Physics II Textbook: Fluid, Thermo, Oscillation, Wave, Sound
advertisement

Fı́sica II (ver. 3)
Tomoi Koide
December 17, 2021
2
Contents
I
Fluido
7
1 O que é “fluido”?
2 Variáveis de fluido
2.1 Densidade de massa
2.2 Força externa . . . .
2.3 Pressure . . . . . . .
2.4 Velocity field . . . .
2.5 Hydrodynamics . . .
9
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
11
11
11
12
14
15
3 Static fluid
3.1 Variation of pressure under gravitational force
3.2 Variation of pressure under centrifugal force .
3.3 Variation of pressure between two fluids . . .
3.4 Arquimedes’s principle . . . . . . . . . . . . .
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
17
17
18
19
20
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
4 Stationary fluid
23
4.1 Conservation of mass (equation of continuity) . . . . . . . . . . . 23
4.2 Bernoulli’s theorem . . . . . . . . . . . . . . . . . . . . . . . . . . 24
4.3 Torricelli’s theorem . . . . . . . . . . . . . . . . . . . . . . . . . . 26
5 Viscosity
29
5.1 Definition . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
5.2 Hagen-Poiseuille’s law . . . . . . . . . . . . . . . . . . . . . . . . 30
II
Thermodynamics
6 Variables of thermodynamics
6.1 What is temperature . . . .
6.2 Heat . . . . . . . . . . . . .
6.2.1 Thermal conduction
6.3 Dilatação térmica . . . . . .
33
.
.
.
.
.
.
.
.
7 First law of thermodynamics
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
35
36
37
40
41
43
3
4
CONTENTS
8 Vários Processos Termodinâmicos
8.1 Banho Térmico . . . . . . . . . . . . . . .
8.2 Processo . . . . . . . . . . . . . . . . . . .
8.2.1 Processos Reversı́vel e Irreversı́vel
8.2.2 Processo Quase-Estático . . . . . .
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
45
45
45
46
46
9 Properties of Ideal Gas
9.1 Equation of state . . . . . . . . . . . . . .
9.2 Volume and energy in ideal gas . . . . . .
9.3 heat capacities of ideal gas . . . . . . . . .
9.4 Quasi-static adiabatic process of ideal gas
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
51
51
52
52
54
10 Second law of thermodynamics
10.1 Princı́pios . . . . . . . . . . . .
10.2 Ciclo de Carnot . . . . . . . . .
10.3 Eficiência do Calor . . . . . . .
10.3.1 ciclo de Carnot . . . . .
10.3.2 Máximo da Eficiência .
10.4 Desigualdade de Clausius . . .
10.5 Entropia . . . . . . . . . . . . .
10.5.1 Processo Reversı́vel . . .
10.5.2 Processo Irreversı́vel . .
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
57
57
58
60
60
62
64
65
65
67
11 Kinetic theory of gas
11.1 kinetic representation of pressure
11.2 specific heat of ideal gas . . . . .
11.3 Maxwell-Boltzmann distribution
11.4 Mean free path . . . . . . . . . .
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
69
69
71
71
73
12 Real gas and phase transition
75
12.1 van der Waals equation of state . . . . . . . . . . . . . . . . . . . 75
12.2 Phase transition . . . . . . . . . . . . . . . . . . . . . . . . . . . 76
12.3 Latent heat . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 77
III
Oscillation, Wave and Sound
81
13 Mathematical preparation
83
13.1 Second-order homogeneous differential equation . . . . . . . . . . 83
13.2 Second-order inhomogeneous differential equation . . . . . . . . . 84
13.3 Triangle functions . . . . . . . . . . . . . . . . . . . . . . . . . . 85
14 Oscillation
14.1 Simple harmonic motion . .
14.2 Period . . . . . . . . . . . .
14.3 Energy and averaged energy
14.4 Pendulum . . . . . . . . . .
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
87
87
88
89
89
CONTENTS
14.5 Spring . . . . . . . . . . . . . .
14.6 Superposition principle . . . . .
14.7 Damped oscillation . . . . . . .
14.7.1 Supercritical γ 2 > 4ω02 .
14.7.2 Subcritical γ 2 < 4ω02 . .
14.7.3 Critical γ 2 = 4ω02 . . . .
14.8 Forced oscillation . . . . . . . .
14.8.1 ω ̸= ω0 . . . . . . . . . .
14.8.2 ω = ω0 . . . . . . . . . .
14.9 Forced oscillation with damping
5
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
90
92
92
92
93
93
93
94
94
95
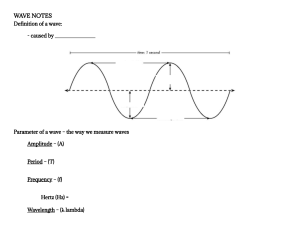
15 Wave
15.1 Fundamental properties of wave . .
15.2 Wave length and period . . . . . .
15.3 velocity . . . . . . . . . . . . . . .
15.4 String . . . . . . . . . . . . . . . .
15.5 Superposition . . . . . . . . . . . .
15.5.1 stationary wave . . . . . . .
15.5.2 Beat . . . . . . . . . . . . .
15.6 Normal mode . . . . . . . . . . . .
15.7 Reflection . . . . . . . . . . . . . .
15.7.1 Fixed (Dirichlet) boundary
15.7.2 Free (Neumann) boundary .
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
97
97
99
99
99
101
101
103
103
104
104
106
16 Sound
16.1 Dynamics of sound (I) . . . . . . .
16.2 Dynamics of sound (II) . . . . . . .
16.3 Relation between (I) and (II) . . .
16.4 Energy . . . . . . . . . . . . . . . .
16.5 Air column . . . . . . . . . . . . .
16.6 Interference in 2 dimension . . . .
16.7 Doppler effect . . . . . . . . . . . .
16.7.1 Moving source . . . . . . .
16.7.2 Moving observer . . . . . .
16.7.3 Moving source and observer
16.8 Shock wave . . . . . . . . . . . . .
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
111
111
113
114
115
116
118
119
119
120
120
121
6
CONTENTS
Part I
Fluido
7
Chapter 1
O que é “fluido”?
Vamos considerar áqua como um exemplo. Experimantalmente, sabemos que a
água se comporta de maneira diferente dependendo a temperatura: gás, lı́quido
e sólido. Diferentemente de gelo, que é a água de sólido, vapor e água lı́quida
pode facilmente mudar a sua forma, ou seja, estes não perder a sua mobilidade.
O palavra de “fluido” indica gás e lı́quido e as suas dinâmicas são descritas pela
teoria chamada hidrodinâmica.
Na mecâmica classica de Newton, a dinâmica de uma partı́cula é determinada pelos quatro quantidades: massa, velocidade, forças externa e interno. A
equação fundamental é
dv
m
= Fin + Fex .
(1.1)
dt
Por outro lado, na discussão de hidridinâmica, usamos a densidade de massa,
campo de valocidade, força externa e gradiente de pressão. A relação entre a
dinâmica de partı́cula e a fluido é resumida na Tabela 1.1.
partı́cula
massa
velocidade
força externa
força interna
−→
−→
−→
−→
fluido
mass density
velocity field
força externa
gradiente de pressão
Table 1.1:
9
ρ(x)
v(x)
−∇P
10
CHAPTER 1. O QUE É “FLUIDO”?
Chapter 2
Variáveis de fluido
Neste capı́tulo , definimos variáveis apparecendo na hidridinâmica.
2.1
Densidade de massa
Consideramos uma região pequena em torno de um ponto x em um fluido, cujo
volume é dado por d3 x e a massa observada do fluido dentro esta região pequena
é dm. Logo definimos a densidade de massa ρ(x) pela equação seguinte,
ρ(x) ≡ 3lim
d x→0
dm
.
d3 x
(2.1)
Equivalentement, sabendo que a densidade de massa ρ(x), a massa desta regiz ao
é dada por
dm = ρ(x)d3 x.
(2.2)
2.2
Força externa
In the application of hydrodynamics, we consider various kinds of external
forces, which are essentially the same as the ones in particle systems. For example, let us consider the gravitational force applied to a fluid of a small volume
d3 x. Then, by using the mass density, the gravitational force is expressed by
Fgra (x) = dm(x)g = ρ(x)d3 xg,
with g being the gravitational constant.
This result is easily extended to a system with a finite volume V as
Z
Fgra =
d3 xρ(x)g.
V
11
(2.3)
(2.4)
12
CHAPTER 2. VARIÁVEIS DE FLUIDO
Figure 2.1:
2.3
Pressure
Let us consider a cylinder with a piston which is fulfilled with a gas. When we
push the piston and compress the gas, we observe that the gas also push the
piston to our side. This is because, fluid consists of the ensemble of microscopic
particles and these collide with the piston. To represent this nature of fluid, we
introduce the quantity called Pressure.
The pressure is characterized by the following four properties.
1) The pressure distributes throughout fluids. Therefore the pressure is given
by a function of position,
P = P (x).
(2.5)
For example, as is well-known in files, the submarine explodes if it goes too
much underwater. This is because the pressure of the water becomes larger at
a deeper position.
2) The direction of the pressure is always orthogonal to the surface which
we consider. This is explained by Fig. 2.2, where the direction of the pressure
is orthogonal to the plate at the position x.
3) The pressure is given by the force per unit area,
P = lim
dS→0
|F|
.
dS
(2.6)
2.3. PRESSURE
13
Figure 2.2:
The typical unit to measure the pressure is
1 bar = 105 N/m2 = 105 Pa.
(2.7)
4) However, the magnitude of the pressure is independent of the direction.
This can be shown as follows. Let us consider a static fluid. For the sake of
simplicity, we consider the 2-dimensional fluid, but it is easy to extend this
argument to 3 dimension. We choose a certain point A and consider a triangle
boundary around A. The length of the two sides of the triangle is given by a
and b, and one of the angle is α. See Fig. 2.3.
This region is pushed from the water around. From the definition 2), these
pressures are orthogonal to the each side and we suppose that these are given
by P , Px and Py . Because we consider the static fluid, the forces realized to
this region should cancel each other. From the definition 3), the balance of the
forces are calculated as
Py b =
Px a =
p
a2 + b2 ,
p
P cos α a2 + b2 .
P sin α
(2.8)
(2.9)
14
CHAPTER 2. VARIÁVEIS DE FLUIDO
On the other hand,
cos α
=
sin α
=
a
,
a 2 + b2
b
√
.
2
a + b2
√
(2.10)
(2.11)
Therefore
P = Px = Py .
(2.12)
From the action-reaction law in classical mechanics, the pressure of the fluid in
this region has the same magnitude independently of the direction.
Taking limit of a, b −→ 0 fixing a/b, this region is converges to the point
A. That is, the magnitude of the pressure at the point A is independent of the
direction.
Figure 2.3:
Even if there are external forces, the above conclusion is not changed. In
particular, 3) and 4) are sometimes called the principle of Pascal.
2.4
Velocity field
To define the velocity field, we need to introduce a concept called fluid particle
(fluid element). Let us separate a fluid into small elements. Then the fluid is
2.5. HYDRODYNAMICS
15
represented by the ensemble of the elements and thus the dynamics of the fluid
can be expressed as the sum of the motions of the elements. See Fig. 2.4. Such
en element is called fluid particle. During the time evolution, the mass of each
fluid particle is conserved.
Let us consider a fluid particle at the position x and that the change of the
position after dt is denoted by dr(x, dt). Then the velocity of the fluid at the
position x is defined by
v(x) = lim
dt−→0
dr(x, dt)
.
dt
(2.13)
See Fig. 2.5. This velocity distributes in all region of a fluid. Thus we call it
velocity field.
Figure 2.4:
2.5
Hydrodynamics
Roughly speaking, there are two different fluids; one is the perfect (ideal) fluid
and the other viscous fluid. Almost all fluids around us behave as viscous
fluids and the dynamics of the perfect fluid is given by the simplified version
of the Navier-Stokes-Fourier equation which describes (a part of) viscous fluids.
Nevertheless, it is still worth studying perfect fluid dynamics, because there are
many situations where the ideal fluid behaviors coincide with those of vicious
fluids even quantitatively.
From the correspondence in Table 1.1, the equation of the perfect fluid is
obtained as the generalization of the Newton equation,
dv
∂
m
= Fin + Fex −→ ρ
+ v · ∇ v = −∇P + Fex .
(2.14)
dt
∂t
16
CHAPTER 2. VARIÁVEIS DE FLUIDO
Figure 2.5:
Here we used the fact that the time derivative for fields is given by
X
d
∂
∂
−→
+
vi i .
dt
∂t i=1,2,3 ∂x
(2.15)
This equation of the perfect fluid is called Euler equation. The equation with
viscosity, which is called Navier-Stokes-Fourier equation, is discussed later.
Any phenomenon of (Newtonian) fluids can be described by solving this
equation. However, in this course, we study simple situations where we can find
solutions without solving this complex equation directly.
Chapter 3
Static fluid
The simplest case is static fluid where v = 0 and there is no time dependence
for hydrodynamic variables. Is such a case, any problem can be solved by
considering
the balance of pressure and external forces.
Later, we find various theorems and principles of static fluid like Arquimedes’s
principle, but all these can be derived from the above important law!
To apply this idea, we first need to know the behavior of the pressure of
fluid.
3.1
Variation of pressure under gravitational force
Now we consider three points A, B and C inside the fluid and consider each
pressure, applying gravitational force. See Fig. 3.1. The mass density of the
fluid is constant and given by ρ. First we consider the points A and B. To
discuss the balance of the forces, we consider a virtual region between the two
pints and consider the forces realized to it. The point A pushes the region to
right and the point B to left. Because we consider the static fluid, the total
force realized to this region should vanishes,
PA ds − PB ds = 0,
(3.1)
where ds is the cross-section of the region. Thus the pressures of A and B are
equivalent, Pa = PB .
Now we consider the points B and C. The pressure of B push down the
region while that of C push up it. Furthermore, the total mass of the fluid
in this region is ρdsH and thus we have the downward force attributed to the
gravitational force, ρdsHg. Again, because we consider the static fluid, the total
force vanishes,
PB ds + ρdsHg − PC ds = 0.
