stripping column • feed gas enters the bottom column recovers CH3OH from aqueous solution • water recovered from feed gas is recovered at bottom of the contactor • gas is chilled due to required specifications for its end use: • by expanding it through an expansion valve • by external refrigeration cycle • by turbine expander • by combination of refrigeration cycle • by turbine expander on natural gas stream. THE IFPEXOL PROCESS (2) • contain of gases are such as acid gas, S, CO2,H2S,COS and RSH • gas routed from cold separator to absorption contactor • cold CH3OH solution flows counter-currently to gas and absorbs acid gases and S • CH3OH rich in acid gases is regenerated in conventional manner by expansion phases and stripping solution Types of Industrial Solvent 1. Dimethylether of Polyethylene Glycol - (DMPEG) • • • • can be called as Selexol Process used in natural gas and synthesis gas processing enables simultaneous or selective absorption of acid gases final regeneration phase of solvent differs according to acid gases present in feed gas as function of acid gas specification of treated gas feed gas contains of CO2 and traces of H2S, final expansion phase suffice and part of solvent reheated before last expansion for regeneration satisfactory feed gas contains mainly H2S and little CO2, final stripping with air required to remove the H2S absorbed by solvent. gas contain of large amounts of H2S and CO2, 2 stage scheme used with air stripping part of solvent operating temperature of DMPEG is at 25oC and –15oC • • • • • Types of Industrial Solvent (2) 2. Cold Methanol • • • • • can be called as IFPEXOL & Rectisol Process CH3OH used in absorber at temperature below 0oC in range of –30oC minimizes loss CH3OH of through vaporization in treated gas promote solution of acid gases in solvent cold CH3OH used for processing natural gas rich in acid gases and synthesis gas uses of CH3OH are such as: • dehydration • acid gas removal • natural gas liquid recovery • Types of Industrial Solvent (3) 3. n- Methyl Pyrrolidone (NMP) • • • can be called as Purisol Process has property in common with polyethylene glycol dimethylether high selectivity of H2S with respect to CO2 4. Propylene Carbonate (PC) • • was developed by Flour Corporation final regeneration phase of solution consists in stripping by air. Types of Industrial Solvent (4) 5. Sulfinol Process • • was developed by Shell a solution contains sulfolane (physical solvent) and di-isopropanolamine (chemical solvent) proportions of sulfolane, DIPA and water are adjusted in case depend on treatment to be performed use of this solvent is to remove small quantities of degradation products from DIPA has lesser tendency to form and remove RSH and COS effectively in presence of physical solvent, means significant absorption of heavy HC (aromatics) acid gas specifications required for natural gas liquefaction plant feeds can easily be met. • • • • • ADSORPTION PROCESS Dehydration by Adsorption Principle: • A physical process whereby a suitable porous solid with specific property is able to fix water molecules on the surface of pores where water vapor condensed • Characteristic of Adsorbents – have very large internal contact, 250-850 m2/g – Possess a strong affinity for water vapour and a high capacity for adsorption – Be easily and economically regenerable – Undergo slight pressure drop under flow of gas – Possess good mechanical strength DEHYDRATION Dehydration by Adsorption 1. Adsorption Phase • gas flow through drier from top to bottom • adsorbent saturated with water • halted phase first before reach breakthrough point • polarity of water much stronger attraction on adsorbent • ejects hydrocarbon molecules that less stronger 2. Regeneration Phase • regenerate bed of adsorbent • performed operation by increase temperature or lower the pressure • can be performed by heating bed of adsorbent • 2 stage of regeneration of drier: heating phase & cooling phase DEHYDRATION Dehydration by Adsorption Adsorbent: • • • • • should posses strong affinity for water vapor high capacity for adsorption process easily and economically re-generable undergo little drop in pressure posses good mechanical strength Types of Adsorbent: • • • • activated alumina silica gel molecular sieves activated carbon DEHYDRATION Dehydration by Adsorption Adsorbent Characteristics: • capacity of adsorbents depends on their nature • low value of relative humidity gives high capacity of molecular sieve • (ex: adsorbent) • low dew point is required for cryogenic treatment of natural gas • ability to adsorb heavy HC can show the selectivity of adsorbent DEHYDRATION UNITS ADSORPTION PHASE: Gas Flow: From top to bottom Adsorbent : Saturated with H2O Polarity of H2O: Stronger HC Molecule: Less