(3.2)
17
18
CHAPTER 3. STATIC FLUID
Figure 3.1:
Therefore PC = PB + ρHg.
In short,
PA = PB = PC − ρgH.
3.2
(3.3)
Variation of pressure under centrifugal force
Figure 3.2:
Now let us consider the centrifugal force around the axis y as an external
force whose angular velocity is ω as is shown in Fig. 3.2. The balance between
3.3. VARIATION OF PRESSURE BETWEEN TWO FLUIDS
19
A and B becomes
ρω 2 2
(l + 2al) = PB .
2
Here the second term is calculated as
Z l+a
1
(ρdsdx)xω 2 = ρω 2 ds (l2 + 2al).
2
a
PA +
3.3
(3.4)
(3.5)
Variation of pressure between two fluids
Let us consider the pressure between the two points, one is at air (mass density
ρa ) and the other at water (mass density ρw ), as is shown in Fig. 3.3.
Figure 3.3:
Even in this case, the argument is essentially the same as before. By preparing the point C which is located at the surface of the two fluids, we find
PA = PC − ρa ag and PC = PB − ρw Hg. Therefore,
PA = PB − (ρa a + ρw H)g.
(3.6)
At the normal condition,
ρa
=
1.293 kg/m3 ,
(3.7)
ρw
=
999.7 kg/m3 .
(3.8)
20
CHAPTER 3. STATIC FLUID
That is, the mass density of water is extremely huge comparing to that of air.
Therefore when a and H are the quantities with the same order, the expression
of the pressure given above is approximately given by
PA = PB − ρw Hg.
(3.9)
In other words, we can often
ignore the change of the atmospheric pressure in practical applications.
3.4
Arquimedes’s principle
By applying the above argument, we can derive Arquimedes’s principle.
Let us consider a body whose cross-section is given by S. The mass density
is homogeneous but the value is not known. See Fig. 3.4.
Figure 3.4:
First we need to know the pressure of the bottom of the body inside the
fluid. As we have learned, it is equivalent to the pressure of the point A, which
is calculated as
PA = Pair + ρw Hg,
(3.10)
3.4. ARQUIMEDES’S PRINCIPLE
21
where Pair is the atmospheric pressure. On the other hand, the pressure at
the top of the body is given by Pair . Remember the discussion in the previous
subsection.
Then the balance of the forces to the body is
Pair S + ρbody (a + H)Sg = PA S −→ ρbody = ρw
H
.
a+H
(3.11)
That is, the mass density of the body must be smaller than that of the liquid.
Note that we cannot consider a negative value of a in the above result.
The buoyancy is defined by the upward force against the gravitational force .
In this case, it is given by
Fbuo = (PA − Pair )S = (ρw SH)g ≡ M g.
(3.12)
This mass is equivalent to that of the liquid substituted by the body. This is
known as Arquimedes’s principle.
This conclusion is applicable to any form of bodies. For example, the buoyancy realized to the body shown in Fig. 3.5 is given by the gravitational force
with the mass of the liquid, whose mass corresponds to the volume of the body
under the liquid (shadow part). This can easily show by applying the argument
similar to the above.
22
CHAPTER 3. STATIC FLUID
Figure 3.5:
Chapter 4
Stationary fluid
So far, we have discussed the static fluids. Now we consider the stationary fluids
where there is a finite fluid velocity, v ̸= 0 which is time independent.
For a static fluid, the balance of forces plays a central role in the argument.
On the other hand, there are two important laws for a stationary fluid:
the conservation of mass (equation of continuity) and Bernoulli’s theorem.
4.1
Conservation of mass (equation of continuity)
Let us consider the flow of water of a hose pipe which is connected to a tap.
Then the amount of water going out from the hose is the same amount of the
incoming from the tap. One can easily find that this invariance of the amount
of the water is independent of the cross-section of the hose.
See Fig. 4.2, where the cross-section of the hose is changed. For unit time,
the mass of the water coming into the hose from the left corresponds to ρA vA SA ,
while that leaving to the right is ρB vB SB . These are the same amount,
ρA vA SA = ρB vB SB .
(4.1)
This relation is satisfied for any points inside the hose. The flow of fluid in
the hose is called stream tube, where the following conservation law is always
satisfied,
ρvS = const .
(4.2)
This is the reflection of the conservation of the mass of fluid. This equation is
sometimes called equation of continuity, because the conservation of the mass
can be expressed as the form of the equaiton of continuity,
∂t ρ + ∇ · (ρv) = 0.
One can easily confirm that this equation reproduce Eq. (4.2).
23
(4.3)
24
CHAPTER 4. STATIONARY FLUID
Figure 4.1:
Water is called incompressible fluid where the mass density is independent
of x, ρ = const. Then the mass conservation is simplified as
vS = const.
(4.4)
Inversely, air is an example of compressible fluid and then we cannot use the
above simplified equation.
4.2
Bernoulli’s theorem
Roughly speaking, Bernoulli’s theorem corresponds to the energy conservation
of fluid.
Let us consider a stream tube shown by the left panel of Fig. 4.3. After a
small time evolution, it is changed as is shown by the right panel. However, the
energy of the fluid is conserved during this time evolution.
First let us consider the left hand side of the flux. Then we find that the
energy corresponding to the cylinder A is lost after the time evolution. The
4.2. BERNOULLI’S THEOREM
25
Figure 4.2:
corresponding kinetic and potential energies are given by
KA
=
VA
=
1
2
(ρA SA vA dt)vA
,
2
(ρA SA vA dt)gzA .
(4.5)
(4.6)
On the other hand, the energy corresponding to the cylinder B is gained,
KB
=
VB
=
1
2
(ρB SB vB dt)vB
,
2
(ρB SB vB dt)gzB .
(4.7)
(4.8)
In short, the change of the total energy is
1
1
2
2
(ρB SB vB dt)vB
− (ρA SA vA dt)vA
+ (ρB SB vB dt)gzB − (ρA SA vA dt)gzA .
2
2
(4.9)
Moreover, this flux is pushed by the surrounded fluid and the work given to the
flux is
∆W = PA SA vA dt − PB SB vB dt.
(4.10)
∆E =
Because
∆E = ∆W,
(4.11)
26
CHAPTER 4. STATIONARY FLUID
Figure 4.3:
we find
1
1
2
2
ρB SB vB vB
− ρA SA vA vA
+ ρB SB vB gzB − ρA SA vA gzA = PA SA vA − PB SB vB
2
2
1
1
2
2
−→ ρA SA vA vA
+ ρA SA vA gzA + PA SA vA = ρB SB vB vB
+ ρB SB vB gzB + PB SB vB .
2
2
(4.12)
Note that we have the equation of continuity,
ρA SA vA = const.
(4.13)
Therefore, the following quantity is conserved,
1 2
1
v + gz + P = const .
2
ρ
(4.14)
In short, this quantity should be conserved for each flux. This conservation law
is called Bernoulli’s theorem.
More general expression of Bernoulli’s theorem can be derived directly from
the Euler equation.
4.3
Torricelli’s theorem
As a typical example of the application of Bernoulli’s theorem, let us consider
a large container which has a very small hole from which the inside liquid goes
out. To apply Bernoulli’s theorem, we consider a stream tube which connects
the surface of the liquid and the hole, as is shown in Fig. 4.4. Because the size
of the container is much larger than that of the hole, the velocity of the fluid at
the surface (point A) is zero. This can be shown by the equation of continuity.
4.3. TORRICELLI’S THEOREM
27
When the fluid velocity at the point B is denoted by v, Bernoulli’s theorem
leads to
v2
P0 + ρHg = ρ + PB ,
(4.15)
2
where ρ is the mass density of the liquid and PB is the pressure of the fluid at
B. We further used the liquid is incompressible.
To estimate the velocity v, we need to know PB . Because the fluid flows
in the present case and thus we cannot apply the argument for calculating the
pressure developed in the previous section. However, when we consider that the
pressure of the fluid balances the atmospheric one, we can set
PB = P0 .
Substituting it into Bernoulli’s theorem, we find
p
v = 2Hg.
(4.16)
(4.17)
This is called Torricelli’s theorem.
Figure 4.4:
<Comment>
So far, we have discussed various aspects of the perfect fluid. As was pointed out
before, however, all these results can be obtained directly from the fundamental
equations of hydrodynamics,
∂
ρ
+ v · ∇ v = −∇P + Fex ,
(4.18)
∂t
∂t ρ + ∇ · (ρv) = 0.
(4.19)
The second equation comes form the equation of continuity.
28
CHAPTER 4. STATIONARY FLUID
The second equation is satisfied for any kind of fluids and thus represents
more universal property of fluid. However, the first equation (Euler equation)
is not applicable to the real fluid because the Euler equation does not contains
the effect of viscosity. Then what is viscosity?
Chapter 5
Viscosity
5.1
Definition
So far, we have discussed the properties of perfect fluid. However, as we have
already discussed, usual fluids has viscosity.
As we have studied, the forces realized each part of fluids are represented
as the pressure and external forces. However, we observe another force between fluid particles moving with different velocities in real fluids. This is called
viscosity.
Figure 5.1:
Let us consider two plates and water (Newtonian fluid) is fulfilled between
them. When the upper plate is pulled with a constant velocity v0 , we observe
29
30
CHAPTER 5. VISCOSITY
that the velocity of the water is expressed as
vx (y) =
y
v0 .
D
(5.1)
This can be expressed as
∂vx
v0
= .
∂y
D
(5.2)
On the other hand, to keep a constant velocity of the plate v0 , the force is
proportional to the magnitude of the velocity v0 and the inverse of the distance
of the plates D,
1
|F|
∝ v0 .
(5.3)
τ≡
A
D
The right hand side is nothing but the gradient of the velocity and thus we have
τ =µ
∂vx
.
∂y
(5.4)
This τ is the (shear) viscosity and η is a proportional constant called viscosity
coefficient. The direction of this force is shown by Fig. 5.2. Here we have two
stream tubes with different velocities. The viscosity realizes at the boundary of
the two tubes and has a direction so as to make the velocity difference vanish.
That is, the slow tube is accelerated and the fast one is decelerated by the
viscosity.
There is another viscosity called bulk viscosity, but we do not discuss it in
this course.
5.2
Hagen-Poiseuille’s law
As an example of the application of viscosity, let us consider a cylinder which
is fulfilled by a viscous liquid and calculate the velocity field. The both sides of
the cylinder is pushed by the pressure PA and PB satisfying PA > PB . Because
of the cylindrical symmetry, the velocity field is a function of the distance from
the center of the cylinder, v(y). Then let us consider a virtual cylinder with
the radius y (y < r). The flow of the cylinder is stationary and the total forces
cancel each other. As the forces realized to the virtual cylinder, there are two
contributions; the pressure and viscosity.
PA (πy 2 ) − PB (πy 2 ) − τ (2πyL) = (PA − PB )πy 2 − µ
∂v(y)
(2πyL) = 0. (5.5)
∂y
Here we consider the case where the velocity is decelerated approaching toward
the cylinder. Then ∂v/∂y < 0, thus
(PA − PB ) + µ
∂v(y) 2L
∂v(y)
PA − PB
= 0 −→
=−
y.
∂y y
∂y
2Lµ
(5.6)
5.2. HAGEN-POISEUILLE’S LAW
31
Figure 5.2:
Suppose that v(r) = 0. Then the solution of the differential equation becomes
1 PA − PB 2
v(y) =
(r − y 2 ).
(5.7)
4µ
L
The total flow of the liquid per unit time is defined by
Z
Q≡
r
dr2πyv(y) =
0
πr4 PA − PB
.
8µ
L
(5.8)
Thus the viscosity coefficient ζ can be calculated as
µ=
πr4 PA − PB
.
8Q
L
(5.9)
32
CHAPTER 5. VISCOSITY
Figure 5.3:
This result is useful to evaluate the magnitude of the viscous coefficient from
experimental data.
<Comment>
Including the shear viscosity, the equations of hydrodynamics is modified as
X
∂
ρ
+ v · ∇ vi = −∇i P +
∇j π ij + Fiex ,
(5.10)
∂t
j=1,2,3
∂t ρ + ∇ · (ρv) = 0,
(5.11)
where
π ij = µ(∂j v i + ∂i v j ).
The first equation is called Navier-Stokes-Fourier equation.
(5.12)
Part II
Thermodynamics
33
Chapter 6
Variables of
thermodynamics
For example, let us consider a bottle of Coca-cola. From the point of view of
classical mechanics, fluids like Coca-cola consists of huge number of microscopic
particles (molecules). However, when we explain the state of Coca-cola to our
friends, probably we do not explain the state of molecules inside the Coca-cola,
the positions and momenta of each molecules. Instead, we will explain example,
the size, cool or hot, taste and so on.
The variables which characterize such a macroscopic state are called thermodynamic variables and normally we consider the following 6 quantities,
• volume V
• pressure P
• temperature T
• particle (molecule) number N
• energy E
• entropy S
• (chemical potential µ)
The last chemical potential is not discussed in this course.
There are many variables but fortunately, these are not independent. The
number of independent variables is
THREE .
For example, when we know E, V and N of a thermodynamic (macroscopic) system, other thermodynamic variables are given by functions of theses variables,
P (E, V, N ), T (E, V, N ) and so on.
35
36
CHAPTER 6. VARIABLES OF THERMODYNAMICS
Moreover, there is another important concept, heat. However, heat cannot
be used to characterizes macroscopic states. Thus heat is not included as a
thermodynamic variable. However, it is still important to discuss the transition
from one thermodynamic state to the other.
6.1
What is temperature
Figure 6.1:
Among six variables, only two variables are not physically familiar, temperature and entropy. The entropy is introduced later. Here we discuss the
properties of temperature. There are two important aspects.
Maybe some people consider that temperature characterizes the energy of
a system. That is, the system with higher temperature has a higher energy.
6.2. HEAT
37
However, this intuition does not necessarily correct. For example let us compare
boiled water (100 0 C) with fluorescent lamp (20000 0 C). The temperature of
the fluorescent lamp is much higher than that of boiled water, but what we can
touch is not the boiled water but the fluorescent lamp.
This is because N of the fluorescent lamp is much smaller than that of the
boiled water. For example, when we ignore the “idealized” ensemble of particles
where the effect of interaction and the finiteness of particles are ignored. Such
an ensemble is called ideal gas. The energy of the ideal gas is expressed as
E ∝ N T.
(6.1)
Thus both of N and T are important for energy. Because of the smallness of
N for the fluorescent lamp, its energy is much smaller than that of the boiled
water. This is the reason why we can touch the fluorescent lamp without any
damage.
Then what is the most important property of temperature? This becomes
clear when we consider the two thermodynamic system. Let us consider a Cocacola and a box with a gas whose energies are given by EA and EB , respectively.
When we put the Coca-cola inside the box, then, for example, is the energy of
the Coca-cola changed? In general, for example, in the framework of classical
mechanics, it is not easy to answer the question. However, if we introduce the
concept of temperature, we can say that if the temperatures of the two objects
are different, the energy is changed. In this manner, the concept of the temperature is useful to see the thermodynamic state among different thermodynamic
systems.
The other aspect is the unit to measure temperature. There are two famous
choice of this unit, Celsius 0 C and Kelvin K. Historically the Celsius temperature is defined through the property of water, that is, 00 C = freezing point and
1000 C = boiling point.
On the other hand, the absolute (Kelvin) temperature is positive definite
and there is a following relation,
T [K] = T [0 C] + 273.15.
(6.2)
The important property of the absolute temperature is that it does not take any
negative value. This is clear from the above expression because the absolute
zero of the temperature is given by Tmin = 273.150 C. That is the minimum
temperature in Kelvin is Tmin = 0K.
In the argument of thermodynamics, we normally use the absolute temperature.
6.2
Heat
Let us consider two thermodynamics systems whose temperature are given by
TA and TB satisfying TA > TB . Then from our experience, we know that the
temperature TA decreases and TB increases when the two systems are connected.
38
CHAPTER 6. VARIABLES OF THERMODYNAMICS
We express this process as “a positive heat is transferred from TA side to TB
side.” In this manner, heat is introduced to express the change of thermodynamic states.
Figure 6.2:
The heat is sometimes expressed as a function of the change of temperature.
For example, let us consider a cooling process where the temperature of the
system A is changed from TA to TF as is shown in Fig. 6.2. Then the amount
of the heat lost from the system A is expressed as
dQ
= mA cmass
(TA − TF )
A
=
CA (TA − TF ).
(6.3)
Here cmass
and CA are called specific heat per mass and heat capacity, respecA
6.2. HEAT
39
tively. Similarly, the system B is heated and the heat gained is given by
dQ = mB cmass
(TF − TB )
B
= CB (TF − TB ).
(6.4)
Suppose all heats lost from the system A is used for heating the system B,
then the final temperature is calculated as
mA cmass
(TA − TF ) = mB cmass
(TF − TB ) −→ TF =
A
B
mA cmass
TA + mB cmass
TB
A
B
.(6.5)
mass
mA cmass
+
m
c
B B
A
As a special case, let us consider the systems A and B are the same material
and have the same size. Then mA cA = mB cB and then
TF =
TA + TB
.
2
(6.6)
Figure 6.3:
As an example, let us consider the emission of heat from our body. Let us
consider a person whose weight is 70 kg and the body temperature is 36 0 C.
The specific heat per unit mass of the body is cmass = 3.47 × 103 J/(K · kg).
Our body emits heat to environment. When the room temperature is given by
40
CHAPTER 6. VARIABLES OF THERMODYNAMICS
20 0 C, the emitted heat is estimated by
Q = mcmass ((36 + 273.15) − (20 + 273.15))
=
70 × 3.47 × 103 × 16
=
925 kcal,
(6.7)
where 1 cal = 4.2 J = 4.2 kg m2 s2 . Normally, we take 2000 kcal per day. The
half of what we ate is consumed to maintain the temperature of the body.
As another definition, we can consider the specific heat assiciated with the
particle number. Because the number of the particle of gas and fluid is huge,
so we measure it by using the Avogadro constant, NA = 6.02 × 1023 . Then we
can define mol by
n=
N
,
NA
(6.8)
where N is the number of particles. Then the specific heat per unit mol is given
by
C = ncmol .
6.2.1
(6.9)
Thermal conduction
Let us consider the time dependence of the conduction of the heat from A to
B through a material which has a quantity called heat conductivity λ as is
shown in Fig. 6.4. Then, as an experimental law called Fourier’s law of thermal
conduction, the conduction of the heat per unit time between A and B is given
by
dQ
TA − TB
= λA
,
(6.10)
dt
L
where A and L are the area and length of the material, respectively. Here
TA > TB as before.
As another application, let us consider the conduction with two different
materials and calculate the temperature at the boundary, as is shown in Fig.
6.5.
We consider the stationary process. Then the heat conduction per unit time
in both material should be the same,
λ1 A
TA − T
T − TB
(λ1 /L1 )TA + (λ2 /L2 )TB
= λ2 A
−→ T =
.
L1
L2
λ1 /L1 + λ2 /L2
(6.11)
<Comment>
We have so far used the fact the the heat flows from from higher temperature
to lower one. Then can this fact be derived from thermodynamics or we should
introduce it as a principle? As we will see later, it is actually derived from the
second law of thermodynamics, Clausius’s inequality. Thus, for the construction
of the theory of thermodynamics, we do not need assume this fact.
6.3. DILATAÇÃO TÉRMICA
41
Figure 6.4:
6.3
Dilatação térmica
Have you ever seen the rail line? It is not one continuous line, but there are
several spaces. These spaces are prepared to avoid the deformation of the rail
line by thermal expansion of iron. This phenomenon is called thermal expansion.
There is a linear relation between the temperature and length changes as
∆l = αl∆T,
(6.12)
where l is the length of the bar of material and α is the coefficient of linear
expansion. That is, when the temperature increases by ∆T , the length total of
the bar is given by
l = l0 (1 + α∆T ),
(6.13)
where l0 is the length at ∆T = 0.
This is the expansion of bars. Let us consider a expansion of the material
with the volume V = l1 l2 l3 at T = T0 . When the temperature becomes T = T1
(T1 > T0 ), each side of the volume expands and the volume becomes V ′ =
l1 l2 l3 (1 + α∆T )3 where ∆T = T1 − T0 . Therefore the change of the volume is
dV = V ′ − V ≈ 3αV ∆T.
(6.14)
42
CHAPTER 6. VARIABLES OF THERMODYNAMICS
Figure 6.5:
That is the coefficient of the volume expansion dV /V is three times larger than
that of linear expansion.
Chapter 7
First law of
thermodynamics
Vamos considerar o aumento de temperatura de água dentro de um copo. Existem dois métodos para isso. Por exemplo, usamos o fogo para fazer comidas.
Ou seja, a temperatura da água é aumentada por conectar o sistema em questão
com outro sistema mais quente. Neste caso um calor Q se propaga do fogo ao
nosso sistema.
Por outro lado, você já assistiu em filmes que o fogo pode ser feito por fricção
de pedaços de madeira. Ou que o prego esquenta quando batido pelo martelo.
Ou seja, o trabalho dinâmico se torna o calor. Desta forma, a temperetura da
água é aumentada ao ser agitada por uma colher cujo trabalho é W . Portanto,
ambos o calor e o trabalho são, em certo sentido, equivalentes,
Q = W.
(7.1)
Por outrolado, na mecânica clássica, é sabido que o trabalho muda a energia.
Vamos considerar a evolução de tempo de tA até tB . A partir da equação de
Newton, obtemos
d 1
mv 2 = F · v
dt 2
−→ ∆E = EA − EB = W ′ =
Z
A
F · dx,
(7.2)
B
2
2
onde EA = mvA
/2 e EB = mvB
/2. Aqui supomos que não existe a força
conservadora.
Devido à equivalência do calor e trabalho, podemos trocar a parte do trabalho W ′ pelo calor como W ′ = W + W ′′ = W + Q. Substituindo na equaçâo
acima, obtemos
∆E = Q + W.
(7.3)
Isso é a primeira lei da termodinâmica. É facı́l saber que isso é a generalização
da conservação da energis da mecânica clássica.
43
44
CHAPTER 7. FIRST LAW OF THERMODYNAMICS
Figure 7.1: A primeira lei de termodinámica.
Até agora, consideramos que o trabalho positivo è realizado para o sistema.
Mas nas seguintes discussões, usamous a definição de que o trabalho dado pelo
sistema é positivo. Para isso, a primeira lei é re-expressa por
∆E = Q − W.
(7.4)
As direcões do calor e o trabalho são dadas por Fig. 8.2. Por exemplo, consideramos que o sistema recebe o trabalho positivo W e emite o calor positive Q.
Indica que o sistema realiza o trabalho negativo −W e recebe o calor negativo
−Q e a primeira lei è escrito como
∆E = −Q + W.
(7.5)
Chapter 8
Vários Processos
Termodinâmicos
Para estudar a segunda lei da termodinâmica, consideremos as mudanças das
variáveis termodinâmicas. Neste capı́tulo introduziremos conceitos para isso.
8.1
Banho Térmico
Vamos preparar dois sistemas A e B cujas temperaturas são TA e TB respectivamente e TA < TB . Quando estão em contato, os sistemas atingem o estado de
equilı́brio. Neste caso os sistemas não realizam trabalhos. Desta forma, a partir
da primeira lei, o calor Q > 0 é produzido por consumir a energia ∆EB > 0 do
sistema B.
Q é transferido ao sistema A e ele usa o
calor para aumentar a energia. Portanto
o estado do sistema B muda como
(EB , VB , NB ) −→ (EB −∆EB , VB , NB ).
(8.1)
Consideramos que a energia do sistema
EB é tão grande que
EB − ∆E = EB (1 − ∆E/EB ) ≈ EB .
(8.2)
A temperatura é a função de
8.2
(EB , VB ,Processo
NB ), ou seja a temperatura do sistema B não muda antes e
A
mudança
um estado Este
de equilı́brio
depois
destadeevolução.
sistema para ontro é chamada de processo. Na
termodinâmica,
há
vários
processos.
especial é chamado de banho térmico.
45
46
8.2.1
CHAPTER 8. VÁRIOS PROCESSOS TERMODINÂMICOS
Processos Reversı́vel e Irreversı́vel
Vamos considerar dois estados de equilı́brio, e mudança de um para o outro,
como
(E, V, N ) −→ (E ′ , V ′ , N ′ ).
(8.3)
Se um processo inverso existe
(E ′ , V ′ , N ′ ) −→ (E, V, N ),
(8.4)
o processo (8.3) é reversı́vel. Se o processo que corresponde à Eq. (8.4) não
existe, o processo (8.3) é irreversı́vel. (o estado de ambiente tambèm não pode
ser mudado.)
Entretanto, a maioria dos processos no mundo são irreversı́veis. Por exemplo,
vamos considerar uma coca-cola gelada na cima da mesa. Nos sabemos bem que
a temperatura da coca-cola esquenta até a da sala,
(Tcoca , V, N ) −→ (Tf ora , V, N ).
(8.5)
Se este processo seja reversı́vel, existe a procersso onde a temperatura da cocacola resfria de Tf ora para Tcoca . Mas sabemos nunca observer tal processo. Veja
tambèm Fig. 8.1.
8.2.2
Processo Quase-Estático
Como discutimos, embora a maioria dos processos na termodinâmica são irreversı́veis, ainda podemos considerar o processo reversı́vel, chamado de quaseestático. Na verdade, esse tipo de processo è mais importante na aplicação da
termodinâmica.
Vamos considerar, por exemplo, água numa caixa cuja parede não é fixada.
Puxando ou Empurrando a parede, a superfı́cie da água não é homogênea e o
8.2. PROCESSO
47
seu estado desvia o equilı́brio. Mas quando a parede se move bastante lento,
a sua superfı́cie mantem homogênea. Assim, podemos considerar que a água
fica no estado de equilı́brio durante o movimento e o estado termodinâmico è
mantido. Esse processo é o processo de quase-estático.
O processo de quase-estático tem três propriedades.
• os estados durante o processo podem ser caracterizados pelas variáveis
termodinâmicas.
R
• o trabalho deste processo é expresso como P dV .
• o processo reversı́vel (normalmente).
O segundo item é provado assim. Consideramos um gás confinado dentro
um recipiente com pistão. Por definição, o trabalho feito por gás é dado por
Z x1
P Sdx,
(8.6)
x0
onde S é a área da pistão, e x0 e x1 representam as posições inicial e final da
pistão. Neste caso, o elemento de volume é expresso como dV = Sdx. Logo
podemos deduzir o segundo item. Note que esta expressão é aplicável para
expansão e compressão. Para expansão, o sistema realiza o trabalho positivo, e
para compressão, o negativo.
Quando a mudança de volume é pequena, a pressão pode ser considerada
constante. Então a primeira lei no processo quase-estático é expressa como
∆E = Q − P ∆V.
(8.7)
Note que esta expressão è satisfeito sò para os processos reversı̀veis.
Há vários processos quase-estàticos.
Processo Quase-Estático Adiabático
Neste processo, não existe a troca do calor. Logo a primeira lei do processo è
dada por
∆E = Q − W = −W = −P ∆V.
(8.8)
Quando um sistema se move de (E, V, N ) a (E ′ , V ′ , N ′ ) através do processo
quase-estático adiabático, isso é representado por
qa
(E, V, N ) −→ (E ′ , V ′ , N ′ ).
(8.9)
Processo Quase-Estático Isotérmico
Neste processo, consideramos que um sistema está em contato com um banho
térmico de temperatura T . Durante este processo, a temperatura do sistema
é fixada com T , se o mudança do sistema é bastante devagar. Quando um
sistema se move de (E, V, N ) a (E ′ , V ′ , N ′ ) através da processo isotérmico, isso
é representado por
it
(E, V, N ) −→ (E ′ , V ′ , N ′ ).
(8.10)
É claro que T (E, V, N ) = T (E ′ , V ′ , N ′ ).