Stronger REGENERATION PHASE: T: 200-300oC P: Low Adsorbent : Activated Al, Si Gel, Mol Sieves Stages of Regeneration: Heating Phase Cooling Phase Dehydration by Adsorption Process Description: • using 2 columns called dryers packed with solid adsorbent • involve 2 phase: adsorption phase & regeneration phase 1. • • • • • Adsorption Phase gas flow through drier from top to bottom adsorbent saturated with water halted phase first before reach breakthrough point polarity of water much stronger attraction on adsorbent ejects hydrocarbon molecules that is less stronger 2. Regeneration Phase • regenerate bed of adsorbent • performed operation by increasing in T (200-300 oC) or lowering the P, or by a combination of both • can be performed by heating bed of adsorbent • 2 stage of regeneration of drier: heating phase & cooling phase – Heating Phase: Hot air desorb the water from the adsorbent – Cooling Phase: Drier is cooled at the end of the heating phase to the initial condition Types of Adsorbent • activated alumina – SA=280 m2/g; pore volume=0.4 m3/g; pore diameter=2-4 nm; density= 720820 kg/m3) – Reduce content by 1 ppmv – Regeneration T= 150-220 oC • silica gel – SA=550-800 m2/g; pore volume=0.35-0.5 m3/g; pore diameter=2.5 nm; density= 720-800 kg/m3) – Reduce content by 10 ppmv – Regeneration T= 150-250 oC • molecular sieves (or zeolites) – Composition oxides of (Si, Al) and Na or K or Ca – Zeolite 3A (K), Zeolite 4A(Ca); Zeolite 5A(Na); Zeolite X (10 A diameter) – SA=650-800 m2/g; pore volume=0.27 m3/g; pore diameter=3-5 nm; density= 690-720 kg/m3) – Reduce content by 1 ppmv – Regeneration T= 200-300 oC • activated carbon Design of Adsorption Dehydration Units Choice of Adsorbent Dryer Arrangement • • • • • should posses strong affinity for water vapor high capacity for adsorption process easily and economically re-generable undergo little drop in pressure posses good mechanical strength • capacity of adsorbents depends on their nature • low value of relative humidity gives high capacity of molecular sieve • (ex: adsorbent) • low dew point is required for cryogenic treatment of natural gas • ability to adsorb heavy HC can show the selectivity of adsorbent ADSORPTION Description of Process: • • • • • • process flow scheme is identical to dehydration, with exception of regeneration gas circuit gas is contain of acid gases and mercaptans cannot be recycled back in regeneration phase of dehydration, water desorbed from molecular sieves by hot regeneration gas can condensed and removed in acid gas removal, acid gas more difficult to extract from regeneration gas regeneration gas contain CO2, can be injected into fuel gas network regeneration gas contain sulfur, can be incinerated or routed to treatment unit ADSORPTION (2) Utilization: • • • Molecular sieves have strong affinity for polar compounds Polar compounds such as water,H2S, mercaptans and CO2 Feed gas contain of water adsorb Selection of Molecular Sieves: • • • • • depends on compounds to be removed from feed gas types of molecular sieve • 4A type sieves are used for CO2 • 5A type sieves are used for CO2, H2S, COS and light mercaptans • 13X types sieves are used for CO2, H2S, COS and light & heavy RSH the gas is simultaneously complete dehydrated molecular sieve can determine optimum solution complexity of co-adsorption phenomena is related to: • Composition of the feed gas • Specification of the sales gas PERMEATION PROCESS PERMEATION Definition: Permeation is • a technique that used in separating He and H2 from other gases • develop extensively for natural gas treatment • based on difference in permeation rate that characterizes the speed of diffusion of various constituents through membrane • separation takes place through very fine hollow fibers of micro porous membrane. • ‘fast gases’ like H2, He and less content of CO2 and H2S are diffused through membrane • ‘slow gases’ cannot pass through membrane, only constitute residual gas PERMEATION (1) • a gas in permeator composed of fine hollow fibers • located in shell • one end fiber closed off by epoxy resin plug and the other end enables recovery the gas that passes through membrane • on top, tube sheet close off the hollow fibers while at same time allow evacuation of residual gas • at bottom, tube sheet is contained of ends of fibers through which permeate gases passes • process is developed for H2 recovery • applied in natural gas treatment is limited at present • concern re-treatment for CO2 re-injection of gas from oil reservoir PERMEATION (1) advantages: • in treating NG very rich in CO2 (over 20%) • contains little or no H2S • use lower CO2 content to around 2% disadvantage: • cannot be widely used until improve in permeability and selectivity of membrane and resistance to deterioration