48
CHAPTER 8. VÁRIOS PROCESSOS TERMODINÂMICOS
Processo Quase-Estático Isovolumétrico
Neste processo, consideramos que a mudança fixando o volume. Quando um
sistema se move de (E, V, N ) a (E ′ , V, N ) através da processo isotérmico, isso é
representado por
iv
(E, V, N ) −→ (E ′ , V, N ).
(8.11)
Processo Quase-Estático Isobárico
Neste processo, consideramos que a mudança fixando a presssão. Quando um
sistema se move de (E, V, N ) a (E ′ , V ′ , N ) através da processo isotérmico, isso
é representado por
ib
(E, V, N ) −→ (E ′ , V ′ , N ).
(8.12)
É claro que P (E, V, N ) = P (E ′ , V ′ , N ′ ).
8.2. PROCESSO
49
Figure 8.1: Os processos irreversı́veis
50
CHAPTER 8. VÁRIOS PROCESSOS TERMODINÂMICOS
Figure 8.2: Os processos quase-estático
Chapter 9
Properties of Ideal Gas
9.1
Equation of state
The argument in thermodynamics is sometimes too much abstract and we cannot calculate anything. Then to deeper our understanding of thermodynamics,
it is sometimes better to apply the general argument to a certain example. The
simplest example is the ensemble of particles called ideal gas, where
• there is no interaction between particles .
• the size of each particle is extremely small and ignored .
In this case, it is known that there is a famous relation between thermodynamic variables,
P V = kB N T,
(9.1)
where kB = 1.38 × 10−23 J/K is called Boltzmann constant. Or this can be
re-expressed as
P V = nRT ,
(9.2)
where n = N/NA is the mole number and R is the universal constant of gas,
R = kB NA = 8.314J/(molK). These relations are called equation of state
(EoS).
From EoS, we can derive the two typical behaviors of the ideal gas. When
T, N = const, then
1
P ∝ .
(9.3)
V
This is known as Boyle’s law. And when P, N = const, we obtain
V ∝ T.
(9.4)
This is Charles’s law.
Using this EoS of the ideal gas, we can calculate concretely works for various
situations.
51
52
CHAPTER 9. PROPERTIES OF IDEAL GAS
1) Quasi-static isothermal process, (T, Vi , N ) → (T, Vf , N )
Z
Vf
W =
Z
Vf
dV P =
Vi
Vi
nRT
= nRT
dV
V
Z
Vf
dV
Vi
Vf
1
= nRT ln
.
V
Vi
(9.5)
2) Quasi-static isobaric process, (P, Vi , N ) → (P, Vf , N )
Z
Vf
dV P = P (Vf − Vi ).
W =
(9.6)
Vi
3) Quasi-static isovolumetric process, (Pi , V, N ) → (Pf , V, N )
Z
V
W =
dV P = 0.
(9.7)
V
9.2
Volume and energy in ideal gas
In the beginning of the discussion of thermodynamics, we learned that we need
to specify three variables to chose a thermodynamic state uniquely. Let us now
choose (T, V, N ) and then the energy is a function of these variables,
E = E(T, V, N ).
(9.8)
On the other hand, as an experimental fact (Joule-Thomson), it is known
that the temperature of the gas ideal does not change in the free expansion.
Uring this process, the energy is not changed. Therefore we can drop this
volume dependence,
Eideal = E(T, N ).
That is, the energy of the ideal gas does not depends on the volume. This is
reasonable behavior because the ideal gas, by definition, does not contain the
contribution from interaction between particles.
Moreover, by using the concept of entropy, first law and EoS, we can show
∂E
= 0.
(9.9)
∂V T,N
This is the mathematical representation of the above statement.
9.3
heat capacities of ideal gas
In the previous section, we introduce the concept of the heat capacity (specific
heat). However, such a definition is still incomplete because the definition depends on thermodynamic processes. Let us consider that the temperature of a
9.3. HEAT CAPACITIES OF IDEAL GAS
53
system is changed as T −→ T + dT . Typically, then, we have two definitions;
one is for a quasi-static isobaric process
mass
dQ = CP dT = ncmol
dT,
P dT = mcP
(9.10)
and the other an isovolumetric process,
mass
dQ = CV dT = ncmol
dT,
V dT = mcV
(9.11)
where n denotes the mole number.
For the ideal gas, we can derive an important relation between CP and CV .
Let us consider the processes shown in Fig. 9.1.
Figure 9.1:
Let us consider the quasi-static process A → C. Then we can calculate
dE
= Ec − Ea = E(T + dT, N ) − E(T, N ),
(9.12)
dW
=
(9.13)
0,
dQ = CV dT.
(9.14)
From the first law,
dE = dQ − dW −→ E(T + dT, N ) − E(T, N ) = CV dT.
(9.15)
54
CHAPTER 9. PROPERTIES OF IDEAL GAS
On the other hand, from the process A → B,
dE
dW
= EB − Ea = E(T + dT, N ) − E(T, N ),
Z V +dV
=
dV P = P dV,
(9.16)
(9.17)
V
dQ = CP dT,
(9.18)
leading to
E(T + dT, N ) − E(T, N ) = CP dT − P dV.
(9.19)
Therefore, we find the following relation between CV and CP ,
CP dT − P dV = CV dT.
(9.20)
Next, we consider EoS at the points A and B, which are given by
P V = nRT,
P (V + dV ) = nR(T + dT ).
(9.21)
From these two, we find
P dV = nRdT.
(9.22)
Substituting this into Eq. (9.20), we obtain
cmol
= cmol
+ R.
P
V
(9.23)
That is, for the ideal gas, CP is always larger than CV .
9.4
Quasi-static adiabatic process of ideal gas
In the quasi-static adiabatic process of the ideal gas, we can derive a special
relation. The first law of this process is written by
dE = dQ − dW = −dW = −P dV.
(9.24)
From Eq. (9.15),
CV dT + P dV = 0 −→ CV dT +
nRT
dV = 0.
V
(9.25)
Let us the process, (T1 , V1 , N ) → (T2 , V2 , N ). Then the above equation
becomes
Z T2
Z V2
CV
nR
dT
+
dV
=0
T
V
T1
V1
V2
T2
+ nR ln
=0
−→ CV ln
T1
V1
T2
V2
−→ ln
+ (γ − 1) ln
=0
T1
V1
γ−1
T2 V2
−→ ln
=0
T1 V1
−→ T V γ−1 = const.
(9.26)
9.4. QUASI-STATIC ADIABATIC PROCESS OF IDEAL GAS
55
Here γ ≡ CP /CV and we used that CV is independent of T for the ideal gas.
This can be re-expressed, by using EoS, as
P V γ = const.
(9.27)
That is, this quantity is conserved during quasi-static adiabatic process.
Then we can calculate the work for this quasi-static adiabatic process, (P1 , V1 , N ) →
(P2 , V2 , N ) as
Z
W
V2
=
P dV
V1
Z V2
P1 V1γ
dV
Vγ
V1
1
γ
= P1 V1 −
(V2−γ+1 − V1−γ+1 )
γ−1
"
γ−1 #
V1
P1 V1
1−
.
=
γ−1
V2
=
(9.28)
56
CHAPTER 9. PROPERTIES OF IDEAL GAS
Chapter 10
Second law of
thermodynamics
A primeira lei da termodinâmica mostra que o calor é um tipo de energia. Mas
o comportamento do calor não pode ser exatamente equivalente à energia. Se
fosse verdade, nós poderiamos asistir televisão pelo esfriamento da temperatura
do quarto. Isso nunca acontece. A segunda lei da termodinâmica explica a
diferença entre a energia e o calor.
10.1
Princı́pios
Há várias expressões para representar a segunda lei. Uma delas é conhecida
como o princı́pio de Clausius:
Há dois sistemas A e B, e a temperatura de A está maior do que a
de B. Quando um calor se transfere de B para A, o estado do
sistema externo deve ser mudado.
Outra é o princı́pio de Kelvin (Thomson):
Há só um sistema com temperatura finita. É impossivel trocar todos
os calores do sistema para o trabalho.
Os comportamentos dos princı̀pios são mostrados na Fig. 10.1. Os dois são
equivalentes; se o Clausius for satisfeito, o Kelvin è deduzido e vice versa.
Utilizando estes princı́pois, podemos deduzir
• a teoria de Carnot
• a introdução de conceito de entropia e a lei do aumento de entropia
57
58
CHAPTER 10. SECOND LAW OF THERMODYNAMICS
Figure 10.1: O princı́pio de Clausius (o lado esquedo) e o de Kelvin (o lado
direito)
• a definição de temperatura absoluta
Para deduzi-los, primeiro discutiremos o ciclo de Carnot.
10.2
Ciclo de Carnot
O ciclo de Carnot é construı́do pela combinação de quatro processos quaseestáticos. Por isso, preparamos dois banhos térmicos 1 e 2 cujas temperaturas
são T1 e T2 , e T1 > T2 . Além disso, o estado inicial do ciclo de Carnot é
dado por (T1 , VA , N ). Deste estado, vamos considerar os seguintes processos
quase-estáticos,
1. Absorver o calor Q1 > 0 do banho térmico 1, utilizando o processo
isotérmico.
qit
(T1 , VA , N ) −→ (T1 , VB , N )
2. Resfriar a temperatura até T2 , utilizando o processo quase-estático adiabático.
qa
(T1 , VB , N ) −→ (T2 , VC , N )
3. Emitir o calor Q2 > 0 ao banho térmico 2, utilizando o processo isotérmico.
qit
(T2 , VC , N ) −→ (T2 , VD , N )
4. Aquecer a temperatura até T1 , utilizando o processo quase-estático adiabático.
qa
(T2 , VD , N ) −→ (T1 , VA , N )
Depois destes processos, o estado volta ao inı́cio (T1 , VA , N ).
Vamos considerar o trabalho pelo ciclo de Carnot. Nos dois primeiros processos, o gás realiza os trabalhos W1 e W2 para fora, respectivamente. Nos dois
próximos processos, ele recebe os trabalhos W3 e W4 de fora, respectivamente.
Desta forma, a soma de tudo é
W = W1 + W2 − W3 − W4 .
(10.1)
10.2. CICLO DE CARNOT
59
Figure 10.2: O ciclo de Carnot (o lado esquedo) e o ciclo inverso de Carnot (o
lado direito)
Cada valor dos trabalhos depende da natureza do ciclo de Carnot. O fato
importante é que não tem a mudança da energia depois de um ciclo porque o
estado volta ao inicial. Então, a partir da primeira lei, obtemos
∆E = Q1 − Q2 − W −→ W = Q1 − Q2 .
(10.2)
O ciclo de Carnot possui a seguinte natureza.
1. ∆E = 0.
2. o ciclo de Carnot é reversı́vel.
3. o trabalho W é maior do que zero.
O segundo item é trivial por causa da construição do ciclo. Portanto, se
existir o ciclo de Carnot representado pelo lado esquedo da Fig. 10.2, sempre
hà o ciclo inverso de Carnot, onde o ciclo recebe o calor Q2 do banho térmico 2
e emite Q1 para 1, produzindo o trabalho −W como mostrtado no lado direito
da Fig. 10.2.
O terceiro item pode ser provado da seguinte forma. Vamos considerar o
ciclo inverso de Carnot. Se W é zero, o ciclo inverso transfere o calor do banho
2 a 1. Entretanto, isso é inconsistente com o princı́pio de Clausius e não pode
acontecer.
Se W é negativo, o ciclo inverso realiza o trabalho positivo −W > 0 transferindo o calor do banho 2 a 1. Este trabalho −W > 0 pode ser utilizado para
produzir um calor que é dado para o banho térmico 1. Isso é inconsistente com
o principı́o de Clausius. Finalmente, W é o valor positivo exceto por zero.
Similarmente, podemos provar que o processo mostrado na Fig. 10.3 não
pode acontecer.
60
CHAPTER 10. SECOND LAW OF THERMODYNAMICS
Figure 10.3: Esse processo não pode acontecer.
10.3
Eficiência do Calor
10.3.1
ciclo de Carnot
O ciclo de Carnot é construı́do só dos processos quase-estáticos. Mas podemos
pensar ciclos mais gerais. Estes ciclos podem voltar a estados iniciais antes de
um ciclo, mas são irreversı́veis.
Nos ciclos, podemos introduzir uma quantidade chamada de eficiência do
calor. Quando um ciclo recebe o calor total Q = Q1 − Q2 de banhos térmicos,
e realiza um trabalho durante um ciclo, a eficiência é dada por
η=
W
Q1 − Q2
Q2
=
=1−
.
Q1
Q1
Q1
(10.3)
O fato muito importante para esta quantidade é que
1) Todos os ciclos de Carnot têm a mesma eficiência.
2) No ciclo de Carnot, a eficiência do calor é dada por uma função
de temperatura.
Isso é provado da seguinte forma. Vamos considerar o ciclo de Carnot A
que absorve o calor Q1 > 0 do banho térmico 1 da temperatura T1 e emite o
calor Q2 > 0 ao banho 2 da temperatura T2 . O trabalho realizado pelo ciclo de
Carnot é W > 0.
Além disso, introduzimos o outro ciclo de Carnot B que absorve o calor
Q′1 > 0 do banho 1 e emite o calor Q′2 > 0 ao banho 2, produzindo o trabalho
W . Combinando o ciclo de Carnot A e o ciclo inverso de Carnot, podemos
construir o ciclo nove que absorve o calor Q1 − Q′1 do banlho 1 e emite Q2 − Q′2
do banho 2. Como este ciclo não realiza nenhum trabalho, obtemos
Q1 − Q′1 = Q2 − Q′2 .
(10.4)
10.3. EFICIÊNCIA DO CALOR
61
Figure 10.4: Para ser consistente com o princı́pio de Clausius, Q1 = Q′1 e
Q2 = Q′2 .
Para o ciclo ser consistente com o princı́pio de Clausius,
Q1 − Q′1 = Q2 − Q′2 ≥ 0.
(10.5)
Por definição, ele também é reversı́vel. Outra vez, para o ciclo inverso ser
consistente com o princı́pio de Clausius,
Q1 − Q′1 = Q2 − Q′2 ≤ 0.
(10.6)
Finalmente, obtemos
Q1 = Q′1 ,
Q2 = Q′2 .
(10.7)
Portanto a taxa de calor é
Q2
Q′
= 2′ .
Q1
Q1
(10.8)
Ou seja, esta taxa pode ser dependente das naturezas dos banhos térmicos,
mas independente da do ciclo de Carnot. Em outras palavras, a eficiência é
igual a todos os ciclos de Carnot e uma função só da temperatura dos banhos,
QB /QA = f (T1 , T2 ).
Ademais podemos provar o seguinte fato,
Q2
Q1
= f (T1 , T2 ) =
θ(T2 )
θ(T1 ) .
A prova é seguinte. Preparamos três banhos térmicos,1,2 e 3 com tempereturas T1 , T2 e T3 , respectivamente. O ordem da tempereturas é T1 > T2 > T3 .
Entre banhos 1 e 2 e entre banhos 2 e 3, há dois ciclos de Carnot A e B. As
taxas de calor são dadas por
Q2
= f (T1 , T2 ),
Q1
Q3
= f (T2 , T3 ),
Q2
(10.9)
(10.10)
62
CHAPTER 10. SECOND LAW OF THERMODYNAMICS
Figure 10.5: O sistema do lado esquedo é equivalente ao do lado direito.
respectivamente. Além disso, consideramos que os ciclos A e B e o banho 2
formam um novo ciclo de Carnot. Assim, esta taxa é
Q3
= f (T1 , T3 ).
Q1
(10.11)
Q3
Q3 Q2
=
.
Q1
Q2 Q1
(10.12)
f (T1 , T3 ) = f (T1 , T2 )f (T2 , T3 ).
(10.13)
O lado esquedo é equivalente a
Portanto,
Para satisfazer esta expressão, obtemos
f (T1 , T2 ) =
θ(T2 )
.
θ(T1 )
(10.14)
A forma da função θ(T ) pode ser determinada por experimentos. Utilizando
a tempereruta de Celsius, θ é expresso como
θ = t + 273.15.
(10.15)
Portanto, θ é equivalente à temperetura absoluta,
ηc = 1 −
T2
T1 .
Aqui, o ı́ndice c indica a eficiência do ciclo de Carnot.
10.3.2
Máximo da Eficiência
A seguir, vamos discutir a eficiência de ciclos gerais, denotada por η. Então, há
a seguinte desigualdade,
10.3. EFICIÊNCIA DO CALOR
63
η ≤ ηc = 1 −
T2
T1 .
Só quando o ciclo reversı́vel, η = ηc .
A prova é dada da seguinte forma. Imagine um ciclo geral que pode ser
irreversı́vel, o ciclo C. Ele absorve o calor Q1 do banho 1 e emite Q2 a 2, onde
as temperaturas são dadas por T1 e T2 , respectivamente e T1 > T2 . O trabalho
produzido por este ciclo A é W . Além disso, introduzimos um ciclo inverso
de Carnot B que absorve Q′2 do banho 2 e emite Q′1 a 1 utilizando o trabalho
produzido pelo ciclo geral. Em resumo, os dois ciclos absorvem Q1 − Q′1 do
banho 1 e emitem Q2 − Q′2 a 2. Como ∆U = 0 e W = 0,
Q1 − Q′1 = Q2 − Q′2 .
(10.16)
Para satisfazer o princı́pio de Clausius, esta quantidade deve ser positiva,
Q1 − Q′1 ≥ 0.
(10.17)
Então a eficiência do ciclo A é
η=
W
.
Q1
(10.18)
Por outro lado, a eficiência do ciclo de Carnot que corresponde ao ciclo inverso
de Carnot A é dada por
W
(10.19)
ηc = ′ .
Q1
Portanto, obtemos
η < ηc .
(10.20)
Nós podemos considerar o ciclo inverso do ciclo combinado de A e B só
quando o ciclo A é reversı́vel. Assim, para satisfazer o princı́pio de Clausisus
para este ciclo inverso, Q1 = Q′1 . Finalmente, obtemos
η =1−
Q2
T2
≤ ηc = 1 − .
Q1
T1
(10.21)
A partir desta equação, deduzimos que
Q2
T2
Q1
Q2
≥
−→
−
≤ 0.
Q1
T1
T1
T2
(10.22)
A seguir, definimos que o calor absorvido por ciclos é positivo. Desta forma,
obtemos
Q1
Q2
+
≤ 0.
(10.23)
T1
T2
64
CHAPTER 10. SECOND LAW OF THERMODYNAMICS
Figure 10.6: Para ser consistente com o princı́pio de Clausius, Q1 > Q′1 e
Q2 > Q′2 .
10.4
Desigualdade de Clausius
A eq. (10.23) é o resuldato do caso com dois banhos térmicos. Vamos considerar
M banhos. Quando o nosso sistema absorve o calor Qi do banho i com a
temperatura Ti , há a seguinte relação,
PM
Qi
i=1 Ti
≤ 0.
Isso é conhecido como a desigualdade de Clausius. A igualdade desta equação
é satisfeita quando este processo é reversı́vel. Note que isso é satisfeito só por
ciclos.
Isso é provado da seguinte forma. Vamos preparar M ciclos de Carnot, um
ciclo geral e M+1 banhos térmicos. O calor total obtido dos banhos é dado por
Q=
M
X
Q′i
(10.24)
i=1
Devido à primeira lei,
Q = W,
(10.25)
onde W indica o trabalho total realizado pelo ciclos.
Para satisfazer o princı́pio de Kelvin, Q ≤ 0. Note que a igualdade é satisfeita
s’o para o ciclo reversı́vel. Além disso, a partir da eficiência do ciclo de Carnot,
Qi
Ti
= ′.
′
Qi
T
(10.26)
Portanto obtemos
M
X
Qi
i=1
Ti
=
M
X
Q′
i=1
i
T′
M
1 X ′
= ′
Q ≤ 0.
T i=1 i
(10.27)
10.5. ENTROPIA
65
Figure 10.7: O sistema para deduzir a desigualdade de Clausius.
Figure 10.8: Processos reversı́veis podem ser expressos pelas combinações dos
processos adiabaticos e quasi-estáticos térmicos.
10.5
Entropia
Extendendo a ideia da desigualdade de Clausius, podemos introduzir uma nova
variável termodinâmica, a entropia.
10.5.1
Processo Reversı́vel
Vamos considerar um ciclo no diagrama de (T, V ). No caso reversı́vel, todos
os processos do ciclo podem ser expressos no diagrama. Quando a trajetória
é dividida em pequenas partes, elas podem ser expressas pela combinação dos
processos quase-estáticos adiabáticos e isotérmicos.
Por exemplo, na parte de (Tk−1 , Vk−1 ) até (Tk , Vk ), para mudar o volume,
o ciclo absorve um calor Qk−1 do banho de Tk−1 . Então, a desigualdade de
Clausius deste caso é expressa como
Z ¯
dQ
= 0,
c T
(10.28)
66
CHAPTER 10. SECOND LAW OF THERMODYNAMICS
Figure 10.9: Podemos aplicar a desigualdade de Clausius para este sistema.
onde
Z ¯
M
X
dQ
∆Qk
≡ lim
.
M →∞
Tk
c T
i=1
(10.29)
Aqui, usamos o fato de o ciclo ser reversı́vel.
Este ciclo pode ser interpretado como a combinação das duas trajetórias
Γ1 e Γ2 que têm mesmos pontos inicial e final. Desta forma, a desigualdade é
re-escrita como
Z B
Z B
¯
¯
dQ
dQ
=
.
(10.30)
A(Γ2 ) T
A(Γ1 ) T
Ou seja, esta quantidade é independente da escolha da trajetótia das integrais e é
uma função só dos estados inicial e final. Portanto esta quantidade é uma função
de variáveis termodinânicas. Assim, ela também é uma variável termodinânica,
chamada de entropia,
S(E, V, N ) ≡
R (E,V,N )
o
¯
dQ
T .
Aqui, o ponto inicial o é o ponto padrão para definir a entropia. Existe a
ambiguidade para escolher este ponto. Mas a escolha dele não é irrelevante
para desenvolver a teoria da termodinâmica.
A partir desta definição, a mudança da entropia de quando um sistema
absorve um calor ∆Q de um banho de T é
dS =
∆Q
T .
10.5. ENTROPIA
67
Figure 10.10: Podemos aplicar a desigualdade de Clausius para este sistema. A
linha pontilhada indica o processo irreversı́vel.
10.5.2
Processo Irreversı́vel
Até agora, consideramos dois caminhos reversı́veis entre os pontos A e B. Vamos
trocar um dos caminhos com o processo irreversı́vel. Assim, a desigualdade de
Claudius é
Z B ¯
Z B ¯
dQ
dQ
−
≤ 0.
(10.31)
T
A irr
A Γ T
Portanto
Z
B
A irr
¯
dQ
≤ S(B) − S(A) ≡ ∆S.
T
(10.32)
Em especial, os dois pontos A e B podem ser conectados por processos
(quase-estáticos) adiabáticos,
∆S ≥ 0.
Ou seja, a entropia sempre aumenta pelos processos adiabáticos.
68
CHAPTER 10. SECOND LAW OF THERMODYNAMICS
Chapter 11
Kinetic theory of gas
11.1
kinetic representation of pressure
So far, we have developed theory of macroscopic systems without using the
detailed knowledge of classical mechanics. Then it is natural to ask how we can
derive the results of thermodynamics from classical mechanics.
Let us consider a box which is fulfilled by a huge number of particles. For
simplicity, we consider the ideal gas where we can ignore interactions. To simplify the description of the state of these particles, let us introduce a velocity
distribution function f (vx , vy , vz ) as
dN
= f (vx , vy , vz )dvx dvy dvz .
V
(11.1)
Here dN/V is the particle number per unit volume, whose velocity is given
by the value between (vx , vy , vz ) and (vx + dvx , vy + dvy , vz + dvz ) for each
component.
Then we consider the collision of particles with the wall of the box, in the x
direction. By this collision, the velocity is changed as
(vx , vy , vz ) −→ (−vx , vy , vz ).
(11.2)
That is, by this collision, the wall receives the momentum 2mvx .
Let us consider particles with the velocity around (vx , vy , vz ) and their collisions with the wall during a small time interval dt. Then the particles which can
collide with the wall should be included in the volume dV = Svx dt as is shown
in Fig. 11.1. Therefore, the number of the particles which can collide with
the wall is f (vx , vy , vz )dvx dvy dvz Svx dt. For each collision, the wall receives the
69
70
CHAPTER 11. KINETIC THEORY OF GAS
Figure 11.1:
momentum 2mvx . Thus the total momentum becomes
Z
dP
=
∞
Z
∞
dvx
Z
∞
dvy
dvz 2mvx f (vx , vy , vz )Svx dt
−∞
Z−∞
1
d3 vvx2 f (vx , vy , vz )
dt2mS
2
Z
dtmS
d3 vv2 f (vx , vy , vz ).
3
0
=
=
(11.3)
By definition, the force is the change of momentum per unit time and the
pressure is a force per unit area. In short, the pressure can be expressed as
m
P =
3
Z
d3 vv2 f (vx , vy , vz ).
(11.4)
11.2. SPECIFIC HEAT OF IDEAL GAS
On the other hand, the energy is expressed as
Z
m
E=V
d3 v v2 f (vx , vy , vz ),
2
71
(11.5)
where V is the volume. Therefore, the pressure can be re-expressed as
P =
11.2
2E
.
3V
(11.6)
specific heat of ideal gas
By using Eq. (11.6) and EoS of the ideal gas, the energy of the ideal gas per
unit particle is given by
3
E
= kB T.
(11.7)
ε=
N
2
This result means that each degree of freedom has an energy corresponding to
kB T /2 par unit particle. For example, the two dimensional ideal gas, ε = kB T
and the diatomic molecule is ε = 5kB T /2. In short, the energy of the ideal gas
per unit particle is summarized as
f
k
T
,
B
2
where f is the number of degrees of freedom.
For the ideal gas, the change of energy is expressed by using the specific heat
at constant volume. Using the above results, then it can be calculated as
d(εN )
CV =
= f2 kB N .
(11.8)
dT V
On the other hand, using Eq. (9.23), the specific heat at constant pressure is
f
f
CP = kB N + nR = kB N + kB N = f +2
k
N
.
(11.9)
B
2
2
2
11.3
Maxwell-Boltzmann distribution
The velocity distribution f is given by the so-called Maxwell-Boltzmann distribution function,
q
3
2
N
m
−β m
v
f (v) = V 2πkB T e 2
,
where
β=
1
.
kB T
(11.10)
To discuss the property of this distribution function, we consider the two
different temperatures, which are shown in Fig. 11.2. Then, from Eq. (11.3),
72
CHAPTER 11. KINETIC THEORY OF GAS
Figure 11.2:
one can see that T2 > T1 . Increasing temperature, the width of the distribution
function becomes larger.
We are sometimes interested only in the magnitude of the particle velocity.
Then the particle number which satisfies the velocity v ≤ |v| ≤ v + dv is given
by
g(v) = 4πv 2 f (v),
(11.11)
because
Z
d3 vf (v) =
Z
∞
dv4πv 2 f (v) ≡
0
Z
∞
dvg(v).
(11.12)
0
The form of g is expressed by Fig. 11.3. In this figure, the most of particles
has a velocity around CM . This is calculated as
r
∂g
2kB T
.
(11.13)
= 0 −→ CM =
∂v v=CM
m
On the other hand, the averaged velocity, which is denoted by CA , is given
by
3
kB N T
2
Z
=
−→
m 2
m
v f ≡ V ⟨v2 ⟩
2
2
r
p
3kB T
CA ≡ ⟨v2 ⟩ =
.
m
V
d3 v
(11.14)
11.4. MEAN FREE PATH
73
Figure 11.3:
Here we used
Z
2
dxe−ax =
r
π
.
a
(11.15)
That is, CA is larger than CM , independently of teh temperature.
11.4
Mean free path
So far we have considered ideal gas, but when is this approximation justified? To
see it, it is convenient to consider the concept called mean free path lmf p , which
gives the averaged distance between particles. For example, let us consider one
particle which collides with other particles for N times and the distances among
each collisions are given by li . Then
lmf p =
N
1 X
li .
N i=1
(11.16)
This is the exact definition but it is difficult to calculate following this definition.
Thus we adapt another definition, which is not exact but relatively easy to
estimate. Suppose there is a particle whose velocity is v and it collides with
other particles for z times per unit time. Then approximately the mean free
74
CHAPTER 11. KINETIC THEORY OF GAS
Figure 11.4:
path will be characterized by
v
.
(11.17)
z
Let us denote that the radius of a particle is given by d. After a small time
evolution dt, the volume which was occupied by the particle is given by
lmf p ≈
dV = πd2 vdt,
(11.18)
and the particle included in this volume is ρdV . During this time interval, the
number of collisions is approximately proportional to the number of particle
inside this volume,
Z ≈ ρdV,
(11.19)
where ρ is the particle number density. Substituting into Eq. (11.17), we have
1
lmf p ∝ ρπd
2 .
To justify the ideal gas picture, this mean free path should be sufficiently
large. That is, this picture is justified for very dilute gas, ρ << 1.
Chapter 12
Real gas and phase
transition
12.1
van der Waals equation of state
Here let us improve ideal EoS by including the effects which are not considered
in teh ideal gas. As we have emphasized so often, there are two effects which
are ignored.
The finite size effect can be included as follows. Let us consider a box whose
volume is given by V and there are many particles inside the box. If there is
no size, the volume in which the particles can move is V itself, but if there
is a finite size, this volume should decrease because a particle cannot occupy
the places which have already occupied by other particles. To represent this
effect, we introduce a positive parameter b and the volum in the EoS should be
replaced by V − b,
Videal = V − b.
(12.1)
As the interaction between molecules, let us consider an attractive interaction. Because of this effect, the momentum transfer through the collisions with
walls will be reduced. This reduction is proportional to ρ2 = N 2 /V 2 . In the
present case, we do not consider the change of the particle number. Thus the
important point is that this modification is proportional to 1/V 2 . Thus the
pressure is replaced by
a
P = Pideal − 2 .
(12.2)
V
Substituting these modifications into the ideal EoS, we obtain
a Pideal Videal = nRT −→ P + 2 (V − b) = nRT.
V
This is called van der Waals equation of state.
75
(12.3)
76
12.2
CHAPTER 12. REAL GAS AND PHASE TRANSITION
Phase transition
It is considered that this EoS is a good model to describe the phase transition.
For example, the water shows different behaviors depending on the thermodynamic variables. At a small temperature, it behaves as ice while it becomes gas
a a high temperature. The state characterized by thermodynamic variables is
called phase and ice and gas belong to different phases.
Figure 12.1:
The structure of the phase of the water is shown in Fig. 12.1. The change of
the phase is called phase transition. For example, the phase transition from ice
(solid phase) to liquid water (liquid phase) occurs at the P = 1 atom and T = 0
0
C. There is a point where the three phase transition lines meet. This point
is called triple point, where solid, liquid and gas coexist. On the other hand,
the transition line between liquid and gas vanishes at a point which is called
critical point. Beyond this point, there is no clear difference between liquid and
gas and this state of water is called supercritical fluid.
The behavior of the liquid-gas phase transition can be described by the van
der Waals equation of state qualitatively. In the left panel of Fig. 12.2, the EoS
is plotted for various different temperatures. where Tc is the temperature of
the critical point. At the region with higher temperature than Tc , the pressure
is monotonically decrease as the volume increased. This is the behavior of
the supercritical fluid. This behavior continues until the critical temperature
12.3. LATENT HEAT
77
Figure 12.2:
T = Tc . However, when the temperature becomes lower than Tc , there appears
the region where the pressure increases as the volume increases. Such a region
is thermodynamically unstable and is not reliable. That is, this is the limitation
of the applicability of the van der Waals EoS.
However, there is a method to improve the behavior. By using the method
called Maxwell construction, we can effectively replaced this unphysical region
by a flat line. As is shown in the right panel of Fig. 12.2, this flat line is plotted
so as to satisfy SA = SB . Then the left and right hand sides of the flat region
corresponds to liquid and gas phases, respectively. The flat line represents the
coexisting phase where gas and liquid exist concurrently.
12.3
Latent heat
In the discussion of heat, we have already considered the transfer of heat without
the phase transition. Now we generalize the argument to the case with the phase
transition.
Let us consider the melting of ice as is shown in Fig. 12.3. The water is
cooled down up to T2 and then the heat emitted during this process is
Q = M cmass
(T1 − T2 ).
L
(12.4)
On the other hand, the heat which is used to heat up the ice up to the phase
transition temperature is
q1 = mcmass
(TL − T0 ),
S
(12.5)
where TL is the transition temperature. The heat used for the phase transition
is called latent heat. In this case, we need additional heat to transform from
78
CHAPTER 12. REAL GAS AND PHASE TRANSITION
phase transition
solid −→ liquid
liquid −→ solid
liquid −→ gas
gas −→ liquid
latent heat
melting heat (absorption)
heat of liquefaction (emission)
vaporization heat (absorption)
heat of solidification (emission)
Table 12.1:
the solid phase to the liquid phase. By using the latent heat per unit mass Lm ,
it is expressed as
q2 = mLm .
(12.6)
Afterward, it is heated up to T2 ,
q3 = mcmass
(T2 − TL ).
L
(12.7)
All heat produced by the water Q is absorbed by the ice. Thus
M cmass
(T1 − T2 ) = q1 + q2 + q3 = mcmass
(TL − T0 ) + mLm + mcmass
(T2 − TL ).
L
S
L
(12.8)
Therefore the latent heat per unit mass is given by
Lm =
M mass
c
(T1 − T2 ) − cmass
(TL − T0 ) − cmass
(T2 − TL ).
S
L
m L
(12.9)
There are several types of the latent heat which are summarized in Table
12.1.
12.3. LATENT HEAT
79
Figure 12.3:
80
CHAPTER 12. REAL GAS AND PHASE TRANSITION
Part III
Oscillation, Wave and
Sound
81
Chapter 13
Mathematical preparation
13.1
Second-order homogeneous differential equation
Let us consider the following differential equation,
d2 y
dy
+
− 12y = 0.
2
dx
dx
(13.1)
To solve this differential equation, we assume that y is given by an exponential
function,
y = eDx ,
(13.2)
where D is a constant.
Substituting it, we find
D2 + D − 12 = 0 −→ D = −4, 3.
(13.3)
Then the solution of this differential equation is given by
y = C1 e−4x + C2 e3x ,
(13.4)
where C1 and C2 are parameters which are determined by initial conditions of
the differential equation.
As another example, we consider
d2 y
dy
+ 49y = 0.
− 14
2
dx
dx
(13.5)
Substituting the exponential function, we have
D2 − 14D + 49 = 0 −→ D = 7.
(13.6)
y = Ce7x .
(13.7)
Thus
83
84
CHAPTER 13. MATHEMATICAL PREPARATION
We can confirm that this is the solution of the above differential equation, but
still not most general one. In fact, the general solution of the second order
differential equation should contains two parameters, but the above solution
has only one parameter C. To obtain more precise solution, we assume that the
parameter C is a function of x and substitute Eq. (13.7) into the differential
equation. Then we find
d2 C
= 0 −→ C = C1 + C2 x.
dx2
(13.8)
Finally, the general solution is
y = (C1 + C2 x)e7x .
13.2
(13.9)
Second-order inhomogeneous differential equation
Let us consider the following differential equation,
dy
d2 y
+
− 12y = −e2x .
dx2
dx
(13.10)
The term on the right hand side does not depend on y. To solve this inhomogeneous equation, we first ignore this term,
d2 y0
dy0
+
− 12y0 = 0.
dx2
dx
(13.11)
The solution of this differential equation can be obtained by using the method
which we have used so far,
y0 = C1 e−4x + C2 e3x .
(13.12)
Moreover, to find a particular solution to Eq. (13.10), let us assume
y1 = Ae2x ,
(13.13)
and substitute it into Eq. (13.10). Then we have
A=
1
.
6
(13.14)
The general solution is given by the sum of y0 and y1 as
1
y = C1 e−4x + C2 e3x + e2x .
6
(13.15)
13.3. TRIANGLE FUNCTIONS
13.3
85
Triangle functions
There are several formulas for the sum and product of triangle functions. However, these can be derived from Euler’s formula,
eiθ = cos θ + i sin θ.
(13.16)
To derive the various formulas of the triangle functions, let us consider
ei(α+β) = eiα eiβ ,
(13.17)
and apply Euler’s formula to both sides,
cos(α + β) + i sin(α + β) = (cos α + i sin α)(cos β + i sin β).
(13.18)
Comparing both sides, we find the following formulas,
cos(α + β)
=
cos α cos β − sin α sin β,
(13.19)
sin(α + β)
=
cos α sin β + sin α cos β.
(13.20)
Other formulas can be obtained from these. For example, from Eq. (13.19), we
have
cos(α + β) + cos(α − β)
.
(13.21)
cos α cos β =
2
< Comment >
Euler’s formula can be derived as follows. Let us consider the following function
f (x) = cos x + i sin x.
(13.22)
This quantity satisfy the following differential equation,
df
= if.
dx
(13.23)
The solution of this equation is f = eiAx , where A is a constant. On the other
hand, there is a condition f (0) = 1. Therefore
cos x + i sin x = f (x) = eix .
(13.24)
86
CHAPTER 13. MATHEMATICAL PREPARATION
Chapter 14
Oscillation
The purpose of this part is to study the behaviors of wave. Wave means the
propagation of a spatial pattern such as density distribution, displacement and
so on. As the simplest case of such a complex wave behavior, let us first learn
oscillation.
14.1
Simple harmonic motion
Let us consider the so-called harmonic oscillator potential,
V (x) =
1 2
kx ,
2
(14.1)
where k is a parameter. Then, the corresponding Newton’s equation of motion
is
dV (x)
= −kx −→ ẍ = ω 2 x,
(14.2)
mẍ = −
dx
where
r
ω=
k
.
m
(14.3)
This ω is called angular velocity.
The solution of this differential equation is expressed by
x(t) = A sin(ωt + B),
(14.4)
where A and B are parameters. Because this is the solution of the second order
differential equation, it contains two parameters in the solution. The argument
of the triangle function is called phase and A is amplitude.
The parameters are fixed by initial conditions. Let us consider the following
conditions,
x(t = 0) = x0 ,
ẋ(t = 0) = 0.
(14.5)
87
88
CHAPTER 14. OSCILLATION
On the other hand, from the solution (14.4),
x(t = 0) = A sin(B),
ẋ(t = 0) = Aω cos B.
(14.6)
Form the condition of ẋ, there are two possibilities; one is
A = 0,
and the other is
cos B = 0 −→ B =
(14.7)
π
(2n + 1),
2
where n = 0, ±1, ±2, · · · .
If A = 0, then we cannot find the condition for x(t = 0). Thus B =
Substituting this into the condition of x(t = 0), we find
x0 = A sin
(14.8)
π
2 (2n+1).
π
(2n + 1) = A(−1)n .
2
(14.9)
π
(2n + 1),
2
(14.10)
Therefore
A = x0 (−1)n ,
B=
and hence
n
o
π
x(t) = x0 (−1)n sin ωt + (2n + 1) = x0 cos(ωt).
2
14.2
(14.11)
Period
An important quantity which characterizes the behavior of the simple harmonic
motion is the concept of period. The solution is oscillating around x = 0, thus
there is a quantity τ̃ which satisfies the following condition
x(t) = x(t + τ̃ ).
(14.12)
Substituting the solution, we find
cos(ωt) = cos(ω(t + τ̃ )).
(14.13)
Therefore
2π
n,
(14.14)
ω
where n = 0, ±1, ±2, · · · . The positive but finite minimum value of τ̃ is called
period. In the present example, it is given by
ωτ̃ = 2nπ −→ τ̃ (n) =
τ ≡ τ̃ (n = 1) =
2π
.
ω
(14.15)
See also Fig. 14.1.
From the period, the frequency of oscillation is defined by
ν≡
1
.
τ
(14.16)
14.3. ENERGY AND AVERAGED ENERGY
89
Figure 14.1:
14.3
Energy and averaged energy
In classical mechanics, the conserved energy is defined by the sum of the kinetic
energy T and the potential energy V . In the simple harmonic motion,
E
T
V
= T + V,
m 2
=
ẋ ,
2
k 2
=
x .
2
(14.17)
(14.18)
(14.19)
Substituting the solution (14.4), the energy is expressed as
E=
1 2
1
kA = mω 2 A2 .
2
2
(14.20)
This is time independent and conserved.
For the periodic motion such as an oscillation, we can discuss the averaged
energy for one period. For example, the averaged kinetic energy is
⟨T ⟩ ≡
1
τ
τ
Z
dt
0
m 2
mω 2 2
ẋ =
A .
2
4
(14.21)
Similarly,
⟨V ⟩ =
mω 2 2
A .
4
(14.22)
That is, the average kinetic and potential energies takes the same value,
⟨T ⟩ = ⟨V ⟩.
14.4
(14.23)
Pendulum
The concrete example of simple harmonic motion is the motion of pendulum.
The forces realized to the pendulum shown in Fig. 14.2 are the gravitational
force (mg) and the string tension (T ). Let us consider a coordinate located at
90
CHAPTER 14. OSCILLATION
Figure 14.2:
the pendulum which has two axes: one is parallel to the string and the other is
orthogonal as is shown in the figure. Then we have two equations,
T
mLθ̈
= mg cos θ + mLθ̇2 ,
(14.24)
= −mg sin θ.
(14.25)
The first equation determines the string tension and we do not discuss it.
To solve the second equation, let us assume θ << 1. Then we can use the
approximation,
sin θ ≈ θ.
(14.26)
Then the second equation can be expressed as the form of the simple harmonic
motion as
θ̈ = −ω 2 θ,
(14.27)
where
r
ω=
14.5
g
.
L
(14.28)
Spring
Another important example is the motion of spring. Let us consider a spring
which has the spring constant k and the length L. The force of the spring with
14.5. SPRING
91
Figure 14.3:
the spring constant k is expressed as
F = −k(x − L).
(14.29)
The definition of x follows the definition of the axis in Fig. 14.3. If x > L, the
direction is up and it becomes down when x < L.
When the mass m is suspended, the length of the spring is changes to L+dL,
where
mg
mg − k(L + dL − L) = 0 −→ dL =
.
(14.30)
k
The equation of motion of the spring is given by
mẍ = mg − k(x − L) = −k(x − L − dL).
(14.31)
Let us introduce a new variable,
y ≡ x − L − dL.
(14.32)
Then the equation of motion of spring becomes
ÿ = −ω 2 y,
where
r
ω=
k
.
m
(14.33)
(14.34)
92
CHAPTER 14. OSCILLATION
14.6
Superposition principle
Let us consider two solutions of the simple harmonic motion x1 and x2 . Then the
linear combination of these solutions is again a solution of the simple harmonic
motion. Such a solution is expressed as
x3 (t) = Ax1 (t) + Bx2 (t),
(14.35)
where A and B are arbitrary constants. This satisfies the differential equation
of teh simple harmonic motion
ẍ3 + ω 2 x3 = A(ẍ1 + ω 2 x1 ) + B(ẍ2 + ω 2 x2 ) = 0.
(14.36)
This is a common feature of the solution of the linear differential equation and
called superposition principle.
14.7
Damped oscillation
Let us remember the motion of pendulum. We argued that this is an example of
the simple harmonic motion, but not exactly. The real pendulum cannot keep
oscillating and finally stops because of the friction with air. This damping effect
can be introduced as
mẍ = −kx − ρẋ −→ ẍ + γ ẋ + ω02 x = 0,
(14.37)
where
ρ
γ= ,
m
r
ω0 =
k
.
m
(14.38)
To solve this equation, let us assume the following form as the solution,
x(t) = Aeλt .
(14.39)
Substituting into Eq. (14.37), we find
2
λ + γλ +
ω02
= 0 −→ λ =
−γ ±
p
γ 2 − 4ω02
.
2
(14.40)
The behavior of this solution is classified to three categories.
14.7.1
Supercritical γ 2 > 4ω02
In this case, the solution is given by
√ 2
√ 2
2
2
x(t) = e−γt/2 (Ae γ −4ω0 t/2 + Be− γ −4ω0 t/2 ),
(14.41)
where A and B are parameters. Then x goes to zero without showing oscillation.
14.8. FORCED OSCILLATION
14.7.2
93
Subcritical γ 2 < 4ω02
In this case, the solution is given by
x(t) = Ae−γt/2 cos(Ωt + B),
where
Ω=
q
(14.42)
ω02 − γ 2 /4.
(14.43)
Now x goes to zero with showing oscillation.
The period of the oscillation can be defined even in this case as
τ=
14.7.3
2π
.
Ω
(14.44)
Critical γ 2 = 4ω02
This case corresponds to the boundary of the supercritical and subcritical regions and the solution of λ is single. Then we cannot construct a solution with
two parameters and thus we need to modify our strategy.
Now we assume the following solution
x(t) = A(t)e−γt/2 .
(14.45)
Substituting into Eq. (14.37), we have
Ä(t) = 0 −→ A(t) = c1 t + c2 .
(14.46)
Therefore the solution is
x(t) = (c1 t + c2 )e−γt/2 .
(14.47)
We can see that this solution contains two parameters as we expected.
14.8
Forced oscillation
In the case of the simple harmonic motion, the one side of the spring is fixed.
Now let us consider that a hand which fix the spring oscillate with a constant
frequency as is shown in Fig. 14.4.
The differential equation is
mẍ = −kx + F sin(ωt) −→ ẍ + ω02 x =
F
sin(ωt).
m
(14.48)
The particular solution of this differential equation is found by assuming
xp (t) = A sin(ωt).
(14.49)
Then we find
F
.
m
To solve this equation, we need to consider the two different cases.
A(ω02 − ω 2 ) =
(14.50)
94
CHAPTER 14. OSCILLATION
Figure 14.4:
14.8.1
ω ̸= ω0
Then the particular solution is expressed as
xp (t) =
F
sin(ωt).
m(ω02 − ω 2 )
(14.51)
One can see that the amplitude of the particular solution becomes extremely
huge when ω is close to ω0 . This is called resonance.
This phenomenon is important to construct a building. In the case of the
earthquake, a building starts to oscillate with a certain frequency. If its frequency is close to that of the earthquake, the amplitude of the oscillation of the
building becomes very large and it will be destroyed.
14.8.2
ω = ω0
In this case, let us assume the following form as the particular solution,
xp (t) = A(t) sin(ωt + B).
Ten we find
δ=
π
,
2
Ä = 0,
Ȧ = −
F
.
2mω
(14.52)
(14.53)
14.9. FORCED OSCILLATION WITH DAMPING
Therefore the particular solution is
F
xp (t) = c −
t cos(ωt).
2mω
14.9
95
(14.54)
Forced oscillation with damping
Let us introduce a damping effect to the forced oscillator. Then the equation
which we should solve is given by
ẍ + γ ẋ + ω02 x =
F
sin ωt.
m
(14.55)
To find a particular solution, we assume
xp = A sin(ωt + δ),
(14.56)
where A is a constant. Substituting into the differential equation, we have
F
sin(ωt).
A (ω02 − ω 2 ) sin(ωt + δ) + γω cos(ωt + δ) =
m
(14.57)
Let us choose the parameter δ as
tan(−δ) =
γω
.
ω02 − ω 2
(14.58)
Then the particular solution is given by
1
F
F
1
p
p
sin(ωt + δ) =
sin(ωt + δ),
2
2
m (ω0 − ω) + (γω)
mω0 (1 − x)2 + Q2 x2
(14.59)
where x = ω/ω0 and Q = γ/ω0 .
If there is no damping term, Q = 0, we observe the divergence of the amplitude as we have observed in the previous section. However, for a finite Q, even
if the amplitude is emphasized for a certain value of x, it never diverge. That
is, the damping term softens the singularity of the beat. The position of the
maximum is located at
1
x=
.
(14.60)
1 + Q2
xp =
96
CHAPTER 14. OSCILLATION
Chapter 15
Wave
15.1
Fundamental properties of wave
Figure 15.1:
When we drop a stone to a pond as is shown in Fig. 15.1, we observe a certain
pattern of the water surface is formed. The propagation of this pattern is called
wave and water is called medium. In this case, the wave means the propagation
of the position of the water surface. That is, the direction of the oscillation and
that of the wave is orthogonal. This kind of wave is called transverse wave. If
both are parallel, it is called longitudinal wave.
The dynamics of the wave obeys very simple equation similar to the simple
harmonic motion. Let us consider a transverse wave whose form is initially given
by y(x, t = 0) = f (x) as is shown in Fig. 15.1. When this wave has the velocity
97
98
CHAPTER 15. WAVE
v, the form of the wave at the time t is given by
y(x, t) = f (x − vt).
(15.1)
Then we can see that this is the solution of the following differential equation
(∂t2 − v 2 ∂x2 )y(x, t) = 0.
(15.2)
This is known as wave equation.
Figure 15.2:
The solution of the differential equation is expressed as
y(x, t) = A sin(kx − ωt + δ),
(15.3)
where k is the wave number and ω is the angular frequency defined by
ω = kv.
A and δ are parameters.
(15.4)
15.2. WAVE LENGTH AND PERIOD
15.2
99
Wave length and period
The definition of period of wave is the same as the oscillation. By definition,
the period should satisfy
|θ(x, t) − θ(x, t + τ )| = 2π −→ τ =
1
2π
= .
ω
ν
(15.5)
where ν is the frequency and the phase is denoted by
θ(x, t) = kx − ωt + δ
(15.6)
In this definition, the value of x is fixed.
Then we can apply the same argument for x by fixing the value of t. That
is, we define the wave length λ by
|θ(x, t) − θ(x + λ, t)| = 2π −→ λ =
2π
.
k
(15.7)
It is also noted that there is the following relation,
λ = vτ,
(15.8)
That is, the wave length can be interpreted that the distance of the wave which
moves during one period.
15.3
velocity
From Eq. (15.4), we can see that the wave velocity is given with the angular
frequency and the wave number by
v=
ω
.
k
(15.9)
This definition is called phase velocity.
On the other hand, when ω has a k dependence, we can define another
definition
∂ω
vG =
.
(15.10)
∂k
This is called group velocity. This velocity plays a important role in the discussion of the beat.
15.4
String
The motion of string is an typical example of wave. Let us consider the motion
of the string shown in Fig. 15.4. Suppose that the line density of the string is
σ and the string tension is T . Then the equation of motion of the small part of
the string at x is given by
σdxÿ = T sin(θ + dθ) − T sin(θ) = T cos θdθ.
(15.11)
100
CHAPTER 15. WAVE
Figure 15.3:
Let us consider the case where θ << 1. Moreover,
θ ≈ tan θ ≈
∂y
.
∂x
(15.12)
Therefore the equation of motion becomes
σ ÿ = T ∂x2 y −→ (∂t2 − v 2 ∂x2 )y = 0,
where
(15.13)
r
T
.
σ
This is nothing but the equation of wave. The solution is
v=
(15.14)
y(x, t) = A sin(kx − ωt + B),
(15.15)
p
where ω = kv = k T /σ. A and B are parameters.
Let us discuss the work done by the tension T . Because there is no displacement long the x direction, it is sufficient to consider the y-force,
Fy = −T
∂y
.
∂x
(15.16)
15.5. SUPERPOSITION
101
The work per uinit time is then
P (x, t) = Fy vy = Fy
∂y
= T ωkA2 cos2 (kx − ωt + B).
∂t
(15.17)
This quantity is called potencia instantanea. The average for the period is
I = P̄ =
1
T
T ωkA2 = k 2 A2 v.
2
2
(15.18)
This quantity is called intensity.
The same result can be obtained as follows. Let us define the kinetic and
potential energy densities of the string by
2
∂y
1
σ
,
(15.19)
K(x, t) =
2
∂t
2
∂y
1
T
,
(15.20)
V (x, t) =
2
∂x
respectively. Substituting the solution, we have
K(x, t)
=
V (x, t)
=
1 2 2
σA ω cos2 (kx − ωt + B),
2
1
T A2 k 2 cos2 (kx − ωt + B).
2
(15.21)
(15.22)
Then, the averaged energy per unit period is a conserved quantity,
Z
1 τ
Ū ≡
dt(K(x, t) + V (x, t))
τ 0
T 2 2
=
k A .
(15.23)
2
When the wave moves, we consider that the energy of the wave also transfers.This energy tansfer is characterized by the intensity which is defined by
I ≡ Ū v =
15.5
T 2 2
k A v.
2
(15.24)
Superposition
As was discussed in oscillation, the superposition principle is satisfied even for
the wave. Here we discuss two typical examples.
15.5.1
stationary wave
Let us consider the following two waves,
y1
= A cos(kx − ωt),
(15.25)
y2
= A cos(kx + ωt).
(15.26)
102
CHAPTER 15. WAVE
Note that the direction of the propagation is opposite although the frequency
and wave length is same.
The sum of these waves is expressed as
y1 + y2 = 2A cos(kx) cos(ωt).
(15.27)
One can see that the x and t dependences are factored out. There is a point
where the wave does not oscillate. This is defined by
cos(kx) = 0 −→ x =
π
(2n + 1).
2k
(15.28)
This point is called node, as is shown in Fig. 15.5.1. On the other hand, the
point which has the maximum amplitude is called antinode and defined by
cos(kx) = ±1 −→ x =
π
n.
k
This wave does not move and called stationary (standing) wave.
Figure 15.4:
(15.29)
15.6. NORMAL MODE
15.5.2
103
Beat
Let us consider the following two waves,
y1
= A cos(k1 x − ω1 t),
(15.30)
y2
= A cos(k2 x + ω2 t),
(15.31)
ω1
= ω̄ + ∆ω,
(15.32)
ω2
= ω̄ − ∆ω,
(15.33)
k1
= k̄ + ∆k,
(15.34)
k2
= k̄ − ∆k.
(15.35)
where
Let us assume ω̄ > ∆ω and k̄ > ∆k.
Then the sum of these waves is
y1 + y2 = 2A cos(∆kx − ∆ωt) cos(k̄x − ω̄t).
(15.36)
It seems that the solution is given by the product of two different waves. The
behavior is shown in Fig. 15.5.2. We observe that there coexists two different
patterns: one is much faster oscillation than the other. That is, the superposition of the two waves which have slightly different k and ω leads to very slow
wave. This phenomenon is called beat.
The velocity of the beat is given by the group velocity,
v=
ω1 − ω2
∂ω
∆ω
=
= vG .
=
∆k
k1 − k2
∂k
(15.37)
Here we used that ∆k ∼ 0. On the other hand, the beat frequency N is
calculated as
1
|ω1 − ω2 |
N= =
.
(15.38)
τ
2π
15.6
Normal mode
The stationary solutions of the string oscillation is called normal modes.
Let us consider the following boundary conditions
y(0, t) = y(l, t) = 0.
(15.39)
We are interested in stationary solutions and then we assume the following
form as the solution,
y(x, t) = f (x)g(t).
(15.40)
Then the differential equation is separated into the two forms,
(∂t2 + v 2 k 2 )g(t) =
=
0,
(15.41)
(∂x2 + k 2 )f (x)
=
0.
(15.42)
104
CHAPTER 15. WAVE
Therefore the solution is expressed as
y(x, t) = (A cos(kx) + B sin(kx)) sin(kvt + δ).
(15.43)
To satisfy the boundary conditions,
A = 0,
A + B sin(kl) = 0.
(15.44)
In short, the solution is given by
y(x, t) = B sin(kx) sin(kvt + δ),
(15.45)
where
π
n (n = 0, 1, 2, · · · ).
(15.46)
l
Then the stationary solution is characterized by the integer n as is shown in
Fig. 15.6.
k=
15.7
Reflection
15.7.1
Fixed (Dirichlet) boundary
Let us produce a stationary wave to the direction of a wall as is shown in Fig.
15.7.1
As an incident wave, we assume
fI (x, t) = g(x − vt).
(15.47)
On the other hand, the reflection wave is assumed to be
fR (x, t) = h(x + vt).
(15.48)
Note that the direction of the velocity is opposite to the incident wave. The
solution of the wave including the effect of the reflaction is given by the sum of
these solutions,
f (x, t) = fI (x, t) + fR (x, t) = g(x − vt) + h(x + vt).
(15.49)
By the reflection, we consider the boundary condition where there is no
oscillation ta the wall,
f (0, t) = 0 −→
g(−vt) = −h(vt).
(15.50)
To satisfy this,
h(q) = −g(−q).
(15.51)
f (x, t) = g(x − vt) − g(−vt − x).
(15.52)
Therefore
15.7. REFLECTION
105
The another explanation is as follows.
As the incident wave, we consider
fI = A sin(kx − ωt).
(15.53)
On the other hand, the reflection wave is assumed to be
fR = B sin(k ′ x − ω ′ t + δ).
(15.54)
By the reflection,
the angular frequency is not changed .
Thus
ω ′ = ω.
(15.55)
On the other hand, the sign of the velocity of the reflection wave is opposite to
that of the incident wave,
ω′
ω
= − −→ k ′ = −k.
′
k
k
(15.56)
The wave realized on the left hand side of the wall is given by the linear
combination of the incident and reflection waves as
f (x, t) = A sin(kx − ωt) + B sin(−kx − ωt + δ).
(15.57)
At the wall, there is no oscillation,
f (0, t) = 0 −→
−→
A sin(−ωt) + B sin(−ωt + δ) = 0
(A + B cos δ) sin(ωt) + B sin δ cos(ωt) = 0. (15.58)
This boundary condition is called Dirichlet boundary condition.
To satisfy this condition for any time t, the following conditions should be
satisfied at the same time,
A + B cos δ
=
0,
(15.59)
B sin δ
=
0.
(15.60)
Then there are two possibilities; one is
A = B = 0,
(15.61)
and the other is
δ = πn,
(n = 0, ±1, ±2, · · · )
n
A + B(−1) = 0.
(15.62)
(15.63)
In short, the reflection wave is expressed as
fR = −A sin(−kx − ωt)
(15.64)
106
CHAPTER 15. WAVE
15.7.2
Free (Neumann) boundary
Let us consider another important boundary condition, Neumann condition,
where the medium of the wave (string) can move freely at the boundary as is
shown in Fig. 15.7.2.
Then, instead of the condition (15.58), we apply
∂f
∂x
= 0.
(15.65)
x=0
This means that the wave forms an antinode at the boundary.
By repeating the same argument, we find the following reflection wave
∂f
∂x
= 0 −→ g ′ (−vt) + h′ (vt) = 0,
(15.66)
d
g(q).
dq
(15.67)
x=0
where
g ′ (q) =
This condition leads to
h(q) = g(−q),
(15.68)
h′ (q) = −g ′ (−q).
(15.69)
f (x, t) = g(x − vt) + g(−x − vt).
(15.70)
because
Therefore
15.7. REFLECTION
107
Figure 15.5:
108
CHAPTER 15. WAVE
Figure 15.6:
15.7. REFLECTION
109
Figure 15.7:
110
CHAPTER 15. WAVE
Figure 15.8:
Chapter 16
Sound
We have discussed so far transverse wave. In this chapter, we study a typical
example of longitudinal wave, sound.
16.1
Dynamics of sound (I)
The sound is a wave where the fluctuation of the mass density propagates, as
is shown in Fig. 16.1. In this case, the direction of the wave propagation and
the oscillation of the mass density is parallel each other, thus the sound is a
longitudinal wave.
As we have studied, the dynamics of gas is described by hydrodynamics,
1
= − ∇P,
ρ
= −∂x (ρv).
(∂t + v∂x )v
∂t ρ
(16.1)
(16.2)
Let us consider the fluctuation of the mass density from its equilibrium value
ρ0 ,
ρ̄ ≡ ρ − ρ0 ,
(16.3)
which is much smaller than ρ0 . We further assume that the fluid velocity v is
small. Then the above hydrodynamic equations are reduced to
∂t v
1
∂x P,
ρ0
−ρ0 ∂x v.
≈
−
∂t ρ̄ ≈
(16.4)
(16.5)
Eliminating v from the above, we have
∂t ρ̄ = ∂x2 P.
(16.6)
The pressure is a function of three thermodynamic variables. Let us choose
(N, V, s). Because we do not consider the change of the particle number, we can
111
112
CHAPTER 16. SOUND
Figure 16.1:
ignore the N dependence. We can further replace the V dependence by the ρ
dependence,
P (ρ, s).
(16.7)
Then the derivative can be calculated as
∂P
∂P
∂x P =
∇ρ +
∇s
∂ρ
∂s
For the adiabatic process, ∇s = 0. Thus
∂P
∂x P ≈
∇ρ.
∂ρ ρ=ρ0
(16.8)
(16.9)
Therefore we obtain the following wave equation,
(∂t2 − v 2 ∂x2 )ρ̄ = 0,
(16.10)
where
s
v=
∂P
∂ρ
.
ρ=ρ0
(16.11)
16.2. DYNAMICS OF SOUND (II)
113
Figure 16.2:
For the ideal gas, this sound velocity can be calculated as follows. For the
adiabatic process, we have
P V γ = const. −→ P ∝ ργ0 .
Thus
∂P
∂ρ
=γ
ρ=ρ0
That is
P
kB T
=γ
.
ρ0
M
(16.13)
r
kB T
,
M
where M is the mass of the particles of the gas and γ = cP /cV .
v=
16.2
(16.12)
γ
(16.14)
Dynamics of sound (II)
The dynamics can be derived in a more phenomenological fashion. Let us consider the tube of a gas whose cross-section is S and we discuss the small portion
between x and x + dx. Because of the sound, the gas of the tube oscillates as a
function of x and t. Let us express this displacement from the equilibrium position by ξ. Then the left and right sides of the portion are changed to x + ξ(x)
114
CHAPTER 16. SOUND
and x + dx + ξ(x + dx), respectively. The force realized to this part is just
pressure and the equation of motion is expressed as
ρSdxξ¨ = S{P (x+ξ(x))−P (x+dx+ξ(x+dx))} ≈ S(P (x)−P (x+dx)). (16.15)
Here we assume that the variation of the pressure is small and we can approximately set p(x + ξ) ≈ P (x). We further consider the deviation of the pressure
from the equilibrium value as
P (x) = P0 + ∆P (x).
(16.16)
Then the equation of motion becomes
1
(∆P (x) − ∆P (x + dx)).
ρ0 ξ¨ =
dx
(16.17)
In the present case, it is natural to consider that the pressure variation
is induced by that of the volume. Thus, for ∆P , let us apply the following
assumption,
Vf − Vi
∆P (x) = −B
.
(16.18)
V
Here B is called bulk modulus of elasticity. Note that
Vf − Vi = S(x + dx + ξ(x + dx) − x − ξ(x)) − S(x + dx − x) = Sdξ. (16.19)
Therefore
∆P (x) = −B
dξ
.
dx
(16.20)
In short, the equation of motion is
ρ0 ξ¨ = B∂x2 ξ.
16.3
(16.21)
Relation between (I) and (II)
In the derivation of (I), the sound is expressed as the equation for the mass
density, while it is given by the evolution of displacement in (2). These are,
however, equivalent.
From Eq. (16.19),
Vf = S(dx + ξ(x + dx) − ξ(x)) = Vi
dξ
1+
dx
.
(16.22)
That is, the variation of the displacement can be expressed as
dξ
Vf − Vi
=
.
dx
Vi
(16.23)
16.4. ENERGY
115
On the other hand, the mass density is proportional to the inverse of the
volume. Therefore,
ρf − ρi
ρi
Vi
−1
Vf
−1
Vf − Vi
=
1+
−1
Vi
dξ
Vf − Vi
=− .
≈ −
Vi
dx
=
(16.24)
Here we used (Vf − Vi )/Vi << 1. Therefore
ρ̄ = −ρ0
dξ
.
dx
(16.25)
That is, the displacement is essentially equivalent to the mass density in the
sound.
16.4
Energy
Figure 16.3:
116
CHAPTER 16. SOUND
The energy of the sound is defined by
E = K + V,
(16.26)
where
K
=
U
=
1 ˙2
ρ0 ξ ,
2
1
B(∂x ξ)2 .
2
(16.27)
(16.28)
Let us consider the wave given by ξ = A sin(kx − ωt + δ). Then the averaged
energy per unit period is
⟨E⟩ =
1
1
ρ0 ω 2 A2 = Bk 2 A2 .
2
2
This is a constant and this energy is a conserved quantity. Note that
s
B
ω
v=
= .
ρ0
k
(16.29)
(16.30)
The deviation of the pressure in this case can be calculated as
∆P = −B
∂ξ
= −Pmax cos(kx − ωt + δ),
∂x
(16.31)
where
Pmax = BKA.
(16.32)
By using this parameter, the intensity of the sound is expressed as
I
≡
=
Ēv
2
1 Pmax
1
ρ 0 ω 2 A2 v =
.
2
2 ρ0 v
(16.33)
To represent the sound intensity is sometimes represented by the level of the
sound intensity defined by
β ≡ 10 ln10
I
.
10−12 (w/m2 )
(16.34)
The unite is decibel (dB). The typical values is shown in Fig. 16.4.
16.5
Air column
Imagine that we play whistle. The sound of whistle changes by the hole which
we push. This can be explained by the formation of stationary wave in the air
column.
16.6. INTERFERENCE IN 2 DIMENSION
117
Figure 16.4:
Let us consider an air column where one of the edge is closed. As was
discussed, there is no oscillation at the fixed boundary while antinodes is formed
at the free boundary. Therefore, the stationary waves such as the left panel of
Fig. 16.5 are formed and the wave length is given by
λ=
4L
2n + 1
(n = 0, 1, 2, · · · ).
(16.35)
When there are a hole, an antinode is formed there and thus we can change the
n of the stationary wave by choosing holes which we push. This is the structure
of whistle.
Similarly, we can discuss an air column where both of edges are fee. Then
the both of the edges of the stationary waves are antinodes and the wave length
is given by
λ=
2L
n
(n = 1, 2, 3, · · · ),
as is shown in the right panel of Fig. 16.5.
(16.36)
118
CHAPTER 16. SOUND
Figure 16.5:
Figure 16.6:
16.6
Interference in 2 dimension
We consider the emissions of the same wave from two different points O1 and
O2 as is shown in Fig. 16.6. The wave is described by
f (r, t) = a cos(kr − ωt),
(16.37)
where r denotes the distance between the position of the wave source and the
observation point of the wave.
Then the wave observed at the point P is described by
fT (P ) = a cos(kr1 − ωt) + a cos(kr2 − ωt).
(16.38)
(Rtanθ − d/2)2 = r12 − R2 .
(16.39)
Note that
16.7. DOPPLER EFFECT
119
Because we keep the contribution of θ upto the first order, the above equation
leads to
r1 = R −
d
sin θ.
2
(16.40)
r2 = R +
d
sin θ.
2
(16.41)
Similarly,
Using these,
fT (P ) = 2a cos(kR − ωt) cos(
kd sin θ
).
2
(16.42)
Therefore the combined wave is enhanced at the position satisfying
kd sin θ
= nπ,
2
(16.43)
where n = 0, ±1, · · · . It is cancelled when
kd sin θ
n+1
=
π.
2
2
16.7
(16.44)
Doppler effect
The nature of wave is changed by the states of the source and observer, moving
or stopped. This effect is called Doppler effect.
16.7.1
Moving source
Let us consider that the source of the sound moves toward the observer with a
velocity us as is shown by Fig. 16.7. Suppose that the frequency and the wave
length of the wave produced by the source are given by ν and λ, respectively
when the source is stopped. That is, the velocity of the sound is
v = νλ.
(16.45)
On the other hand, when it moves, it is clear that the wave length which
is observed by the observer is changed as is shown by Fig. 16.7. Let us go to
a frame where the source is stopped. Then, the wave moves with the velocity
v − us . Here we assume |v| > |us |. On the other hand, for the source,
the frequency is not changed .
Therefore the wavelength becomes
λ′ =
v − us
.
ν
(16.46)
120
CHAPTER 16. SOUND
Figure 16.7:
This wavelength is observed by the observer.
Then what is the frequency observed by the observer? Because the velocity
of the wave for the observer is still v itself. Thus the observed frequency is given
by
v
v
ν′ = ′ =
ν.
(16.47)
λ
v − us
When the source approaches to us, the frequency of the sound becomes larger,
while it becomes smaller when the source leaves from us.
16.7.2
Moving observer
Now we consider the case where the observer moves toward the source with the
velocity uo as is shown in Fig. 16.8. In the previous case, what is not changed
is the frequency. However, now
the wave length is not changed ,
as is shown in the figure. For the observer, the velocity of the wave seems to be
v + uo . Here we assume |v| > |u0 |. Then the observed frequency becomes
ν′ =
v + uo
v + uo
=
ν.
λ
v
(16.48)
In a similar way to the previous case, the observed frequency of the sound
becomes larger when we approach the source approaches.
16.7.3
Moving source and observer
As the most general case, let us consider both of the source and the observer
move toward the directions of each other with the velocities us and uo , respec-
16.8. SHOCK WAVE
121
Figure 16.8:
tively, which is shown in Fig. ??. First let us go to the rest frame of the source.
The wavelength is then given by
λ′ =
v − us
.
ν
(16.49)
On the other hand, the velocity of the wave form the observer is v+uo . Therefore
the frequency observed by the observer is
ν′ =
16.8
v + uo
v + uo
=
ν.
′
λ
v − us
(16.50)
Shock wave
So far, we have assumed |v| > |us |. If this condition is violated, we observe a
phenomenon called shock wave.
When |v| = |us |, we observe the wavelength becomes zero as is shown in Fig.
16.10. When the velocity of the source becomes faster than the sound velocity,
we observe a cone structure, which is called Mach cone. The angle of the cone
α is calculated by
v
sin α =
.
(16.51)
us
122
CHAPTER 16. SOUND
Figure 16.9:
The Mach cone expands with the velocity of sound.
16.8. SHOCK WAVE
123
Figure 16.10: